Thermophysical Properties of Cement Mortar Containing Waste Glass Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
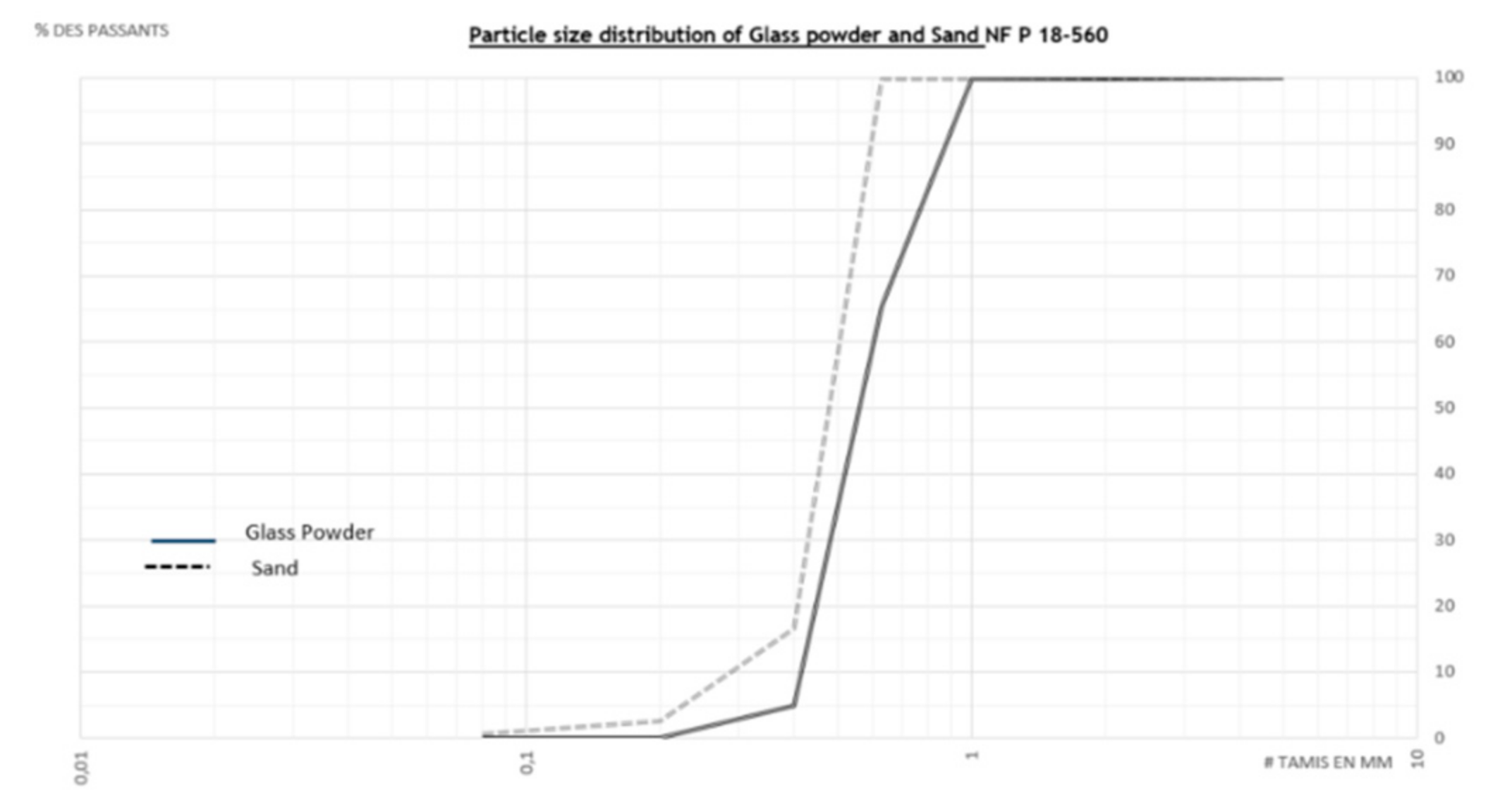
2.2. Samples Preparation
2.3. Methods
3. Results and Discussions
3.1. Scanning Electron Microscopy (SEM) and Microanalysis by (EDX)
3.2. Thermo-Physical Properties
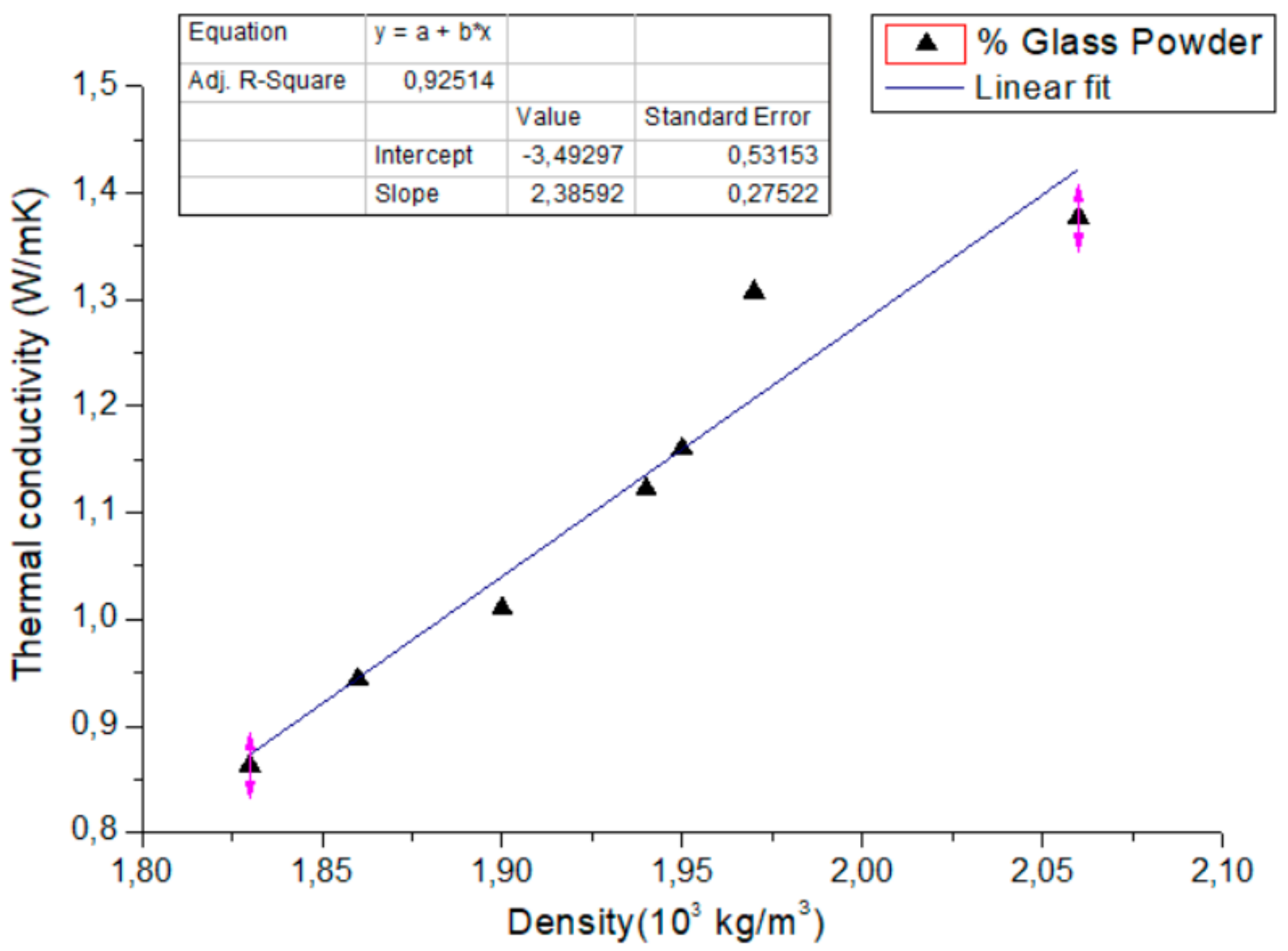
3.2.1. Relationship between Density and Thermal Conductivity
3.2.2. Effect of Glass Powder Content on Thermal Conductivity and Specific Heat
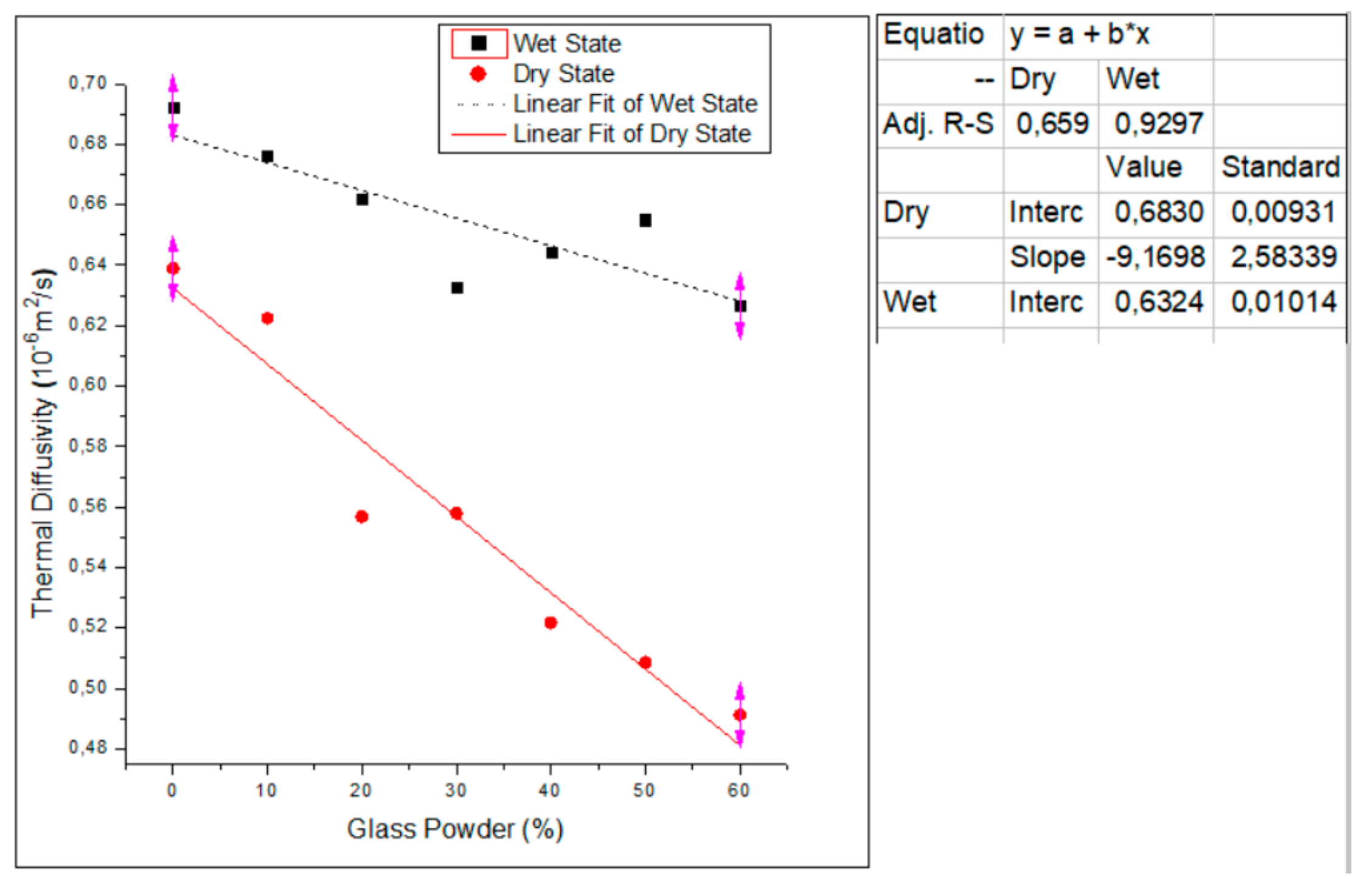
3.2.3. Effect of Glass Powder Content on Thermal Diffusivity
3.3. Influence of Temperature on Thermal Conductivity and Specific Heat
4. Conclusions
- The incorporation of waste glass powder contents into the cement mortar reduced the thermophysical properties’ values by 37, 18 and 22% respectively for thermal conductivity, volumetric specific heat and thermal diffusivity. In a dry state, the increase in the substitution rate of the glass powder leads to an increase in the volume of the pores, which causes a lower density and consequently, the thermal conductivity and volumetric specific heat of the mortars decreases. This could be explained by the low absorption of water in the glass. In a saturated state, the increase in the volumic mass of the samples could be explained by water occupating the empty pores, which subsequently induces the increase of the density of the mortars.
- According to SEM results, the chemical composition of the glass powder makes this material competitive as a pozzolanic material. The glass powder addition decreases the portlandite quantity and develops C-S-H gels. The use of glass powder in the mortar leads to modifications of the pore size.
- The density of the new mortar was also reduced by approximately 11% in the dry state and 5%in the wet state. This decrease in density, dry and/or wet, has a significant effect on the thermo-physical parameters of this composite compared to the reference mortar.
- Incorporation glass powder also reduces the specific heat for the two hydric states discussed (dry and saturated). This can be explained by the low specific thermal value of the glass (913 KJ/m3K), which is located in pores.
- The thermal conductivity increases when the temperature increases and/or the substitution rate decreases. Thus, at 60% glass powder, the thermal conductivity increases by 22.25% when the temperature varies from 20 °C to 50 °C. This result can be explained in part by the fact that an increase in temperature causes an expansion of the grains and, therefore, a decrease in porosity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, M.; Bhattacharyya, S.; Barai, S.V. Thermal and mechanical properties of concrete and its constituents at elevated temperatures: A review. Constr. Build. Mater. 2021, 270, 121398. [Google Scholar] [CrossRef]
- Asadi, I.; Shafigh, P.; Hashemi, M.; Akhiani, A.R.; Maghfouri, M.; Sajadi, B.; Mahyuddin, N.; Esfandiari, M.; Talebi, H.R.; Metselaar, H.S.C. Thermophysical properties of sustainable cement mortar containing oil palm boiler clinker (OPBC) as a fine aggregate. Constr. Build. Mater. 2021, 268, 121091. [Google Scholar] [CrossRef]
- Oren, O.H.; Gholampour, A.; Gencel, O.; Ozbakkaloglu, T. Physical and mechanical properties of foam concretes containing granulated blast furnace slag as fine aggregate. Constr. Build. Mater. 2020, 238, 117774. [Google Scholar] [CrossRef]
- Tekin, I.; Gencel, O.; Gholampour, A.; Oren, O.H.; Koksal, F.; Ozbakkaloglu, T. Recycling zeolitic tuff and marble waste in the production of eco-friendly geopolymer concretes. J. Clean. Prod. 2020, 268, 122298. [Google Scholar] [CrossRef]
- Belouadah, M.; Rahmouni, Z.E.A.; Tebbal, N. Influence of the addition of glass powder and marble powder on the physical and mechanical behavior of composite cement. Procedia Comput. Sci. 2019, 158, 366–375. [Google Scholar] [CrossRef]
- Mohammadyan-Yasouj, S.E.; Ghaderi, A. Experimental investigation of waste glass powder, basalt fibre, and carbon nanotube on the mechanical properties of concrete. Constr. Build. Mater. 2020, 252, 119115. [Google Scholar] [CrossRef]
- Hwang, S.S.; Cortés, C.M.M. Properties of mortar and pervious concrete with co-utilization of coal fly ash and waste glass powder as partial cement replacements. Constr. Build. Mater. 2021, 270, 121415. [Google Scholar] [CrossRef]
- Jani, Y.; Hogland, W. Waste glass in the production of cement and concrete—A review. J. Environ. Chem. Eng. 2014, 2, 1767–1775. [Google Scholar] [CrossRef]
- Kamali, M.; Ghahremaninezhad, A. Effect of glass powders on the mechanical and durability properties of cementitious materials. Constr. Build. Mater. 2015, 98, 407–416. [Google Scholar] [CrossRef]
- Nahi, S.; Leklou, N.; Khelidj, A.; Oudjit, M.N.; Zenati, A. Properties of cement pastes and mortars containing recycled green glass powder. Constr. Build. Mater. 2020, 262, 120875. [Google Scholar] [CrossRef]
- Elaqra, H.; Rustom, R. Effect of using glass powder as cement replacement on rheological and mechanical properties of cement paste. Constr. Build. Mater. 2018, 179, 326–335. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Properties of high volume glass powder concrete. Cem. Concr. Compos. 2017, 75, 22–29. [Google Scholar] [CrossRef]
- Somé, S.C.; Ben Fraj, A.; Pavoine, A.; Chehade, M.H. Modeling and experimental characterization of effective transverse thermal properties of hemp insulation concrete. Constr. Build. Mater. 2018, 189, 384–396. [Google Scholar] [CrossRef]
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Lu, J.-X.; Zhan, B.-J.; Duan, Z.-H.; Poon, C.S. Using glass powder to improve the durability of architectural mortar prepared with glass aggregates. Mater. Des. 2017, 135, 102–111. [Google Scholar] [CrossRef]
- Kalakada, Z.; Doh, J.; Zi, G. Utilisation of coarse glass powder as pozzolanic cement—A mix design investigation. Constr. Build. Mater. 2020, 240, 117916. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.-C.; Mo, K.H.; Shi, C. A critical review of waste glass powder—Multiple roles of utilization in cement-based materials and construction products. J. Environ. Manag. 2019, 242, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Ez-Zaki, H.; El Gharbi, B.; Diouri, A. Development of eco-friendly mortars incorporating glass and shell powders. Constr. Build. Mater. 2018, 159, 198–204. [Google Scholar] [CrossRef]
- Islam, G.S.; Rahman, M.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Murphy, T.; Wuhrer, R. High temperature performance of mortars containing fine glass powders. J. Clean. Prod. 2017, 162, 16–26. [Google Scholar] [CrossRef]
- Zheng, K. Pozzolanic reaction of glass powder and its role in controlling alkali–silica reaction. Cem. Concr. Compos. 2016, 67, 30–38. [Google Scholar] [CrossRef]
- Matos, A.M.; Sousa-Coutinho, J. Waste glass powder in cement: Macro and micro scale study. Adv. Cem. Res. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Shafaatian, S.M.H.; Wright, J.R.; Rajabipour, F. Performance of recycled soda–lime glass powder in mitigating alkali–silica reaction. Green Mater. 2019, 7, 28–39. [Google Scholar] [CrossRef]
- Bignozzi, M.; Saccani, A.; Barbieri, L.; Lancellotti, I. Glass waste as supplementary cementing materials: The effects of glass chemical composition. Cem. Concr. Compos. 2015, 55, 45–52. [Google Scholar] [CrossRef]
- Omran, A.; Soliman, N.; Zidol, A.; Tagnit-Hamou, A. Performance of Ground-Glass Pozzolan as a Cementitious Material—A Review. Adv. Civ. Eng. Mater. 2018, 7, 237–270. [Google Scholar] [CrossRef]
- Sinha, D.D.A. Thermal properties of concrete. PARIPEX-Indian J. Res. 2014, 3, 90–91. [Google Scholar] [CrossRef]
- Nguyen, L.; Beaucour, A.-L.; Ortola, S.; Noumowé, A. Experimental study on the thermal properties of lightweight aggregate concretes at different moisture contents and ambient temperatures. Constr. Build. Mater. 2017, 151, 720–731. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Yang, X. Changes in color and thermal properties of fly ash cement mortar after heat treatment. Constr. Build. Mater. 2018, 165, 72–81. [Google Scholar] [CrossRef]
- Liu, S.; Xie, G.; Wang, S. Effect of glass powder on microstructure of cement pastes. Adv. Cem. Res. 2015, 27, 259–267. [Google Scholar] [CrossRef]
- Zhang, L.; Gustavsen, A.; Jelle, B.P.; Yang, L.; Gao, T.; Wang, Y. Thermal conductivity of cement stabilized earth blocks. Constr. Build. Mater. 2017, 151, 504–511. [Google Scholar] [CrossRef]
- Sikora, P.; Horszczaruk, E.; Skoczylas, K.; Rucinska, T. Thermal Properties of Cement Mortars Containing Waste Glass Aggregate and Nanosilica. Procedia Eng. 2017, 196, 159–166. [Google Scholar] [CrossRef]







| Formula | Glass powder (%atom) | Cement (%atom) |
|---|---|---|
| C | 12.68 | 20.29 |
| O | 55.4 | 55.99 |
| Na | 7.07 | 0.20 |
| Mg | 0.17 | 0.47 |
| Al | 0.50 | 1.43 |
| Si | 20.69 | 3.93 |
| Ca | 3.59 | 15.73 |
| S | - | 0.89 |
| K | - | 0.53 |
| Ti | - | 0.05 |
| Fe | - | 0.47 |
| Tot | 100.00 | 100.00 |
| Formula | (a) %atom | (b) %atom | (c) %atom |
|---|---|---|---|
| C | 9.32 | 10.07 | 8.71 |
| O | 65.95 | 66.84 | 65.43 |
| Na | 0.15 | 0.33 | 1.45 |
| Mg | 0.51 | 0.45 | 0.42 |
| Al | 1.11 | 1.10 | 1.23 |
| Si | 7.47 | 7.88 | 10.49 |
| Ca | 14.34 | 12.31 | 11.38 |
| S | 0.41 | 0.49 | 0.45 |
| K | 0.22 | 0.16 | 0.13 |
| Fe | 0.51 | 0.37 | 0.30 |
| Tot | 100.00 | 100.00 | 100.00 |
| MGP (%) | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
|---|---|---|---|---|---|---|---|
| Wet | 2.1 | 2.07 | 2.05 | 2.03 | 2.01 | 2.00 | 1.98 |
| Dry | 2.06 | 1.97 | 1.95 | 1.94 | 1.90 | 1.86 | 1.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasry, O.; Samaouali, A.; Belarouf, S.; Moufakkir, A.; Sghiouri El Idrissi, H.; Soulami, H.; El Rhaffari, Y.; Hraita, M.; Fertahi, S.E.D.; Hafidi-Alaoui, A. Thermophysical Properties of Cement Mortar Containing Waste Glass Powder. Crystals 2021, 11, 488. https://doi.org/10.3390/cryst11050488
Nasry O, Samaouali A, Belarouf S, Moufakkir A, Sghiouri El Idrissi H, Soulami H, El Rhaffari Y, Hraita M, Fertahi SED, Hafidi-Alaoui A. Thermophysical Properties of Cement Mortar Containing Waste Glass Powder. Crystals. 2021; 11(5):488. https://doi.org/10.3390/cryst11050488
Chicago/Turabian StyleNasry, Oumaima, Abderrahim Samaouali, Sara Belarouf, Abdelkrim Moufakkir, Hanane Sghiouri El Idrissi, Houda Soulami, Younes El Rhaffari, Mohamed Hraita, Saïf Ed Dîn Fertahi, and Adil Hafidi-Alaoui. 2021. "Thermophysical Properties of Cement Mortar Containing Waste Glass Powder" Crystals 11, no. 5: 488. https://doi.org/10.3390/cryst11050488
APA StyleNasry, O., Samaouali, A., Belarouf, S., Moufakkir, A., Sghiouri El Idrissi, H., Soulami, H., El Rhaffari, Y., Hraita, M., Fertahi, S. E. D., & Hafidi-Alaoui, A. (2021). Thermophysical Properties of Cement Mortar Containing Waste Glass Powder. Crystals, 11(5), 488. https://doi.org/10.3390/cryst11050488







