The Role of Astaxanthin on Chronic Diseases
Abstract
1. Introduction
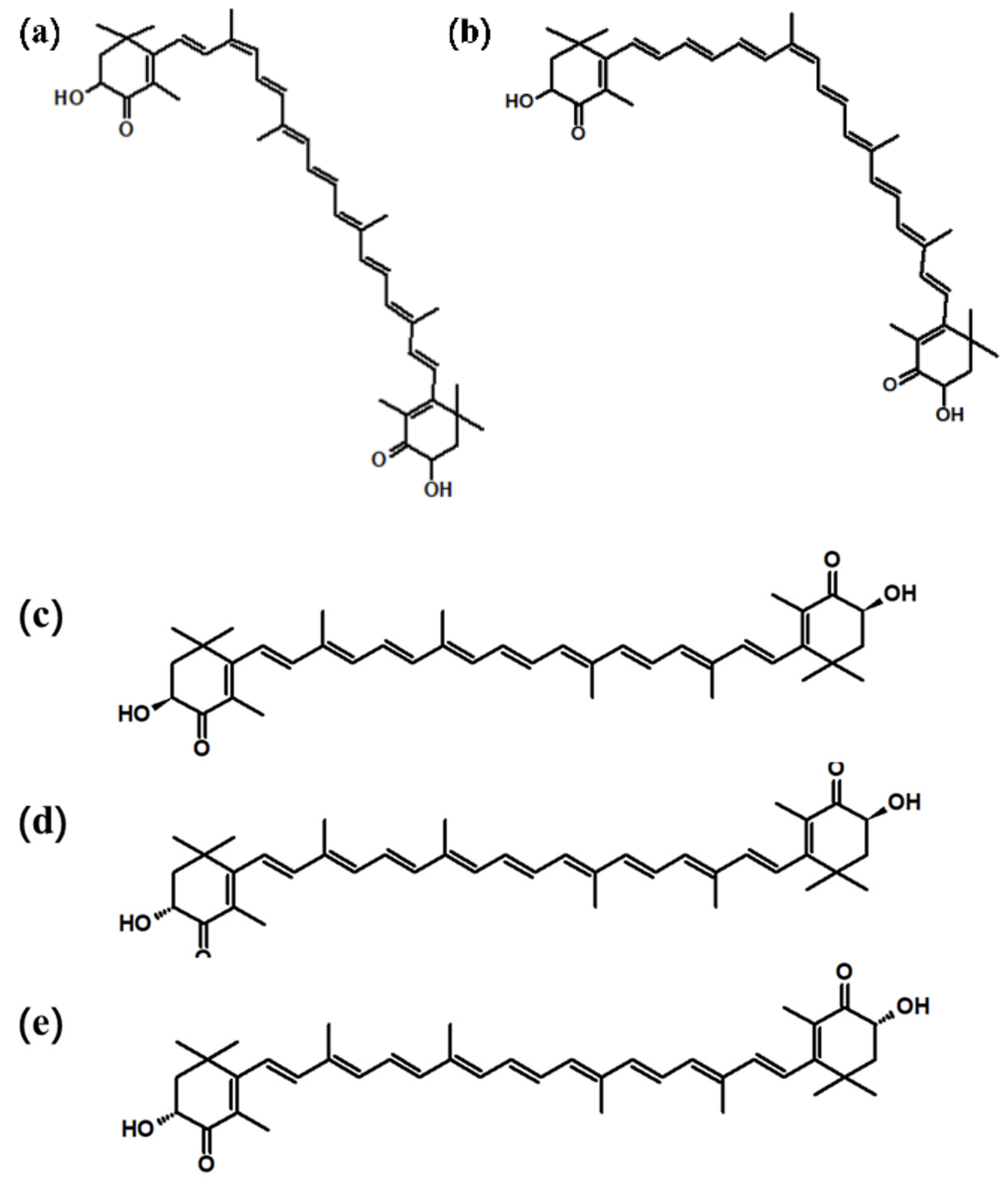
2. The Structure of Astaxanthin
3. Source of Astaxanthin
4. The Function of Astaxanthin
5. Diabetes and Cardiovascular Disease
5.1. Astaxanthin and Diabetes
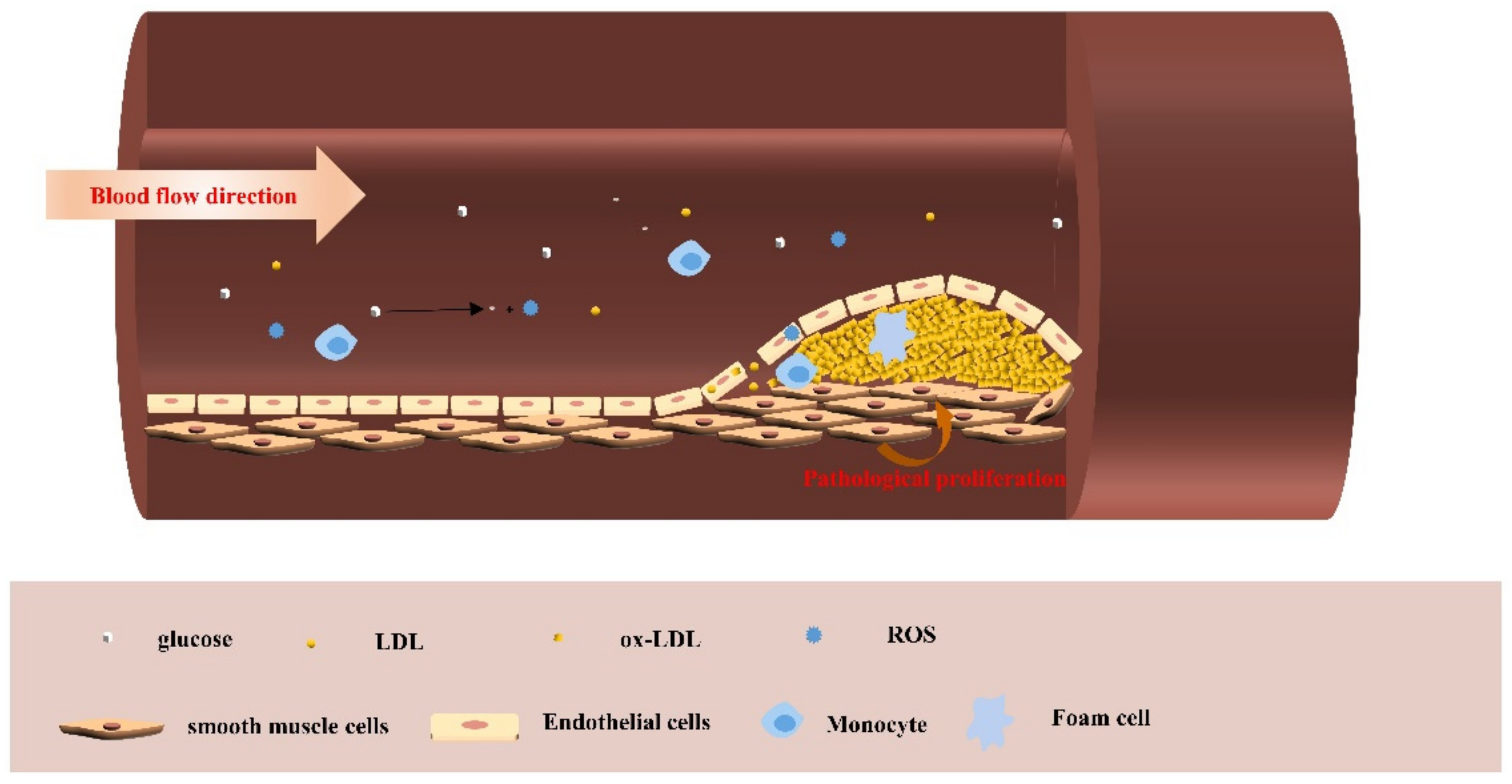
5.2. Astaxanthin and Cardiovascular Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDM | Gestational diabetes mellitus |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| ADA | American Diabetes Association |
| AHA | American Heart Association |
| LDL | Low-density lipoprotein |
| ox-LDL | oxidized low-density |
| HO-1 | Heme oxygenase-1 |
| Nrf2 | Nuclear factor erythroid-2-related factor 2 |
| IL-6 | Interleukin 6 |
| HUVEC | Human umbilical vein endothelial cells |
| VSMC | Vascular smooth muscle cells |
| eNOS | Endothelial nitric oxide synthase |
| GFBS | Glycated fetal bovine serum |
| TNF-α | Tumor necrosis factor alpha |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Protein kinase B |
| COX-2 | Cyclooxygenase-2 |
| BDNF | Brain-derived neurotrophic factor |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting kinase-3 |
| ROS | Reactive oxygen species |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| JNK | C-Jun N-terminal kinase |
References
- Manunta, C. Astaxanthin in insects and other terrestrial arthropods. Nature 1948, 162, 298. [Google Scholar] [CrossRef]
- Naguib, M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.L.; Pogson, B.J. Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci. 2020, 25, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wade, N.M.; Gabaudan, J.; Glencross, B.D. A review of carotenoid utilisation and function in crustacean aquaculture. Rev. Aquacult. 2017, 9, 141–156. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, J. Astaxanthin isomers: Selective distribution and isomerization in aquatic animals. Aquaculture 2020, 520, 734915. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 10. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Ryden, L.; Standl, E.; Bartnik, M.; Van den Berghe, G.; Betteridge, J.; De Boer, M.J.; Cosentino, F.; Jönsson, B.; Laakso, M.; Malmberg, K.; et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary-The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2007, 28, 88–136. [Google Scholar]
- Ford, E.S.; Zhao, G.X.; Li, C.Y. Pre-diabetes and the risk for cardiovascular disease A systematic review of the evidence. J. Am. Coll. Cardiol. 2010, 55, 1310–1317. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ Br. Med. J. 2020, 370, 29. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 11. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; Rafieian-Kopaei, M. Oxidative stress and antioxidants in diabetes mellitus. Asian Pac. J. Trop. Med. 2020, 13, 431–438. [Google Scholar]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Evans, J.; Goldfine, I.; Maddux, B.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Kim, B.; Farruggia, C.; Ku, C.S.; Pham, T.X.; Yang, Y.; Bae, M.; Wegner, C.J.; Farrell, N.J.; Harness, E.; Park, Y.K.; et al. Astaxanthin inhibits inflammation and fibrosis in the liver and adipose tissue of mouse models of diet-induced obesity and nonalcoholic steatohepatitis. J. Nutr. Biochem. 2017, 43, 27–35. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Yogalakshmi, B.; Sreeja, S.; Anuradha, C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-kappa B-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress 2014, 19, 183–191. [Google Scholar] [CrossRef]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. BBA Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef]
- Yuan, J.P.; Chen, F. Isomerization of trans-astaxanthin to cis-Isomers in organic solvents. J. Agric. Food Chem. 1999, 47, 3656–3660. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 2015, 81, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Grung, M.; D’Souza, F.M.L.; Borowitzka, M.; Liaaen-Jensen, S. Algal carotenoids 51. secondary carotenoids 2.Haematococcus pluvialis aplanospores as a source of (3S, 3′S)-astaxanthin esters. J. Appl. Phycol. 1992, 4, 165–171. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, X.; Liu, D. Preparation of (3R, 3′ R)-astaxanthin monoester and (3R, 3′ R)-astaxanthin from Antarctic krill (Euphausia superba Dana). Eur. Food Res. Technol. 2015, 240, 295–299. [Google Scholar] [CrossRef]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613–1622. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ooi, C.W.; Chew, K.W.; Foo, S.C.; Lim, J.W.; Tao, Y.; Jiang, N.; Ho, S.H.; Show, P.L. Permeabilization of Haematococcus pluvialis and solid-liquid extraction of astaxanthin by CO2-based alkyl carbamate ionic liquids. Chem. Eng. J. 2021, 411, 128510. [Google Scholar] [CrossRef]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and β-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheroscier Thromb 2000, 7, 216–222. [Google Scholar] [CrossRef]
- Shen, H.; Kuo, C.C.; Chou, J.; Delvolve, A.; Jackson, S.N.; Post, J.; Woods, A.S.; Hoffer, B.J.; Wang, Y.; Harvey, B.K. Astaxanthin reduces ischemic brain injury in adult rats. FASEB J. 2009, 23, 1958–1968. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.W.; Kim, B.W.; Kim, W.J.; Choi, Y.H. Astaxanthin improves the proliferative capacity as well as the osteogenic and adipogenic differentiation potential in neural stem cells. Food Chem. Toxicol. 2010, 48, 1741–1745. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Ma, H.; Chen, S.; Xiong, H.; Wang, M.; Hang, W.; Zhu, X.; Zheng, Y.; Ge, B.; Li, R.; Cui, H. Astaxanthin from Haematococcus pluvialis ameliorates the chemotherapeutic drug (doxorubicin) induced liver injury through the Keap1/Nrf2/HO-1 pathway in mice. Food Funct. 2020, 11, 4659–4671. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.T.; Yeh, H.Y.; Tsai, Y.T.; Yuan, T.H.; Liao, M.T.; Huang, J.W.; Chen, H.W. Astaxanthin counteracts vascular calcification in vitro through an early up-regulation of SOD2 based on a transcriptomic approach. Int. J. Mol. Sci. 2020, 21, 8530. [Google Scholar] [CrossRef]
- Yuan, L.; Qu, Y.; Li, Q.; An, T.; Chen, Z.; Chen, Y.; Deng, X.; Bai, D. Protective effect of astaxanthin against La2O3 nanoparticles induced neurotoxicity by activating PI3K/AKT/Nrf-2 signaling in mice. Food Chem. Toxicol. 2020, 144, 111582. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wanga, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014, 5, 158–166. [Google Scholar] [CrossRef]
- Yuan, L.; Liang, P.; Qu, Y.; An, T.; Wang, J.; Deng, X.; Bai, L.; Shen, P.; Bai, D. Protective effect of astaxanthin against SnS2 nanoflowers induced testes toxicity by suppressing RIPK1-RIPK3-MLKL signaling in mice. Food Chem. Toxicol. 2020, 145, 111736. [Google Scholar] [CrossRef]
- Peng, Y.J.; Lu, J.W.; Liu, F.C.; Lee, C.H.; Lee, H.S.; Ho, Y.J.; Hsieh, T.H.; Wu, C.C.; Wang, C.C. Astaxanthin attenuates joint inflammation induced by monosodium urate crystals. FASEB J. 2020, 34, 11215–11226. [Google Scholar] [CrossRef]
- Ozbeyli, D.; Gurler, E.B.; Buzcu, H.; Çilingir-Kaya, Ö.T.; Çam, M.E.; Yüksel, M. Astaxanthin alleviates oxidative damage in acute pancreatitis via direct antioxidant mechanisms. Turk. J. Gastroenterol. 2020, 31, 706–712. [Google Scholar] [CrossRef]
- Xue, X.-L.; Han, X.-D.; Li, Y.; Chu, X.F.; Miao, W.M.; Zhang, J.L.; Fan, S.J. Astaxanthin attenuates total body irradiation-induced hematopoietic system injury in mice via inhibition of oxidative stress and apoptosis. Stem Cell Res. Ther. 2017, 8, 7. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Bi, J.; Gu, J.; Deng, Y.; Liu, C. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int. Immunopharmacol. 2017, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Monoe, F.; Ohsawa, I.; Ohta, S.; Miyamoto, T. Astaxanthin improves osteopenia caused by aldehyde-stress resulting from Aldh2 mutation due to impaired osteoblastogenesis. Biochem. Biophys Res. Commun. 2020, 527, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Y.; Jin, J.; Lu, G.; Kang, X.L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Hou, C.; Li, J.; Peng, H.; Wang, Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 2020, 197, 108–113. [Google Scholar] [CrossRef]
- Ghlissi, Z.; Hakim, A.; Sila, A.; Mnif, H.; Zeghal, K.; Rebai, T.; Bougatef, A.; Sahnoun, Z. Evaluation of efficacy of natural astaxanthin and vitamin E in prevention of colistin-induced nephrotoxicity in the rat model. Environ. Toxicol. Pharmacol. 2014, 37, 960–966. [Google Scholar] [CrossRef]
- Sila, A.; Ghlissi, Z.; Kamoun, Z.; Makni, M.; Nasri, M.; Bougatef, A.; Sahnoun, Z. Astaxanthin from shrimp by-products ameliorates nephropathy in diabetic rats. Eur. J. Nutr. 2015, 54, 301–307. [Google Scholar] [CrossRef]
- Wu, L.; Sun, Z.; Chen, A.; Guo, X.; Wang, J. Effect of astaxanthin and exercise on antioxidant capacity of human body, blood lactic acid and blood uric acid metabolismEffet de l’astaxanthine et de l’exercice sur la capacité antioxydante, la lactatémie, et le métabolisme de l’acide urique. Sci. Sports 2019, 34, 348–352. [Google Scholar] [CrossRef]
- Shokri, M.N.; Tahmasebi, M.; Mohammadi, A.J.; Zakerkish, M.; Mohammadshahi, M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14022. [Google Scholar]
- Hayashi, M.; Kawamura, M.; Kawashima, Y.; Uemura, T.; Maoka, T. Effect of astaxanthin-rich extract derived from paracoccus carotinifaciens on the status of stress and sleep in adults. J. Clin. Biochem. Nutr. 2020, 66, 92–102. [Google Scholar] [CrossRef]
- Talbott, S.M.; Hantla, D.; Capelli, B.; Ding, L.; Li, Y.; Artaria, C. Effect of astaxanthin supplementation on psychophysiological heart-brain axis. Dyn. Healthy Subj. 2019, 9, 521–531. [Google Scholar]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Food Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef]
- Buse, J.B.; Ginsberg, H.N.; Bakris, G.L.; Clark, N.G.; Costa, F.; Eckel, R.; Fonseca, V.; Gerstein, H.C.; Grundy, S.; Nesto, R.W.; et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus-A scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007, 30, 162–172. [Google Scholar] [CrossRef]
- Grundy, S.M. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Nentwich, M.M.; Ulbig, M.W. Diabetic retinopathy-ocular complications of diabetes mellitus. World J. Diabetes 2015, 6, 489–499. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Haffner, S.M. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am. J. Cardiol. 2006, 97, 3–11. [Google Scholar] [CrossRef]
- Taborsky, M.; Linhart, A.; Rosolova, H.; Spinard, J. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Summary of the document prepared by the Czech Society of Cardiology. Cor. Vasa 2020, 62, 105–138. [Google Scholar]
- Selvin, E.; Marinopoulos, S.; Berkenblit, G.; Rami, T.; Brancati, F.L.; Powe, N.R.; Golden, S.H. Meta-analysis: Glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann. Intern. Med. 2004, 141, 421–431. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Münzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef]
- Fiorello, M.L.; Treweeke, A.T.; Macfarlane, D.P.; Megson, I.L. The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes. Sci. Rep. 2020, 10, 19547. [Google Scholar] [CrossRef]
- Thiel, W.H.; Esposito, C.L.; Dickey, D.D.; Dassie, J.P.; Long, M.E.; Adam, J.; Streeter, J.; Schickling, B.; Takapoo, M.; Flenker, K.S.; et al. Vascular smooth muscle cell RNA aptamers for the treatment of cardiovascular disease. Mol. Ther. 2015, 23, 27. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Riches, K.; Alshanwani, A.R.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Wood, I.C.; Turner, N.A.; Porter, K.E. Elevated expression levels of miR-143/5 in saphenous vein smooth muscle cells from patients with type 2 diabetes drive persistent changes in phenotype and function. J. Mol. Cell Cardiol. 2014, 74, 240–250. [Google Scholar] [CrossRef]
- Casella, S.; Bielli, A.; Mauriello, A.; Orlandi, A. Molecular pathways regulating macrovascular pathology and vascular smooth muscle cells phenotype in type 2 diabetes. Int. J. Mol. Sci. 2015, 16, 24353–24368. [Google Scholar] [CrossRef]
- Villeneuve, L.M.; Reddy, M.A.; Lanting, L.L.; Wang, M.; Meng, L.; Natarajan, R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc. Natl. Acad. Sci. USA 2008, 105, 9047–9052. [Google Scholar] [CrossRef]
- Ehses, J.A.; Perren, A.; Eppler, E.; Ribaux, P.; Pospisilik, J.A.; Maor-Cahn, R.; Gueripel, X.; Ellingsgaard, H.; Schneider, M.K.; Biollaz, G. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 2007, 56, 2356–2370. [Google Scholar] [CrossRef]
- Mirza, R.E.; Fang, M.M.; Ennis, W.J.; Koh, T.J. Blocking interleukin-1 beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Honold, L.; Nahrendorf, M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ. Res. 2018, 122, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Mechanisms of disease-Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin improves glucose metabolism and reduces blood pressure in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar]
- Feng, W.; Wang, Y.; Guo, N.; Huang, P.; Mi, Y. Effects of Astaxanthin on Inflammation and Insulin Resistance in a Mouse Model of Gestational Diabetes Mellitus. Dose-Response 2020, 18, 1559325820926765. [Google Scholar] [CrossRef]
- Uchiyama, K.; Naito, Y.; Hasegawa, G.; Nakamura, N.; Takahashi, J.; Yoshikawa, T. Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002, 7, 290–293. [Google Scholar] [CrossRef]
- Li, Y.-C.; He, Q.-H.; Liu, R.-X.; Zhang, B.; Yang, Z.-X.; Zhou, M. Effects of Haematococcus pluvialis astaxanthin on diabetes mice for decreasing blood glucose and its mechanisms. Sci. Technol. Food Ind. 2016, 37, 355–359. [Google Scholar]
- Chen, Y.; Tang, J.; Zhang, Y.; Du, J.; Wang, Y.; Yu, H.; He, Y. Astaxanthin alleviates gestational diabetes mellitus in mice through suppression of oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 2517–2527. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a molecular therapeutic target for Astaxanthin. Biomed. Pharmacother 2021, 137, 111374. [Google Scholar] [CrossRef]
- Landon, R.; Gueguen, V.; Petite, H.; Letourneur, D.; Pavon-Djavid, G.; Anagnostou, F. Impact of astaxanthin on diabetes pathogenesis and chronic complications. Mar. Drugs 2020, 18, 357. [Google Scholar] [CrossRef]
- Ishiki, M.; Nishida, Y.; Ishibashi, H.; Wada, T.; Fujisaka, S.; Takikawa, A.; Urakaze, M.; Sasaoka, T.; Usui, I.; Tobe, K. Impact of divergent effects of astaxanthin on insulin signaling in L6 cells. Endocrinology 2013, 154, 2600–2612. [Google Scholar] [CrossRef]
- Zhuge, F.; Ni, Y.; Wan, C.; Liu, F.; Fu, Z. Anti-diabetic effects of astaxanthin on an STZ-induced diabetic model in rats. Endocr. J. 2020, 68, EJ20-0699. [Google Scholar]
- Alderman, M.H. Prediabetes: An unexplored cardiovascular disease risk factor. J. Hypertens 2021, 39, 42–43. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Li, J.A.; Chen, L.; Zhang, X.Q.; Guan, S.K. Enhancing biocompatibility and corrosion resistance of biodegradable Mg-Zn-Y-Nd alloy by preparing PDA/HA coating for potential application of cardiovascular biomaterials. Mat. Sci. Eng. C Mater. 2020, 109, 110607. [Google Scholar] [CrossRef]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef]
- Donato, A.J.; Morgan, R.G.; Walker, A.E.; Lesniewski, L.A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell Cardiol. 2015, 89, 122–135. [Google Scholar] [CrossRef]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative stress and vascular smooth muscle cell growth: A mechanistic linkage by cyclophilin A. Antioxid. Redox Signal. 2010, 12, 675–682. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M. Astaxanthin Protects Ochratoxin A-Induced Oxidative Stress and Apoptosis in the Heart via the Nrf2 Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Sun, C.; Hao, Q.; Cheng, J. Astaxanthin ameliorates cardiomyocyte apoptosis after coronary microembolization by inhibiting oxidative stress via Nrf2/HO-1 pathway in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Wang, H.; Lin, J.; Chen, Z.; Chang-Sheng, X.; Huang, D.; Zhang, L.; Liang, J. The protective effect and related mechanisms of astaxanthin on endothelial function in diabetic rats. Chin. J. Hypertens 2015, 23, 530–536. [Google Scholar]
- Nishigaki, I.; Rajendran, P.; Venugopal, R.; Ekambaram, G.; Sakthisekaran, D.; Nishigaki, Y. Cytoprotective role of astaxanthin against glycated protein/iron chelate-induced toxicity in human umbilical vein endothelial cells. Phytother. Res. 2010, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; Chen, S.C.; Chen, P.C. Astaxanthin attenuated thrombotic risk factors in type 2 diabetic patients. J. Funct. Food 2019, 53, 22–27. [Google Scholar] [CrossRef]
| Species | Organ | Method | Dose | Function | Researcher |
|---|---|---|---|---|---|
| mice | Hippocampus | oral | 60 mg/kg/day | PI3K/AKT/Nrf-2↑ | Yuan Lu et al. [45] |
| rat | Brain | oral | 60 mg/three times a week | COX-2↓, BDNF levels↑ | Wanqiang Wu et al. [46] |
| mice | Testis | oral | 60 mg/kg/day | RIPK1-RIPK3-MLKL↓ | Yuan Lu et al. [47] |
| rat | joint | injection | 50 mg/kg/day | COX-2/IL-6↓ | Yi-Jen Peng et al. [48] |
| rat | pancreas | oral | 40 mg/kg/day | ROS↓, GSH↑ | Dilek Özbeyli et al. [49] |
| mice | Lung, thymus | injection | 50 mg/kg/day | ROS↓, Nrf-2↑ | Xiaolei Xue et al. [50] |
| mice | Liver | injection | 60 mg/kg/day | TNF-α /JNK↓ | Jingyao Zhang et al. [51] |
| mice | Bone | oral | 4 μM/mL | Improve mitochondrial function of MC3T3-E1 cells | Hiroko Hoshi et al. [52] |
| mice | Eye | oral | 50 mg/kg/day | Reduced apoptosis of retinal ganglion cells | LingYan Dong et al. [53] |
| 5 μM/mL | TNF-α/IL-1β↓ | Hui Li et al. [54] | |||
| rat | kidney | oral | 20 mg/kg/day 20 mg/kg/day | SOD/CAT/GPX/GSH↑ | Zohra Ghlissi et al. [55] Assaâd Sila et al. [56] |
| Project | Dose | Time | Function | Researcher |
|---|---|---|---|---|
| Male | 12 mg/day | 4 week | Maintain the body’s antioxidant capacity after high-intensity exercise | Wu L. et al. [57] |
| 2 diabetes mellitus | 8 mg/day | 8 week | MDA/IL-6↓ | Shokri M. N. et al. [58] |
| Random | 12 mg/day | 8 week | Improve sleep quality of depressed people | Masahiro H. et al. [59] |
| Male/Female | 12 mg/day | 8 week | Reduce average heart rate at submaximal endurance intensities | Shawn M. T. et al. [60] |
| Overweight | 12 mg/day | 12 week | LDL/MDA/ApoA1↓ | Choi H. D. et al. [61] |
| Smokers | 5/20/40 mg /day | 3 week | MDA↓, SOD↑ | Kim J. H. et al. [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hou, Y.; Li, J.; Wang, J. The Role of Astaxanthin on Chronic Diseases. Crystals 2021, 11, 505. https://doi.org/10.3390/cryst11050505
Zhang X, Hou Y, Li J, Wang J. The Role of Astaxanthin on Chronic Diseases. Crystals. 2021; 11(5):505. https://doi.org/10.3390/cryst11050505
Chicago/Turabian StyleZhang, Xueqi, Yachen Hou, Jingan Li, and Jianfeng Wang. 2021. "The Role of Astaxanthin on Chronic Diseases" Crystals 11, no. 5: 505. https://doi.org/10.3390/cryst11050505
APA StyleZhang, X., Hou, Y., Li, J., & Wang, J. (2021). The Role of Astaxanthin on Chronic Diseases. Crystals, 11(5), 505. https://doi.org/10.3390/cryst11050505







