3.1. Microstructure of DZ40M in As-Cast and Aged State
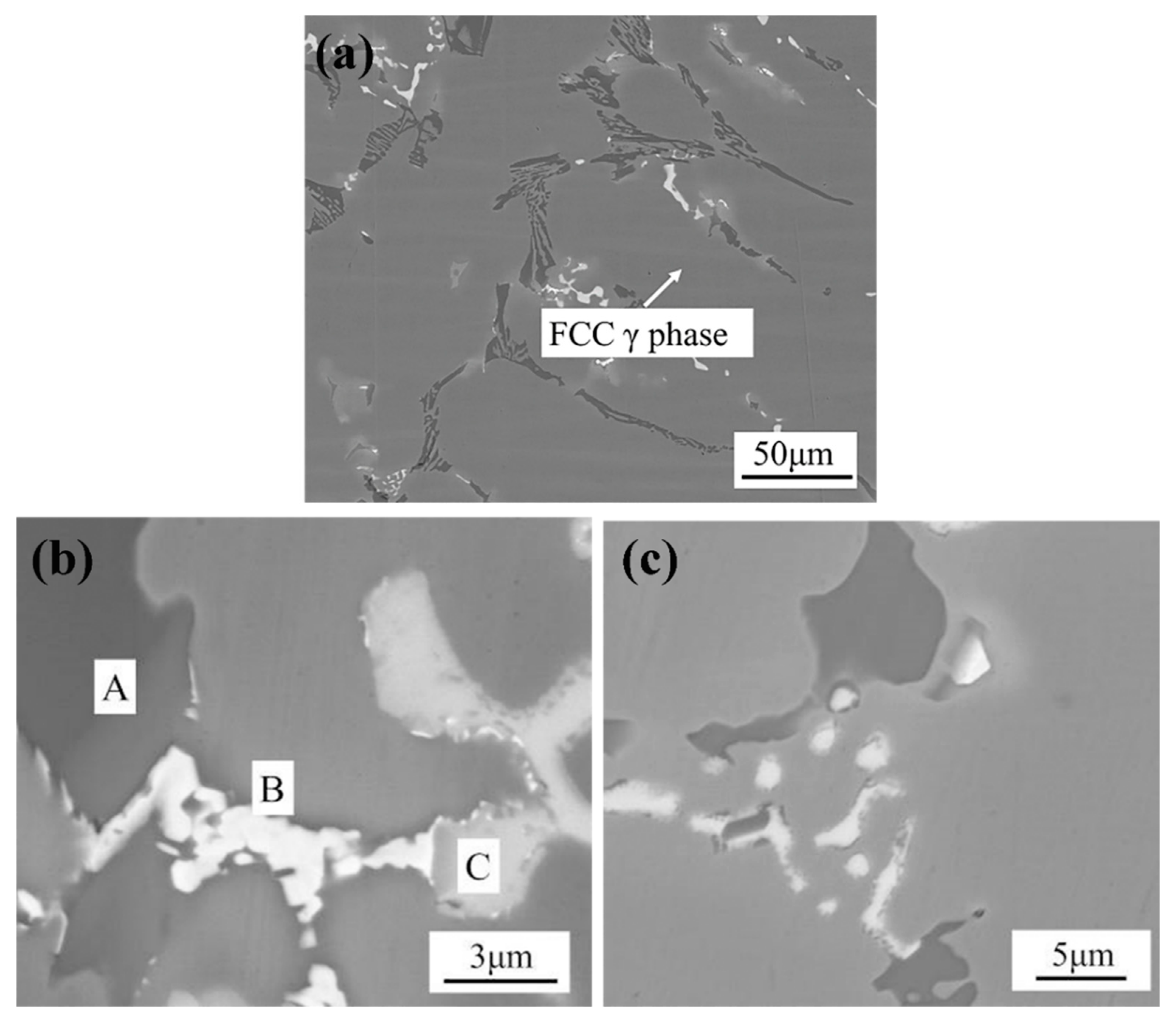
Figure 2 shows the typical microstructure of DZ40M in as-cast. It shows from
Figure 2a that the carbide skeleton structure is formed in the matrix of FCC cobalt-based solid solution phase, which plays a bearing role on the alloy matrix. The EDS data show that the carbide is composed of the Phase A, B, and C. As shown in
Table 2, Phase A is rich in Cr and has a small amount of Co. According to the previous study, it can be known that Phase A is the primary carbide of M
7C
3. M
7C
3 is a metastable phase with a triangular lattice of simple orthogonal structure, easy to decompose at high temperature. Phase B contains a certain amount of W, so it is presumed to be M
6C carbide. M
6C carbide has three kinds of morphology: granular, acicular and thin film. When it precipitates in the grain boundary or inside grain, it can improve the durability of the alloy, as shown in
Figure 2c. Phase C contains a considerable amount of Ta, Ti, and Zr, which can be speculated to be MC carbide. It is the important second phase in DZ40M.
According to Sims [
1], under high temperature conditions, some primary carbides in cobalt-based superalloys are prone to thermodynamic instability and decomposition, and fine secondary carbides are precipitated at the same time. The coarse primary carbides limit the plastic toughness of DZ40M, while the fine carbides can strengthen the fine crystal and ensure the plasticity of the base material. Therefore, in this paper, pre-bonding heat treatment is carried out on DZ40M at 1120 °C/5 h.
Figure 3 shows the typical microstructure of DZ40M in aged state. The coarse M
7C
3 carbide disappeared and there was white meshed phase in the matrix. M
23C
6 carbides distributed along grain boundaries and white spotty M
6C carbides were observed in
Figure 3b.
3.2. Typical Microstructure of TLP-Bonded Joints of DZ40M
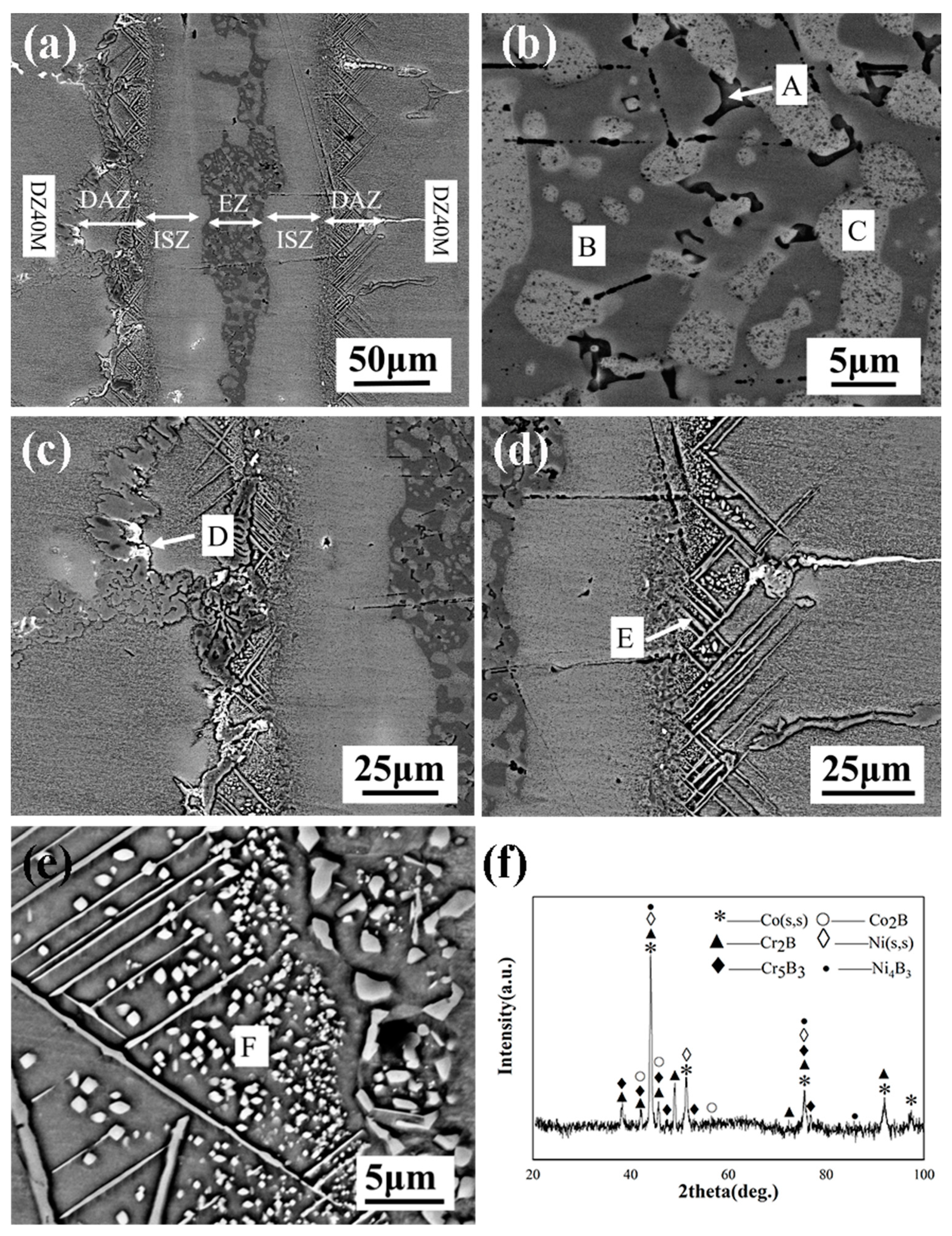
Figure 4 shows the typical microstructure of TLP-bonded joints of DZ40M at 1120 °C/30 min. From
Figure 4a, a large number of eutectic structures remain in the centerline of the joint, indicating that complete isothermal solidification of the joint has not been achieved under this processing parameter. 3 typical areas can be observed, i.e., eutectic zone (EZ), isothermal solidification zone (ISZ) and diffusion affected zone (DAZ). In EZ, dark gray Phase B was observed, in which light gray Phase C and lamellar black Phase A were distributed, which exhibits a binary segregation morphology nature [
12].
EDS compositional analysis of other elements (
Table 3) suggests that the intermetallic phase is a Ni rich boride, and the second phase is identified as a Ni-solid solution. According to Bakhtiari [
16], Phase A may be Cr-Ni rich boride, and Phase B may be Ni rich boride. The initial forming phase of the center line of the joint during cooling is a nickel-based solid solution (Phase C). Insufficient time for the diffusion of boron from the liquid interlayer to the base metal caused B element to gather near the centerline. Because the solubility of B in Ni is very low (only 0.3 at.%), B is easily precipitated in the form of borides. Near DAZ, flocculent Phase D was observed in
Figure 4c. In the process of bonding nickel-based superalloy by Zhang [
17], the precipitation phase of M
23(C,B)
6, which is similar to that of Phase D, was formed near DAZ. Therefore, it is speculated that Phase D is Cr rich boride and Co rich boride. DAZ mainly consists of acicular phase E and granular phase F. The EDS data show that the acicular phase is Co rich boride and the granular phase is Cr rich boride referring to [
18]. In order to further determine the phase composition of the welded joint, X-ray diffraction tests were carried out for fractures with a connection temperature of 1120 °C and a connection time of 30 min as shown in
Figure 4f. The results show that Co(s,s), Ni(s,s), Cr
2B, Cr
5B
3, Ni
4B
3, and Co
2B appeared in the joint. Analyzed with EDS results, Phase E may be Co
2B, and Phase F may be Cr
5B
3 and Cr
2B.
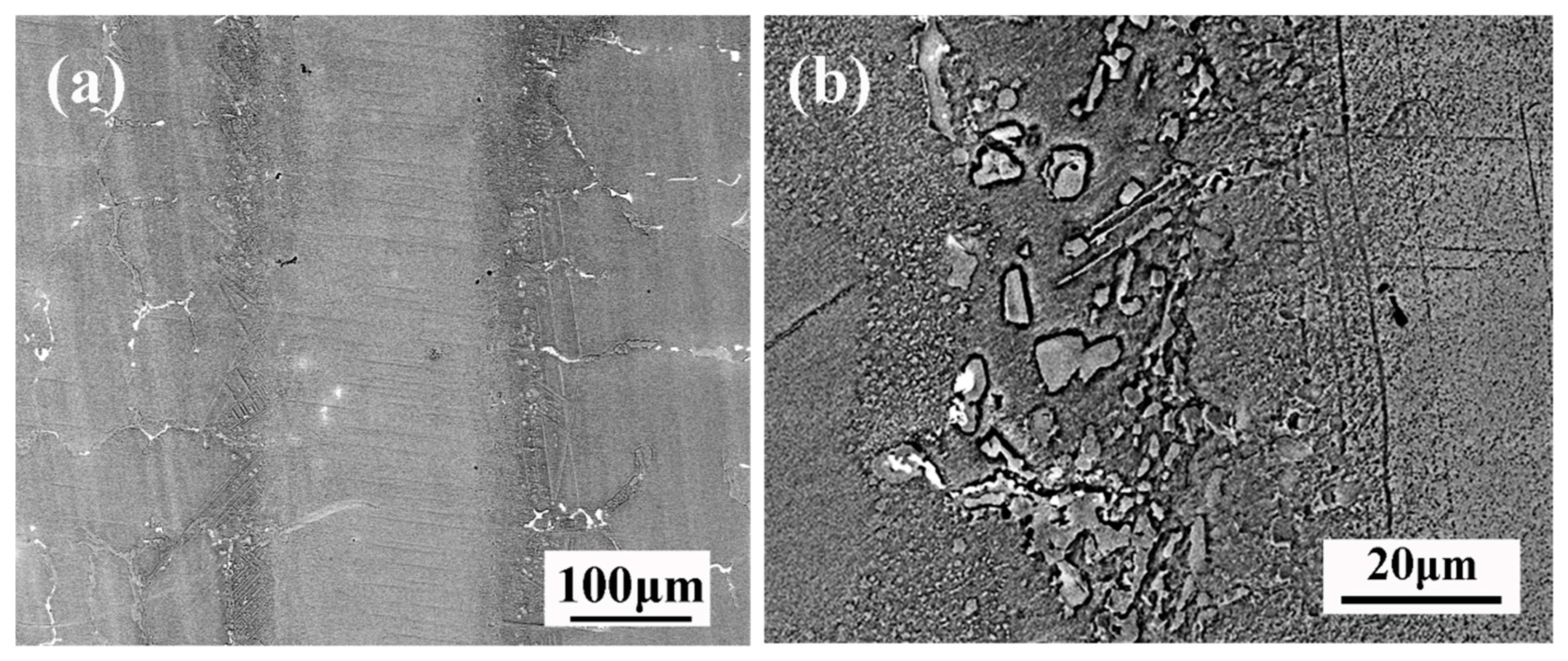
Figure 5 shows the typical microstructure of TLP-bonded joints of DZ40M at 1160 °C/30 min. From
Figure 5a, there is no eutectic phase in the joint. There are mainly some granular phases in DAZ as shown in
Figure 5b. According to
Table 3 and relevant literature, it can be judged that the phase is Cr rich boride. This indicates that the needle phase Co rich boride gradually disappears when the bonding temperature rises to 1160 °C. The melting point of Cr-B compound is higher than that of Co-B compound indicated by the Co-B and Cr-B phase diagram, which explains why the needle phase Co rich boride gradually dissolves while the granular phase Cr rich boride still exists after the temperature rises.
3.3. Microstructural Evolution of the Brazed Joint
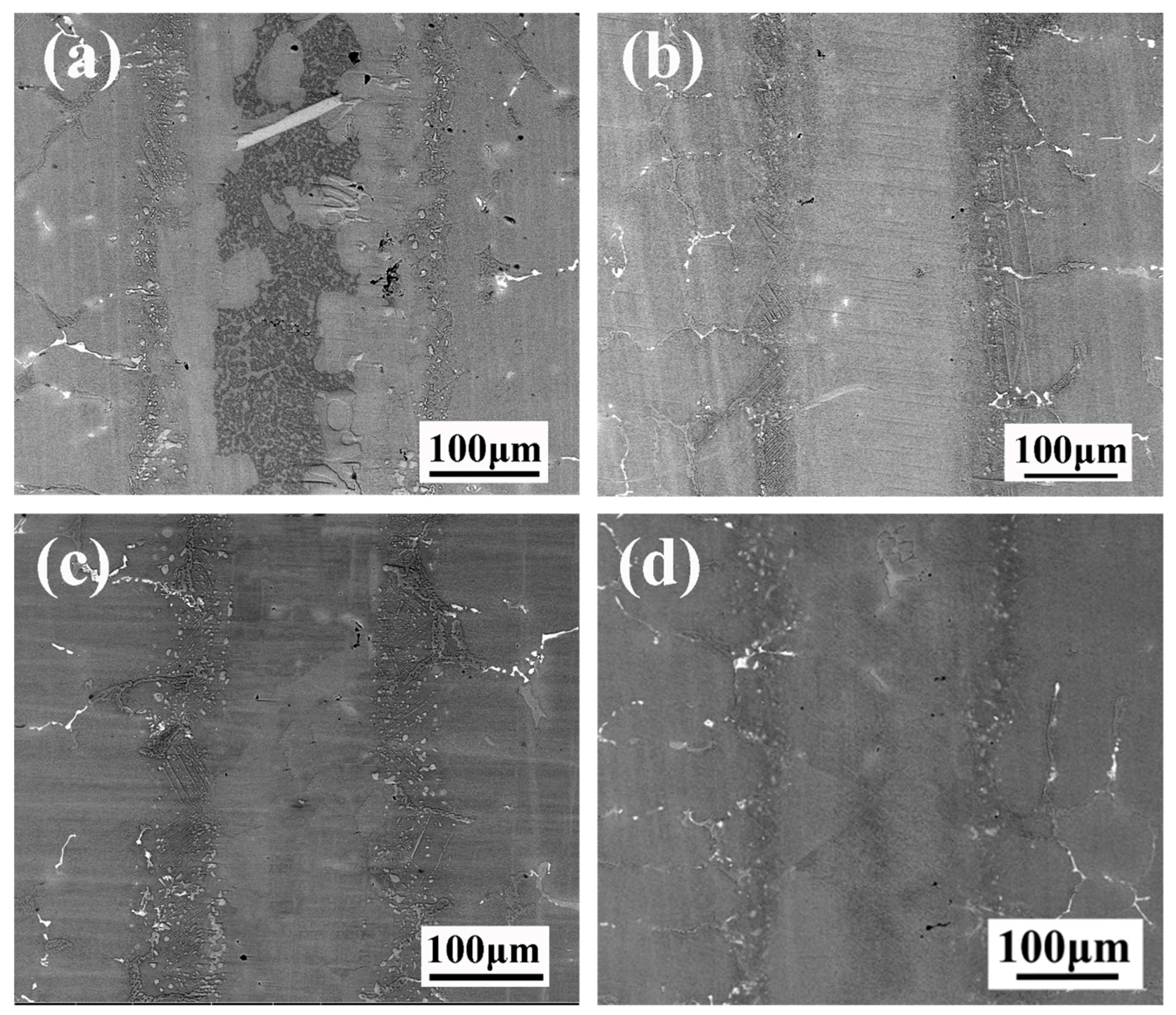
Figure 6 shows the microstructural evolution of TLP-bonded joints of DZ40M brazed for various durations. When the holding duration was 30 min, the commensal phase at the interface disappeared completely. With the extension of insulation time, the thickness of the diffusion zone tends to increase. The thickness of the diffusion zone is mainly related to the degree of element diffusion in the middle layer, and the diffusion distance of B dominates the formation of the diffusion zone. The diffusion coefficient is a commonly used index to measure the diffusion ability of a solute in a matrix which can be represented by the following equation [
19]:
where
D0 is the diffusion constant,
Q is the diffusion activation energy of solute in the matrix,
R is Avogadro’s constant, and
T is the temperature.
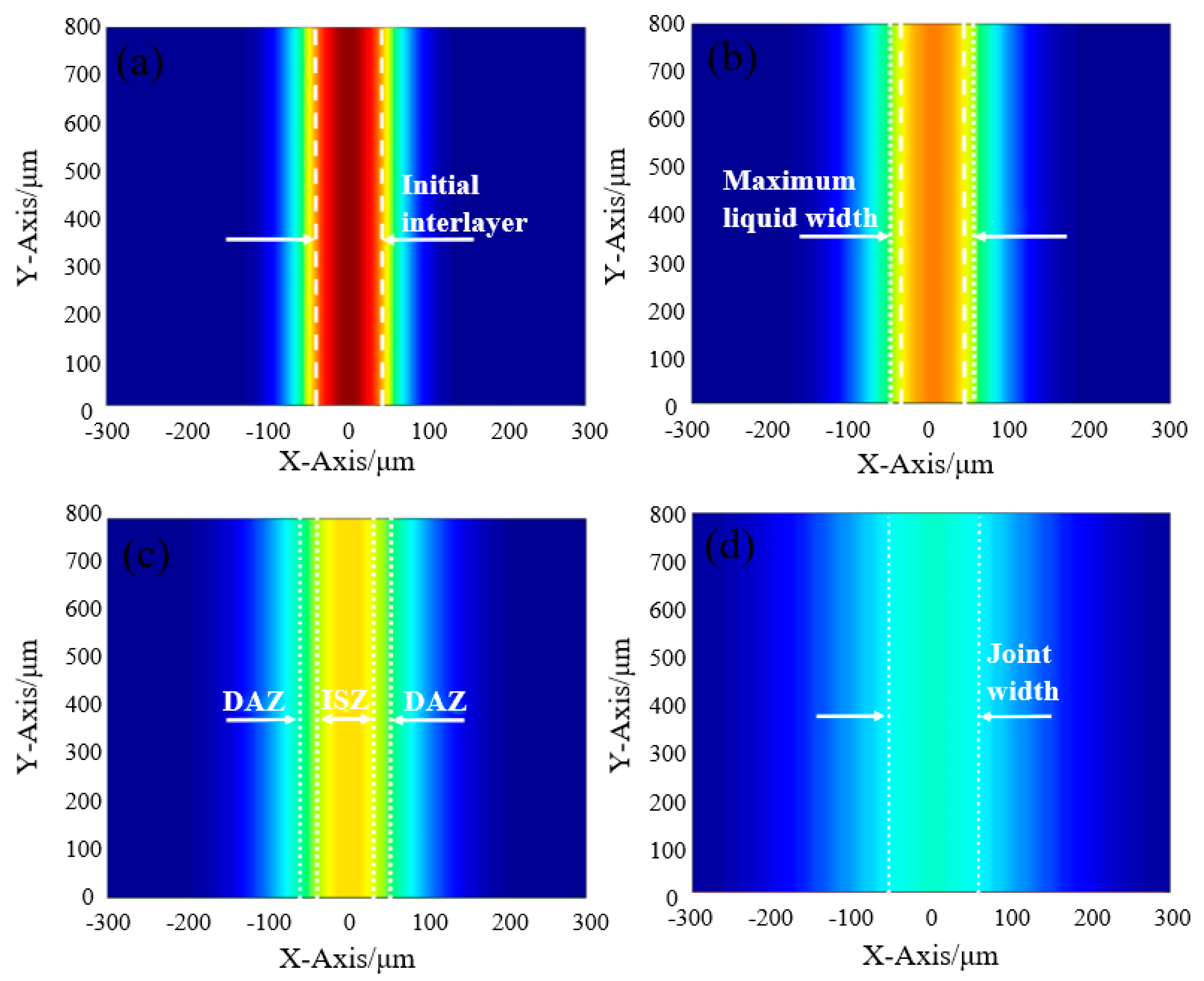
Based on the results above, we tried to propose the bonding mechanism of TLP-bonded joints of DZ40M, as shown in
Figure 7. In the initial stage, interlayer the concentration of B in the interlayer was approximately uniformly distributed, but B near the base metal diffused into the base metal, resulting in a high melting point on both sides of the intermediate layer. Therefore, melting occurred first in the center of the intermediate layer, and then the liquid phase spread to both sides until the mesosphere was completely melted. Afterwards, B diffused from liquid intermediate layer to solid base material.
However, because the diffusion rate of B in the liquid phase is much higher than that in the solid phase, a large amount of B was enriched on the solid base material surface. At the solid-liquid interface, the melting point of the base material decreased and melted, and the solid-liquid boundary expanded towards the base material as shown in
Figure 7b. When the content of B on the solid base material surface was not enough to reduce the melting point of the base material, the diffusion reached equilibrium and the solid-liquid interface did not expand. At this point, the intermediate layer reached the maximum liquid width. At the same time, the high melting point elements in the base material (such as Co, Cr, W, etc.) also diffused into the liquid phase, leading to the melting point of the intermediate layer liquid increasing continuously. As the intermediate layer near the base material contained more elements with high melting point and its melting point was higher, so it was preferred to solidify, as shown in
Figure 7c. The solid–liquid interface advanced from the base material to the middle, isothermal solidification began, and the cobalt solidified isothermally from the faying surfaces into the melt. Boron was gradually segregated due to the low solubility of cobalt base solid solution. When the segregation of B reached a certain extent of concentration, the elements of Co and Cr will react with B to needle and spherical borides.
If the holding time was not enough, the residual liquid phase in the joint were converted into eutectic layer, as shown in
Figure 7d. The microstructure of EZ can be understood by considering the solidification sequence of the remaining liquid during cooling. In the present system, on cooling, the initially formed phase is the Nickel-based solid solution in the form of dendrites growing from liquid/solid interface. During this process, B, solute elements with partition coefficient less than unity, are rejected into the liquid, enriching the liquid composition to the point where the primary solidification path intersects the moncovariant eutectic line separating solid solution and Ni boride. The solidification path then follows the eutectic line as the solid solution and nickel boride form simultaneously from the liquid by a monovariant eutectic reaction. Because the eutectic temperature of Cr-B binary eutectic system exceeded 1600 °C, it is impossible to form Cr-rich borides as the microcosmic component of binary eutectic system. The formation of nickel boride lead to the precipitation of Cr, which increased the content of Cr in the liquid phase. This is because the solubility of Cr in nickel boride (~10.11 at-%) is lower than that in solid solution (~18 at-%). Therefore, the liquid will concentrate to the ternary eutectic point and form ternary Cr boride, Ni boride, and C solid solution eutectic components at this point during the subsequent cooling process. This solidification path can be summarized as follows:
If enough, the joint completed isothermal solidification as shown in
Figure 7e. At this point, some Co rich boride granular phase and black secondary M
23C
6 precipitated phase appear in the joint.
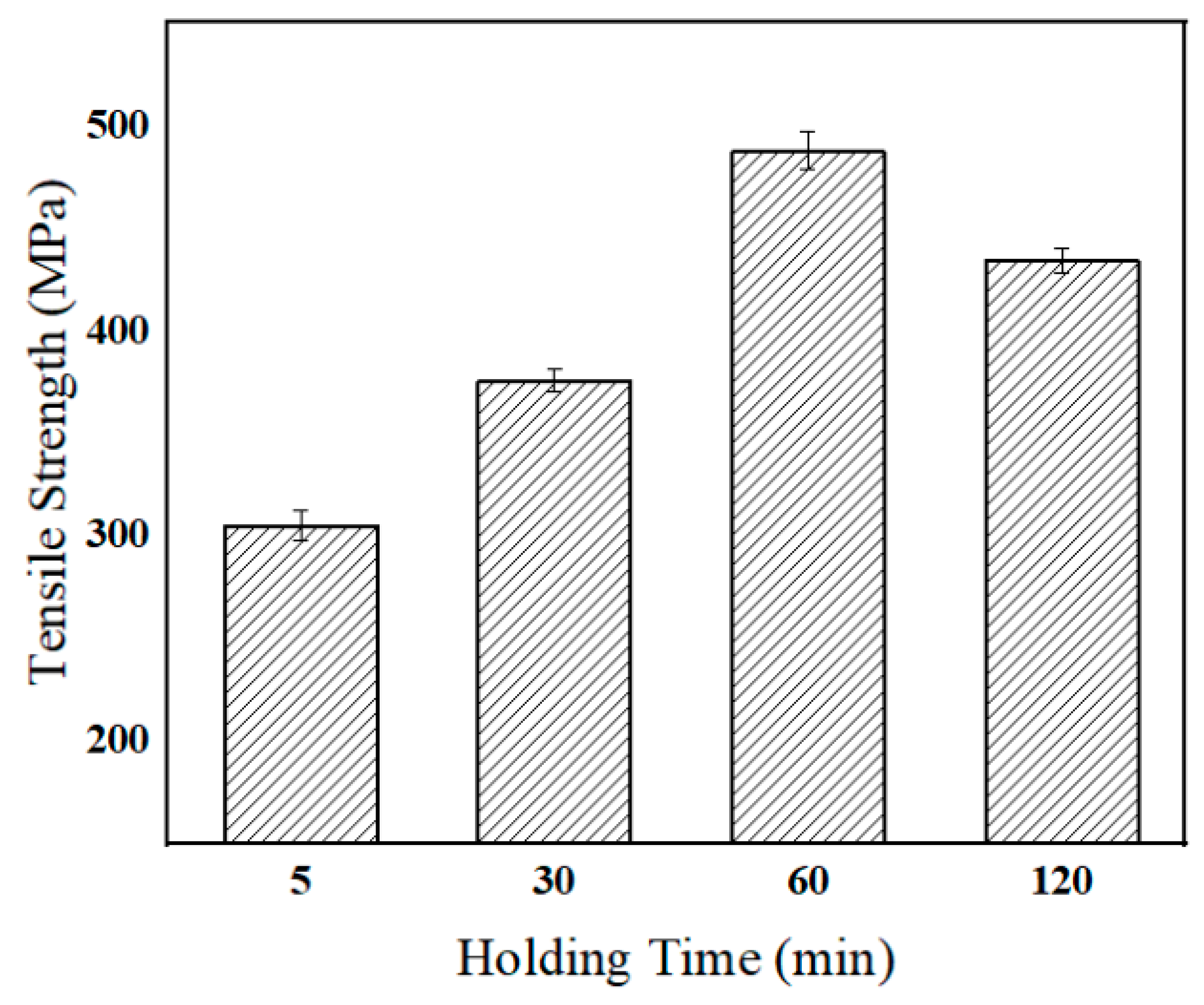
Figure 8 shows the tensile strength variation brazed with various holding duration at 1160 °C. With the increase of holding duration, the tensile strength of the joint tends to increase. When the holding duration was short, too many eutectic phases formed in the joint. When extending the holding duration, the eutectic phase gradually disappeared, which led to the increase of the joint property. When the heat preservation time was 60 min, the joint realized isothermal solidification and achieved the maximum tensile strength of 487 MPa. Theoretically speaking, the longer the holding time was, the more beneficial it was to realize the uniformity of joint tissue, and the higher tensile strength was. However, when the holding time was 120 min, the tensile strength of the joint decreased slightly to 434 MPa. This can be due to grain coarsening when the holding duration extended to 120 min, which normally weakens the joint strength [
20].
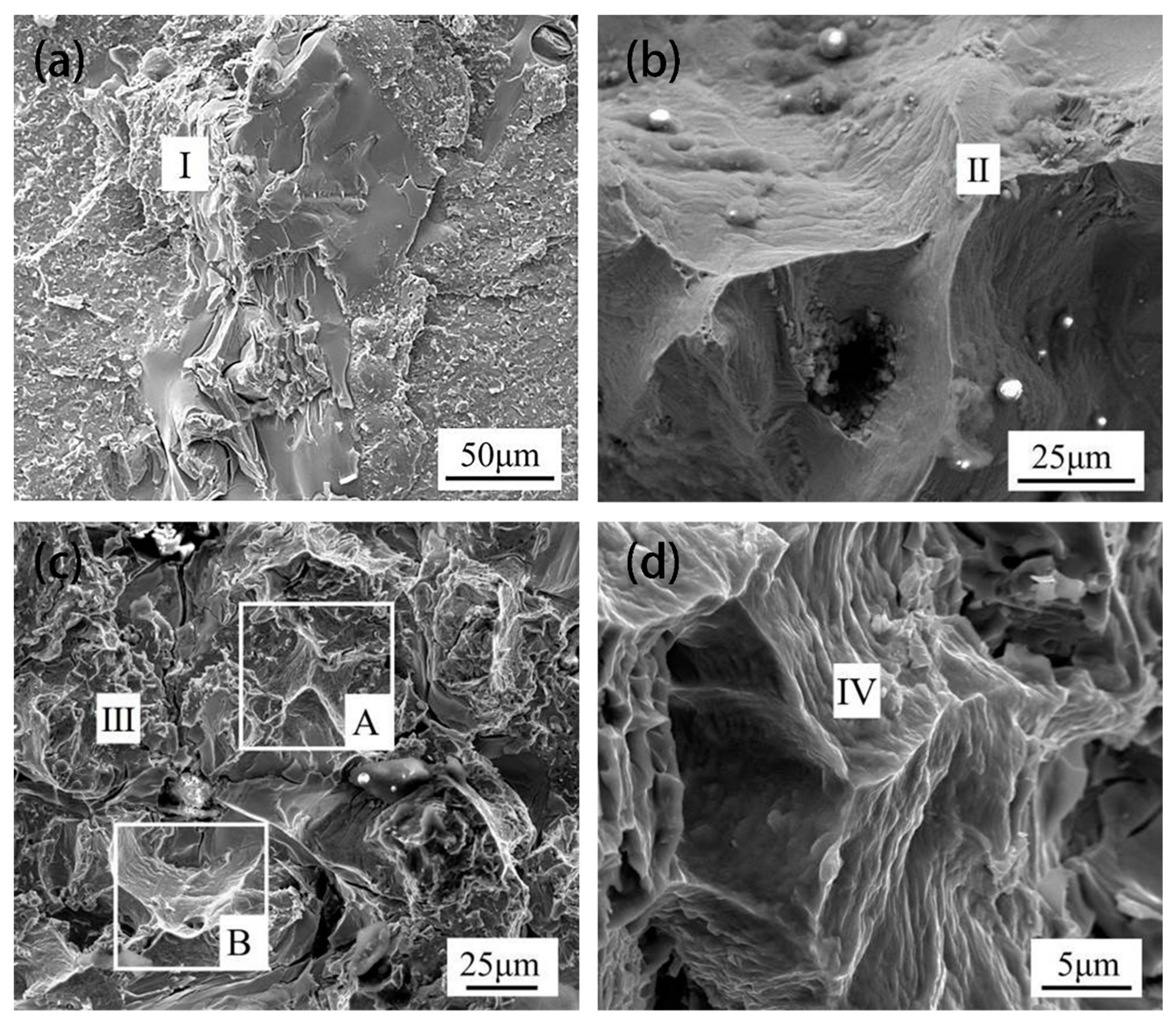
Figure 9 shows the fracture morphologies of TLP-bonded joints of DZ40M brazed with different holding duration at 1160 °C. In
Figure 9a, when the holding duration was 5 min, the cleavage plane appeared, accompanied by many rock-like patterns, indicating a brittle fracture. Binary eutectic phase is the weak part in the joint indicated by the EDS data shown in
Table 4. When holding for 30 min, there were many river patterns in the joint fracture, indicating that the joint was quite brittle as shown in
Figure 9b. The EDS data show that there were many borides located in DAZ in the fracture, making the DAZ a weak location for the joint. A small part of dimple morphology was observed in the fracture with holding time of 60 min, as shown in
Figure 9c at position A and radial patterns were observed at position B. There were rock-like patterns of intergranular fracture at other locations. It could be judged that there was a mixed ductile and brittle fracture for this case. Meanwhile, when holding for 120 min, a herringbone ridge pattern was observed in the fracture, which shows a ductile fracture nature.
3.4. Modeling Isothermal Solidification
The brittle eutectic phases with low melting point in the joint will damage the mechanical properties of the joint. Therefore, it is the focus of TLP diffusion bonding of DZ40M to determine the appropriate process to achieve complete isothermal solidification of the joint. Isothermal solidification depends on the diffusion of melting point depressant elements in the solid phase, so it is slow and usually takes several hours or even a day to complete.
In this study, the thickness and isothermal solidification time of EZ were analyzed by using a computational modeling method. To build a mathematical model, a few assumptions were made [
21]:
(1) TLP bonding is a one-dimensional diffusion process and the diffusion direction is perpendicular to the interface between the middle layer and the base material.
(2) The diffusion coefficient is constant and is not affected by the change of solute concentration during bonding.
(3) The solid/liquid phase interface maintains local equilibrium and the concentration follows the concentration of the equilibrium phase diagram.
(4) The solid/liquid phase interface remains stable and flat, and no phase precipitation is formed.
(5) Since the solute diffuses slowly in the solid phase, the base material is assumed to be a semi-infinite solid.
Fick’s second law was used to analyze solute diffusion in TLP [
22]:
According to initial conditions (
x > 0,
t = 0,
C = 0) and boundary conditions (
x = 0,
t > 0,
C =
C0), the general form of solute distribution [
23] can be solved:
where
A and
B are constants determined by specific boundary conditions;
D is the diffusion coefficient of solute in the matrix; t stands for isothermal solidification time. For the TLP system of DZ40M, when
x → ∞, erf(∞) = 1
where
is the initial solute concentration in the base metal. Let us assume that the solid/liquid interface moves to
x =
X(
t) after time
t. Hence,
where
is the solute concentration of the solid phase at the solid/liquid interface. According to the hypothesis (3), the above equation is true for all values of
t. Therefore,
X(
t) must be proportional to
t1/2 [
24]:
where
k is a constant that accounts for the moving boundary. Solving Equations (3)–(6) results in the following relation:
Since
k is an unknown constant, more equations are needed to solve
k. The mass balance at the solid/liquid interface gives the following relationship [
24]:
where (
) is the concentration difference between the liquid side and the solid side of the solute element and can be approximated as the concentration difference within the distance of
Δx.
represents the moving speed of the solid-liquid interface. Since
Δx is small, the process can be approximately uniform.
is the diffusion flux of the solute element within
Δx. Solving Equations (3)–(6) and Equation (8) results in the following relation that can be used to determine the dimensionless constant
k [
24]
From the base material composition of DZ40M,
CM = 0.015%. The
and
of MPD elements can be obtained from the phase diagram, as shown in
Table 5. The interface constant k was calculated, and the results are shown in
Table 5. Therefore, from Equation (7), the variation rule of the concentration distribution of B in the joint with time was obtained and the cloud diagram of B concentration change at 1160 °C is shown in
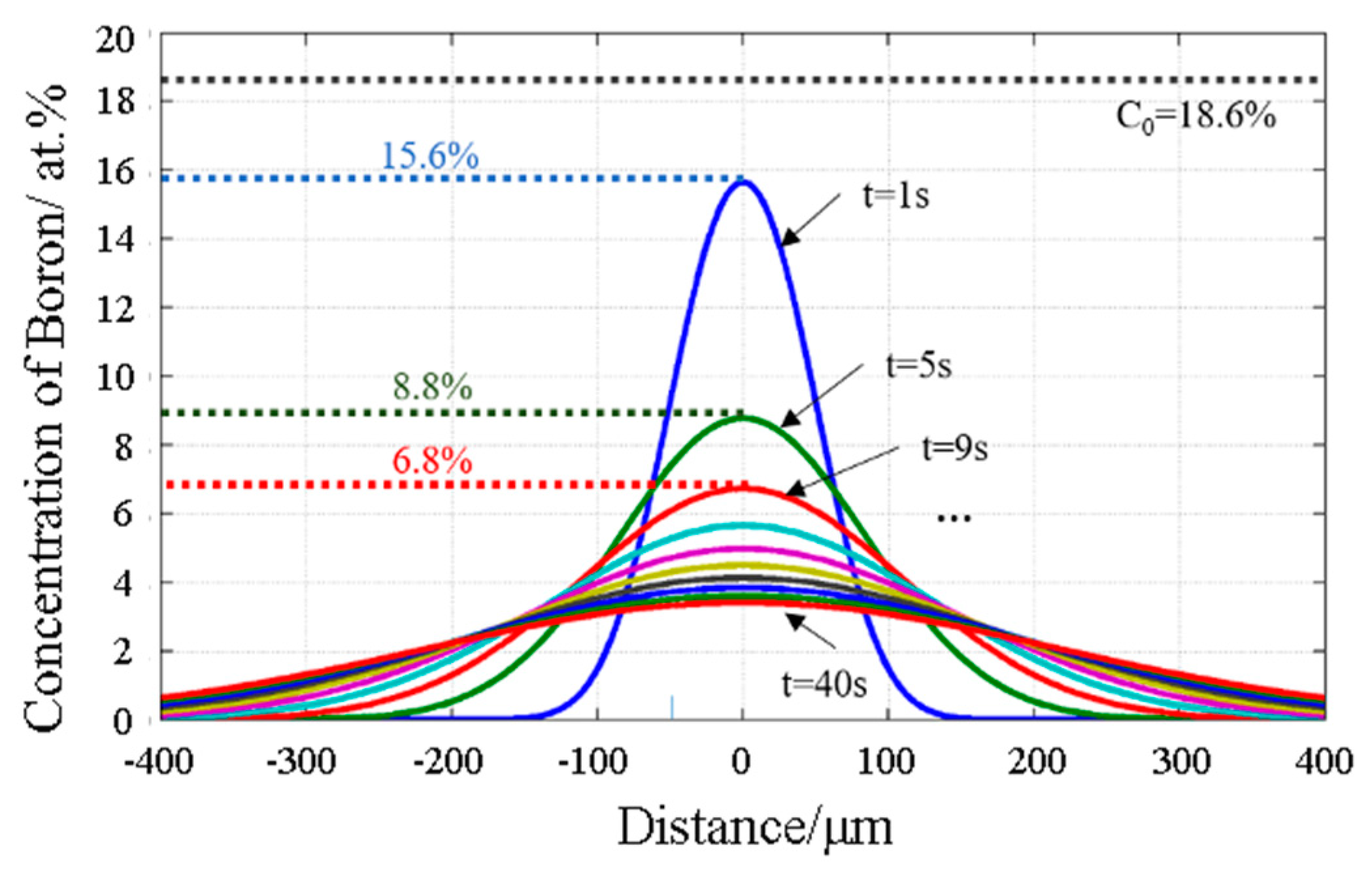
Figure 10.
The concentration of B in the joint center decreased rapidly within 1–10 s. At the 100 s, B in the joint dropped to a certain value, and the scope of the diffusion layer was expanded, but homogenization was not fully realized.
Figure 11 shows the concentration distribution curve of B at a time interval of 4 s at 1160 °C. The concentration of B in the joint center decreased rapidly at the initial stage of the insulation time, but decreased slowly after 20 s.
Zhou et al. [
25] proposed that the maximum liquid phase width had the following relationship with the initial thickness of the mesosphere:
where
and
are the densities of the intermediate layer and base material;
C0 is the initial concentration of B in the intermediate layer, which is 4 wt.% as shown in
Table 1; 2
d0 is the initial thickness of the intermediate layer and its size is 100 μm. Therefore, the maximum liquid phase thickness at 1160 °C were obtained, as shown in
Table 5. Assuming that the holding time of each test was
ts, the distance
X(
ts) moved by the solid/liquid phase interface was the thickness of ISZ, while the remaining liquid phase solidified into eutectic phase during the cooling process and formed EZ. Therefore, the thickness of EZ,
WEZ, was calculated by the following equation:
where
D is the diffusion coefficient, which can be represented by Equation (1). According to Khazaei et al. [
26], the diffusion constant is approximately 0.14 m
2/s and the diffusion activation energy is 229 KJ/mol. Therefore, the diffusion coefficient of B in cobalt-based superalloy at 1160 °C was calculated, as shown in
Table 5.
Figure 12a shows the rate of movement at the solid/liquid interface, namely the rate of isothermal solidification, obtained by taking the derivative of
X(
t) with respect to
t.
The rate of isothermal solidification dropped to a small value after a certain time and remained stable. The variation curve of EZ thickness with holding time at 1160 °C was drawn, as shown in
Figure 12b. With the increase of time, the slope absolute value of the curve was smaller, which means that the liquid phase isothermal solidification rate decreased. From Equation (6), the isothermal solidification time can be expressed as follows:
According to the data in the
Table 5, the time required to complete isothermal solidification of DZ40M joint were obtained. The calculation results show that when the bonding temperature was 1160 °C, the isothermal solidification time was 27.9 min. The experimental results show that there were a lot of eutectic phases in the joint when the holding time was 5 min. When the holding time was 30 min, the joint completed isothermal solidification, which indicated that the calculated results were within the reasonable range of the practical results.