Recent Developments in Carbon Nanotubes-Reinforced Ceramic Matrix Composites: A Review on Dispersion and Densification Techniques
Abstract
1. Introduction
2. Critical Challenges
2.1. Homogenous Dispersion
2.2. Suitable Interfacial Adhesion between CNTs and Ceramic
2.3. Thermal Degradation
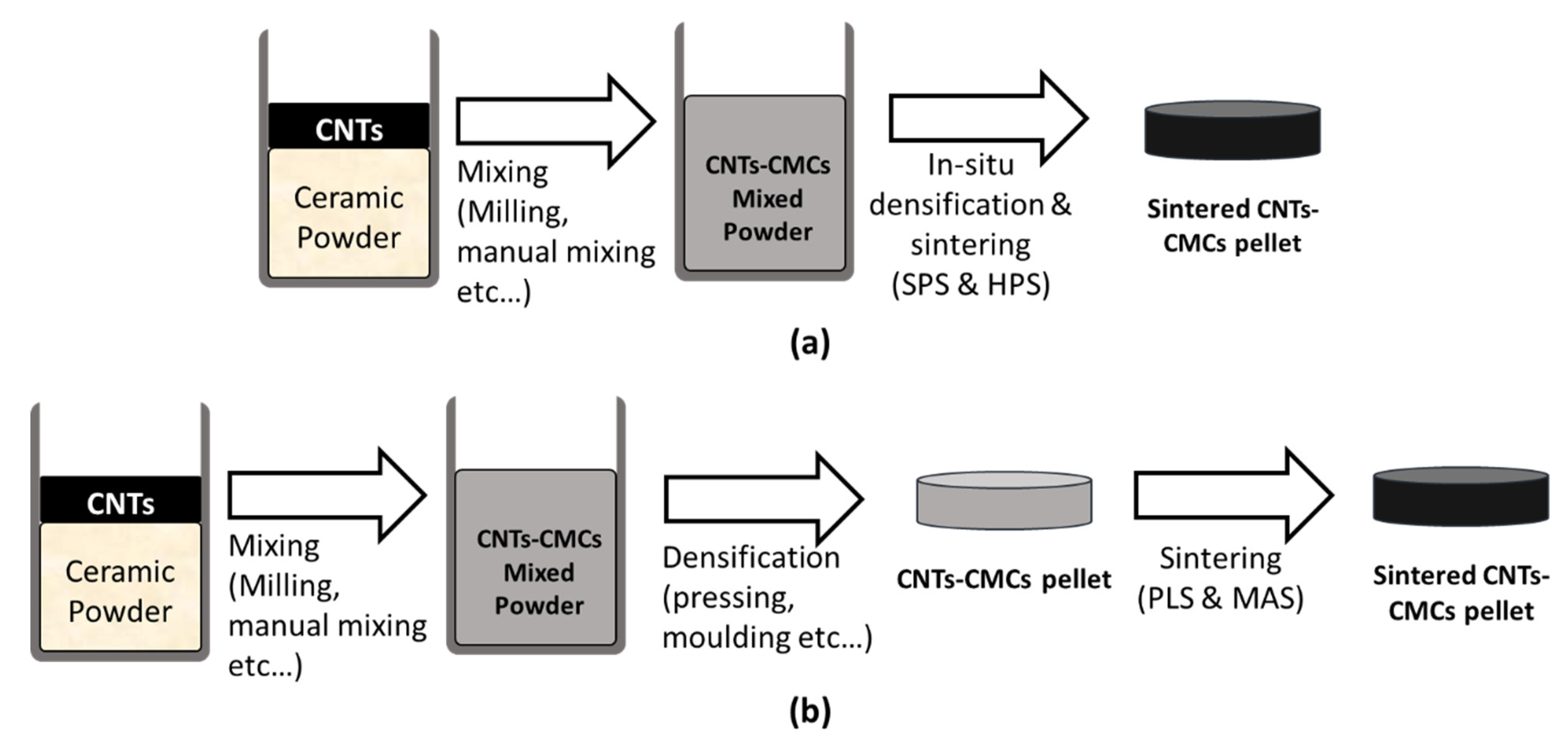
3. Processing Methods
3.1. Powder
3.2. Colloidal
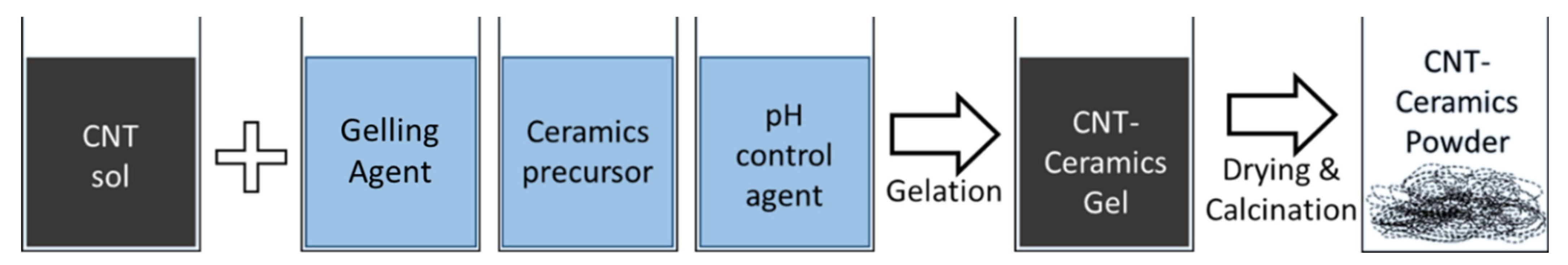
3.3. Sol–Gel
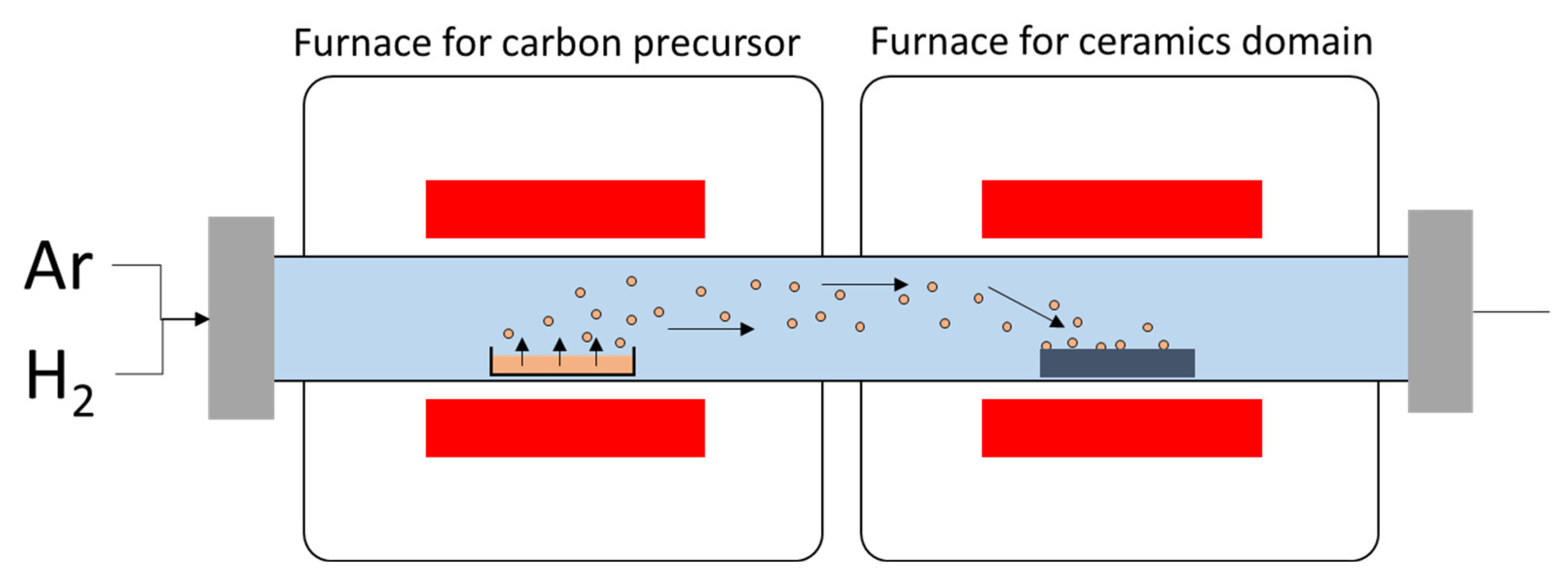
3.4. In Situ CNTs Growth
3.5. Hydrothermal/Solvothermal Processes
4. Densification and Sintering Techniques
4.1. Spark Plasma Sintering (SPS)
4.2. Hot-Press Sintering (HPS)
4.3. Pressureless Sintering (PLS)
4.4. Microwave-Assisted Sintering (MAS)
5. Enhanced Properties
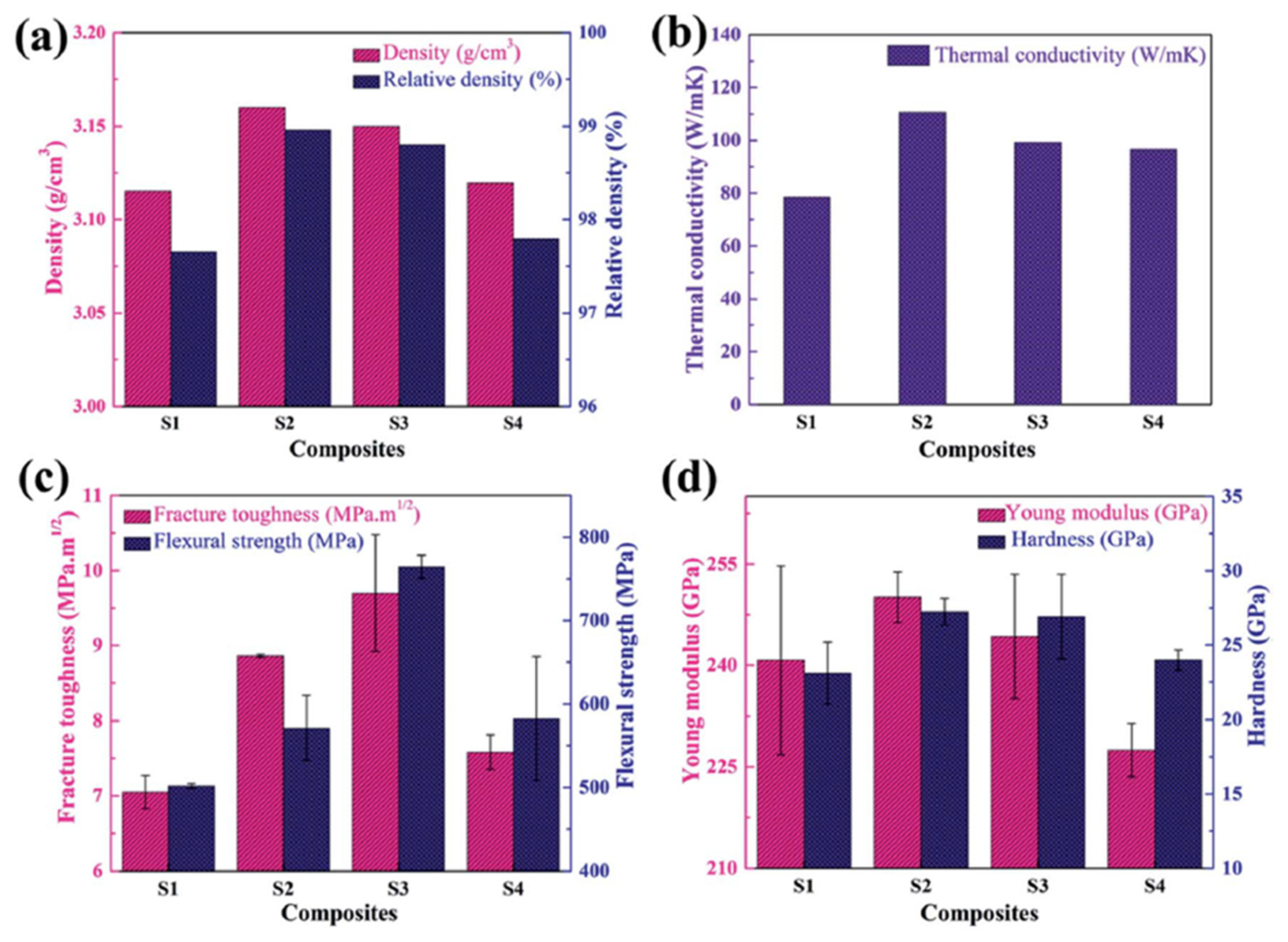
5.1. Microstructural Properties
5.2. Thermal Properties
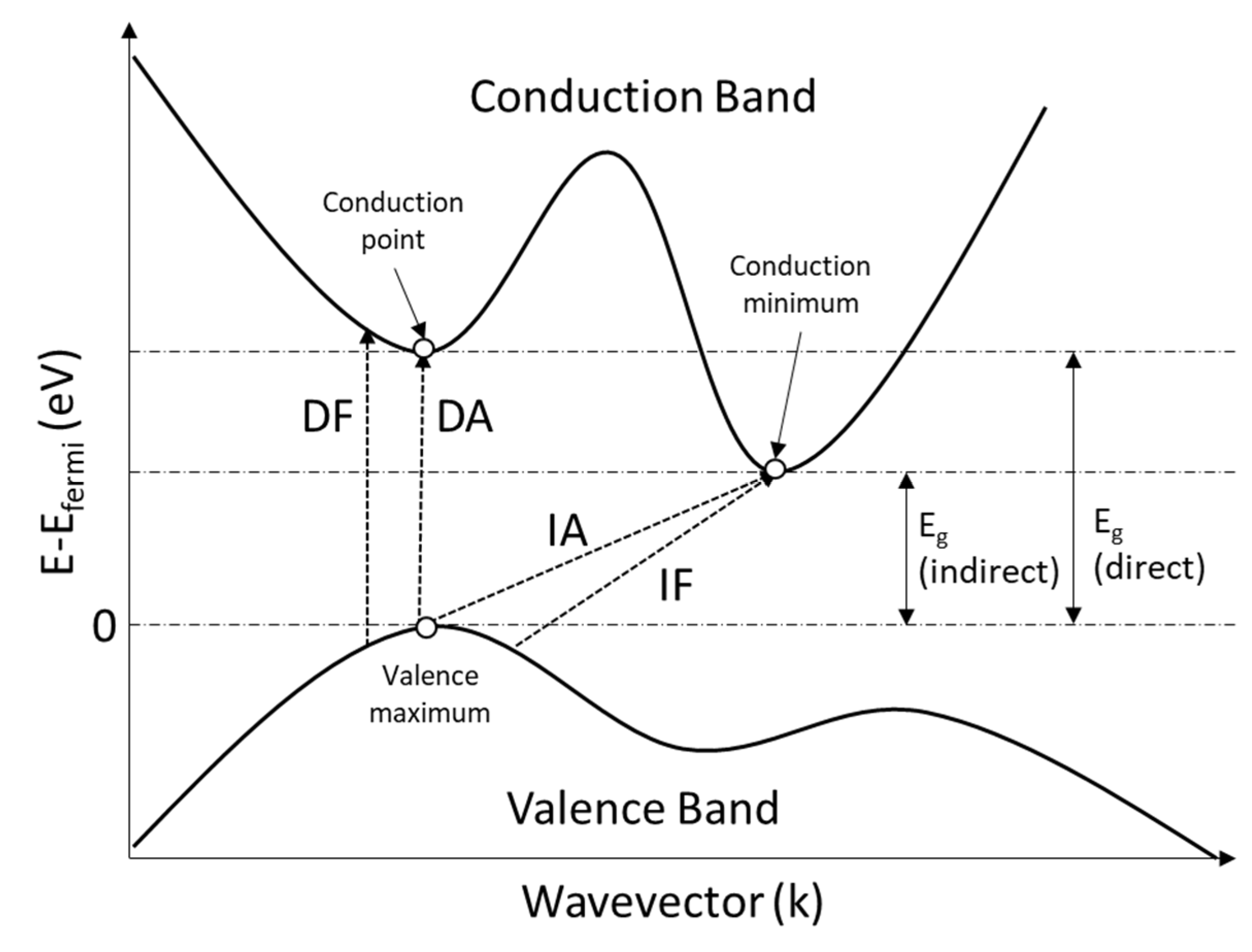
5.3. Optical Properties
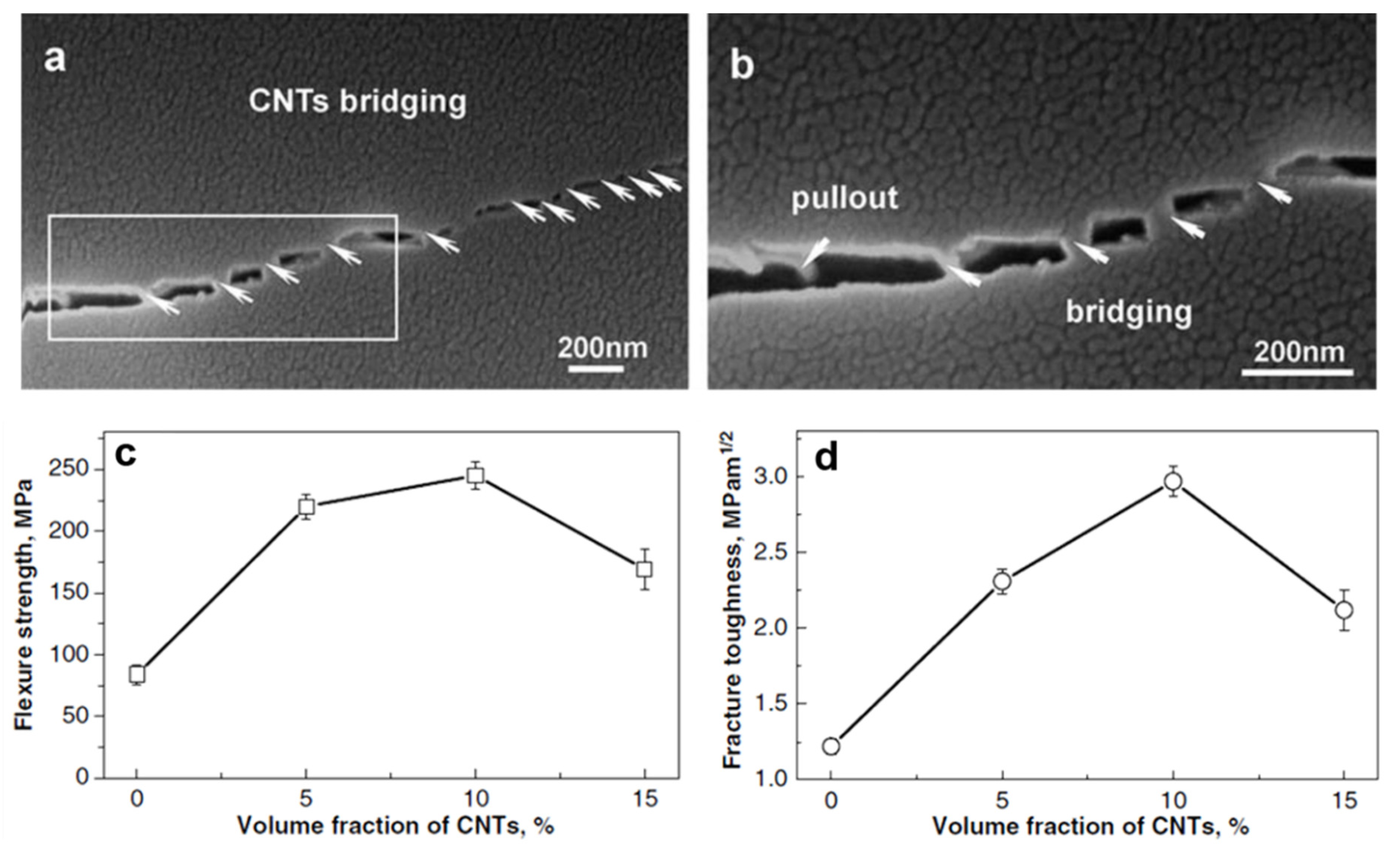
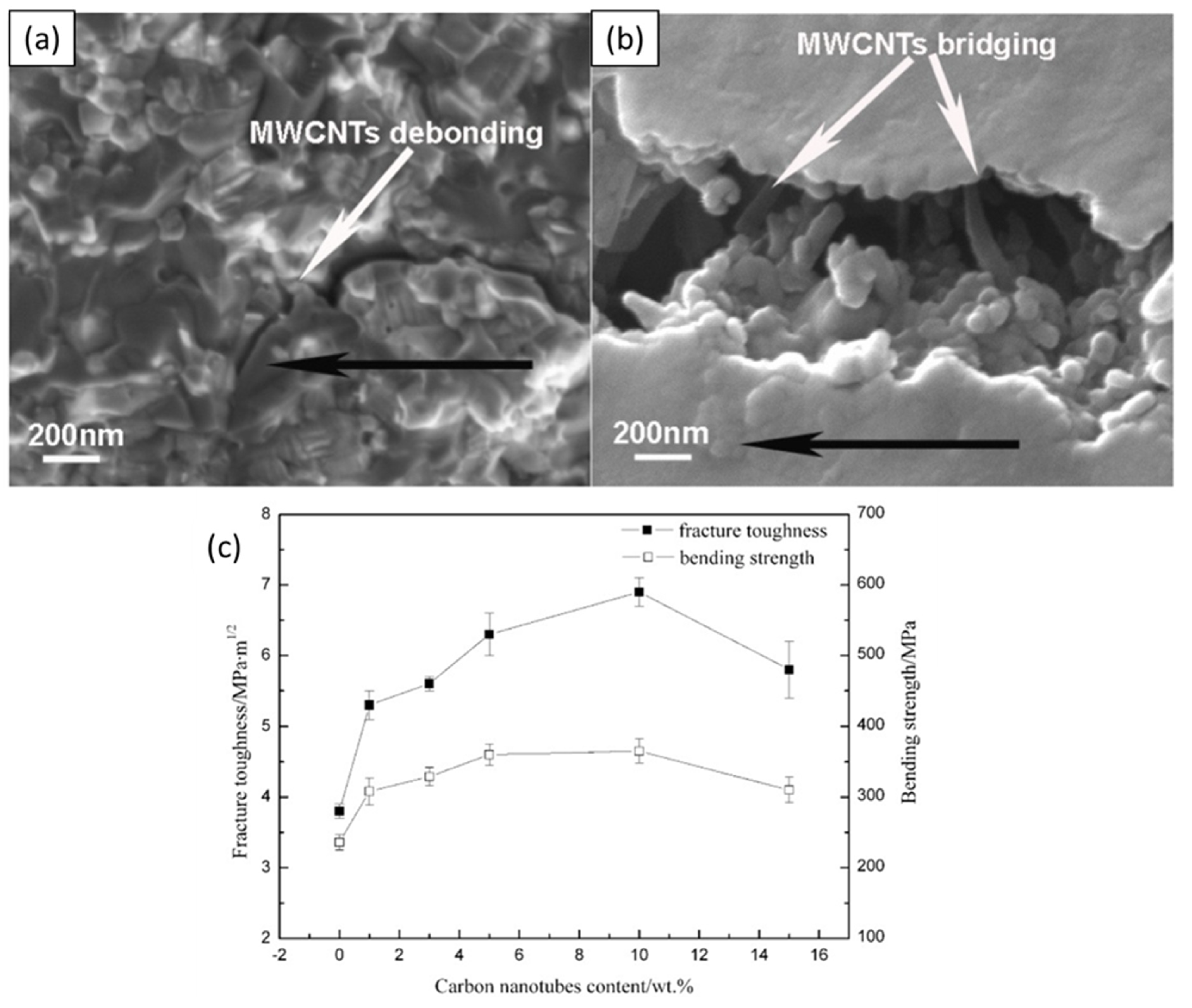
5.4. Mechanical Properties
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCE | Carbon ceramic electrode |
| CMCs | Ceramic matrix composites |
| CNTs | Carbon nanotubes |
| CNTs-CMCs | Carbon nanotube-incorporated ceramic matrix composites |
| CVD | Chemical vapour deposition |
| D | Thermal diffusivity |
| EG | Ethylene glycol |
| Eg | Optical band gap energy |
| HNO3 | Nitric acid |
| HPS | Hot-press sintering |
| HV | Vicker’s surface hardness |
| KIC | Fracture toughness |
| MAS | Microwave-assisted sintering |
| MWCNT | Multi-walled carbon nanotube |
| PLS | Pressureless sintering |
| PVP | Polyvinyl pyrrolidone |
| SPS | Spark plasma sintering |
| SWCNT | Single-walled carbon nanotube |
| κth | Thermal conductivity |
| ρ% | Relative density |
References
- Li, F.; Huang, X.; Liu, J.X.; Zhang, G.J. Sol–gel derived porous ultra-high temperature ceramics. J. Adv. Ceram. 2020, 9, 1–16. [Google Scholar] [CrossRef]
- Gorni, G.; Velázquez, J.J.; Mosa, J.; Balda, R.; Fernández, J.; Durán, A.; Castro, Y. Transparent glass-ceramics produced by Sol–Gel: A suitable alternative for photonic materials. Materials 2018, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, L.; Ferroni, L.; Gardin, C.; Zavan, B.; Secco, M.; Matthews, S.; Bernardo, E. Wollastonite-diopside glass-ceramic foams from supercritical carbon dioxide-assisted extrusion of a silicone resin and inorganic fillers. J. Non Cryst. Solids 2016, 443, 33–38. [Google Scholar] [CrossRef]
- Okada, K.; Hayashi, H.; Takesue, M.; Watanabe, M.; Smith, R.L. Synthesis of ferroelectric K1−xNaxNb1−yTayO3 nanoparticles using a supercritical water flow system. J. Supercrit. Fluids 2017, 123, 101–108. [Google Scholar] [CrossRef]
- Cheong, H.G.; Chu, C.N.; Kwon, K.K.; Song, K.Y. Micro-structuring silicon compound ceramics using nanosecond pulsed laser assisted by hydrothermal reaction. J. Manuf. Process. 2020, 50, 34–46. [Google Scholar] [CrossRef]
- Reka, A.A.; Pavlovski, B.; Makreski, P. New optimized method for low-temperature hydrothermal production of porous ceramics using diatomaceous earth. Ceram. Int. 2017, 43, 12572–12578. [Google Scholar] [CrossRef]
- Ramesh, S.; Sara Lee, K.Y.; Tan, C.Y. A review on the hydrothermal ageing behaviour of Y-TZP ceramics. Ceram. Int. 2018, 44, 20620–20634. [Google Scholar] [CrossRef]
- Palakurthy, S.; Azeem, P.A.; Venugopal Reddy, K.; Penugurti, V.; Manavathi, B. A comparative study on in vitro behavior of calcium silicate ceramics synthesized from biowaste resources. J. Am. Ceram. Soc. 2020, 103, 933–943. [Google Scholar] [CrossRef]
- Devi, K.B.; Lee, B.; Roy, A.; Kumta, P.N.; Roy, M. Effect of zinc oxide doping on in vitro degradation of magnesium silicate bioceramics. Mater. Lett. 2017, 207, 100–103. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, M.; Zhu, Y. Organosilicon polymer-derived ceramics: An overview. J. Adv. Ceram. 2019, 8, 457–478. [Google Scholar] [CrossRef]
- Song, Y.; He, L.; Zhang, X.; Liu, F.; Tian, N.; Tang, Y.; Kong, J. Highly efficient electromagnetic wave absorbing metal-free and carbon-rich ceramics derived from hyperbranched polycarbosilazanes. J. Phys. Chem. C 2017, 121. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. TrAC Trends Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- AfzaliTabar, M.; Alaei, M.; Ranjineh Khojasteh, R.; Motiee, F.; Rashidi, A.M. Preference of multi-walled carbon nanotube (MWCNT) to single-walled carbon nanotube (SWCNT) and activated carbon for preparing silica nanohybrid pickering emulsion for chemical enhanced oil recovery (C-EOR). J. Solid State Chem. 2017, 245, 164–173. [Google Scholar] [CrossRef]
- Nadeem, S.; Khan, A.U.; Hussain, S.T. Model based study of SWCNT and MWCNT thermal conductivities effect on the heat transfer due to the oscillating wall conditions. Int. J. Hydrogen Energy 2017, 42, 28945–28957. [Google Scholar] [CrossRef]
- Cao, R.; Chen, S.; Wang, Y.; Han, N.; Liu, H.; Zhang, X. Functionalized carbon nanotubes as phase change materials with enhanced thermal, electrical conductivity, light-to-thermal, and electro-to-thermal performances. Carbon 2019, 149, 263–272. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Rinzler, A.G.; Tomanek, D.; Colbert, D.T.; Smalley, R.E. Self-assembly of tubular fullerenes. J. Phys. Chem. 1995, 99, 10694–10697. [Google Scholar] [CrossRef]
- Fonseca, A.; Hernadi, K.; Nagy, J.B.; Bernaerts, D.; Lucas, A.A. Optimization of catalytic production and purification of buckytubes. J. Mol. Catal. A Chem. 1996, 107, 159–168. [Google Scholar] [CrossRef]
- Nikolaev, P.; Bronikowski, M.J.; Bradley, R.K.; Rohmund, F.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91–97. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, K.; Verma, V.; Bhatti, H.S. Investigation of optical properties of pristine and functionalized single-walled carbon nanotubes. J. Mater. Sci. Mater. Electron. 2015, 26, 2117–2126. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.C.; Hernández, E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef]
- Wang, Q.; Liew, K.M. Mechanical properties of carbon nanotubes. Carbon Nanotub. New Res. 2009, 260, 157–174. [Google Scholar] [CrossRef]
- Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Endo, M.; Terrones, M.; Dresselhaus, M.S. Thermal stability and structural changes of double-walled carbon nanotubes by heat treatment. Chem. Phys. Lett. 2004, 398, 87–92. [Google Scholar] [CrossRef]
- Aliev, A.E.; Lima, M.H.; Silverman, E.M.; Baughman, R.H. Thermal conductivity of multi-walled carbon nanotube sheets: Radiation losses and quenching of phonon modes. Nanotechnology 2010, 21. [Google Scholar] [CrossRef]
- Leahu, G.; Li Voti, R.; Larciprete, M.C.; Sibilia, C.; Bertolotti, M.; Nefedov, I.; Anoshkin, I.V. Thermal Characterization of Carbon Nanotubes by Photothermal Techniques. Int. J. Thermophys. 2015, 36, 1349–1357. [Google Scholar] [CrossRef]
- Tzounis, L.; Zappalorto, M.; Panozzo, F.; Tsirka, K.; Maragoni, L.; Paipetis, A.S.; Quaresimin, M. Highly conductive ultra-sensitive SWCNT-coated glass fiber reinforcements for laminate composites structural health monitoring. Compos. Part B Eng. 2019, 169, 37–44. [Google Scholar] [CrossRef]
- Subhani, T.; Shaffer, M.S.P.; Boccaccini, A.R. Carbon nanotube (CNT) reinforced glass and glass-ceramic matrix composites. In Ceramic Nanocomposites; Woodhead Publishing: Cambridge, UK, 2013; ISBN 9780857093387. [Google Scholar]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; Ramakrishna, S.; Berto, F. Carbon nanotubes (CNTs)-reinforced magnesium-based matrix composites: A comprehensive review. Materials 2020, 13, 4421. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, L.; Song, W.; Xu, D. Interfacial characteristics of carbon nanotube-polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 149–169. [Google Scholar] [CrossRef]
- Huang, S.J.; Abbas, A.; Ballóková, B. Effect of CNT on microstructure, dry sliding wear and compressive mechanical properties of AZ61 magnesium alloy. J. Mater. Res. Technol. 2019, 8, 4273–4286. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, B.; Zheng, J.C.; Tang, L.B.; An, C.S.; He, Z.J.; Tong, H.; Yu, W.J. Nano-micro structure VO2/CNTs composite as a potential anode material for lithium ion batteries. Ceram. Int. 2018, 44, 13113–13121. [Google Scholar] [CrossRef]
- Peng, T.; Zeng, P.; Ke, D.; Liu, X.; Zhang, X. Hydrothermal Preparation of Multiwalled Carbon Nanotubes (MWCNTs)/CdS Nanocomposite and Its Efficient Photocatalytic Hydrogen Production under Visible Light Irradiation. Energy Fuels 2011, 25, 2203–2210. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, J.; Wang, L.; Peng, F.; Liu, H.; Zhang, J.; Gu, J.; Guo, Z. In-situ pyrolyzed polymethylsilsesquioxane multi-walled carbon nanotubes derived ceramic nanocomposites for electromagnetic wave absorption. Ceram. Int. 2019, 45, 11756–11764. [Google Scholar] [CrossRef]
- Bazli, L.; Siavashi, M.; Shiravi, A. A Review of Carbon nanotube/TiO2 Composite prepared via Sol–Gel method. J. Compos. Compd. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Gao, C.; Feng, P.; Peng, S.; Shuai, C. Carbon nanotube, graphene and boron nitride nanotube reinforced bioactive ceramics for bone repair. Acta Biomater. 2017, 61, 1–20. [Google Scholar] [CrossRef]
- Jin, X.; Fan, X.; Lu, C.; Wang, T. Advances in oxidation and ablation resistance of high and ultra-high temperature ceramics modified or coated carbon/carbon composites. J. Eur. Ceram. Soc. 2018, 38, 1–28. [Google Scholar] [CrossRef]
- Lamnini, S.; Károly, Z.; Bódis, E.; Balázsi, K.; Balázsi, C. Influence of structure on the hardness and the toughening mechanism of the sintered 8YSZ/MWCNTs composites. Ceram. Int. 2019, 45, 5058–5065. [Google Scholar] [CrossRef]
- Jambagi, S.C.; Kar, S.; Brodard, P.; Bandyopadhyay, P.P. Characteristics of plasma sprayed coatings produced from carbon nanotube doped ceramic powder feedstock. Mater. Des. 2016, 112, 392–401. [Google Scholar] [CrossRef]
- Wei, H.; Yin, X.; Li, X.; Li, M.; Dang, X.; Zhang, L.; Cheng, L. Controllable synthesis of defective carbon nanotubes/Sc2Si2O7 ceramic with adjustable dielectric properties for broadband high-performance microwave absorption. Carbon 2019, 147, 276–283. [Google Scholar] [CrossRef]
- Li, H.; Song, X.; Li, B.; Kang, J.; Liang, C.; Wang, H.; Yu, Z.; Qiao, Z. Carbon nanotube-reinforced mesoporous hydroxyapatite composites with excellent mechanical and biological properties for bone replacement material application. Mater. Sci. Eng. C 2017, 77, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Rubel, R.I.; Ali, M.H.; Jafor, M.A.; Alam, M.M. Carbon nanotubes agglomeration in reinforced composites: A review. AIMS Mater. Sci. 2019, 6, 756–780. [Google Scholar] [CrossRef]
- Lanfant, B.; Leconte, Y.; Debski, N.; Bonnefont, G.; Pinault, M.; Mayne-L′Hermite, M.; Habert, A.; Jorand, Y.; Garnier, V.; Fantozzi, G.; et al. Mechanical, thermal and electrical properties of nanostructured CNTs/SiC composites. Ceram. Int. 2019, 45, 2566–2575. [Google Scholar] [CrossRef]
- Rivero-Antúnez, P.; Cano-Crespo, R.; Esquivias, L.; de la Rosa-Fox, N.; Zamora-Ledezma, C.; Domínguez-Rodríguez, A.; Morales-Flórez, V. Mechanical characterization of sol–gel alumina-based ceramics with intragranular reinforcement of multiwalled carbon nanotubes. Ceram. Int. 2020, 46, 19723–19730. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Singh, R.C.; Sharma, S. Hydrothermally synthesized MoS2-multi-walled carbon nanotube composite as a novel room-temperature ammonia sensing platform. Appl. Surf. Sci. 2020, 532, 147373. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Madhar, N.A.; Alzayed, N.S.; Asif, M. Uniform dispersion and exfoliation of multi-walled carbon nanotubes in CNT-MgB2 superconductor composites using surfactants. Materials 2019, 12, 3044. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yang, Y.; Zhang, H.; Lin, Q.; Zhu, B. Synthesis and characterization of in-situ CNTs reinforced TiB2-based composite by CVD using Ni catalysts. Ceram. Int. 2018, 44, 2042–2047. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Pan, B.; Pan, D.; Liu, L.; Hou, X.; Chu, M.; Kondoh, K.; Zhao, M. Regulation of interface between carbon nanotubes-aluminum and its strengthening effect in CNTs reinforced aluminum matrix nanocomposites. Carbon 2019, 155, 686–696. [Google Scholar] [CrossRef]
- Nor, A.F.M.; Sultan, M.T.H.; Jawaid, M.; Azmi, A.M.R.; Shah, A.U.M. Analysing impact properties of CNT filled bamboo/glass hybrid nanocomposites through drop-weight impact testing, UWPI and compression-after-impact behaviour. Compos. Part B Eng. 2019, 168, 166–174. [Google Scholar] [CrossRef]
- Kamalakaran, R.; Lupo, F.; Grobert, N.; Scheu, T.; Jin-Phillipp, N.Y.; Rühle, M. Microstructural characterization of C-SiC-carbon nanotube composite flakes. Carbon 2004, 42, 1–4. [Google Scholar] [CrossRef]
- Sharma, N.; Alam, S.N.; Ray, B.C.; Yadav, S.; Biswas, K. Silica-graphene nanoplatelets and silica-MWCNT composites: Microstructure and mechanical properties. Diam. Relat. Mater. 2018, 87, 186–201. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, W.H.; Xia, H.F.; Wang, Z.T.; Tang, L.B.; An, C.S.; He, Z.J.; Tong, H.; Zheng, J.C. V2(PO4)O/C@CNT hollow spheres with a core-shell structure as a high performance anode material for lithium-ion batteries. Mater. Chem. Front. 2019, 3, 456–463. [Google Scholar] [CrossRef]
- Javed, H.; Islam, M.; Mahmood, N.; Achour, A.; Hameed, A.; Khatri, N. Catalytic growth of multi-walled carbon nanotubes using NiFe2O4 nanoparticles and incorporation into epoxy matrix for enhanced mechanical properties. J. Polym. Eng. 2016, 36, 53–64. [Google Scholar] [CrossRef]
- Theodore, M.; Hosur, M.; Thomas, J.; Jeelani, S. Influence of functionalization on properties of MWCNT-epoxy nanocomposites. Mater. Sci. Eng. A 2011, 528, 1192–1200. [Google Scholar] [CrossRef]
- Shende, R.C.; Ramaprabhu, S. Thermo-optical properties of partially unzipped multiwalled carbon nanotubes dispersed nanofluids for direct absorption solar thermal energy systems. Sol. Energy Mater. Sol. Cells 2016, 157, 117–125. [Google Scholar] [CrossRef]
- Verma, V.; Galaveen, S.C.; Gurnani, L.; Venkateswaran, T.; Mukhopadhyay, A. Development of oxidation resistant and mechanically robust carbon nanotube reinforced ceramic composites. Ceram. Int. 2020, 46, 21784–21789. [Google Scholar] [CrossRef]
- Al-Gharabli, S.; Hamad, E.; Saket, M.; El-Rub, Z.A.; Arafat, H.; Kujawski, W.; Kujawa, J. Advanced material-ordered nanotubular ceramic membranes covalently capped with single-wall carbon nanotubes. Materials 2018, 11, 739. [Google Scholar] [CrossRef]
- Rongli, X.; Bian, D.; Aradhyula, T.V.; Chavali, M.; Zhao, Y. Preparation and corrosion behavior studies of chemically bonded phosphate ceramic coating reinforced with modified multi-walled carbon nanotubes (MWCNTs). Int. J. Appl. Ceram. Technol. 2019, 16, 923–930. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L. Densified multiwalled carbon nanotubes-titanium nitride composites with enhanced thermal properties. Ceram. Int. 2008, 34, 231–235. [Google Scholar] [CrossRef]
- Suslova, E.; Savilov, S.; Egorov, A.; Shumyantsev, A.; Lunin, V. Carbon nanotube frameworks by spark plasma sintering. Microporous Mesoporous Mater. 2020, 293, 109807. [Google Scholar] [CrossRef]
- Ding, M.; Sahebgharani, N.; Musharavati, F.; Jaber, F.; Zalnezhad, E.; Yoon, G.H. Synthesis and properties of HA/ZnO/CNT nanocomposite. Ceram. Int. 2018, 44, 7746–7753. [Google Scholar] [CrossRef]
- Boulerouah, A.; Longuemart, S.; Hus, P.; Sahraoui, A.H. Thermal transport investigation in a CNTs/solid matrix composite. J. Phys. D Appl. Phys. 2013, 46, 055302. [Google Scholar] [CrossRef]
- Esawi, A.; Morsi, K. Dispersion of carbon nanotubes (CNTs) in aluminum powder. Compos. Part A Appl. Sci. Manuf. 2007, 38, 646–650. [Google Scholar] [CrossRef]
- Yoshio, S.; Tatami, J.; Wakihara, T.; Yamakawa, T.; Nakano, H.; Komeya, K.; Meguro, T. Effect of CNT quantity and sintering temperature on electrical and mechanical properties of CNT-dispersed Si3N4 ceramics. J. Ceram. Soc. Jpn. 2011, 119, 70–75. [Google Scholar] [CrossRef][Green Version]
- Tatami, J.; Katashima, T.; Komeya, K.; Meguro, T.; Wakihara, T. Electrically conductive CNT-dispersed silicon nitride ceramics. J. Am. Ceram. Soc. 2005, 88, 2889–2893. [Google Scholar] [CrossRef]
- Mazaheri, M.; Mari, D.; Schaller, R.; Bonnefont, G.; Fantozzi, G. Processing of yttria stabilized zirconia reinforced with multi-walled carbon nanotubes with attractive mechanical properties. J. Eur. Ceram. Soc. 2011, 31, 2691–2698. [Google Scholar] [CrossRef]
- Venkateswarlu, M.; Ashok Kumar, M.; Hema Chandra Reddy, K. Thermal Behavior of Spark Plasma Sintered Ceramic Matrix-Based Nanocomposites. J. Bio-Tribo-Corrosion 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Esawi, A.M.K.; Morsi, K.; Sayed, A.; Taher, M.; Lanka, S. The influence of carbon nanotube (CNT) morphology and diameter on the processing and properties of CNT-reinforced aluminium composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 234–243. [Google Scholar] [CrossRef]
- Popov, O.; Vleugels, J.; Zeynalov, E.; Vishnyakov, V. Reactive hot pressing route for dense ZrB2-SiC and ZrB2-SiC-CNT ultra-high temperature ceramics. J. Eur. Ceram. Soc. 2020, 301, 127065. [Google Scholar] [CrossRef]
- Ye, F.; Liu, L.; Wang, Y.; Zhou, Y.; Peng, B.; Meng, Q. Preparation and mechanical properties of carbon nanotube reinforced barium aluminosilicate glass-ceramic composites. Scr. Mater. 2006, 55, 911–914. [Google Scholar] [CrossRef]
- Chan, K.F.; Mohd Zaid, M.H.; Liza, S.; Matori, K.A.; Mamat, M.S.; Hazan, M.A.; Yaakob, Y. Effect of CNT on microstructural properties of Zn2SiO4/CNT composite via dry powder processing. Mater. Res. Express 2020, 7, 105601. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Gojny, F.H.; Nastalczyk, J.; Roslaniec, Z.; Schulte, K. Surface modified multi-walled carbon nanotubes in CNT/epoxy-composites. Chem. Phys. Lett. 2003, 370, 820–824. [Google Scholar] [CrossRef]
- Manzetti, S.; Andersen, O. A molecular dynamics study of nanoparticle-formation from bioethanol-gasoline blend emissions. Fuel 2016, 183, 55–63. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Adsorption of phenolic compounds by carbon nanotubes: Role of aromaticity and substitution of hydroxyl groups. Environ. Sci. Technol. 2008, 42, 7254–7259. [Google Scholar] [CrossRef]
- Gotovac, S.; Honda, H.; Hattori, Y.; Takahashi, K.; Kanoh, H.; Kaneko, K. Effect of nanoscale curvature of single-walled carbon nanotubes on adsorption of polycyclic aromatic hydrocarbons. Nano Lett. 2007, 7, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, L.; Li, W. Colloidal Processing of Carbon Nanotube/Alumina Composites. Chem. Mater. 2002, 14, 5169–5172. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Shi, L.; Zhang, C.; Yang, X.Z.; Liang, J. Preparation and properties of alumina composites modified by electric field-induced alignment of carbon nanotubes. Appl. Phys. A Mater. Sci. Process. 2007, 89, 761–767. [Google Scholar] [CrossRef]
- Arvanitelis, C.; Jayaseelan, D.D.; Cho, J.; Boccaccini, A.R. Carbon nanotube–SiO2 composites by colloidal processing. Adv. Appl. Ceram. 2008, 107, 155–158. [Google Scholar] [CrossRef]
- Phuoc, T.X.; Massoudi, M.; Chen, R.H. Viscosity and thermal conductivity of nanofluids containing multi-walled carbon nanotubes stabilized by chitosan. Int. J. Therm. Sci. 2011, 50, 12–18. [Google Scholar] [CrossRef]
- Fadhillahanafi, N.M.; Leong, K.Y.; Risby, M.S. Stability and thermal conductivity characteristics of carbon nanotube based nanofluids. Int. J. Automot. Mech. Eng. 2013, 8, 1376–1384. [Google Scholar] [CrossRef]
- Jajja, S.A.; Ali, W.; Ali, H.M. Multiwalled carbon nanotube nanofluid for thermal management of high heat generating computer processor. Heat Transf. Asian Res. 2014, 43, 653–666. [Google Scholar] [CrossRef]
- Hordy, N.; Rabilloud, D.; Meunier, J.L.; Coulombe, S. A Stable Carbon Nanotube Nanofluid for Latent Heat-Driven Volumetric Absorption Solar Heating Applications. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Walvekar, R.; Siddiqui, M.K.; Ong, S.S.; Ismail, A.F. Application of CNT nanofluids in a turbulent flow heat exchanger. J. Exp. Nanosci. 2016, 11, 1–17. [Google Scholar] [CrossRef]
- Rashidi, S.; Rashmi, W.; Abdullah, L.C.; Khalid, M.; Ahmadun, F.R.; Faizah, M.Y. Thermal stability and conductivity of carbon nanotube nanofluid using xanthan gum as surfactant. Sains Malays. 2017, 46, 1017–1024. [Google Scholar] [CrossRef]
- Keinänen, P.; Siljander, S.; Koivula, M.; Sethi, J.; Sarlin, E.; Vuorinen, J.; Kanerva, M. Optimized dispersion quality of aqueous carbon nanotube colloids as a function of sonochemical yield and surfactant/CNT ratio. Heliyon 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Nasirzadehroshenin, F.; Sadeghzadeh, M.; Khadang, A.; Maddah, H.; Ahmadi, M.H.; Sakhaeinia, H.; Chen, L. Modeling of heat transfer performance of carbon nanotube nanofluid in a tube with fixed wall temperature by using ANN–GA. Eur. Phys. J. Plus 2020, 135. [Google Scholar] [CrossRef]
- Poon, R.; Liang, W.; Zhitomirsky, I. Mn3O4 and (ZnFe)OOH Composites for Supercapacitors with High Active Mass. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 855–862. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Shi, J.; Han, R.; Wu, P.; Yue, X.; Zhou, Z.; Chen, G.X.; Li, Q. High dielectric constant composites controlled by a strontium titanate barrier layer on carbon nanotubes towards embedded passive devices. Chem. Eng. J. 2019, 373, 642–650. [Google Scholar] [CrossRef]
- Xu, R.; Bian, D.; Zhao, Y.; Xu, X.; Liu, Y.; Zhou, W. Tribological behavior studies of chemically bonded phosphate ceramic coatings reinforced with modified multi-walled carbon nanotubes (MWCNTs). Int. J. Appl. Ceram. Technol. 2020, 17, 1010–1016. [Google Scholar] [CrossRef]
- Roushani, M.; Karami, M.; Zare Dizajdizi, B. Amperometric NADH sensor based on a carbon ceramic electrode modified with the natural carotenoid crocin and multi-walled carbon nanotubes. Microchim. Acta 2017, 184, 473–481. [Google Scholar] [CrossRef]
- Schebeliski, A.H.; Lima, D.; Marchesi, L.F.Q.P.; Calixto, C.M.F.; Pessôa, C.A. Preparation and characterization of a carbon nanotube-based ceramic electrode and its potential application at detecting sulfonamide drugs. J. Appl. Electrochem. 2018, 48, 471–485. [Google Scholar] [CrossRef]
- Ferrag, C.; Noroozifar, M.; Kerman, K. Thiol functionalized carbon ceramic electrode modified with multi-walled carbon nanotubes and gold nanoparticles for simultaneous determination of purine derivatives. Mater. Sci. Eng. C 2020, 110, 110568. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Nasirizadeh, N.; Mirjalili, M. Enhanced degradation of reactive dyes using a novel carbon ceramic electrode based on copper nanoparticles and multiwall carbon nanotubes. Chin. J. Chem. Eng. 2020, 28, 318–327. [Google Scholar] [CrossRef]
- Mombeshora, E.T.; Simoyi, R.; Nyamori, V.O.; Ndungu, P.G. Multiwalled carbon nanotube-titania nanocomposites: Understanding nano-structural parameters and functionality in dye-sensitized solar cells. S. Afr. J. Chem. 2015, 68, 153–164. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Jeon, S.J.; Kim, H.T. Inexpensive sol–gel synthesis of multiwalled carbon nanotube-TiO2 hybrids for high performance antibacterial materials. Mater. Sci. Eng. C 2016, 68, 780–788. [Google Scholar] [CrossRef]
- Pohl, M.; Kurig, H.; Tallo, I.; Jänes, A.; Lust, E. Novel sol–gel synthesis route of carbide-derived carbon composites for very high power density supercapacitors. Chem. Eng. J. 2017, 320, 576–587. [Google Scholar] [CrossRef]
- Li, W.; Ji, W.; Isfahani, F.T.; Wang, Y.; Li, G.; Liu, Y.; Xing, F. Nano-silica sol–gel and carbon nanotube coupling effect on the performance of cement-based materials. Nanomaterials 2017, 7, 185. [Google Scholar] [CrossRef]
- dos Santos, P.A.M.; de Oliveira Almeida, V.; Benvenutti, E.V.; Balzaretti, N.M.; Gallas, M.R.; Costa, T.M.H. High-grade MWCNT/ZrO2 composites prepared by sol–gel method and high-pressure technique (4.0 GPa): Mechanically resistant, porous, and conductive. J. Sol–Gel Sci. Technol. 2019, 90, 348–358. [Google Scholar] [CrossRef]
- Park, J.E.; Jang, Y.S.; Bae, T.S.; Lee, M.H. Biocompatibility characteristics of titanium coated with multiwalled carbon nanotubes-hydroxyapatite nanocomposites. Materials 2019, 12, 224. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Kang, P.; Wu, P.; Zhou, Z.; Chen, G.-X.; Li, Q. Improve the dielectric property and breakdown strength of composites by cladding a polymer/BaTiO3 composite layer around carbon nanotubes. Polymer 2020, 188, 122157. [Google Scholar] [CrossRef]
- Chen, M.; Yin, X.; Li, M.; Chen, L.; Cheng, L.; Zhang, L. Electromagnetic interference shielding properties of silicon nitride ceramics reinforced by in situ grown carbon nanotubes. Ceram. Int. 2015, 41, 2467–2475. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Synthesis and characterization of ceramic/carbon nanotubes composite adsorptive membrane for copper ion removal from water. Korean J. Chem. Eng. 2015, 32, 292–298. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Xiao, G.; Li, Z.; Bai, B.; Ren, J.; He, G. Enhanced electromagnetic wave absorbing properties of Si-O-C ceramics with in-situ formed 1D nanostructures. Int. J. Appl. Ceram. Technol. 2020, 17, 734–744. [Google Scholar] [CrossRef]
- Rosenburg, F.; Ionescu, E.; Nicoloso, N.; Riedel, R. High-temperature Raman spectroscopy of nano-crystalline carbon in silicon oxycarbide. Materials 2018, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Caņado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size la of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 1–4. [Google Scholar] [CrossRef]
- Mazumder, S.; Sarkar, N.; Park, J.G.; Han, I.S.; Kim, I.J. Carbon nanotubes-porous ceramic composite by in situ CCVD growth of CNTs. Mater. Chem. Phys. 2016, 171, 247–251. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Zhang, H.; Su, B.; Yang, Y. Optimization of CNTs growth on TiB2-based composite powders by CVD with Fe as catalyst. Ceram. Int. 2020, 46, 3837–3843. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, D.; Yu, J.; Xie, Y.; Hu, W.; Jiao, F. Multi-walled carbon nanotubes modified Bi2S3 microspheres for enhanced photocatalytic decomposition efficiency. Ceram. Int. 2017, 43, 15080–15088. [Google Scholar] [CrossRef]
- Li, L.; Sun, Q.; Chen, X.; Jiang, Z.; Xu, Y. Significantly improved dielectric performance of poly(1-butene)-based composite films via filling polydopamine modified Ba(Zr0.2Ti0.8)O3-coated multiwalled carbon nanotubes nanoparticles. Polymers 2021, 13, 285. [Google Scholar] [CrossRef]
- Vadivel, S.; Naveen, A.N.; Theerthagiri, J.; Madhavan, J.; Santhoshini Priya, T.; Balasubramanian, N. Solvothermal synthesis of BiPO4 nanorods/MWCNT (1D-1D) composite for photocatalyst and supercapacitor applications. Ceram. Int. 2016, 42, 14196–14205. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, L. Preparation and Catalytic Activity of M2O3/CNTs (M = Y, Nd, Sm) Nanocomposites by Solvothermal Process. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Khan, M.; Yousaf, A.B.; Chen, M.; Wei, C.; Wu, X.; Huang, N.; Qi, Z.; Li, L. Molybdenum sulfide/graphene-carbon nanotube nanocomposite material for electrocatalytic applications in hydrogen evolution reactions. Nano Res. 2016, 9, 837–848. [Google Scholar] [CrossRef]
- Naoi, K.; Kurita, T.; Abe, M.; Furuhashi, T.; Abe, Y.; Okazaki, K.; Miyamoto, J.; Iwama, E.; Aoyagi, S.; Naoi, W.; et al. Ultrafast Nanocrystalline-TiO2(B)/Carbon Nanotube Hyperdispersion Prepared via Combined Ultracentrifugation and Hydrothermal Treatments for Hybrid Supercapacitors. Adv. Mater. 2016, 28, 6751–6757. [Google Scholar] [CrossRef]
- Jiang, L.; Qiu, Y.; Luo, P.; Yu, Y. Nickel hydroxide-impregnated and -coated carbon nanotubes using an easily manipulated solvothermal route for supercapacitors. Ceram. Int. 2016, 42, 11634–11639. [Google Scholar] [CrossRef]
- Munir, Z.A.; Anselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the spark plasma sintering method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Zhan, G.D.; Kuntz, J.D.; Wan, J.; Mukherjee, A.K. Single-wall carbon nanotubes as attractive toughening agents in alumina-based nanocomposites. Nat. Mater. 2003, 2, 38–42. [Google Scholar] [CrossRef]
- Zhan, G.D.; Kuntz, J.D.; Garay, J.E.; Mukherjee, A.K. Electrical properties of nanoceramics reinforced with ropes of single-walled carbon nanotubes. Appl. Phys. Lett. 2003, 83, 1228–1230. [Google Scholar] [CrossRef]
- Momohjimoh, I.; Saheb, N.; Hussein, M.A.; Laoui, T.; Al-Aqeeli, N. Electrical conductivity of spark plasma sintered Al2O3–SiC and Al2O3-carbon nanotube nanocomposites. Ceram. Int. 2020, 46, 16008–16019. [Google Scholar] [CrossRef]
- Karthiselva, N.S.; Bakshi, S.R. Carbon nanotube and in-situ titanium carbide reinforced titanium diboride matrix composites synthesized by reactive spark plasma sintering. Mater. Sci. Eng. A 2016, 663, 38–48. [Google Scholar] [CrossRef]
- Barmin, A.; Bortnikova, V.; Ivanov, A.; Kornev, V.; Lurie, S.; Solyaev, Y. Microstructure and mechanical properties of silicon carbide ceramics reinforced with multi-walled carbon nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2016, 124. [Google Scholar] [CrossRef]
- Delbari, S.A.; Nayebi, B.; Ghasali, E.; Shokouhimehr, M.; Shahedi Asl, M. Spark plasma sintering of TiN ceramics codoped with SiC and CNT. Ceram. Int. 2019, 45, 3207–3216. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Y.; Qu, Y.; Ma, R.; Fan, R. Radio-frequency negative permittivity of carbon nanotube/copper calcium titanate ceramic nanocomposites fabricated by spark plasma sintering. Ceram. Int. 2020, 46, 2261–2267. [Google Scholar] [CrossRef]
- Wang, J.; Kou, H.; Liu, X.; Pan, Y.; Guo, J. Reinforcement of mullite matrix with multi-walled carbon nanotubes. Ceram. Int. 2007, 33, 719–722. [Google Scholar] [CrossRef]
- Yuan, G.; Li, Y.; Long, X.; Cui, Z.; Dong, Z.; Cong, Y.; Zhang, J.; Li, X. Tuning anisotropic thermal conductivity of unidirectional carbon/carbon composites by incorporating carbonaceous fillers. J. Mater. Sci. 2020, 55, 5079–5098. [Google Scholar] [CrossRef]
- Ding, C.; Gan, W.; Hu, X.; Wu, K.; Wang, X. Investigation into the influence of carbon nanotubes addition on residual stresses and mechanical properties in the CNTs@SiCp/Mg-6Zn hybrid composite using neutron diffraction method. Mater. Sci. Eng. A 2020, 797, 140105. [Google Scholar] [CrossRef]
- Saleem, A.; Zhang, Y.; Gong, H.; Majeed, M.K.; Ashfaq, M.Z.; Jing, J.; Lin, X.; Sheng, M. Carbon nanostructure-reinforced SiCw/Si3N4 composite with enhanced thermal conductivity and mechanical properties. RSC Adv. 2020, 10, 15023–15029. [Google Scholar] [CrossRef]
- Pelleg, J. Mechanism of diffusion. Solid Mech. Its Appl. 2016, 221, 31–39. [Google Scholar] [CrossRef]
- Ahmad, I.; Islam, M.; Dar, M.A.; Xu, F.; Shah, S.I.; Zhu, Y. Magnesia tuned multi-walled carbon nanotubes-reinforced alumina nanocomposites. Mater. Charact. 2015, 99, 210–219. [Google Scholar] [CrossRef]
- Abden, M.J.; Afroze, J.D.; Alam, M.S.; Bahadur, N.M. Pressureless sintering and mechanical properties of hydroxyapatite/functionalized multi-walled carbon nanotube composite. Mater. Sci. Eng. C 2016, 67, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Muccillo, R.; Ferlauto, A.S.; Muccillo, E.N.S. Flash Sintering Samaria-Doped Ceria–Carbon Nanotube Composites. Ceramics 2019, 2, 64–73. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Acevedo, D.R.; Brusatin, G.; Colombo, P. Borosilicate glass matrix composites containing multi-wall carbon nanotubes. J. Eur. Ceram. Soc. 2005, 25, 1515–1523. [Google Scholar] [CrossRef]
- Zhang, S.C.; Fahrenholtz, W.G.; Hilmas, G.E.; Yadlowsky, E.J. Pressureless sintering of carbon nanotube-Al2O3 composites. J. Eur. Ceram. Soc. 2010, 30, 1373–1380. [Google Scholar] [CrossRef]
- Bakhsh, N.; Khalid, F.A.; Hakeem, A.S. Synthesis and characterization of pressureless sintered carbon nanotube reinforced alumina nanocomposites. Mater. Sci. Eng. A 2013, 578, 422–429. [Google Scholar] [CrossRef]
- Aguilar-Elguézabal, A.; Bocanegra-Bernal, M.H. Fracture behaviour of α-Al2O3 ceramics reinforced with a mixture of single-wall and multi-wall carbon nanotubes. Compos. Part B Eng. 2014, 60, 463–470. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.H.; Dominguez-Rios, C.; Echeberria, J.; Reyes-Rojas, A.; Garcia-Reyes, A.; Aguilar-Elguezabal, A. Effect of low-content of carbon nanotubes on the fracture toughness and hardness of carbon nanotube reinforced alumina prepared by sinter, HIP and sinter + HIP routes. Mater. Res. Express 2017, 4. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Xia, J.; Jiang, D. Structure and characteristics of carbon nanotube reinforced SiC composite. Key Eng. Mater. 2017, 726, 127–131. [Google Scholar] [CrossRef]
- Wang, Y.; Iqbal, Z.; Mitra, S. Rapid, low temperature microwave synthesis of novel carbon nanotube-silicon carbide composite. Carbon 2006, 44, 2804–2808. [Google Scholar] [CrossRef]
- Bhandavat, R.; Kuhn, W.; Mansfield, E.; Lehman, J.; Singh, G. Synthesis of polymer-derived ceramic Si(B)CN-carbon nanotube composite by microwave-induced interfacial polarization. ACS Appl. Mater. Interfaces 2012, 4, 11–16. [Google Scholar] [CrossRef]
- Abbaspour, A.; Ghaffarinejad, A. Preparation of a sol–gel-derived carbon nanotube ceramic electrode by microwave irradiation and its application for the determination of adenine and guanine. Electrochim. Acta 2010, 55, 1090–1096. [Google Scholar] [CrossRef]
- Ghobadi, H.; Ebadzadeh, T.; Sadeghian, Z.; Barzegar-Bafrooei, H.; Nemati, A. Microwave-assisted sintering of Al2O3-MWCNT nanocomposites. Ceram. Int. 2017, 43, 6105–6109. [Google Scholar] [CrossRef]
- Hajiaboutalebi, M.; Rajabi, M.; Khanali, O. Physical and mechanical properties of SiC-CNTs nano-composites produced by a rapid microwave process. J. Mater. Sci. Mater. Electron. 2017, 28, 8986–8992. [Google Scholar] [CrossRef]
- Zhao, S.; Xiang, D. A novel route for the synthesis of CNTs/WC composite powders from melamine and metal oxides. Ceram. Int. 2019, 45, 4133–4136. [Google Scholar] [CrossRef]
- Egorov, S.V.; Eremeev, A.G.; Kholoptsev, V.V.; Plotnikov, I.V.; Rybakov, K.I.; Sorokin, A.A.; Balabanov, S.S.; Rostokina, E.Y.; Bykov, Y.V. Rapid microwave sintering of alumina ceramics with an addition of carbon nanotubes. Ceram. Int. 2021, 47, 4604–4610. [Google Scholar] [CrossRef]
- Inam, F.; Yan, H.; Peijs, T.; Reece, M.J. The sintering and grain growth behaviour of ceramic-carbon nanotube nanocomposites. Compos. Sci. Technol. 2010, 70, 947–952. [Google Scholar] [CrossRef]
- Satam, M.K.; Gurnani, L.; Vishwanathe, S.; Mukhopadhyay, A. Development of Carbon Nanotube Reinforced Bulk Polycrystalline Ceramics with Intragranular Carbon Nanotube Reinforcement. J. Am. Ceram. Soc. 2016, 99, 2905–2908. [Google Scholar] [CrossRef]
- Sharma, S.; Kothiyal, N.C. Synergistic effect of zero-dimensional spherical carbon nanoparticles and one-dimensional carbon nanotubes on properties of cement-based ceramic matrix: Microstructural perspectives and crystallization investigations. Compos. Interfaces 2015, 22, 899–921. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Huang, G.; Wu, J. Nucleating effect of multi-walled carbon nanotubes and graphene on the crystallization kinetics and melting behavior of olefin block copolymers. RSC Adv. 2014, 4, 19024–19033. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Einstein, A. Die Plancksche Theorie der Strahlung und die Theorie der spezifischen Wärme. Ann. Phys. 1907, 327, 180–190. [Google Scholar] [CrossRef]
- Debye, P. Zur Theorie der spezifischen Wärmen. Ann. Phys. 1912, 344, 789–839. [Google Scholar] [CrossRef]
- Garai, J. Physics behind the Debye temperature. arXiv 2007, arXiv:physics/0703001. [Google Scholar]
- Ariharan, S.; Nisar, A.; Balaji, N.; Aruna, S.T.; Balani, K. Carbon nanotubes stabilize high temperature phase and toughen Al2O3-based thermal barrier coatings. Compos. Part B Eng. 2017, 124, 76–87. [Google Scholar] [CrossRef]
- Chen, S.; Feng, Y.; Qin, M.; Ji, T.; Feng, W. Improving thermal conductivity in the through-thickness direction of carbon fibre/SiC composites by growing vertically aligned carbon nanotubes. Carbon 2017, 116, 84–93. [Google Scholar] [CrossRef]
- Goyal, K.; Singh, H.; Bhatia, R. Behaviour of carbon nanotubes-Cr2O3 thermal barrier coatings in actual boiler. Surf. Eng. 2020, 36, 124–134. [Google Scholar] [CrossRef]
- Jin, G.; Fang, Y.; Cui, X.; Wang, C.; Zhang, D.; Wen, X.; Mi, Q. Effect of YSZ fibers and carbon nanotubes on bonding strength and thermal cycling lifetime of YSZ-La2Zr2O7 thermal barrier coatings. Surf. Coat. Technol. 2020, 397, 125986. [Google Scholar] [CrossRef]
- Aly, K.; Lubna, M.; Bradford, P.D. Low density, three-dimensionally interconnected carbon nanotube/silicon carbide nanocomposites for thermal protection applications. J. Eur. Ceram. Soc. 2021, 41, 233–243. [Google Scholar] [CrossRef]
- Askari, M.B.; Tavakoli Banizi, Z.; Seifi, M.; Bagheri Dehaghi, S.; Veisi, P. Synthesis of TiO2 nanoparticles and decorated multi-wall carbon nanotube (MWCNT) with anatase TiO2 nanoparticles and study of optical properties and structural characterization of TiO2/MWCNT nanocomposite. Optik 2017, 149, 447–454. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.; Kikuchi, K.; Nomura, N.; Yoshimi, K.; Kawasaki, A. Carbon nanotubes as a unique agent to fabricate nanoceramic/metal composite powders for additive manufacturing. Mater. Des. 2018, 137, 276–285. [Google Scholar] [CrossRef]
- Iftikhar, S.; Warsi, M.F.; Haider, S.; Musaddiq, S.; Shakir, I.; Shahid, M. The impact of carbon nanotubes on the optical, electrical, and magnetic parameters of Ni2+ and Co2+ based spinel ferrites. Ceram. Int. 2019, 45, 21150–21161. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V.; Mallakpour, F. Synthesis of alginate/carbon nanotube/carbon dot/fluoroapatite/TiO2 beads for dye photocatalytic degradation under ultraviolet light. Carbohydr. Polym. 2019, 224, 115138. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, J.; Pan, Y.; Guo, J. Fabrication and mechanical properties of SiO2 matrix composites reinforced by carbon nanotube. Mater. Sci. Eng. A 2003, 357, 392–396. [Google Scholar] [CrossRef]
- Sribalaji, M.; Mukherjee, B.; Bakshi, S.R.; Arunkumar, P.; Suresh Babu, K.; Keshri, A.K. In-situ formed graphene nanoribbon induced toughening and thermal shock resistance of spark plasma sintered carbon nanotube reinforced titanium carbide composite. Compos. Part B Eng. 2017, 123, 227–240. [Google Scholar] [CrossRef]
- Liao, N.; Jia, D.; Yang, Z.; Zhou, Y. Enhanced mechanical properties and thermal shock resistance of Si2BC3N ceramics with SiC coated MWCNTs. J. Adv. Ceram. 2019, 8, 121–132. [Google Scholar] [CrossRef]
- Yamamoto, G.; Shirasu, K.; Nozaka, Y.; Wang, W.; Hashida, T. Microstructure-property relationships in pressureless-sintered carbon nanotube/alumina composites. Mater. Sci. Eng. A 2014, 617, 179–186. [Google Scholar] [CrossRef]
- Song, N.; Liu, H.; Fang, J. Fabrication and mechanical properties of multi-walled carbon nanotube reinforced reaction bonded silicon carbide composites. Ceram. Int. 2016, 42, 351–356. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture Toughness Determinations by Indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Echeberria, J.; Rodríguez, N.; Vleugels, J.; Vanmeensel, K.; Reyes-Rojas, A.; Garcia-Reyes, A.; Domínguez-Rios, C.; Aguilar-Elguézabal, A.; Bocanegra-Bernal, M.H. Hard and tough carbon nanotube-reinforced zirconia-toughened alumina composites prepared by spark plasma sintering. Carbon 2012, 50, 706–717. [Google Scholar] [CrossRef]
- Miyoshi, T.; Sagawa, N.; Sassa, T. Study on fracture toughness evaluation for structural ceramics. Trans. Jpn. Soc. Mech. Eng. Ser. A 1985, 51, 2489–2497. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yoshida, K.; Yano, T. Fabrication of carbon nanotube reinforced boron carbide composite by hot-pressing following extrusion molding. Key Eng. Mater. 2014, 616, 27–31. [Google Scholar] [CrossRef]
- Shetty, D.K.; Wright, I.G.; Mincer, P.N.; Clauer, A.H. Indentation fracture of WC-Co cermets. J. Mater. Sci. 1985, 20, 1873–1882. [Google Scholar] [CrossRef]
- Michálek, M.; Sedláček, J.; Parchoviansky, M.; Michálková, M.; Galusek, D. Mechanical properties and electrical conductivity of alumina/MWCNT and alumina/zirconia/MWCNT composites. Ceram. Int. 2014, 40, 1289–1295. [Google Scholar] [CrossRef]
- Reyes-Rojas, A.; Dominguez-Rios, C.; Garcia-Reyes, A.; Aguilar-Elguezabal, A.; Bocanegra-Bernal, M.H. Sintering of carbon nanotube-reinforced zirconia-toughened alumina composites prepared by uniaxial pressing and cold isostatic pressing. Mater. Res. Express 2018, 5, 105602. [Google Scholar] [CrossRef]
- Popov, O.; Vleugels, J.; Huseynov, A.; Vishnyakov, V. Reactive sintering of TiB2-SiC-CNT ceramics. Ceram. Int. 2019, 45, 22769–22774. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Gao, Z.Q.; Zhang, B.; Hou, Z.P.; Zhang, H.J.; Ye, F.; Wang, W. Fabrication of electrically conductive barium aluminum silicate/silicon nitride composites with enhanced strength and toughness. J. Mater. Sci. 2021, 56, 1221–1230. [Google Scholar] [CrossRef]




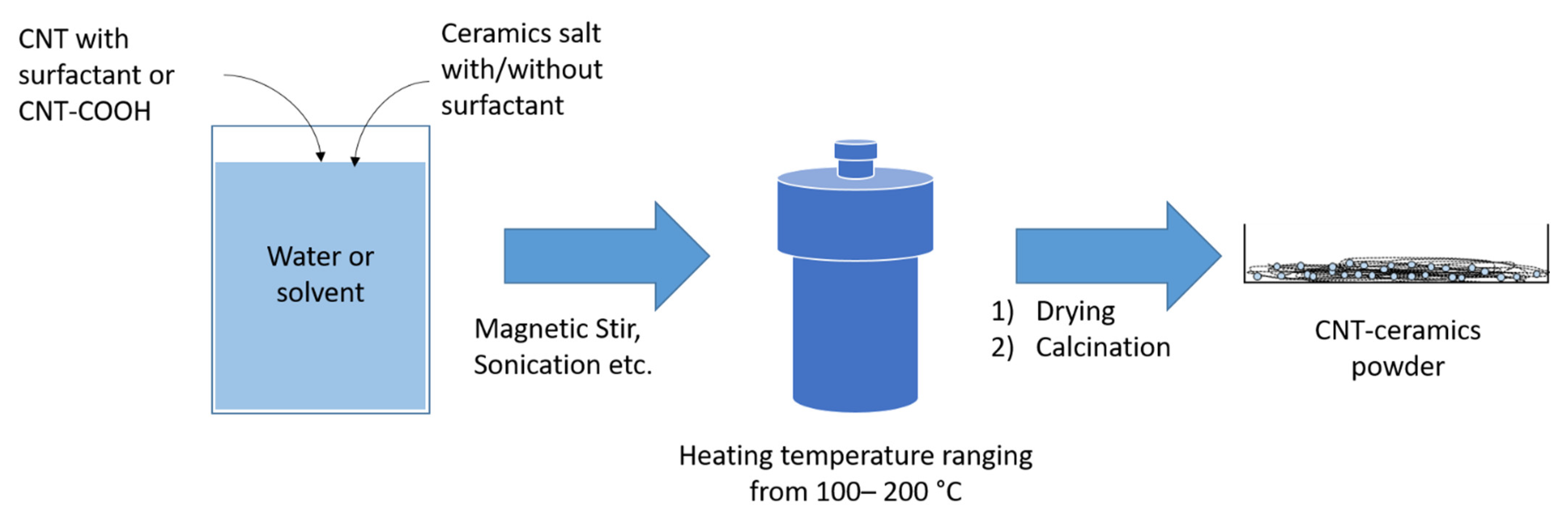
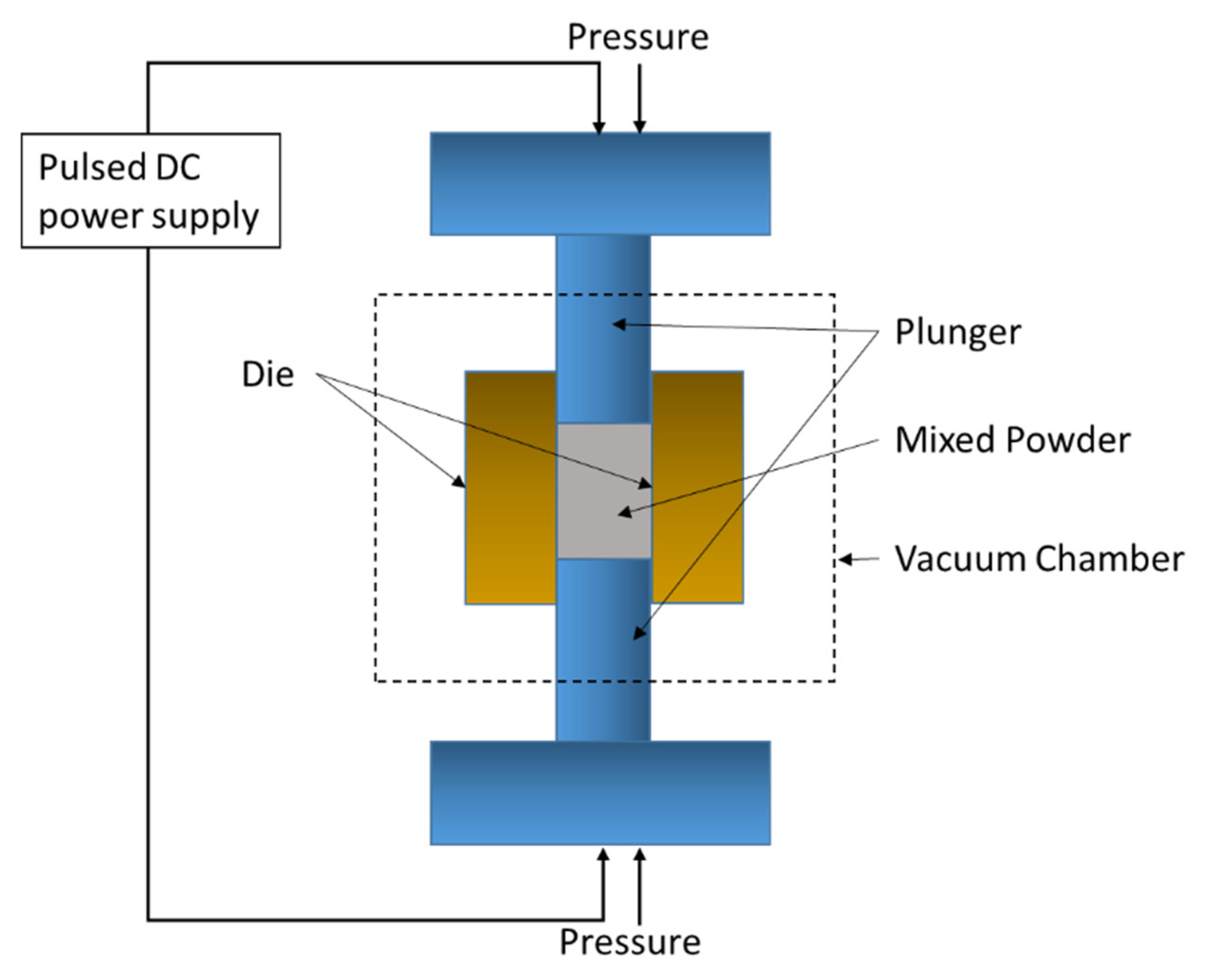
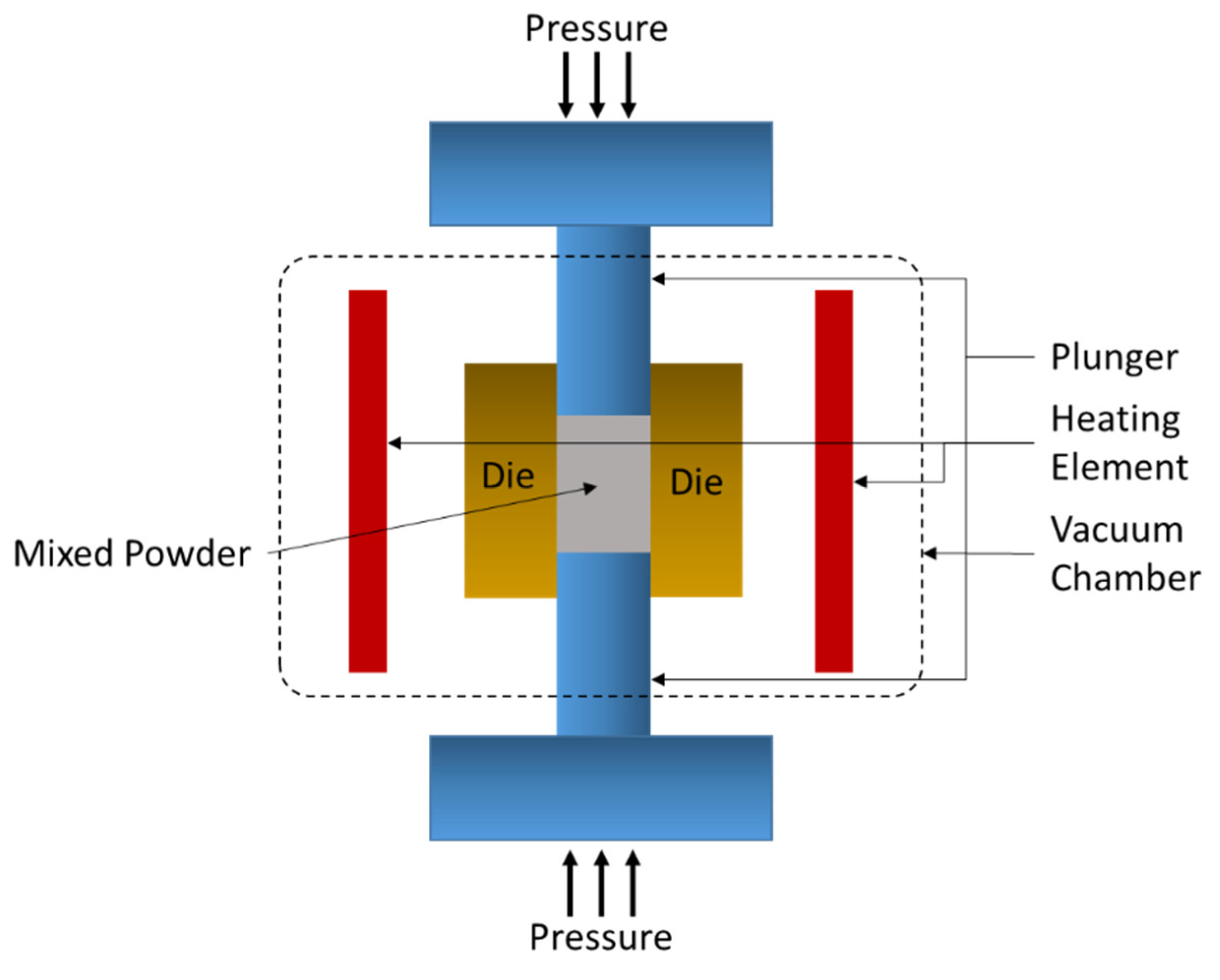
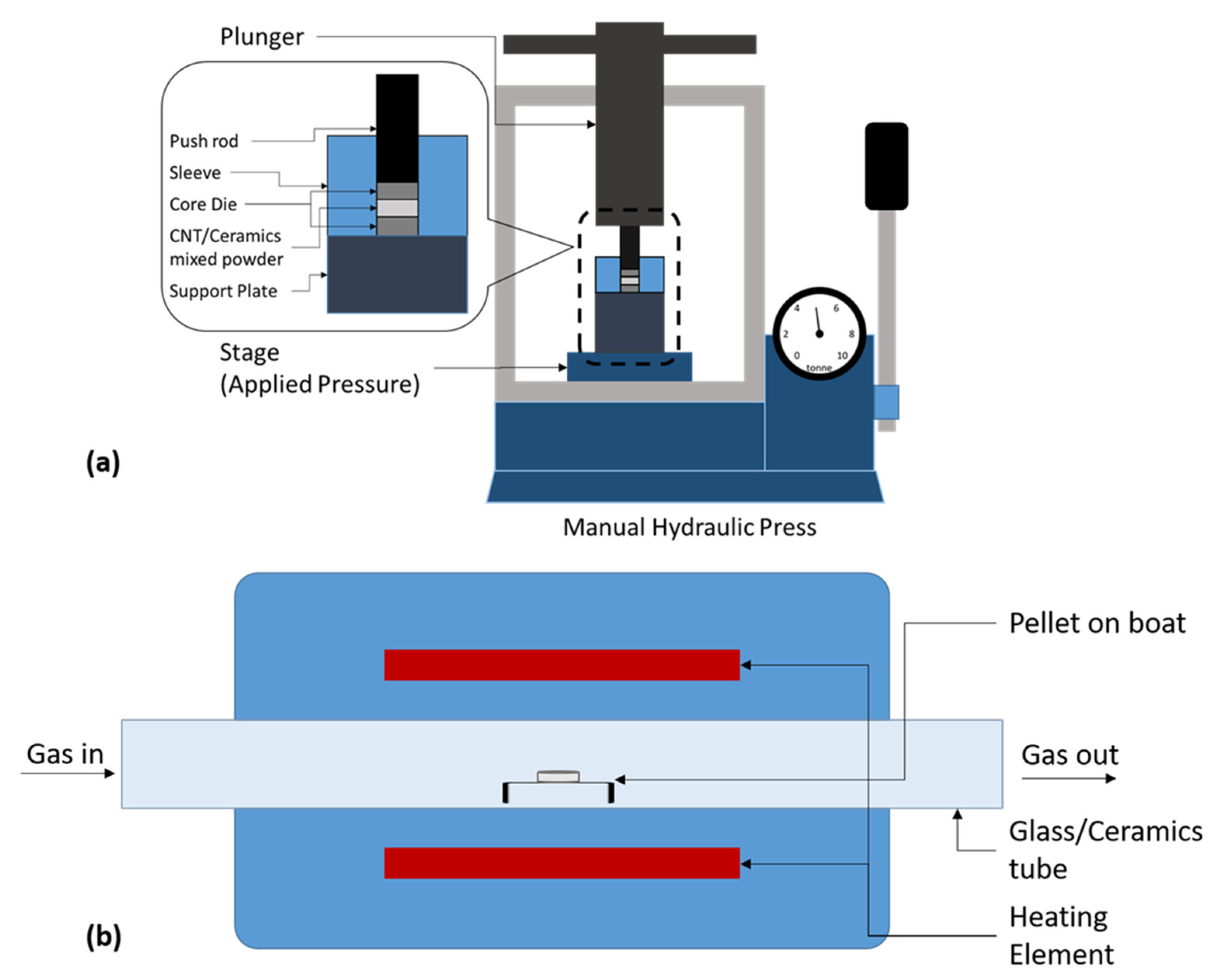
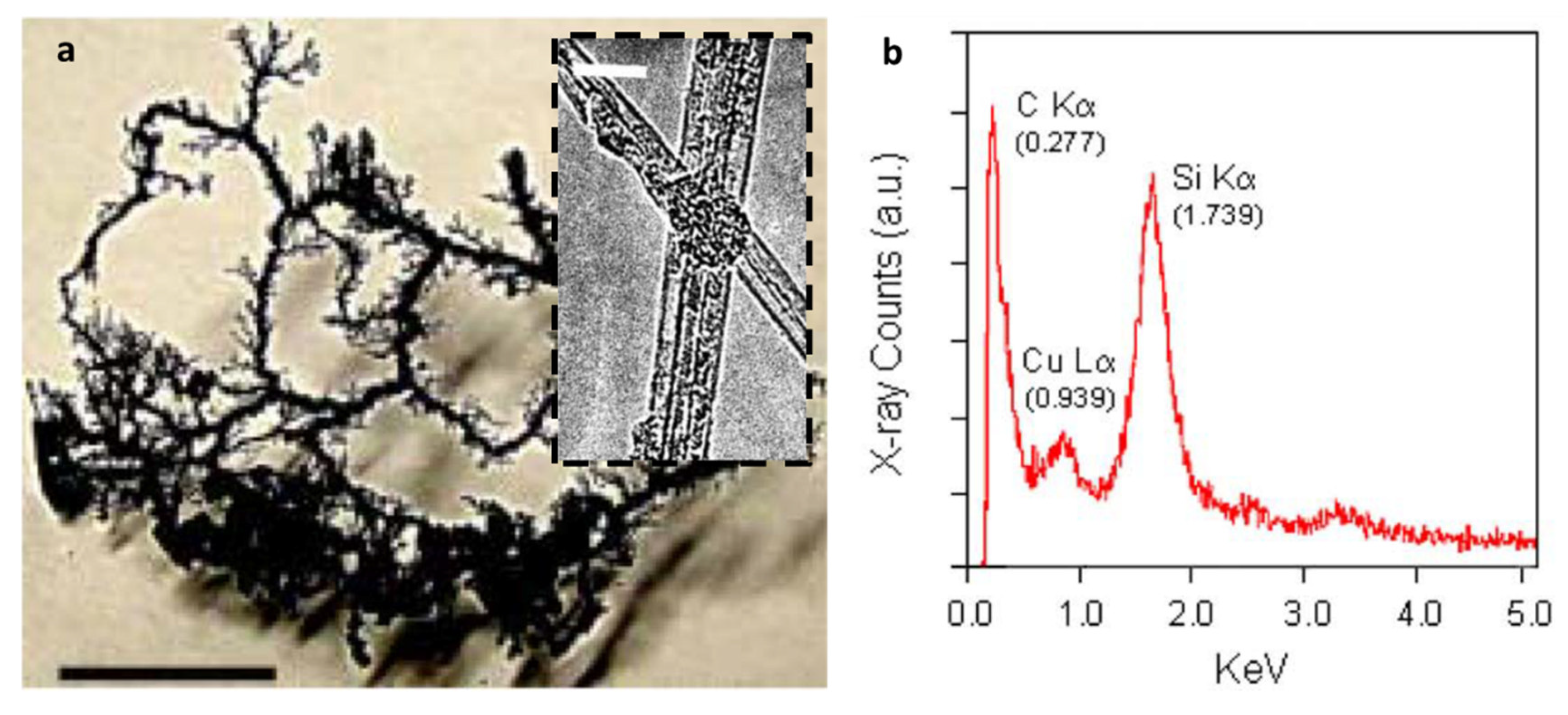
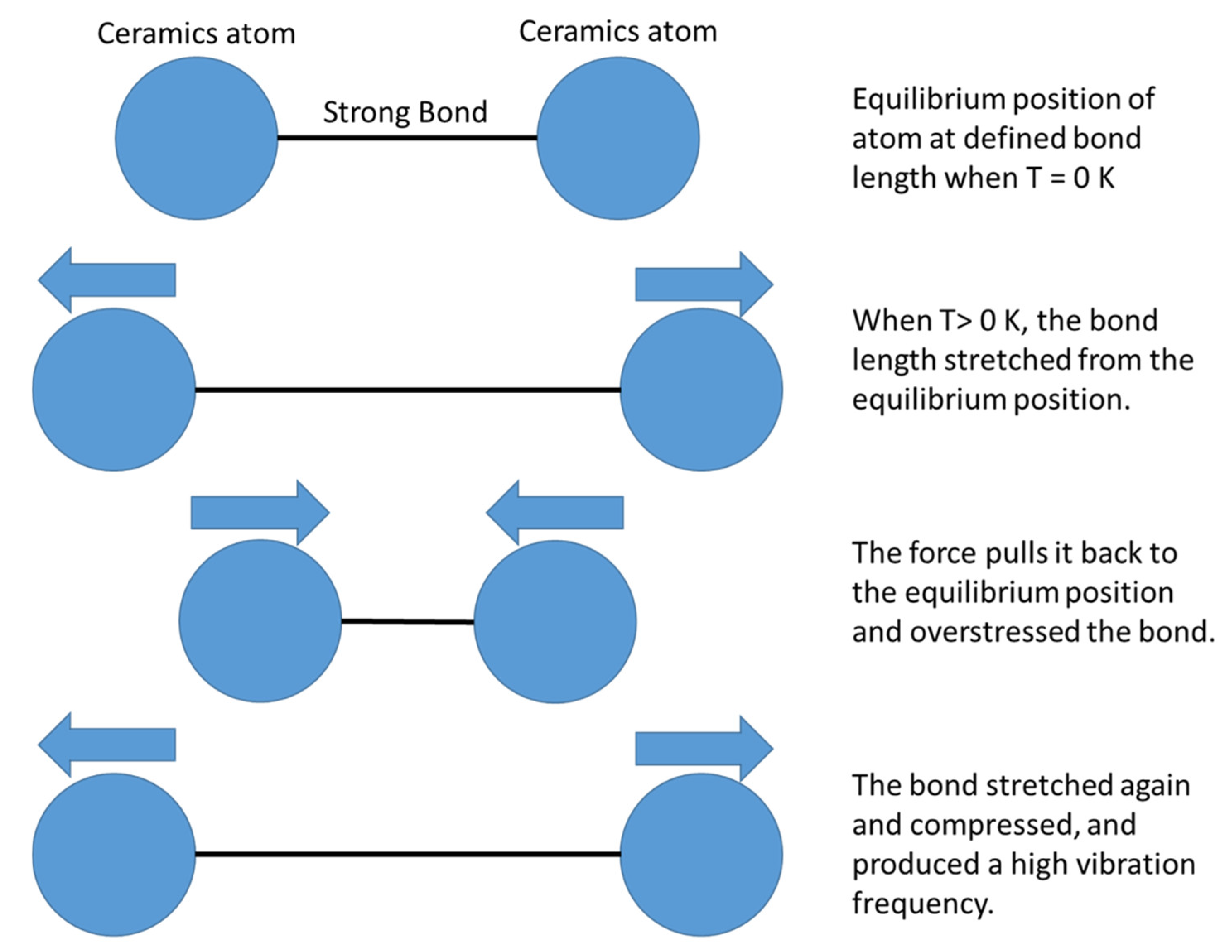
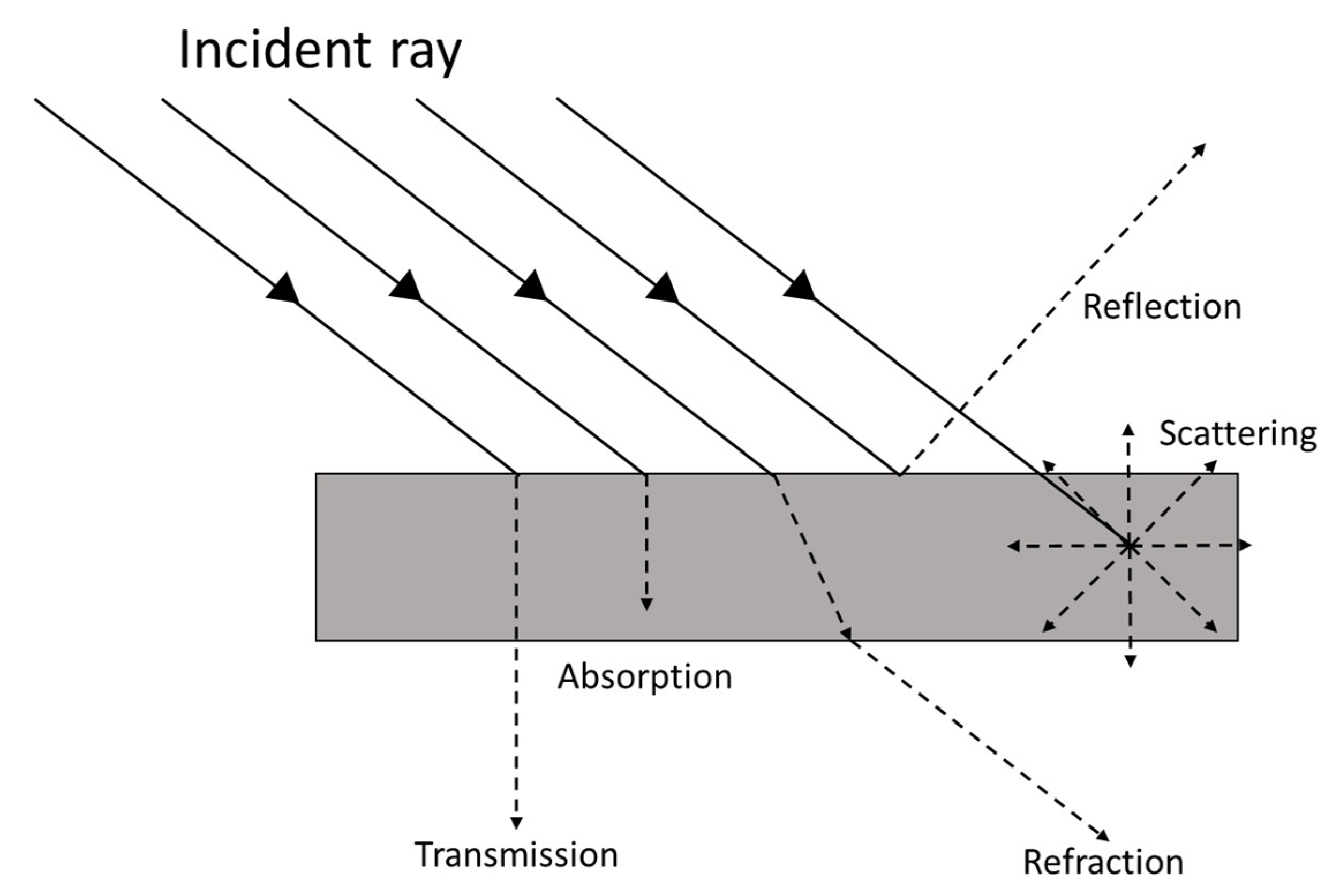
| Dispersion Medium | Additive/Surfactant | Outcome * | Reference |
|---|---|---|---|
| Distilled water | Polyethyleneimine (PEI) | High-stability CNTs nanofluid CNTs-Al2O3 HV: 16.9–17.6 GPa KIC: 3.7–4.9 MPa | [77] |
| Ethanol | n/a | CNTs-Al2O3 Electrical conductivity: 4.5 × 10−15 to 6.2 × 10−2 S/m KIC: 3.78–4.66 MPa·m1/2 Strength: 334–390 MPa | [78] |
| CTAB for CNTs and PAA for SiO2 | n/a | CNTs-SiO2 ceramic 97% relative density (ρ%) | [79] |
| Distilled water | chitosan | Non-Newtonian shear-thinning fluid κth: +13% with 3 wt.% CNT | [80] |
| Distilled water | Polyvinyl pyrrolidone (PVP) | κth: +22.2% (0.5 wt.% CNTs and 0.01 wt.% PVP in water) | [81] |
| Water | n/a | κth: +24.8% (1 wt.% CNT) | [82] |
| Denatured alcohol (85% ethanol and 14% methanol) | n/a | CNTs nanofluid with solar absorption near 100%. | [83] |
| Distilled water | Arabic gum | 0.085 wt.% CNT κth: +67–250% for temperature range of 25–55 °C | [84] |
| Deionized water | Xanthan gum (XG) | 0.1 wt.% CNTs: 0.2 wt.% XG: water κth: 0.65–0.76 W/mK at 70 °C | [85] |
| Deionized water | Triton X-100 Pluronic F-127 Sodium dodecyl sulphate (SDS) CTAB | Stability in the aspect of inertial cavitation activity CNTs-SDS lowest stability | [86] |
| Isopropyl alcohol (IPA) | Polyvinyl alcohol (PVA) | CNTs-MgB2 superconductor JC: 104 A/cm2 at 3.5 T and 20 K 10 times higher than that of the pure sample | [46] |
| Deionized water | Potassium persulfate (K2S2O8) | CNT nanofluid 1.2 vol.% CNTs at 3.4 thermal transfer performance | [87] |
| Distilled water | Toluidine blue (C15H16ClN3S) | CNTs-Mn3O4 Enhanced supercapacitance | [88] |
| Dispersion Medium for CNT Sol | Surfactant/Dispersant for CNT Sol | Gelling Agent | Gelation or Aging Process (after Adding Ceramic Sol) | Reference |
|---|---|---|---|---|
| Ethanol | - | - | Dried overnight | [95] |
| Dimethyl formamide (DMF) | - | Ammonia (NH3) | Aging 80 °C, 2 h | [96] |
| Butanol | PVP | Benzen-1,4-diol a | Heat 105–115 °C, 2.5 h | [97] |
| Water | - | Nano silica sol–gel | Stirred 1 min, molded, and cured at 20 °C | [98] |
| Water | SDBS | Acetyl acetone b | Stir 40 °C, 3 h | [89] |
| Water | Ammonium stearate c | Acetyl acetone | Stir 30 min, ambient | [99] |
| Water | - | NH3 | Stir 3 h and age for 24 h | [100] |
| Water | SDBS d | Acetyl acetone | Stir 40 °C, 2 h | [101] |
| Boehmite sol (AlOOH) | NH3 | In situ gelation (1–2 min) | [44] | |
| Water | SDBS | Aluminium dihydrogen phosphate solution e | Curing 5 h with temperature 50–270 °C | [58,90] |
| Catalyst/CNTs Growth Site | Precursor | Deposition Atmosphere | Deposition Temperature and Duration | Reference |
|---|---|---|---|---|
| Cobalt Acetate tetrahydrate CoAc·4H2O | Acetone (CH3)2CO | Ar | 550 °C, 60 min | [102] |
| Ferrocene Fe(C5H5)2 | Cyclohexanol HOCH(CH2)5 | 400 sccm N2 | 750 °C, 60 min | [103] |
| Iron (III) chloride FeCl3 | Ethanol C2H5OH | Ar | 800–1500 °C, 120 min | [104] |
| Iron (II) chloride tetrahydrate FeCl2·4H2O | Acetylene C2H2 (10 sccm) | 500 sccm N2 | 700 °C, 120 min | [107] |
| Nickel nitrate hexahydrate Ni(NO3)2·6H2O | Methane CH4 (150 sccm) | 300 sccm N2 | 1000 °C, 120 min | [47] |
| Iron (III) nitrate nonahydrate Fe(NO3)3·9H2O | Methane CH4 (100 sccm) | n/a | 800 °C, 150 min | [108] |
| Ni(NO3)2 | Ethylene C2H4 | H2 | 700 °C, 20 min | [40] |
| CNTs Type and Treatment | Dispersion Agent and the Mass Ratio (Dispersion Agent: CNTs) | Autoclave Heating Temperature (°C) | Autoclave Heating Duration (h) | Reference |
|---|---|---|---|---|
| HNO3-treated MWCNTs | - | 210 | 24 | [113] |
| Untreated MWCNTs | Glycolic acid (0.857:0.129) | 200 | 2 | [114] |
| HNO3-treated MWCNTs | - | 120 | 12 | [109] |
| Untreated SWCNTs | - | 180 | 8 | [61] |
| Untreated MWCNTs | Hydrazine monohydrate a (10 mL:3 mg) | 200 | 12 | [45] |
| Untreated MWCNTs | NaOH | 180 | 24 | [110] |
| Dispersion Medium | Dispersion Agent and the Mass Ratio (Dispersion Agent: CNT) | Autoclave Heating Temperature (°C) | Autoclave Heating Duration (h) | Reference |
|---|---|---|---|---|
| Ethanol + water | - | 180 | 24 | [115] |
| Ethylene glycol (EG) | PVP (0.75:0.05) | 160 | 12 | [111] |
| EG | PEG (1:0.03) | 200 | 2.5 | [112] |
| EG | - | 180 | 3 | [32] |
| Raw Material | Dispersion Procedure | SPS Parameter | Outcome * | Reference |
|---|---|---|---|---|
| TiO2, amorphous boron, MWCNTs | Sonication in toluene | 1400 °C, 50 MPa, 10 min | 6 vol.% CNTs-TiB2-TiC composites CTE: −10.64%Grain size reduced 92.93% | [120] |
| SiC, amorphous boron, MWCNTs | Planetary mill | 2000 °C, 50 MPa, 5 min | 1 wt.% CNTs-SiC-B Porosity: −55.56% Density: +3.32% | [121] |
| TiN, MWCNTs | Sonication in ethanol | 1900 °C, 40 MPa, 7 min | 5 wt.% CNTs-TiN Density: −6.13% | [122] |
| CaCO3, CuO, TiO2, MWCNTs | High-energy-vibration ball mill | 1000 °C, 50 MPa, 5 min | 18 wt.% CNTs-CaCu3Ti4O12 Negative permittivity behaviour | [123] |
| Matrix | CNTs Type | Dispersion Procedure | Pelleting and Sintering | Outcome | Reference |
|---|---|---|---|---|---|
| Duran® glass powder | MWCNTs | Colloidal | 10 MPa Two step: 500 °C (30 min) and 750 °C (3 h) | 10 wt.% CNTs-Cristobalite Density: 1.98 g/cm3 Porosity: 11% | [132] |
| Al2O3 | MWCNTs | Chemical mixing and freeze drying | 19 MPa 1500 °C, 2 h | 1 vol.% CNTs-Al2O3 Electrical resistivity: −95.4% Hardness: −3.88% Flexural Strength: +34.74% KIC: +24.24% | [133] |
| Al2O3 | MWCNTs | Ball milling Gas purging sonication | 375 MPa 1600 °C, 15 min | 1 wt.% functionalized CNTs-Al2O3 KIC: +9% Young’s modulus: +7% Shear modulus: +10.66% | [134] |
| Al2O3 | MWCNTs | Wet mixing | 50 MPa 1520 °C, 1 h in air | 0.1 wt.% MWCNTs-Al2O3 ρ%: −1.16% Porosity: +45.93%KIC: −10.18% Grain size reduced 37.23% | [135] |
| Al2O3 | MWCNTs SWCNTs | Sonication | 50 MPa 1520 °C 1 h | 0.1 wt.% SWCNTs-Al2O3 ρ%: −3.46% KIC: +13.79% Grain size increased 56.25% | [136] |
| Silicon coupling agent of KH550 | MWCNTs | Silane coupling reaction | 2050 °C in Ar | Density 3.1–3.02 g/cm3 KIC: 3.8–4.1 MPa·m1/2 | [137] |
| Matrix | CNTs Type | Dispersion Procedure | Microwave Parameter | Outcome | Reference |
|---|---|---|---|---|---|
| Al2O3 | MWCNTs | Colloidal processing | 900 W, 45 min | Al2O3-1 vol.% CNTs KIC: 3.0–4.1 MPam1/2 | [141] |
| SiC | SWCNTs | Wet powder processing | 900 W, 55 min | SiC-0.5 wt.% CNTs Hardness: +23% κth: +50% Electrical conductivity: −27% | [142] |
| Tungsten carbide (WC) | CVD-CNTs | In situ CNTs growth on WO3-Co3O4 | 1200 °C, 10 min | WC and CNTs formed where melamine acted as a C precursor WC attached on the CNTs wall | [143] |
| Al2O3 | CVD-CNTs | Wet powder processing | 1550 °C | Al2O3-1 wt.% CNTs High heating rate (100 °C/min) Mass loss: +0.35% ρ%: −3.4% | [144] |
| Dispersion Process | Densification and Sintering | Outcome * | Reference |
|---|---|---|---|
| Powder | Atmospheric plasma spraying | 4 wt.% MWCNTs-Al2O3 on Inconel 718 substrate κth: +117.7% KIC: +13.1% | [153] |
| Precursor impregnation | Pyrolysis | VACNTs *-CF/SiC κth: +111.6% | [154] |
| Powder | High-velocity oxy-fuel coating | 8 wt.% CNTs-Cr2O3 on T22 steel Corrosion rate: −87.8% | [155] |
| Powder | Atmospheric plasma spraying | 1 wt.% CNTs-YSZ *-La2Zr2O7 Thermal cycling: 126 cycles to 218 cycles | [156] |
| Powder | SPS | 4 wt.% CNTs/SiC-Al2O3 D: 9 mm2/s κth: 30.82 W/mK Cp: 0.9 J/gK | [67] |
| Chemical vapour infiltration | PLS | CNTs-SiC network coated alumina pipe Thermal insulation: 60−12.5 °C/min at 750 °C flame | [157] |
| Dispersion Process | Outcome * | Reference |
|---|---|---|
| Ultrasonication | 20 wt.% MWCNTs-TiO2 Eg: 2.8–3.1 eV (+10.71%) | [158] |
| Hydrothermal | 5 wt.% MWCNTs-Bi2S3 Eg: 1.245–0.875 eV (−29.72%) MB degradation: 60% to 90% Stability after 4 cycles: retain at 75% | [109] |
| Powder | 0.31% CNTs-10% Al2O3-MoTiAl Laser absorptivity: +10.3% | [159] |
| Colloidal | Eg reduction in CNTs- ferrite 0.1 wt.% CNTs-NiFe2O4: −15.17% 0.1 wt.% CNTs-CoFe2O4: −20.0% 0.1 wt.% CNTs-Ni0.4Co0.6Fe2O4: −11.76% | [160] |
| Powder | 45 wt.% CNTs/CQD-FA-TiO2 * Eg: 3.19–3.26 eV (+2.19%) | [161] |
| Dispersion | Densification and Sintering | Outcome * | KIC Characterization Technique | KIC Equation | Reference |
|---|---|---|---|---|---|
| Wet powder | SPS | 0.1 wt.% MWCNTs-Al2O3-MgO-ZrO2 HV: +4.39% from CNTs-free KIC: +3.16% | Vickers indentation | Evans and Charles [167] | [168] |
| Wet powder | HPS and Exclusion | 20 vol.% CNTs-B4C-Al2O3 Bending strength: 380 MPa KIC: 4.19 (0.51) MPa·m1/2 HV: 11.0 (0.21) GPa | Vickers indentation | Miyoshi et al. [169] | [170] |
| Colloidal | HPS | 0.3 wt.% MWCNTs-Al2O3 KIC: +8.0% | Vickers indentation | Shetty et al. [171] | [172] |
| Colloidal | UP-PLS CIP-PLS | 0.01 wt.% CNTs-Al2O3-MgO-Y2O3-ZrO2 UP-PLSed composites KIC: +41.62% HV: −9.68% CIP-PLSed Composites KIC: +41.87% HV: +6.620% | Vickers indentation | Evans and Charles [167] | [173] |
| Powder | HPS | 2.0 wt.% CNTs-SiC-TiB2 KIC: −23.73% HV: +6.93% | Vickers indentation | Evans and Charles [167] | [174] |
| Powder | HPS | 3.85 wt.% CNTs-B4C-ZrC0.8 KIC: +68.14% HV: −35.41% | Vickers indentation | Evans and Charles [167] | [69] |
| Powder | HPS | 2 wt.% MWCNTs-Si3N4-SiC KIC: +37.59% Flexural strength: +52.39% HV: +16.45% | SENB | n/a | [127] |
| Colloidal | SPS | 7 wt.% MWCNTs-BAS-Si3N4 Flexural strength: −22.89% KIC: +31.91% | Vickers indentation | Evans and Charles [167] | [175] |
| Processing Technique | Powder | Colloidal | Sol–Gel | In-Situ CNTs Growth | Hydrothermal |
|---|---|---|---|---|---|
| Duration | Within hours | Within hours | Several hours–several days | Several hours | Several hours–several days |
| Temperature (°C) | Ambient | Ambient | Ambient—200 | >600 | 100–250 |
| Advantage | No or minimum chemical involved | Rapid processing | Forming CNTs network | High interfacial adhesion | Homogenous domain crystal growth |
| Disadvantage | Inhomogeneous CNTs dispersion | CNTs structural defect | Long processing duration | Inconsistent CNTs yield | Long processing duration |
| Densification and Sintering | Spark Plasma Sintering (SPS) | Hot-Press Sintering (HPS) | Pressureless Sintering (PLS) | Microwave-Assisted Sintering (MAS) |
|---|---|---|---|---|
| Duration | Within minutes | Minutes to hours | Several hours | Within 30 min |
| Advantage | Rapid sintering | Short sintering duration | Low setup cost | Rapid sintering |
| Disadvantage | High-energy consumption | High-energy consumption | Inhomogeneous CNTs dispersion | Low CNTs addition only |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.F.; Zaid, M.H.M.; Mamat, M.S.; Liza, S.; Tanemura, M.; Yaakob, Y. Recent Developments in Carbon Nanotubes-Reinforced Ceramic Matrix Composites: A Review on Dispersion and Densification Techniques. Crystals 2021, 11, 457. https://doi.org/10.3390/cryst11050457
Chan KF, Zaid MHM, Mamat MS, Liza S, Tanemura M, Yaakob Y. Recent Developments in Carbon Nanotubes-Reinforced Ceramic Matrix Composites: A Review on Dispersion and Densification Techniques. Crystals. 2021; 11(5):457. https://doi.org/10.3390/cryst11050457
Chicago/Turabian StyleChan, Kar Fei, Mohd Hafiz Mohd Zaid, Md Shuhazlly Mamat, Shahira Liza, Masaki Tanemura, and Yazid Yaakob. 2021. "Recent Developments in Carbon Nanotubes-Reinforced Ceramic Matrix Composites: A Review on Dispersion and Densification Techniques" Crystals 11, no. 5: 457. https://doi.org/10.3390/cryst11050457
APA StyleChan, K. F., Zaid, M. H. M., Mamat, M. S., Liza, S., Tanemura, M., & Yaakob, Y. (2021). Recent Developments in Carbon Nanotubes-Reinforced Ceramic Matrix Composites: A Review on Dispersion and Densification Techniques. Crystals, 11(5), 457. https://doi.org/10.3390/cryst11050457







