The Preparation of High-Volume Fraction SiC/Al Composites with High Thermal Conductivity by Vacuum Pressure Infiltration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Characterization
3. Results and Discussion
3.1. Volume Fraction and Pore Characteristics of SiC Preforms
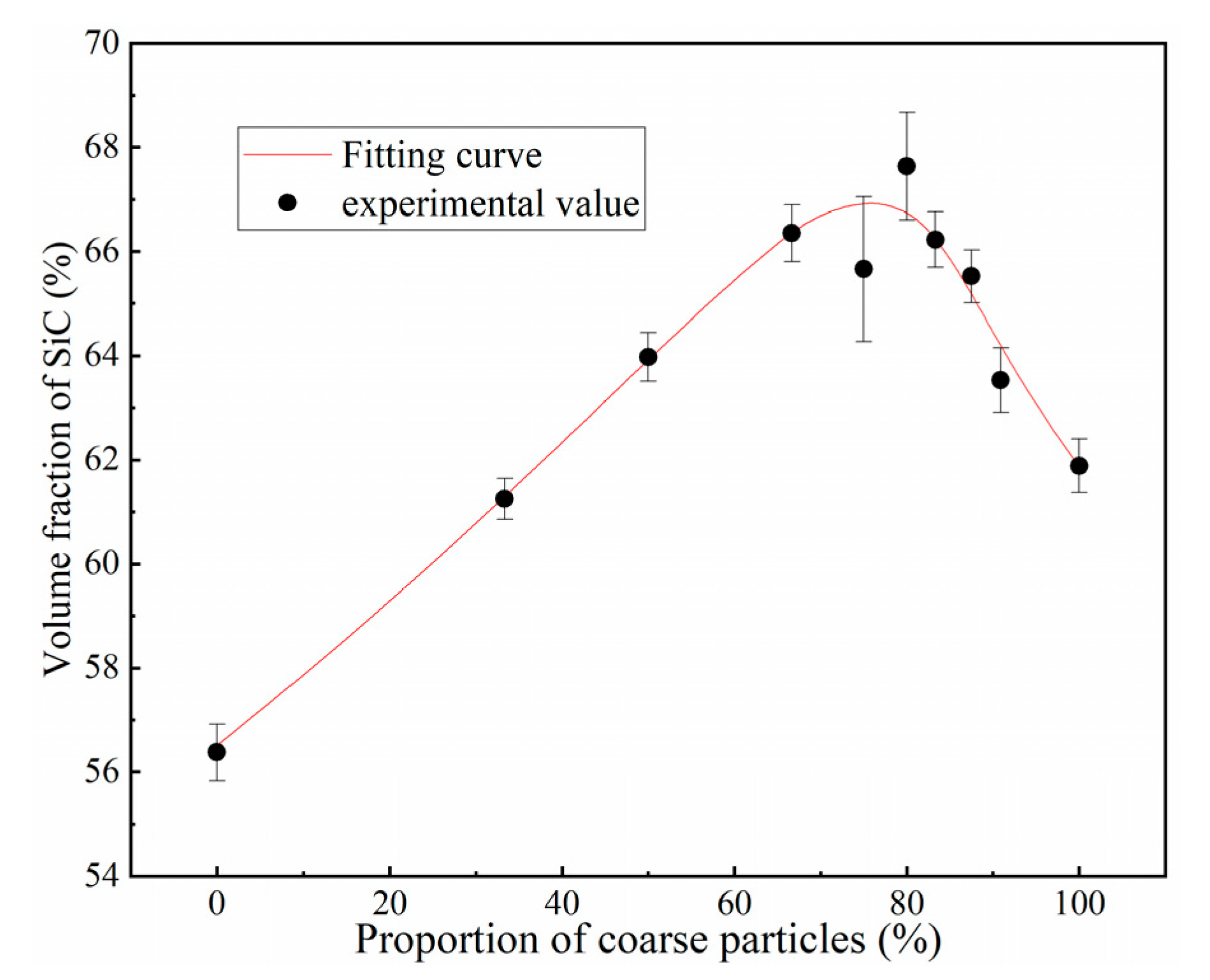
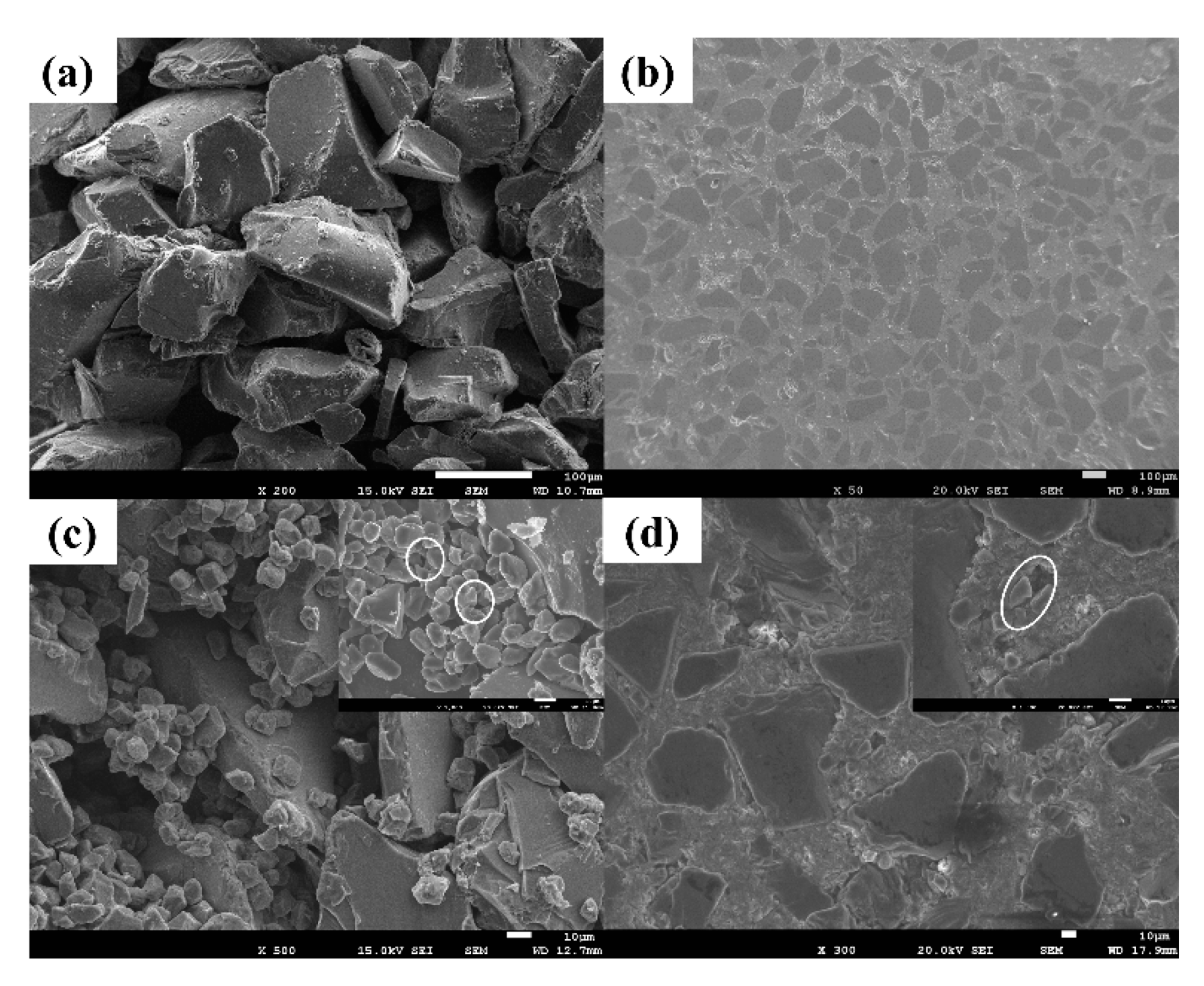
3.1.1. SiC Preform with a Bimodal Size Distribution (12 and 100 μm)
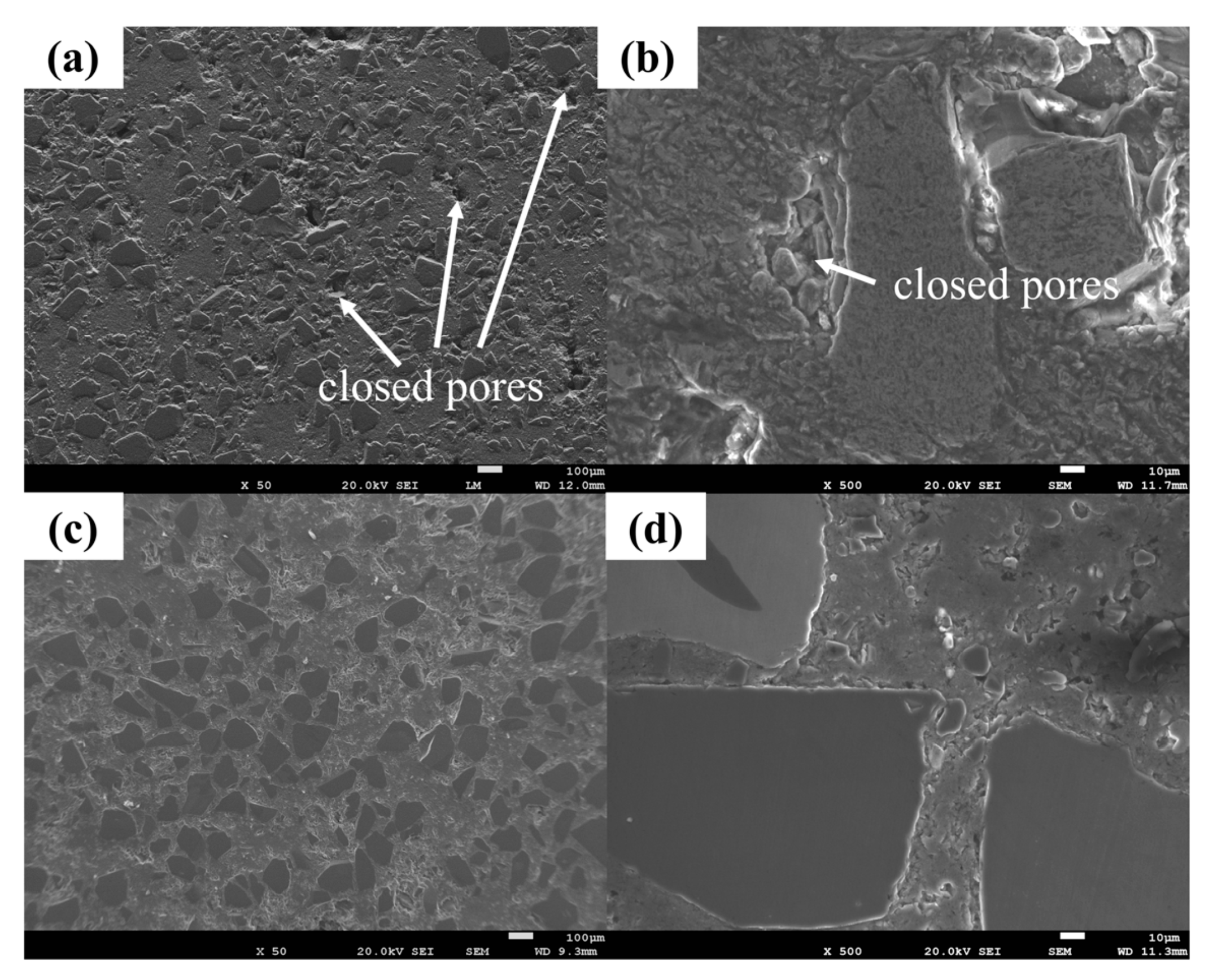
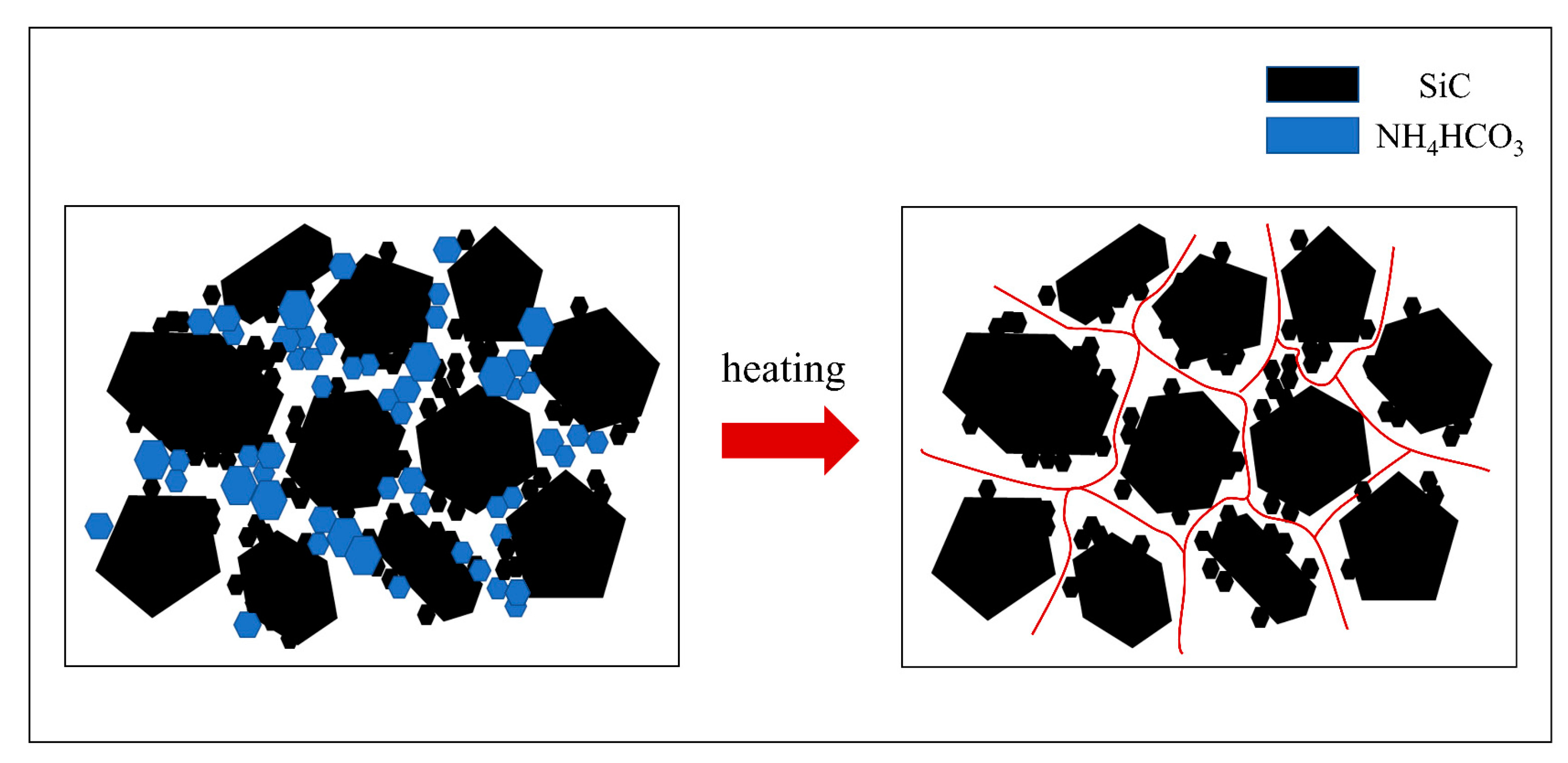
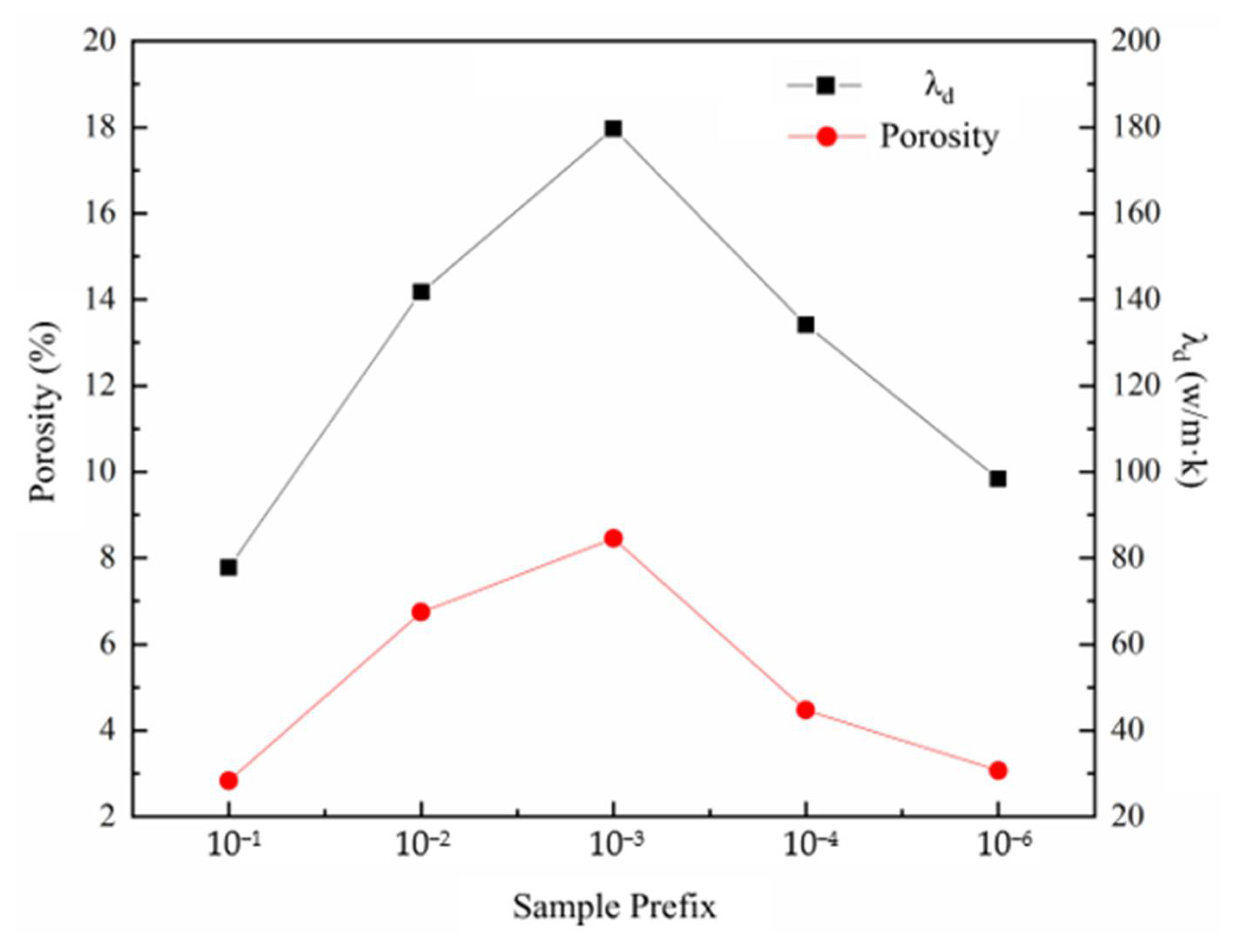
3.1.2. SiC Preform with Different Contents of Pore Forming Agent (NH4HCO3)
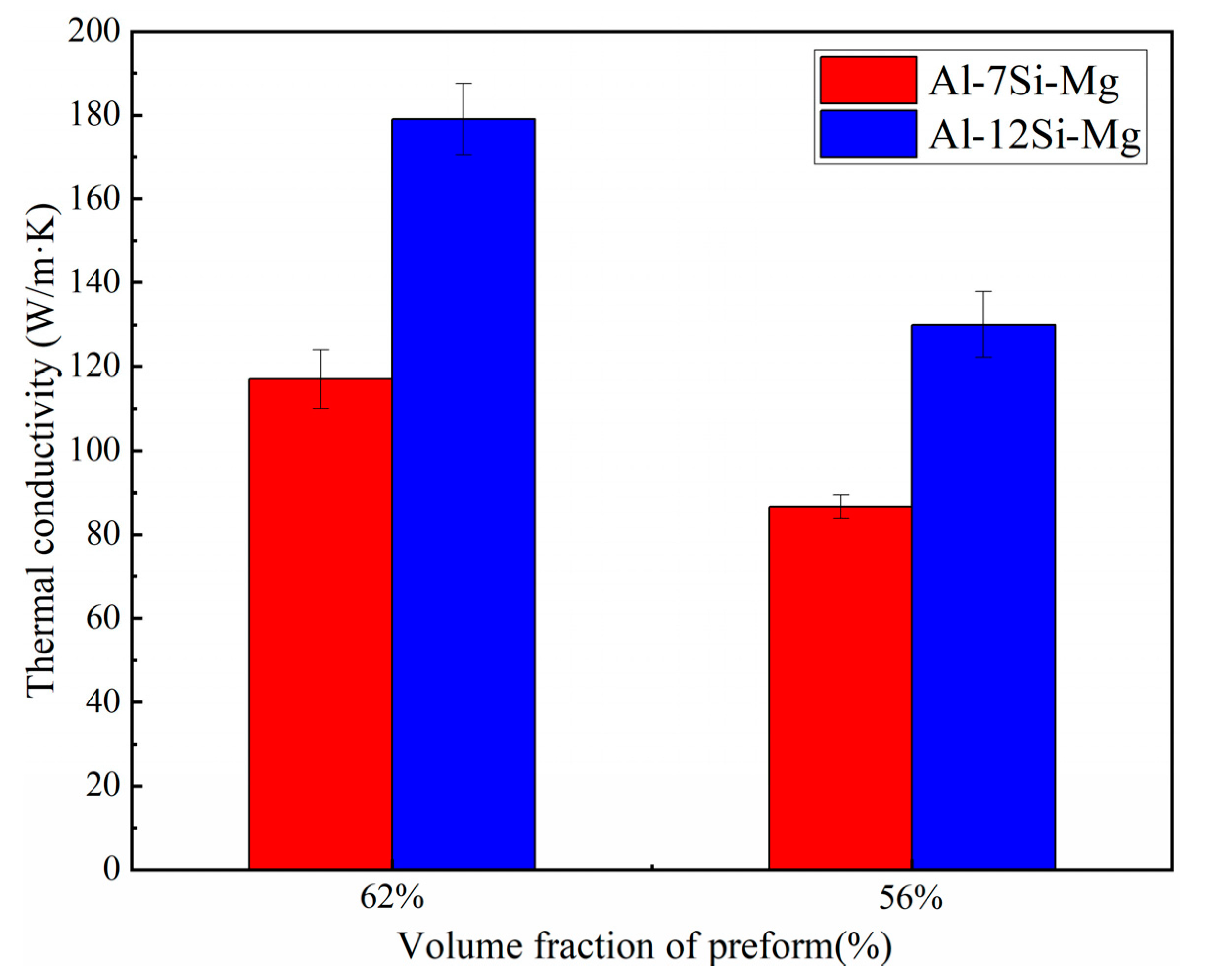
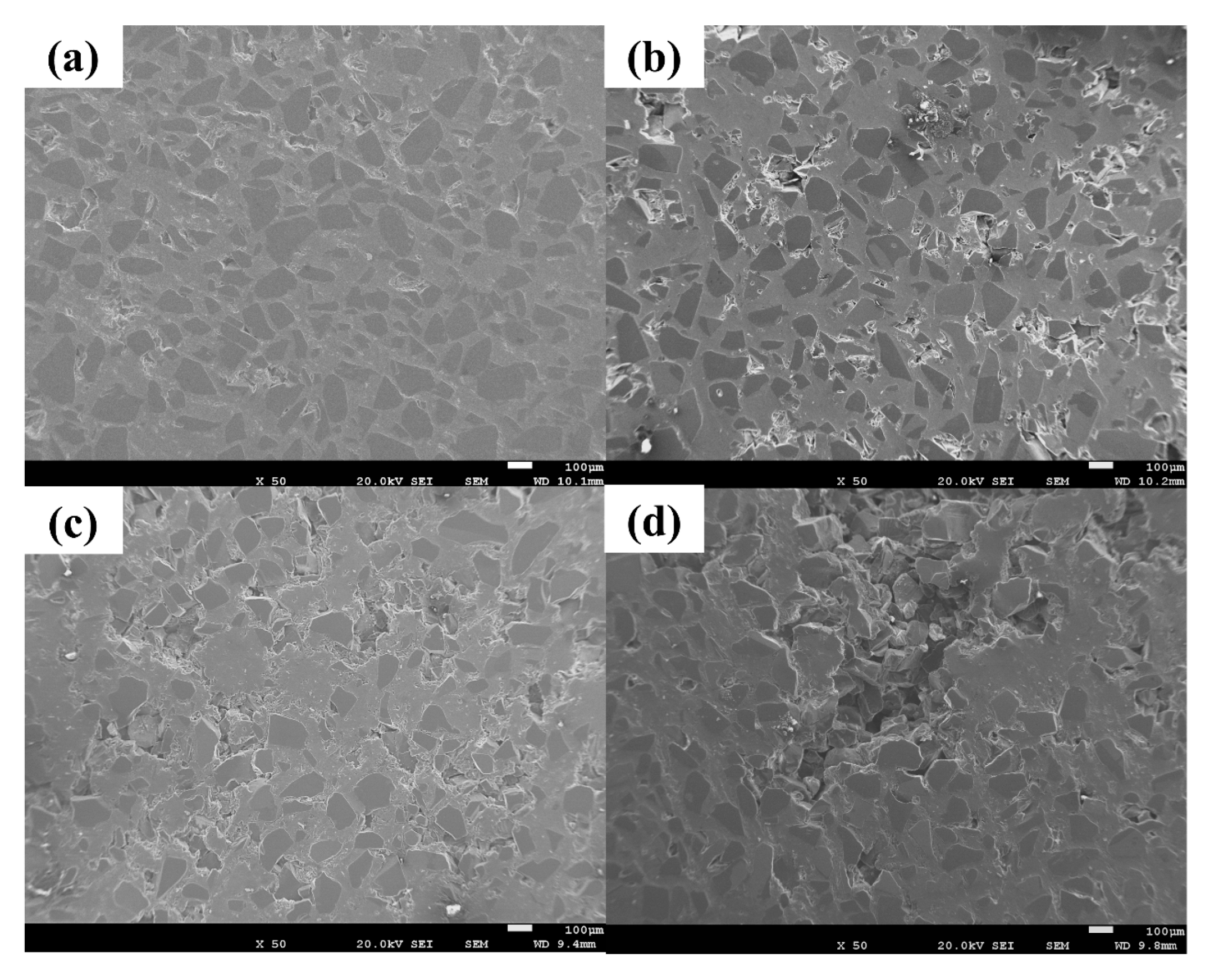
3.2. Infiltration of SiC Preform with Al
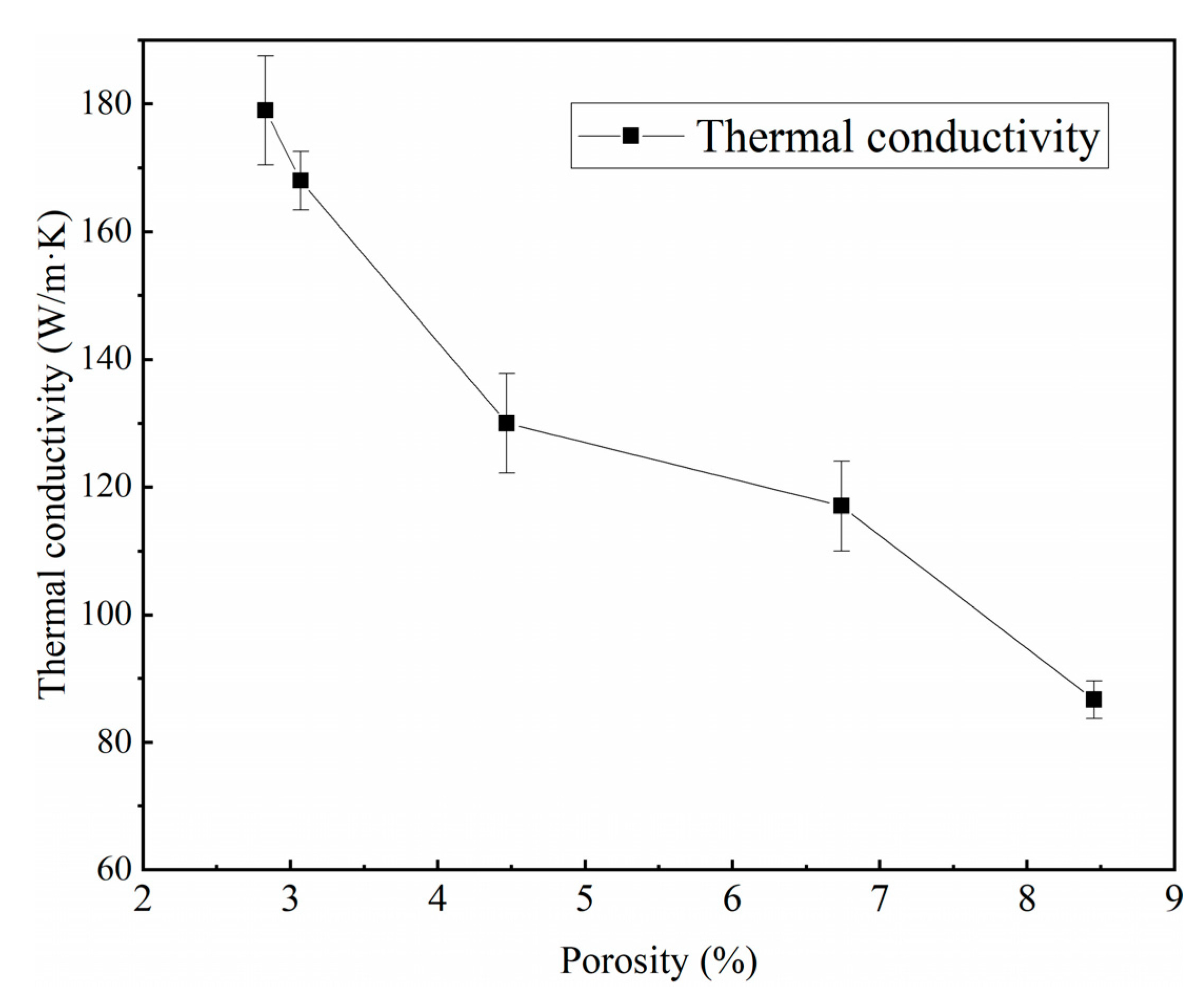
3.3. Thermal Conductivity of Composites and Its Prediction Model
4. Conclusions
- The volume fraction of SiC can be adjusted regularly by using 12 μm and 100 μm SiC particles with different proportions. When the proportion of 100 μm SiC particles is about 77%, the volume fraction of SiC reaches the maximum. The introduction of small particles easily causes the pore size of the SiC preform to shrink, which requires greater infiltration pressure to prepare SiC/Al composites.
- The porosity of the SiC preform can be adjusted stably by adding pore forming agent NH4HCO3, which satisfies the following mathematical model: Y = 26.9 + 0.4X. It is understood that NH4HCO3 acts as a bridge to connect more pores in the preform, which is conducive to the subsequent infiltration process.
- Compared with Al-7Si-Mg, Al-12Si-Mg shows better infiltration results. The main reasons are as follows: First, Al-12Si-Mg is infiltrated fully for the best fluidity and low infiltration resistance. In addition, it is considered that Al-12Si-Mg with high Si content has little tendency to form harmful phase Al4C3, which is beneficial to achieve good infiltration results.
- The thermal conductivity of high-volume fraction SiC/Al is sensitive to their porosity, especially when the porosity is in the range of 2.5–4.5%. Through the newly established λd model and its connection with the porosity, the thermal conductivity of high-volume fraction SiC/Al with 2–8% porosity can be effectively predicted.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Y. Microstructural Characterization and Properties of SiC/Al Composites for Electronic Packaging Fabricated by Pressureless Infiltration. Mater. Sci. Forum 2007, 546–549, 1597–1602. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, K.; Kim, H.; Hong, S. Fabrication process and thermal properties of SiCp/Al metal matrix composites for electronic packaging applications. J. Mater. Sci. 2000, 35, 6231–6236. [Google Scholar] [CrossRef]
- Li, S.; Xiong, D.; Liu, M.; Bai, S.; Zhao, X. Thermophysical properties of SiC/Al composites with three dimensional interpenetrating network structure. Ceram. Int. 2014, 40, 7539–7544. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Z.; Yang, B.; Ge, B.; Zhang, X.; Guo, Y. Preparation and anisotropic thermophysical properties of SiC honeycomb/Al-Mg-Si composite via spontaneous infiltration. Prog. Nat. Sci. Mater. 2019, 2, 177–183. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Z.; Wang, J.; Wu, Y.; Tang, W.; Lv, J. Pressureless infiltration of liquid aluminum alloy into SiC preforms to form near-net-shape SiC/Al composites. J. Alloys Comp. 2008, 465, 239–243. [Google Scholar] [CrossRef]
- Sun, J.; Chen, G.; Wang, B.; Chen, G.; Tang, W. Fabrication, Microstructures, and Properties of 50 vol.% SiCp/6061Al Composites via Hot Pressing. J. Mater. Eng. Perform. 2019, 5, 2697–2706. [Google Scholar] [CrossRef]
- Sijo, M.; Jayadevan, K. Analysis of Stir Cast Aluminium Silicon Carbide Metal Matrix Composite: A Comprehensive Review. Procedia Technol. 2016, 2016, 379–385. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, F.; Wang, Y.; Zhang, B.; Wang, L. Interfacial structure and stability of a co-continuous SiC/Al composite prepared by vacuum-pressure infiltration. Ceram. Int. 2017, 43, 6563–6570. [Google Scholar] [CrossRef]
- Peng, J. Research on Preparation of SiCp Porous Preform and Vacuum Pressure Infiltration of Liquid Aluminum Alloys. Master’s Thesis, South China University of Technology, Guangzhou, China, 2017. [Google Scholar]
- Xie, B.; Zhao, H.; Long, H.; Peng, J.; Liu, R. 3D characteristics of pores in SiC particle preforms with different starch contents by X-ray micro-computed tomography. Ceram. Int. 2019, 18, 23924–23933. [Google Scholar] [CrossRef]
- Long, H.; Zhao, H.; Peng, J.; Liu, R. Effect of particle size on 3D characteristics of pores in SiCp preforms. Acta Mater. Compos. Sin. 2017, 34, 599–607. [Google Scholar]
- Graton, L.; Fraser, H. Systematic Packing of Spheres: With Particular Relation to Porosity and Permeability. J. Geol. 1935, 43, 785–909. [Google Scholar] [CrossRef]
- Yu, A.; Standish, N. Estimation of the porosity of particle mixtures by a linear-mixture packing model. Ind. Eng. Chem. Res. 1991, 30, 1372–1385. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Pan, R. Effect of pore-forming agent on microstructure and thermal conductivity of SiCp/Al composites prepared by pressureless infiltration. Chin. J. Nonferrous Met. 2010, 20, 2162–2167. [Google Scholar]
- Molina, J.; Saravanan, R.; Arpón, R.; García-Cordovilla, C.; Louis, E.; Narciso, J. Pressure infiltration of liquid aluminium into packed SiC particulate with a bimodal size distribution. Acta Mater. 2002, 50, 247–257. [Google Scholar] [CrossRef]
- Liu, T.; Cai, X.; He, L.; Li, G. Effect of Pore-forming Agents Content on Porosity and Experimental Study on Porosity Measurement of Porous SiC Prefrom. Mater. Rev. 2013, 27, 108–112. [Google Scholar]
- Wang, D.; Zheng, Z.; Lv, J.; Xu, G.; Zhou, S.; Tang, W.; Wu, Y. Interface Design in 3D-SiC/Al-Si-Mg Interpenetrating Composite Fabricated by Pressureless Infiltration. Ceram. Int. 2018, 44, 11956–11965. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.; Wu, G. Interfacial microstructure of SiCp/Al composite produced by the pressureless infiltration technique. Ceram. Int. 2013, 39, 4893–4897. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H.; Zhu, M.; Zhu, M.; Deng, L. The Research of β-SiCp/Al Electronic Packaging Composites Fabricated by Pressureless Infiltrating. Adv. Mater. Res 2012, 490–495, 3816–3821. [Google Scholar] [CrossRef]
- Ren, S.; He, X.; Qu, X.; Li, Y. Effect of controlled interfacial reaction on the microstructure and properties of the SiCp/Al composites prepared by pressureless infiltration. J. Alloys Comp. 2008, 455, 424–431. [Google Scholar] [CrossRef]
- Lee, H.; Hong, S. Pressure infiltration casting process and thermophysical properties of high volume fraction SiCp/Al metal matrix composites. Mater. Sci. Tech. 2003, 19, 1057–1064. [Google Scholar] [CrossRef]
- Molina, J.; Prieto, R.; Narciso, J.; Louis, E. The effect of porosity on the thermal conductivity of Al–12wt.% Si/SiC composites. Scripta Mater. 2009, 60, 582–585. [Google Scholar] [CrossRef]
- Shen, Y. Combined effects of microvoids and phase contiguity on the thermal expansion of metal-ceramic composites. Mater. Sci. Eng. A 1997, 237, 102–108. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, K.; Zou, X.; Hua, X.; Que, Y. Effect of porosity on thermal conductivity of SiCP/Al composites. T. Mater. Heat Treat. 2015, 36, 236–240. [Google Scholar]
- Haselman, D.; Donaldson, K. Effect of Reinforcement Particle Size on the Thermal Conductivity of a Particulate-Silicon Carbide-Reinforced Aluminum Matrix Composite. J. Am. Ceram. Soc. 1992, 75, 3137–3140. [Google Scholar] [CrossRef]
- Kawai, C. Effect of Interfacial Reaction on the Thermal Conductivity of Al–SiC Composites with SiC Dispersions. J. Am. Ceram. Soc. 2001, 84, 896–898. [Google Scholar] [CrossRef]
- Hasselman, D.; Johnson, L. Effective Thermal Conductivity of Composites with Interfacial Thermal Barrier Resistance. J. Compos. Mater. 1987, 21, 508–515. [Google Scholar] [CrossRef]
- Hamman, N.; Fleming, J.; Schlueter, E.; Janson, I.; Meridew, J.; Kumar, M. Method and Apparatus for Forming Porous Metal Implants. European Patent EP 1996248B1, 4 April 2016. Available online: https://www.freepatentsonline.com/EP1996248.html (accessed on 27 April 2021).
- Chen, M.; Bai, Y.; Zhang, Z.; Zheng, H.; Zhang, Z. Study on Preparation of SiC Preform for Electronic Packaging Material. In Proceedings of the 2020 21st International Conference on Electronic Packaging Technology (ICEPT), Guangzhou, China, 12–15 August 2020; IEEE: New York, NY, USA, 2020; pp. 1–5. [Google Scholar]
- McGeary, R. Mechanical Packing of Spherical Particles. J. Am. Ceram. Soc. 1961, 44, 513–522. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Z.; Lv, J.; Xu, G.; Zhou, S.; Tang, W.; Wu, Y. Multimodal particle distribution in 3D-SiC/Al-Si-Mg interpenetrating composite fabricated by pressureless infiltration. Ceram. Int. 2018, 16, 19851–19858. [Google Scholar] [CrossRef]
- Molina, J.; Piñero, E.; Narciso, J.; García-Cordovilla, C.; Louis, E. Liquid metal infiltration into ceramic particle preforms with bimodal size distributions. Curr. Opin. Solid State Mater. Sci. 2005, 9, 202–210. [Google Scholar] [CrossRef]
- Delannay, F.; Froyen, L.; Deruyttere, A. The wetting of solids by molten metals and its relation to the preparation of metal-matrix composites. J. Mater. Sci. 1987, 1, 1–16. [Google Scholar] [CrossRef]




| Content Ratio of 100 μm and 12 μm SiC Particles | NH4H2PO4 Content/vol.% | NH4HCO3 Content /vol.% | SiC Content /vol.% |
|---|---|---|---|
| 0:1 | 5 | 25 | 70 |
| 1:2 | 5 | 25 | 70 |
| 1:1 | 5 | 25 | 70 |
| 2:1 | 5 | 25 | 70 |
| 3:1 | 5 | 25 | 70 |
| 4:1 | 5 | 25 | 70 |
| 5:1 | 5 | 25 | 70 |
| 7:1 | 5 | 25 | 70 |
| 10:1 | 5 | 25 | 70 |
| 1:0 | 5 | 25 | 70 |
| NH4HCO3 Content /vol.% | SiC Content /vol.% | Content Ratio of 100 μm and 12 μm SiC Particles |
|---|---|---|
| 0 | 100 | 10:1 |
| 10 | 90 | 10:1 |
| 20 | 80 | 10:1 |
| 30 | 70 | 10:1 |
| Al Alloy | Infiltration Pressure (Mpa) | Infiltration Temperature (°C) | Infiltration Time (min) | Vacuum Degree· (Pa) |
|---|---|---|---|---|
| Al-7Si-Mg | 1.1 | 800 | 10 | 100 |
| Al-12Si-Mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Bai, Y.; Zhang, Z.; Zhao, H. The Preparation of High-Volume Fraction SiC/Al Composites with High Thermal Conductivity by Vacuum Pressure Infiltration. Crystals 2021, 11, 515. https://doi.org/10.3390/cryst11050515
Chen M, Bai Y, Zhang Z, Zhao H. The Preparation of High-Volume Fraction SiC/Al Composites with High Thermal Conductivity by Vacuum Pressure Infiltration. Crystals. 2021; 11(5):515. https://doi.org/10.3390/cryst11050515
Chicago/Turabian StyleChen, Mengqin, Yuelong Bai, Zhifeng Zhang, and Haidong Zhao. 2021. "The Preparation of High-Volume Fraction SiC/Al Composites with High Thermal Conductivity by Vacuum Pressure Infiltration" Crystals 11, no. 5: 515. https://doi.org/10.3390/cryst11050515
APA StyleChen, M., Bai, Y., Zhang, Z., & Zhao, H. (2021). The Preparation of High-Volume Fraction SiC/Al Composites with High Thermal Conductivity by Vacuum Pressure Infiltration. Crystals, 11(5), 515. https://doi.org/10.3390/cryst11050515







