1. Introduction
As the byproduct of human modernization activities, CO
2 is regarded as one of the significant causes of global warming and climate change. One of the main sources of carbon emissions comes from the use of coal as fuel or coke to reduce ores during extraction metallurgy. Taking iron and steel making for example, about 1.3~1.7 tons of CO
2 were exhausted per ton of steel produced in 2020, equating to about 8.0 percent of global carbon dioxide emissions. In the ferrous industry, most of the exhaust gases are reducing the atmosphere and contain the bulk of CO
2 that is emitted at temperatures higher than the ambient temperature. The typical blast furnace gas composition is N
2, CO, CO
2 and H
2 with the temperature of about 473~530 K, and the coke oven gas composition is N
2, CH
4, CO, CO
2 and H
2 with the temperature of about 1000 to 1300 K. CO
2 in the hot exhaust gas is expected to be collected and then reduced to CO that can facilitate the reduction reaction of CO
2 into synthetic fuels for a sustainable environment [
1,
2,
3,
4]. In the process of oxygen-converter steelmaking, CO
2 is a weaker oxidizing agent compared with O
2, and CO
2 can be reduced to CO by the C in the liquid hot metal [
5,
6]. Therefore, it is expected to develop an adsorbent material with the highly selective, controllable, and reversible of CO
2 capture that is high-temperature-resistant in the adsorption/desorption processes to fulfill the requirement of being in the metallurgy area.
Many 2D materials, due to their high surface area for CO
2 capture, have been synthesized or theoretically predicted [
7], such as BN
2 [
8], CN [
9], C
2N [
10], C
3N [
11,
12], PC
n [
13], and Me–N–C (Me = Fe, Cu, Co, etc.) nanosheets [
14,
15], borophene nanosheet [
16,
17], covalent triazine frameworks [
18], nanoporous grapheme [
19], and penta-graphene [
20]. Adding/removing the electrons to/from the adsorbent materials allows alteration of the affinity between the adsorbent materials and gases that is in favor of the CO
2 capture from the gas mixture [
21]. Qin et al. [
10] showed that the interaction between CO
2 and the C
2N nanosheet is enhanced by the negative charges or external electric field, and CO
2 molecules are released from the C
2N nanosheet once the charge state/electric field is switched off. Li et al. [
11] found that CO
2 molecules can be adsorbed and desorbed from the C
3N nanosheet by adjustment of the applying charge density. Tao et al. [
22] found that the charged calcite is highly selective for separating CO
2 from the mixture of N
2, H
2 and CH
4, and the optimal charge density range for CO
2 capture and separation is 8.04~18.56 × 10
13 e
−/cm
2. Other previous studies [
8,
10,
11,
13,
14,
15,
17,
18,
21,
22,
23,
24,
25] also showed that the negative charges could be used as a switch in CO
2 capture and separation techniques by changing the affinity between CO
2 and the substrates, such as penta-BN
2, g-C
3N
4, nitrogen-doped porous carbons, etc.
Recently, works by Li and Shi et al. [
26,
27] showed that the silicon carbon monolayer possesses high thermal stabilities such that the Si
2C
3 and SiC
3 sheets can retain their planar geometries below 3500 K and manifest excellent properties of semiconductivity and elasticity for applications in electronics and optoelectronics. Chabi et al. [
28] found that 2D silicon carbide (Si
xC
y) is a universal material, and exhibits the same properties as graphene, silicon or silicon carbide, depending on the composition (i.e., Si
2C
3, SiC
3 and SiC
4). For extensive exploration of novel nanostructures as potential materials for CO
2 capture, a question raised in this study is whether SiC
3 nanosheets are also a promising material for CO
2 capture, and whether its capture/separation could be efficiently tuned by the charge/electric field.
Therefore, the adsorption behaviors of CO2, H2, N2 and CH4 on the SiC3 nanosheets with and without applying the injecting negative charges were studied by Density Functional Theory (DFT) calculations. Firstly, the stability of SiC3 nanosheet with different gas molecule (i.e., CO2, H2, N2 and CH4) adsorption was explored. Secondly, the effects of the injecting negative charges on the electronic structure, bonding features of adsorption systems and the mechanism of the CO2 adsorption/desorption on the SiC3 nanosheet were studied. Finally, the possibility of CO2 separation from the gas mixture with the assistance of negative charge, and the SiC3 nanosheet as a promising CO2 capture material were confirmed.
2. Calculation Method and Details
The calculations about the adsorption behavior of gas (i.e., CO
2, H
2, N
2 and CH
4) on the SiC
3 nanosheets were performed by using Density Functional Theory (DFT) methods, the implemented Dmol
3 module [
29] with Generalized Gradient Approximation (GGA) and the Perdew–Burke–Ernzerhof (PBE) functional exchange correction functional. The details about the PBE functional have been well documented in the previous paper [
30]. The effects of the core electrons were treated as a single effective potential by using the DFT semi-core pseudopotentials (USPP). Double numerical plus polarization (DNP) with a cutoff radius of 4.7 Å, and the DFT + D method with the weak van der Waals (vdW) correction were adopted in our calculations. Additionally, 4 × 4 × 1 k-points sampling of the SiC
3 nanosheet with 18 Si and 54 C atoms was adopted, and a 20 Å vacuum was set along the normal direction of the nanosheet for the elimination of the interactions between surface atoms. In geometric optimizations, the convergence tolerance was 1.0 × 10
−5 Ha for the total energy, 0.002 Ha/Å for the maximum force, and 0.005 Å for the maximal displacement.
The adsorption energy (
Eads) of gas
i on the SiC
3 nanosheet is described by the following equation [
31,
32]:
where
is the energy of the gas molecule
i adsorbed on the SiC
3 nanosheet,
is the energy of the pure SiC
3 nanosheet,
is the energy of the isolated gas molecule
i, and
i indicates the gas molecule (i.e., CO
2, H
2, N
2 and CH
4). A lower value of
corresponds to the stronger interaction between the SiC
3 nanosheet and gas molecule
i, and thus, represents a more stable molecules adsorption structure.
The influence of the injecting negative charges on the stability of the SiC
3 nanosheet was studied, where the negative charges of numbers 0 to 5 e
− were injected into the SiC
3 nanosheet, equivalent to the negative charge densities of 0~2.06 × 10
14 e
−/cm
2, which were applied on the SiC
3 nanosheet. For characterizing the adsorption of the gas molecule (i.e., CO
2, H
2, N
2 and CH
4) on the different, negatively charged SiC
3 nanosheet, the negative-charge density of the SiC
3 nanosheet (
ρ) was investigated, and the negative charge density was defined as follows [
10,
17].
where
Q is the total negative charges injected into (4 × 4 × 1) the supercell of the SiC
3 nanosheet, and
S is the corresponding surface area (2.43 × 10
−14 cm
2).
To study the structural stability of a SiC
3 nanosheet under different negative-charge conditions, the phonon dispersion spectra and the cohesive energy are calculated. Generally, a larger cohesive energy corresponds to a more stable molecule-adsorption structure; also, no imaginary frequency in the phonon dispersion spectra indicates the stability of a material. The cohesive energy is defined as follows:
where
,
and
are the energies of a single C atom, a single Si atom and the total energy of SiC
3 nanosheet, respectively.
and
are the number of Si and C atoms in the SiC
3 nanosheet. Next, the effect of the injection of negative charges on the adsorption behavior of gas (i.e., CO
2, H
2, N
2 and CH
4) on the SiC
3 nanosheets was studied.
3. Results and Discussion
3.1. Electronic Properties and Stability of SiC3 Nanosheet
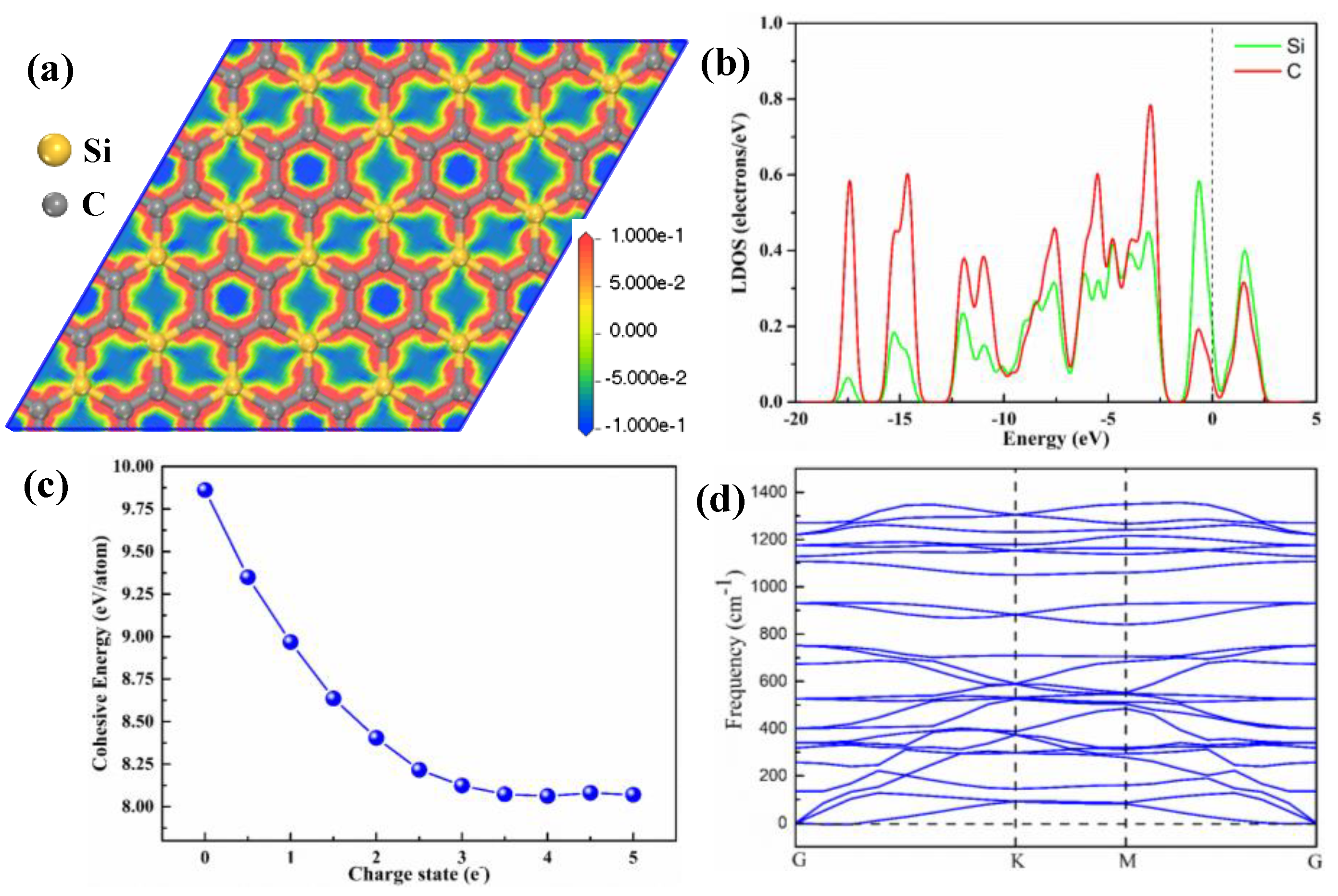
The SiC
3 nanosheet is a graphene-like structure conformation with a P6/mmm space group. The calculated lattice constants of the primitive SiC
3 nanosheet are a = b = 5.584 Å.
Figure 1a shows the deformation electron density of the 4 × 4 × 1 fully relaxed supercell structure of the SiC
3 nanosheet. One can see that electrons are accumulated around the six-membered ring of carbon due to the larger, stronger electronegativity of carbon than that of silicon. The SiC
3 nanosheet has two different bonds, which are Si–C and C–C. The bonds lengths of Si–C and C–C are 1.796 and 1.428 Å, respectively, and the angle of C–C–C, C–C–Si and C–Si–C are all 120°. Those calculated results are in good agreement with the reported value [
33]. For a pure SiC
3 nanosheet, the obtained Local Density of States (LDOS) of C and Si atoms are shown in
Figure 1b. LDOS results show that the SiC
3 nanosheet exhibits a metallic characteristic due to several bands across the Fermi level that might be primarily contributed by the Si-p orbital [
26]. The finding matches with the obtained results by Chabi et al. [
28] that show that the SiC
3 nanosheet is a potential material for use as a semiconductor.
Figure 1c shows the cohesive energy of the SiC
3 nanosheet under different charged conditions. The cohesive energy of a neutral SiC
3 nanosheet is 9.86 eV/atom, which is slightly larger than the reported value of neutral SiC
3 (7.84 eV/atom) [
26]. The cohesive energy of the SiC
3 nanosheet is 9.86 eV/atom without the applied negative charge, and it decreases with increasing the applied negative charge, finally achieving a stable value (8.37 eV/atom) when the applied negative charge exceeds 3 e
−1. It is indicated that the SiC
3 nanosheet is a strongly bonded network and is a stable structure under the negative charge conditions. There is no imaginary frequency in the phonon dispersion spectra (
Figure 1d). Therefore, all these results reflect the dynamic stability of the SiC
3 nanosheet under the condition of injecting negative charges. The SiC
3 nanosheet can withstand a rather high temperature under atmospheric pressure [
26]. Therefore, the SiC
3 nanosheet is a stable material for CO
2 separation application after the injection of negative charges.
3.2. CO2/H2/N2/CH4 Adsorption on Uncharged SiC3 Nanosheet
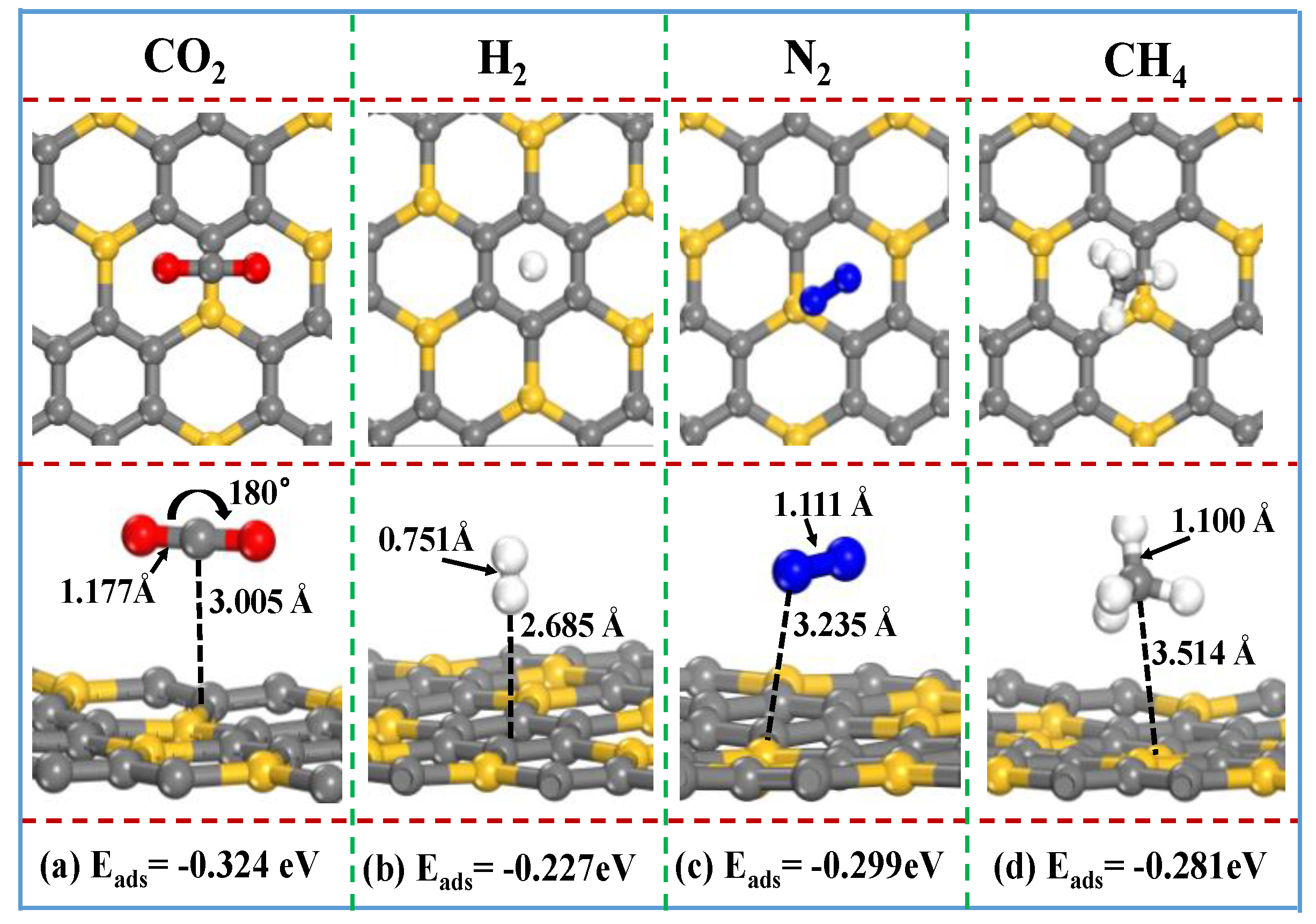
The gas adsorption behaviors of a neutral SiC
3 nanosheet is firstly investigated by analyzing the most stable relaxation configurations of the SiC
3 nanosheet with gas adsorption. Various initial adsorption sites are considered, including the top sites of C and Si atoms and bridge sites of C–C and C–Si bonds, as well as the center of hexagon holes. Stable relaxation configurations of the SiC
3 nanosheet with different gas molecule (i.e., CO
2, H
2, N
2 and CH
4) adsorptions are obtained and shown in
Figure 2. For the most stable relaxation configurations, the CO
2, H
2, N
2 and CH
4 molecule distances from the SiC
3 nanosheet are 3.005, 2.6850, 3.235 and 3. 145 Å, respectively.
For the CO2 adsorbed on the SiC3 nanosheet, the O–C–O angle and the C–O bond length exhibit a minor change in comparison to the isolated carbon dioxide molecule, indicating that the absorption of CO2 is classical physisorption. Similar results are observed for the H2, N2 and CH4 molecule adsorbed on the SiC3 nanosheet. Thus, it could be concluded that the gas molecules (i.e., CO2, H2, N2 and CH4) adsorbed on the SiC3 nanosheet surface are caused by physisorption.
The adsorption energies (Eads) of CO2, H2, N2 and CH4 adsorbed on the SiC3 nanosheet are −0.324, −0.227, −0.299 and −0.281 eV, respectively. Among those four gases, the most stable relaxation configurations of gas molecules adsorbed on SiC3 nanosheet are CO2, followed by N2, CH4 and H2, which indicates that the SiC3 material is a potential material for use as a CO2 adsorbent.
3.3. Adsorption of CO2/H2/N2/CH4 by Charged SiC3 Nanosheet
With the injection of the negative charges with density of 1.23 × 10
14 e
−/cm
2, the stable relaxation configurations of the SiC
3 nanosheet with different gas molecule (i.e., CO
2, H
2, N
2 and CH
4) adsorption are obtained and shown in
Figure 3.
After the injection of negative charges into the SiC
3 nanosheet with CO
2 absorption, the CO
2 molecule strongly interacts with the SiC
3 nanosheet with the adsorption energy
Eads of −1.628 eV; the adsorption energy is significantly smaller than that of the uncharged SiC
3 nanosheet (−0.324 eV). Furthermore, the O–C–O angle changes from 180° to 131°, the C–O bond length increases from 1.117 Å to 1.259 Å, and the distance between CO
2 and the SiC
3 nanosheet decreases from 3.005 Å to 2.002 Å. Obvious electron density distribution overlap is observed between CO
2 and the SiC
3 nanosheet (
Figure 4e), indicating that the strong interaction between CO
2 and the SiC
3 nanosheet, and the absorption of the CO
2 molecule on the SiC
3 nanosheet is due to chemisorption.
The optimized adsorption configurations of H
2, N
2 and CH
4 after the injection of negative charges are shown in
Figure 3b–d. After the injection of negative charges into the SiC
3 nanosheet with H
2 absorption, for the H
2 molecule, the H–H bond length increases slightly from 0.751 to 0.769 Å, the distance between H
2 and the SiC
3 nanosheet increases slightly from 2.685 to 2.964 Å, and the adsorption energy
Eads decreases from −0.227 to -0.499 eV. For the N
2 molecule, the N–N bond length increases slightly from 1.111 to 1.123 Å, the distance between N
2 and the SiC
3 nanosheet decreases slightly from 3.235 to 3.151 Å, and the adsorption energy
Eads decreases from −0.299 to −0.578 eV. For the CH
4 molecule, the C–H bond length increases slightly from 1.100 to 1.102 Å, and the distance between CH
4 and the SiC
3 nanosheet decreases from 3.514 to 2.700 Å, and the adsorption energy
Eads decreases from −0.281 to −0.525 eV. Furthermore, no evident electron density distribution overlaps are observed between gas molecules (H
2, N
2 and CH
4) and the SiC
3 nanosheet after the injection of negative charges (
Figure 4e). Thus, it is suggested that the adsorptions of H
2, N
2 and CH
4 on the SiC
3 nanosheet are due to physisorption with/without the injecting negative charges, which differs from the chemisorption of CO
2 on the SiC
3 nanosheet with the injection of negative charges.
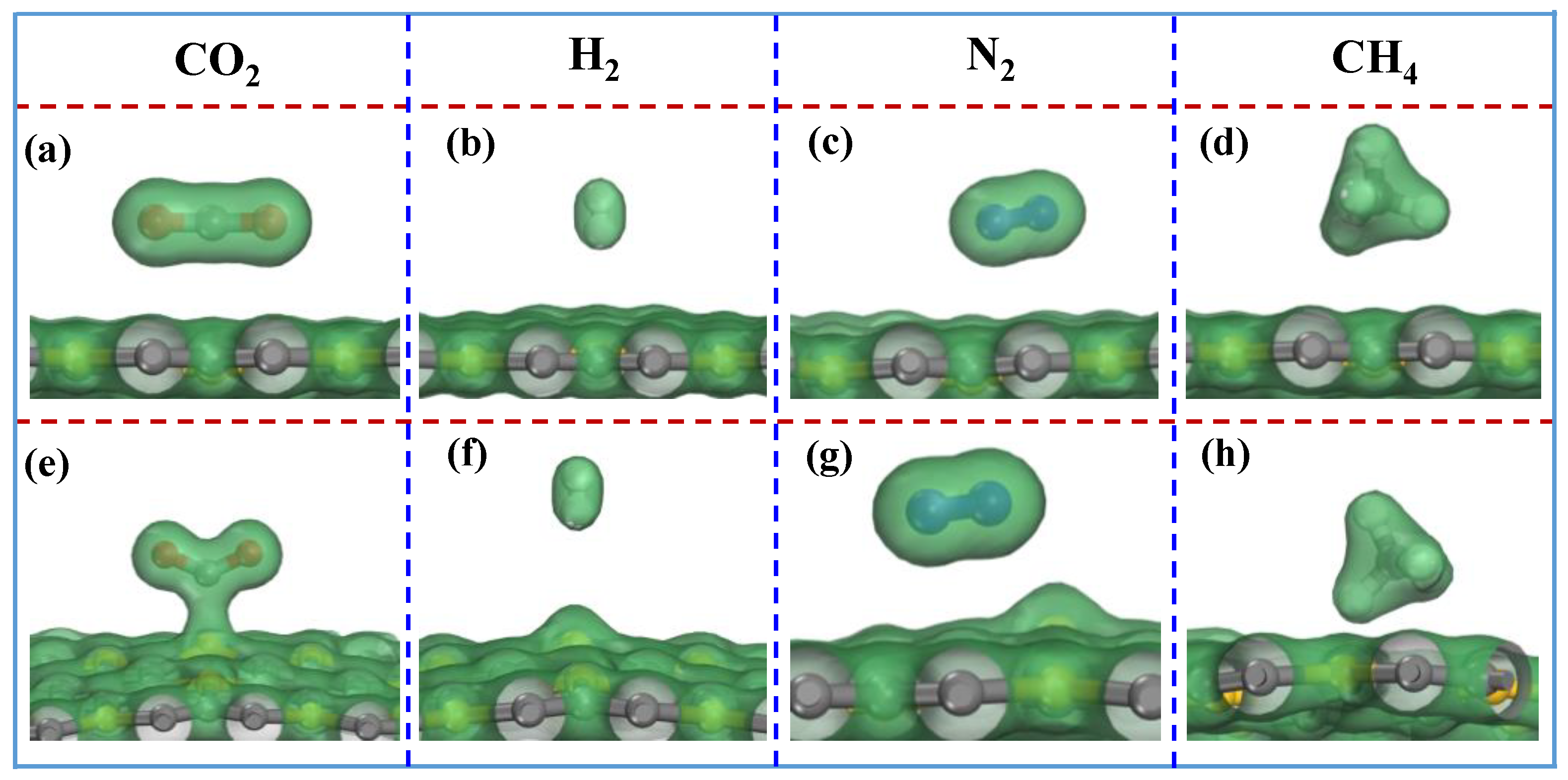
Figure 4 shows no obvious electron density distribution overlaps observed between CO
2/H
2/N
2/CH
4 molecules and the SiC
3 nanosheet without the injecting negative charges. It indicates that CO
2/H
2/N
2/CH
4 interact weakly with the SiC
3 nanosheet without the injecting negative charges. However, after the injection of the negative charge with density of 1.23 × 10
14 e
−/cm
2, obvious electron density distribution overlap between CO
2 and the SiC
3 nanosheet is observed. In other words, after the injection of the negative charges, the adsorptions of H
2, N
2 and CH
4 on the SiC
3 nanosheet are also due to physisorption with weak interactions between these molecules; when the adsorption mechanism of CO
2 on the SiC
3 nanosheet changes from physisorption to chemisorption, the interaction between CO
2 and the SiC
3 nanosheet gets stronger. These results suggest that CO
2 could be separated from the mixture of CO
2, H
2, N
2 and CH
4 that might be tuned by switching on/off the injection of negative charges.
3.4. Mechanism of CO2 Adsorption/Desorption
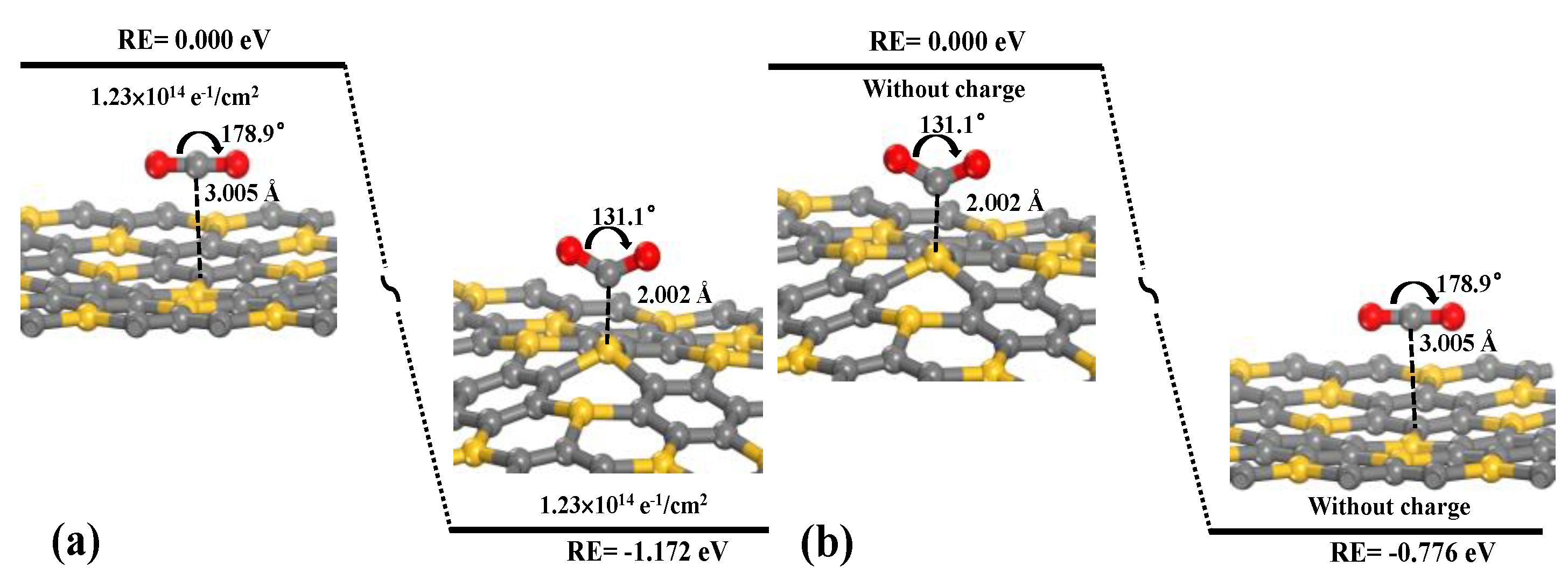
The mechanism of CO
2 adsorption/desorption on the SiC
3 nanosheet are investigated by switching on/off the injection of negative charges.
Figure 5 shows that the adsorption of CO
2 on the SiC
3 nanosheet is due to physisorption without the injection of negative charges. With the injection of negative charges with a density of 1.23 × 10
14 e
−/cm
2, for the CO
2 molecule, the O–C–O angle changes from 180° to 131°, the C–O bond length increases from 1.117 Å to 1.259 Å, and the distance between CO
2 and the SiC
3 nanosheet decreases from 3.005 Å to 2.002 Å. This result implies that the adsorption mechanism changes from physisorption to chemisorption; the analysis is consistent with the obtained results from
Figure 4, and the process is exothermic with a reaction energy of −1.172 eV. After switching off the injection of negative charges, the adsorption mechanism of CO
2 changes from chemisorption to physisorption. The adsorbed CO
2 molecule is rebound to its physisorption state, and the distance between CO
2 and the SiC
3 nanosheet increases from 2.002 to 3.005 Å. This transition is also exothermic, 0.776 eV, without any energy barrier. In conclusion, the processes of CO
2 adsorption/desorption on the SiC
3 nanosheet is reversible, and CO
2 adsorption and separation would be tuned by switching on/off the injection of negative charges.
3.5. Separation of CO2 from H2, N2 and CH4 on Negatively Charged SiC3
The effect of the injection of negative charges on the interactions between gases and the SiC
3 nanosheet is shown in
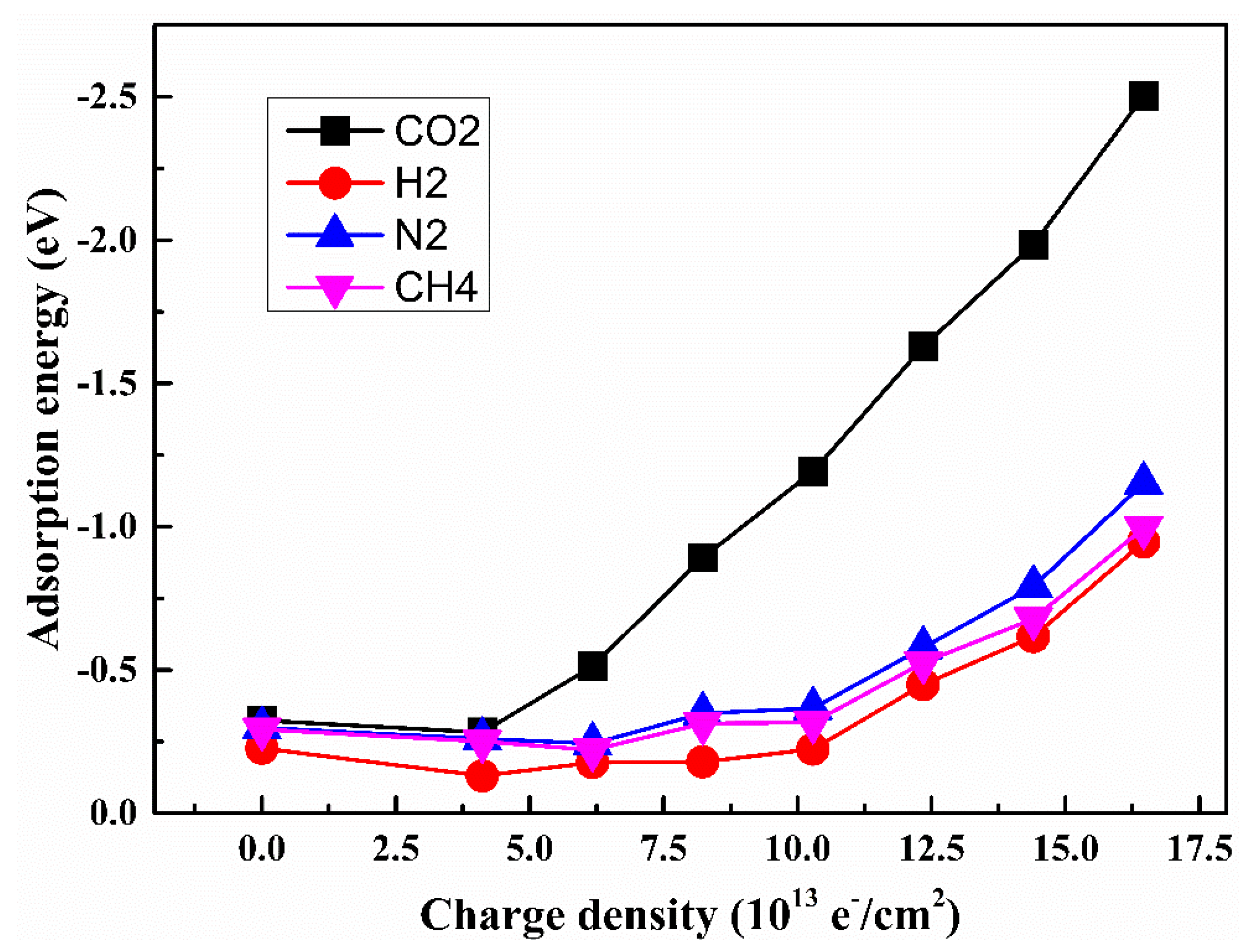
Figure 6. With the increase in negative charge density, the adsorption energy of CO
2 is smaller than those values of N
2, CH
4 and H
2. When the negative charge intensity exceeds 3.75 × 10
13 e
−/cm
2, the adsorption energy of CO
2 adsorption decreases with the negative charge intensity observably, while the decrement of the adsorption energies for H
2, N
2 and CH
4 are relatively small. It is suggested that the CO
2–SiC
3 adsorption structure is more stable than the adsorption structures of N
2–SiC
3, CH
4–SiC
3, and H
2–SiC
3 under different charged conditions.
To further explore the CO
2 separation ability of the SiC
3 nanosheet by switching on/off the injection of negative charges, the co-adsorption behaviors of CO
2, H
2, N
2 and CH
4 on the SiC
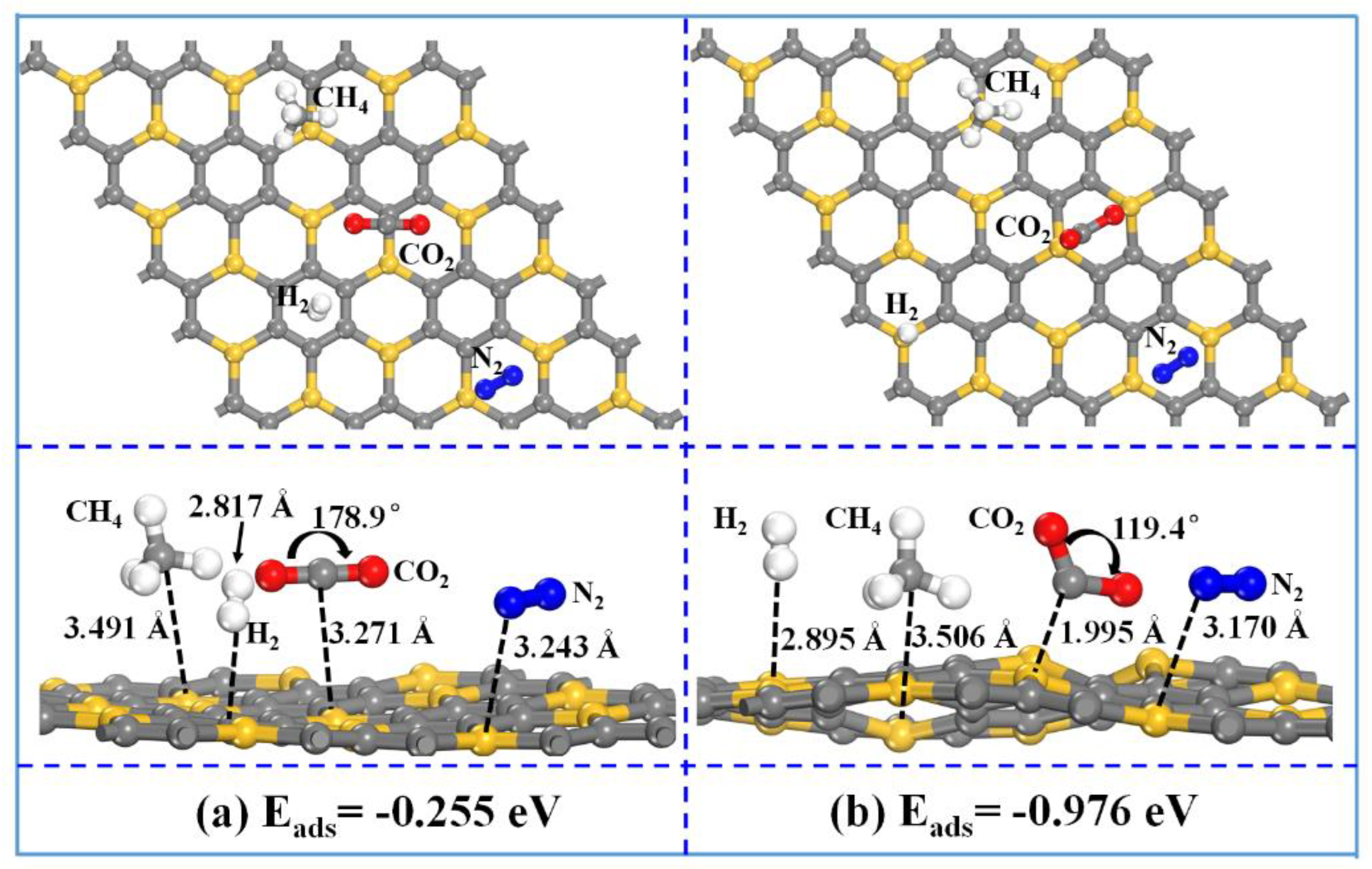
3 nanosheet are investigated. As
Figure 7 shows, after the injecting of the negative charges with a density of 1.23 × 10
14 e
−/cm
2 into the SiC
3 nanosheet with gases co-absorption, the co-adsorption energy decreases from −0.255 to −0.976 eV. It implies that with the injection of negative charges, the SiC
3 nanosheet with gases (i.e., CO
2, H
2, N
2 and CH
4) co-absorption gets more stable in comparison with the one without the injection of negative charges.
Switching on the injection of negative charges, the modules of H
2, N
2 and CH
4 move away from the SiC
3 nanosheet. The distance between the molecule and the SiC
3 nanosheet increases from 2.817 to 3.506 Å for H
2–SiC
3, from 3.243 to 3.170 Å for N
2–SiC
3, and from 3.491 to 3.506 Å for CH
4–SiC
3. No obvious electron density distribution overlaps are observed between H
2/N
2/CH
4 molecules and the SiC
3 nanosheet with/without the injection of negative charges (
Figure 8). It indicates that the effect of the injection of negative charges on the adsorptions of H
2/N
2/CH
4 is not obvious, and H
2/N
2/CH
4 are all physiosorbed on the SiC
3 nanosheet with/without the injection of negative charges.
With the injection of negative charges into the SiC
3 nanosheet with gases co-absorption, the distance between the CO
2 molecule and the SiC
3 nanosheet gets shorter, decreasing from 3.271 to 1.995 Å, and the O–C–O angle of CO
2 changes from 178.9° to 119.4°. No obvious electron density distribution overlap is observed between the CO
2 molecule and the SiC
3 nanosheet without the injection of negative charges, while an obvious electron density distribution overlap is observed with the injection of negative charges (
Figure 8), implying that the absorption mechanism of CO
2 changes from physisorption to chemisorption after the injection of negative charges, which is also consistent with the obtained results from
Figure 5.
In summary, H2/N2/CH4 all interact weakly with the SiC3 nanosheet with/without the injection of negative charges. While the interaction between CO2 and the SiC3 nanosheet can be strengthened by the injection of negative charges, the absorption mechanism of CO2 changes from physisorption to chemisorption after the injection of negative charges. Therefore, it could be concluded that the separation CO2 from the mixture of CO2, H2, N2 and CH4 can be achieved by switching on/off the injection of negative charges. All of the above results demonstrate that the SiC3 nanosheet is a promising material for the separation CO2 from the CO2/H2/N2/CH4 mixture by using the injection of negative charges.