Structural Phase Transition in (NH4)3GeF7–Raman Spectroscopy Data
Abstract
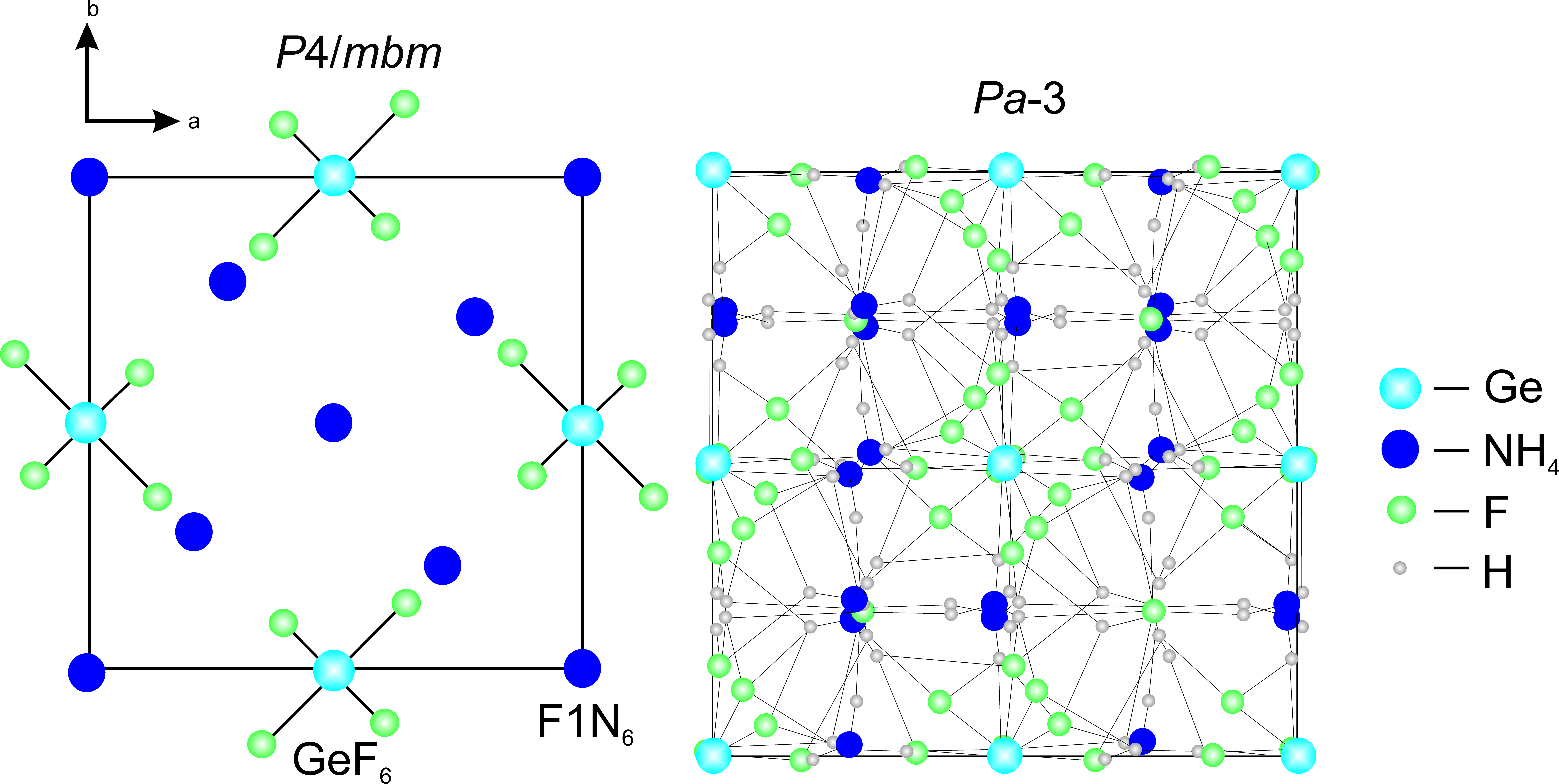
1. Introduction
2. Materials and Methods
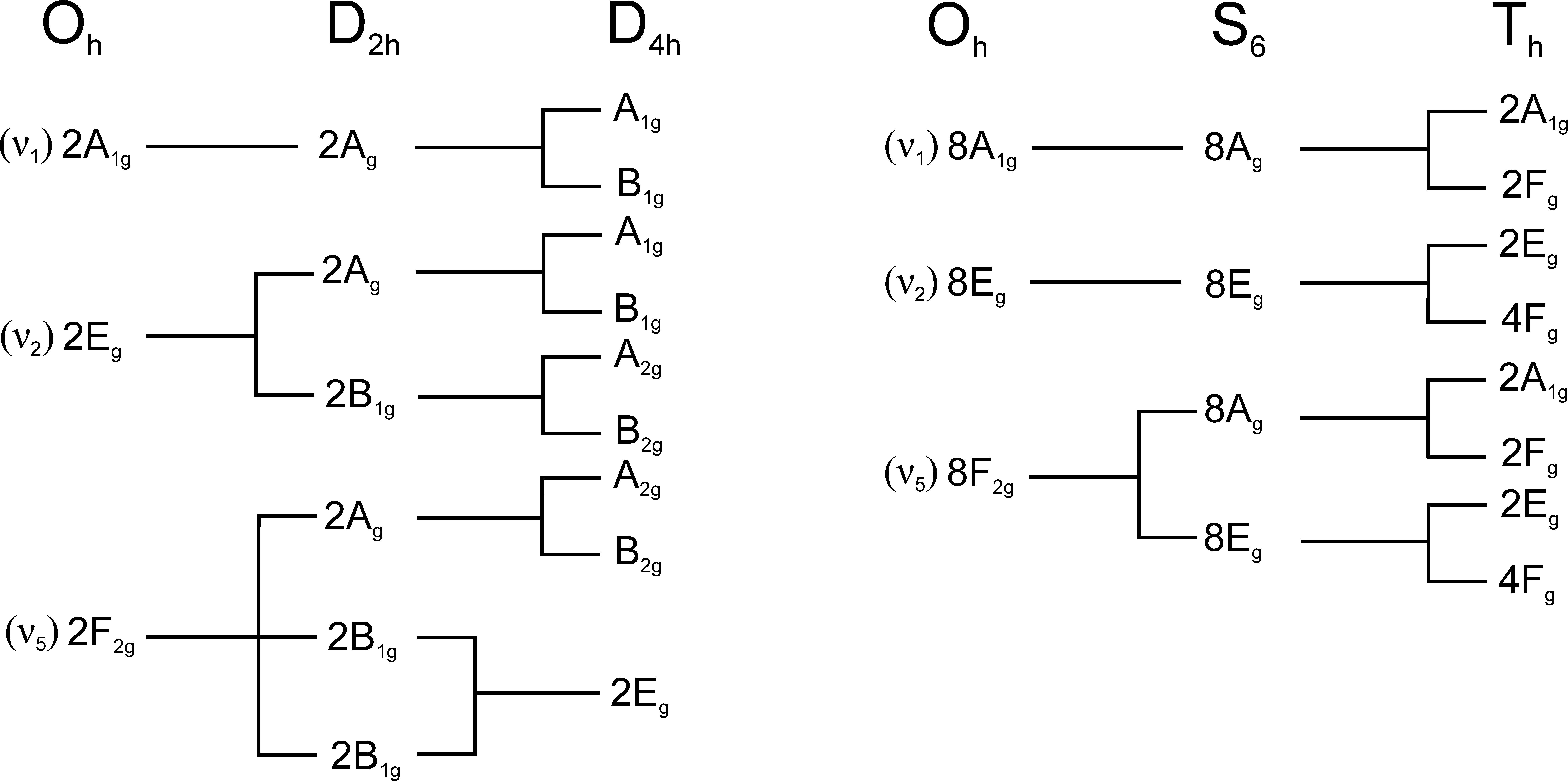
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, J.; Yang, Y.; Zhao, J.P.; Liu, X.S.; Endres, F.; Chi, C.X.; Wang, B.S.; Liu, X.X.; Li, Y. Ionic liquid electrodeposition of strain-released Germanium nanowires as stable anodes for lithium ion batteries. Nanoscale 2017, 9, 8481–8488. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.W.; Cao, M.H. Multidimensional germanium-based materials as anodes for Lithium-ion batteries. Chem. Asian J. 2016, 20, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, Y.; Zhang, X.; Cheng, L.W.; Qian, M.M.; Wei, W.; Wang, H. Germanium-based high-performance dual-ion batteries. Nanoscale 2020, 12, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, L.C.; Monconduit, L.; Seznec, V. Si and Ge-based anode materials for Li-, Na-, and K-ion batteries: A perspective from structure to electrochemical mechanism. Small 2020, 16, e1905260. [Google Scholar] [CrossRef]
- Kalem, S.; Werner, P.; Talalaev, V. Near-IR photoluminescence from Si/Ge nanowire-grown silicon wafers: Effect of HF treatment. Appl. Phys. A 2013, 112, 561–567. [Google Scholar] [CrossRef]
- Kalem, S.; Arthursson, Ö.; Romandic, I. Formation of germanates on germanium by chemical vapor treatment. Thin. Solid Films 2010, 518, 2377–2380. [Google Scholar] [CrossRef][Green Version]
- Yota, J.; Burrows, V.A. An infrared-study of thin-film formation on Si and Gk surfaces treated with aqueous NH4F and HF. J. Appl. Phys. 1991, 69, 7369–7371. [Google Scholar] [CrossRef]
- Plitzko, C.; Meyer, G. Crystal structure of triammonium heptafluorogermanate (NH4)3GeF7. Z. Kristallogr. 1998, 213, 475. [Google Scholar] [CrossRef][Green Version]
- Meyer, G. The Oxidation of metals with Liebig acids. Z Anorg. Allg. Chem. 2008, 634, 201–222. [Google Scholar] [CrossRef]
- D’yachenko, A.N.; Kraidenko, R.I. Fluorination of germanium concentrates with ammonium fluorides. Russ. J. Appl. Chem. 2008, 81, 952–955. [Google Scholar] [CrossRef]
- Mel’nikova, S.V.; Molokeev, M.S.; Laptash, N.M.; Pogoreltsev, E.I.; Misyul, S.V.; Flerov, I.N. Sequence of phase transitions in (NH4)3SiF7. Dalton Trans. 2017, 46, 2609–2617. [Google Scholar] [CrossRef]
- Mel’nikova, S.V.; Molokeev, M.S.; Laptash, N.M.; Misyul, S.V. A non-typical sequence of phase transitions in (NH4)3GeF7: Optical and structural characterization. Dalton Trans. 2016, 45, 5321–5327. [Google Scholar] [CrossRef]
- Molokeev, M.; Misjul, S.V.; Flerov, I.N.; Laptash, N.M. Reconstructive phase transition in (NH4)3TiF7 accompanied by the ordering of TiF6 octahedra. Acta. Cryst. 2014, B70, 924–931. [Google Scholar] [CrossRef]
- Flerov, I.N.; Molokeev, M.S.; Laptash, N.M.; Udovenko, A.A.; Pogoreltsev, E.I.; Mel’nikova, S.V.; Misyul, S.V. Structural transformation between two cubic phases of (NH4)3SnF7. J. Fluorine Chem. 2015, 178, 86–92. [Google Scholar] [CrossRef]
- Pogoreltsev, E.I.; Bogdanov, E.V.; Kartashev, A.V.; Molokeev, M.S.; Flerov, I.N.; Laptash, N.M. Thermal properties of (NH4)2MeF6∙NH4F (Me: Ti, Sn) crystals undergoing transformation between two cubic phases. Ferroelectrics 2016, 501, 20–25. [Google Scholar] [CrossRef]
- Bogdanov, E.V.; Kartashev, A.V.; Pogoreltsev, E.I.; Gorev, M.V.; Laptash, N.M.; Flerov, I.N. Anomalous behaviour of thermodynamic properties at successive phase transitions in (NH4)3GeF7. J. Solid State Chem. 2017, 256, 162–167. [Google Scholar] [CrossRef][Green Version]
- Mel’nikova, S.V.; Pogoreltsev, E.I.; Flerov, I.N.; Laptash, N.M. Unusual sequence of phase transitions in (NH4)3TiF7 detected by optic and calorimetric studies. J. Fluorine Chem. 2014, 165, 14–19. [Google Scholar] [CrossRef]
- Pogoreltsev, E.I.; Flerov, I.N.; Kartashev, A.V.; Bogdanov, E.V.; Laptash, N.M. Heat capacity, entropy, dielectric properties and T–p phase diagram of (NH4)3TiF7. J. Fluorine Chem. 2014, 168, 247–250. [Google Scholar] [CrossRef]
- Kartashev, A.V.; Gorev, M.V.; Bogdanov, E.V.; Flerov, I.N.; Laptash, N.M. Thermal properties and phase transition in the fluoride, (NH4)3SnF7. J. Solid State Chem. 2016, 237, 269–273. [Google Scholar] [CrossRef]
- Gerasimova, Y.V.; Krylov, A.S.; Vtyurin, A.N.; Laptash, N.M.; Krylova, S.N. Ferroelastic phase transition in the family of double fluoride crystals by Raman spectroscopy. Ferroelectrics 2020, 568, 185–190. [Google Scholar] [CrossRef]
- Krylov, A.S.; Kolesnikova, E.M.; Isaenko, L.I.; Krylova, S.N.; Vtyurin, A.N. Measurement of Raman-scattering spectra of Rb2KMoO3F3 crystal: Evidence for controllable disorder in the lattice structure. Cryst. Growth Des. 2014, 14, 923–927. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley: New York, NY, USA, 2009; p. 419. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova, Y.; Laptash, N.; Krylov, A.; Vonog, V.; Vtyurin, A. Structural Phase Transition in (NH4)3GeF7–Raman Spectroscopy Data. Crystals 2021, 11, 506. https://doi.org/10.3390/cryst11050506
Gerasimova Y, Laptash N, Krylov A, Vonog V, Vtyurin A. Structural Phase Transition in (NH4)3GeF7–Raman Spectroscopy Data. Crystals. 2021; 11(5):506. https://doi.org/10.3390/cryst11050506
Chicago/Turabian StyleGerasimova, Yulia, Natalia Laptash, Alexander Krylov, Vita Vonog, and Alexander Vtyurin. 2021. "Structural Phase Transition in (NH4)3GeF7–Raman Spectroscopy Data" Crystals 11, no. 5: 506. https://doi.org/10.3390/cryst11050506
APA StyleGerasimova, Y., Laptash, N., Krylov, A., Vonog, V., & Vtyurin, A. (2021). Structural Phase Transition in (NH4)3GeF7–Raman Spectroscopy Data. Crystals, 11(5), 506. https://doi.org/10.3390/cryst11050506








