1. Introduction
To measure the pH value of concrete, Larbi et al. [
1] compressed the fresh concrete to squeeze the pore solution, and then vacuum filtered it for chemical composition analysis and pH measurement. Nowadays, it is typical to measure the pH value of fresh concrete using hydroxide titration methodology.
However, Behnood et al. [
2] mention that many countries have not developed a set of standardized test procedures for pH measurement in concrete; therefore, they categorized the pH measurement methods of hardened concrete into destructive methods and non-destructive methods. Destructive methods include the expression method, the in-situ leaching method (ISL), and the ex-situ leaching method (ESL). Non-destructive methods include embedded potentiometric electrodes and optical fiber sensors.
In the extraction method, Taylor [
3] obtained the pore solution of hardened concrete pieces through a hydraulic press machine, then measured the pH value of the pore solution. Taylor used this method to extract the pore solution of cement slag paste (water-cement ratio: 0.5); after two years of curing, the pore solution was squeezed with a maximum hydraulic pressure of 343 MPa. To obtain more pore solution of mortar, Barneyback et al. [
4] increased the pressure to 550 MPa. After extracting the concrete pore solution, the pH value could be measured by the commercial electrode pH measurement equipment or could be calculated from the hydroxide ion concentration by titration.
Then, Sagues et al. [
5] developed an in-situ leaching method by drilling holes in concrete specimens with a diameter of nearly 5 mm and a depth of 25 mm. After holes are drilled, the concrete powder is removed. Then 0.4 mL of deionized water is poured into the hole, and MI-405 glass pH microelectrode and Ag/AgCl electrode are used to measure the pH value. Stojanović [
6] also used the ISL method to measure the pH level of powdered concrete samples and in suspension. However, Li [
7] and Cyr [
8] claimed that the ISL method has a variety of limitations such as require moisture saturation, the constituent assumption, and a long testing period. In addition, Paudel [
9] developed a modified in situ leaching method to achieve a more accurate method using for measuring pH value of fly-ash geopolymer concrete.
Another pH measurement method which is called ex situ leaching methods (ESL) is well-known for faster and easier due to its simple procedures and no special or expensive equipment required [
10]. The ex situ leaching method (ESL) is conducted by taking the sample to the laboratory and sealing it in a bag during transportation to prevent it from evaporation and exposure to carbon dioxide in the air. In the laboratory, the specimen is crushed and passed through a #200 (0.074 mm) sieve. After that, 10 g of the specimen is mixed with 10 g of deionized water, with continuous stirring on a magnetic stirrer for 5 min, and then pH is measured. This method is based on the ASTM D4972 for soil pH value measurement, which is typically used to measure the pH value of soil. Behnood et al. [
2] compiled the results of the ex-situ leaching method to determine the pH of concrete under different study conditions. However, there is still no standardized test procedure for measuring the pH of concrete using the ESL method. According to Plusquellec et al. [
11], a majority of ESL methods should include the following steps: (1) ground concrete is powdered, (2) leaching of the powder with a known amount of deionized water in a set time, (3) the solution is filtered, (4) the extracted solution is analyzed. In recent years, the ESL method was used widely for measuring the pH level of the mortar and concrete [
12]
For the pH measurement method for the pore solution of low alkaline cementitious materials, the Spanish National Research Council (CSIC) [
10], mentioned that the repeatability and reproducibility of the ESL method can be more applicable and more accurate than other pH measurement methods. Besides, Xia [
13] stated that the applicability to high performance concretes and short experimentation period are the advantages of the ESL method compared to in situ leaching (ISL) method and pore water expression (PWE) method. However, the accuracy of the ESL method when measurement concretes containing pozzolans or highly soluble inorganic salts should be determined.
Since the pH plays an important role in the durability of the concrete used in the final storage site of radioactive nuclear waste, it is essential to develop a set of specific standard test methods for high accuracy, repeatability, and reproducibility in measuring the pH of the concrete. The current ESL method has two problems. Firstly, the specimen treatment method before crushing is not clear enough, such as whether air-drying or oven-drying is required; the second is that the stirring of solution and the pH measurement need to be in an environment of N2 gas.
In this study, the experiments used the ordinary Portland cement (OPC) paste and OPC mixing with the silica fume (SF) and propose an S-ESL method to measure a pH value. The first consideration is to understand the impact of the processing method of the specimen before crushing on the results of the pH measurement; the second consideration is to understand the influence of the pH measurement process if it is not under the N2 gas protection environment. S-ESL method is according to the above test results, by improving the standard procedures of ESL to simplify the equipment required of test, but still achieve the accuracy of pH measurement.
2. Experimental Methods
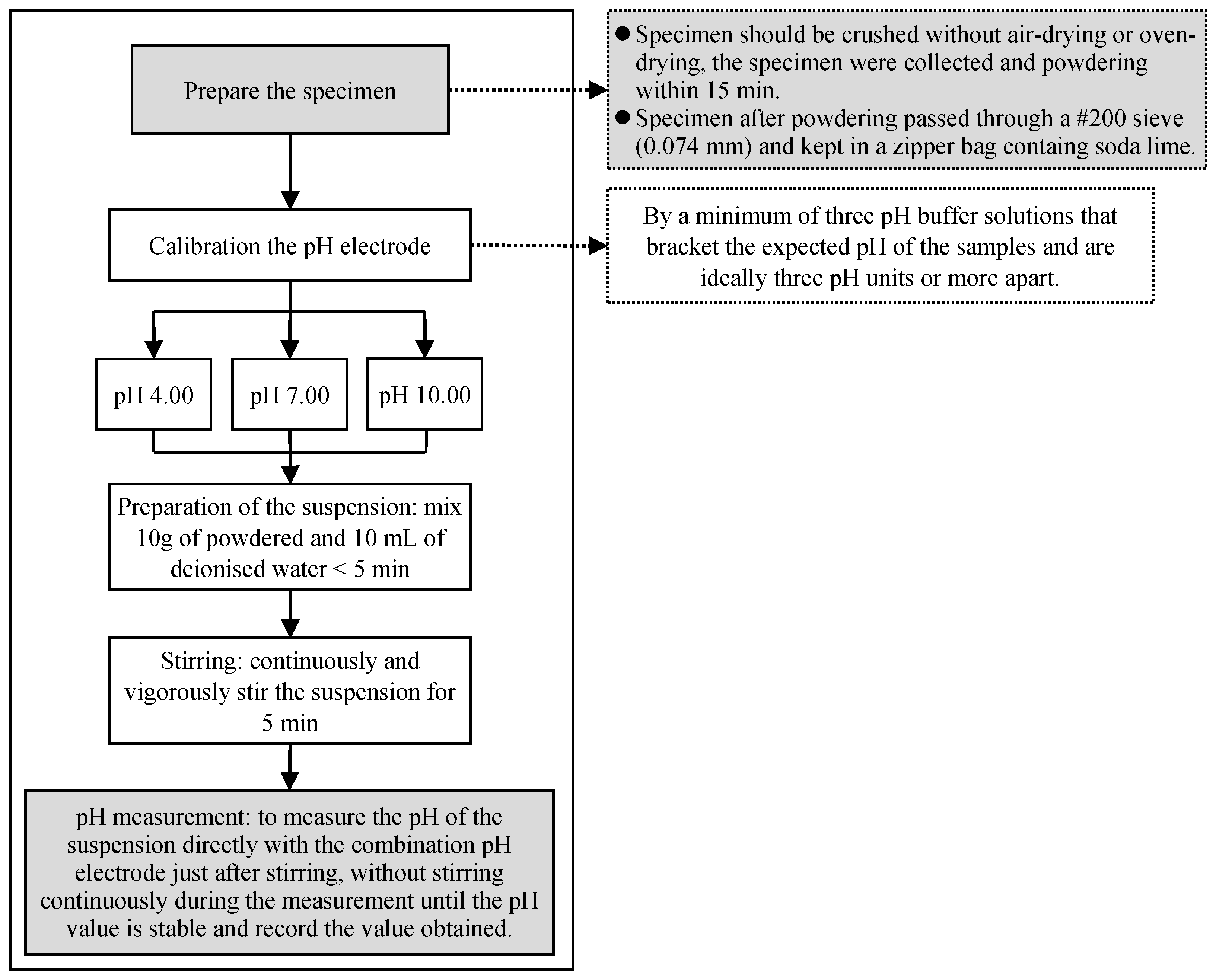
2.1. The Experimental Procedure
Extensive experiments were conducted to examine the S-ESL method for a standard testing procedure using various combinations of the pure cement paste (OPC) as well as 40% silica fume as cement paste replacement (C60-SF40), with a water-binder (W/B) ratio of 0.5, and curing at room temperature for 1 day, 7 days, and 28 days. Due to the characteristics of continuous hydration of the short curing age specimens, the variation of the test results can be amplified. Thus, the S-ESL method can achieve reliable results.
The preliminary test procedure refers to the standard pH test method for the soil ASTM D4972 as in a related report, wherein, the specimen was not dried, and the ground powder was quenched with cold water [
7,
10,
14,
15,
16,
17,
18,
19]. Alonso [
10] recommended that the typical experiment’s parameters should be considered, including specimen’s powder with particle size less than 80 μm, the liquid-to-solid ratio of 1, followed by specimen stirring and quenching in 5 min. ESL process is shown in
Figure 1.
S-ESL method is designed for hard-dissolved solid materials. The preliminary operating procedures of the experiment are described here. The specimen was taken out from the curing room, the surface water was wiped, the specimen was crushed with a hydraulic punch, the specimen’s pieces were collected and ground within 15 min. The specimen powder was passed through a #200 sieve (0.074 mm) and kept in a zipper bag containing soda lime. Then, 10 g of powder was taken out from the zipper bag and put kept in a beaker, mixed with 10 g of deionized water within 5 min, stirred for 5 min on a magnetic stirrer, and then the pH was measured just after stirring, without stirring continuously during the measurement until the pH value is stable and record the value obtained. The temperature of the solution in the experiment should match the room temperature environment. S-ESL process is shown in
Figure 2.
With an aim to evaluate the standard operating procedures of the S-ESL method, variation in various factors were investigated which comprise the specimen, the curing time, the dryness of the specimen before crushing or the drying of the specimen in general, the size of the powder, the air-exposure periods of the powder as well as the solution, and the temperature of solution. The effects of those factors on the pH of the specimen were analyzed to understand the influence of those factors, then the standard operating procedure of the S-ESL method was established for measuring the pH of cement matrix materials.
The experimental procedures for the tests designed in this study are described as follows:
The influence of air-drying before crushing on pH value.
The cement paste was cured for 28 days. After that, the specimen was taken out of the curing room, and the surface water of the specimen was wiped. After 0, 0.25, 0.5, 0.75, 1, 2, 3, 4, and 5 h, the specimen was crushed by a hydraulic punch. The experiment was conducted according to the standard S-ESL procedure.
The influence of oven-drying on the pH value before crushing.
The cement paste was cured for 28 days. After that, the specimen was taken out of the curing room, the surface water of the specimen was wiped, and the specimen was kept in an oven at 105 °C for 0, 1, 3, and 6 h. Then, the specimen was taken out of the oven and cooled for 1 h, then crushed with a hydraulic punch. The experiment was conducted according to the standard S-ESL procedure.
The influence of the air-exposure time of powder on the pH value
Both the OPC and C60-SF40 cement paste were cured for 28 days. After that, the specimen was taken out of the curing room, the surface water of the specimen wiped, and the specimen was crushed and ground with a hydraulic punch. Then, the powder was exposed to the air for 0, 1, 5, 10, 20, 30, 60, and 120 min before mixing with deionized water. The experiment was conducted according to the standard S-ESL procedure.
The influence of the air-exposure time of solution on the pH value
The experiment was divided into two parts and the difference of the results was observed between 1 and 28 days of specimen curing time.
In the first part, the OPC paste was cured for one day, and the powder was obtained according to the standard S-ESL method. The powder was divided into six solution parts at the same time which contained 10 g of powder each in six separate beakers. Then, the solutions in these six beakers were exposed to the air for 0, 5, 12, 20, 30, and 60 min before conducting the pH measurements.
In the second part, both of the OPC and C60-SF40 pastes were cured for 28 days and then divided into eight solution parts at the same time. These eight beakers of solutions were exposed to the air for 1, 5, 10, 20, 30, 45, 60, and 120 min before measuring the pH.
The pH of multiple beaker solutions from the same source were compared.
The OPC paste was cured for seven days, then the powder was obtained according to the standard S-ESL method, then, eight beakers were taken to make eight parts of the solution, and the test was carried out according to the standard S-ESL procedure.
The pH result of the same cup of solution at different measurement time intervals.
The OPC paste was cured for seven days, then the powder was obtained according to the standard S-ESL method. After making one beaker of the solution as an experiment specimen, it was exposed to air and the pH was measured every 5 min for a total of eight times.
Influence of the particle size of the powder on the results of pH value.
OPC and C60-SF40 pastes were cured for 28 days, then the powder was obtained according to the standard S-ESL method. The powder was tested in 4 sizes: #30~#50 (0.595–0.297 mm), #50~#100 (0.297–0.149 mm), #100~#200 (0.149–0.074 mm); <#200 (0.074 mm).
The influence of the solution’s temperature on the pH.
OPC and C60-SF40 pastes were cured for 28 days, and the powder was obtained according to the standard S-ESL method. The temperature of the solution was adjusted to 24 °C, 25 °C, 30 °C, 35 °C, 40 °C, and 45 °C using a magnet heater.
2.2. Materials
The experiment used Portland cement type 1 (Taiwan Cement Company, Taipei, Taiwan), and Microsilica Grade 951-U silica fume (ELKEM, Oslo, Norway). The chemical composition of the cement is mentioned in
Table 1. In addition, the silica fume used was a gray powder with a particle size of 0.1~0.2 μm, a specific gravity of 2.2, the specific surface area variation between 2200 and 2600 m
2/kg and passed through No. 325 sieve > 90%.
2.3. The Experimental Instrument
The experimental equipment were: (1) a pH measurements from the S-ESL method using combination of pH electrode with a resolution of 0.01 pH, accuracy of ±0.1 pH, and automatic temperature compensation (ATC) (Bland: DGWater, model: G59U10-10000, made in Poland), (2) Electronic scale with ±0.1 g accuracy, (3) a magnetic stirrer (model: PC-420D, LED digital display, the heating temperature control ranger is 5~550 °C, and the speed is 60~1150 rmp, Corning, Glendale, AZ, USA), (4) beakers and plastic flasks or cups (can be capped or sealed), and (5) a grinding equipment (working mechanism: pressure, impact and friction, fineness < 0.04 mm (depending on the material), grinding chamber volume: max. 250 mL, input: broken glass < 15 mm).
To calibrate the pH electrode, at least three standard buffer solutions were required. The expected range of pH should be 10.5–12.5 for the low-pH cement material. Ideally, the standard buffer solution should be separated into three pH ranges, for example, pH 7, 10 ± 0.8, and 13–14. All standard buffer solutions for temperature correction and calibration were included, and the pore solution was tested at the same temperature.
3. Results and Discussions
3.1. The Effect of Air-Drying before Crushing on the pH
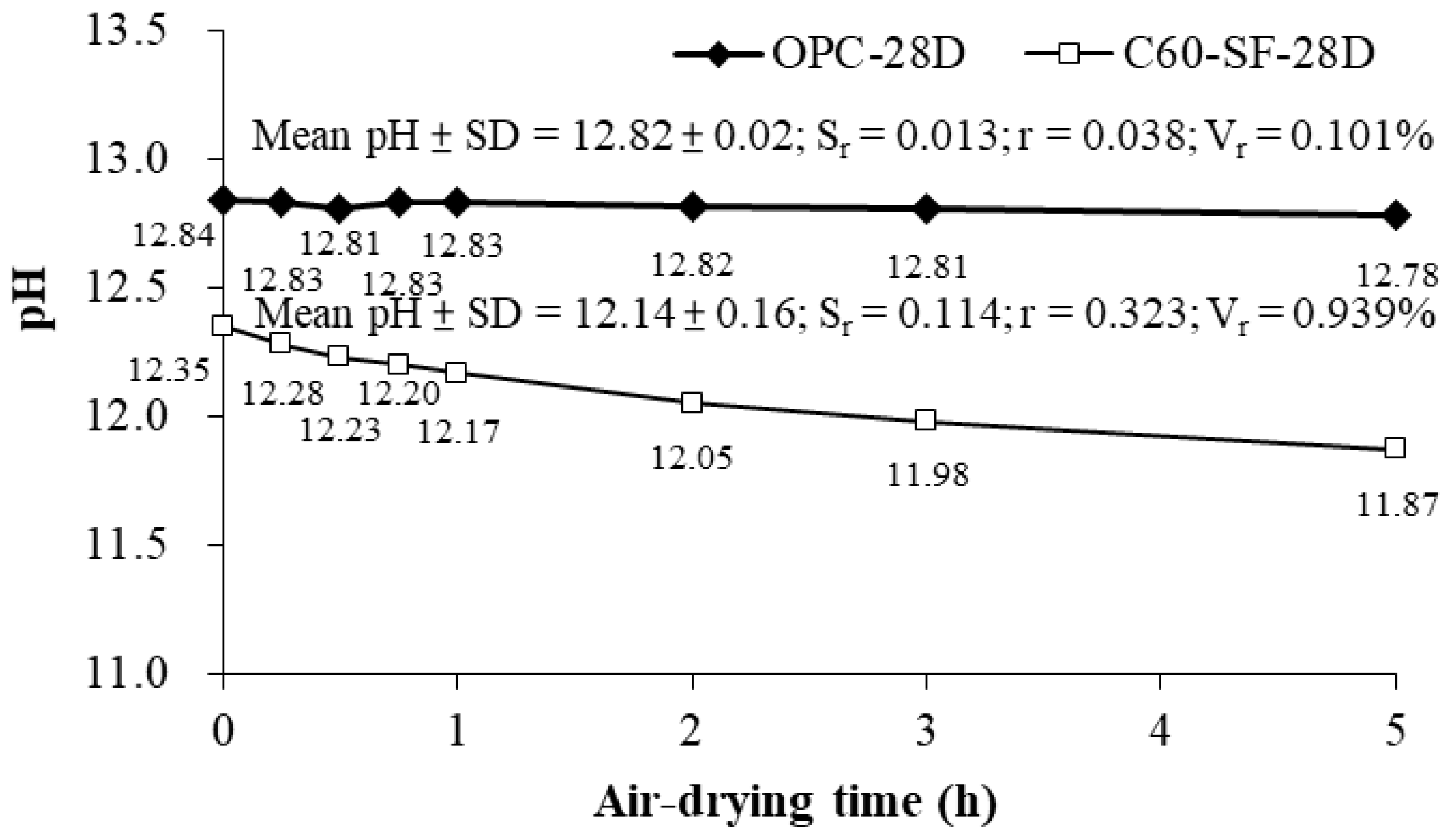
Figure 3 shows that for OPC and C60-SF40 pastes, after 28 days of curing, the pH value of the S-ESL test decreased as the air-drying duration before crushing increased. In addition, the pH value of the C60-SF40 specimen reduced at approximately 0.096 per hour. In contrast, a decrease in pH of the OPC specimen was slower at about 0.012 per hour. The reason for the decrease in pH was related to the hydration of the specimen still.
For the C60-SF40 cement paste, the hydration rate was slower than that of OPC cement paste due to the additional silica fume in the C60-SF40 component. As a result, the hydration percentage of the C60-SF40 specimen after the 28 days was lower than the hydration percentage of the OPC specimen. Thus, changing the specimen’s air-drying duration had a significant effect on the reduction pH value of C60-SF40. Therefore, when the specimen reaches the curing age, it is suggested that the subsequent procedure such as crushing be carried out immediately after air-drying, so as not to underestimate the pH of the concrete.
The pH of OPC and C60-SF40 specimens after 28 days of curing was 12.84 and 12.35, respectively, indicating that adding silica fume to the cement could reduce the pH of the paste. In addition, we also carried out the pH test of C60-SF40 paste after curing for seven days and 28 days, and the results were 13.07 and 12.35, respectively. This indicates that when silica fume is used to replace the cement, silica fume consumes the alkalinity in the cement admixture and reduces the pH of concrete. This is the method used for designing low alkaline cement by adding Pozzolan materials to achieve low alkali properties.
In addition, observation by statistical analysis, the statistical analysis method of the Spanish National Research Council (CSIC) [
10] was used to estimate the variance of the statistical analysis (
), based on the equation:
where:
SSr is the residual, i.e., the sum of the variance of the statistical analysis value in the laboratory;
N is the total number of data points;
p is the number of laboratories;
r is value of repeatability. The uncertainty of between measurements is equal to the confidence interval of differences. By convention, the confidence interval is taken to be 95%,
[
10].
Vr is repeatability variablility;
and m is the gross average value.
As shown in
Figure 3, when the OPC-28D’s air-drying time is under 5 h, the mean and standard deviation (SD) of the measured pH value is 12.82 ± 0.02, the repeatability error S
r = 0.013, the value of repeatability r = 0.038, and repeatability variability V
r = 0.101%. The result shows that the repeatability (r) of the pH measurement is acceptable. The repeatability (r) obtained is lower than ±0.1, which can be assigned to the pH electrode. However, when the C60-SF-28D’s air-drying time is under 5 h, the mean and standard deviation of the measured pH value is 12.14 ± 0.16, the repeatability error S
r = 0.114, value of repeatability r = 0.323, and repeatability variability V
r = 0.939%. The results show that the repeatability (r) of the pH measurement is unacceptable, which is about 3 times higher than the error of the pH electrode (±0.1). Therefore, the pH measurement results will be affected by the air-drying time before the specimen is crushed. In the S-ESL procedure, the specimen should be crushed without air drying.
3.2. The Effect of Oven-Drying before Crushing on the pH Value
Figure 4 shows that for OPC and C60-SF40 pastes, after 28 days of curing, the pH value of the specimen decreased with the increase in the oven-drying duration. In addition, the pH value of the C60-SF40 specimen reduced more at the rate of about 0.093 per hour. The explanation for this reduction is related to the continued hydration of paste. The hydration percentage of C60-SF40 paste after 28 days curing was lower than that of the OPC paste after 28 days curing. Thus, oven-drying the specimen before crushing has a significant impact on the reduction of the C60-SF40 pH. Therefore, when the specimen reaches the curing age, it is suggested that the subsequent procedure such as crushing should be carried out immediately after oven-drying so that the measured pH value of the concrete is not underestimated.
Using Equations (1)–(3), the result is shown in
Figure 4. When the OPC-28D is put under oven-drying within 6 h, the mean and standard deviation of the measured pH value is 12.77 ± 0.06, the repeatability error S
r = 0.046, the value of repeatability r = 0.129, and repeatability variability V
r = 0.360%. For C60-SF-28D, the mean and standard deviation of the measured pH value is 12.06 ± 0.25, the repeatability error S
r = 0.176, the value of repeatability r = 0.499, and repeatability variability V
r = 1.459%. The repeatability (r) of two specimens uses oven-drying before crushing is higher than two specimens using air-drying before crushing. Therefore, for incomplete hydration’s specimen, the pH results will be affected by the oven-drying period before the specimen is crushed. Therefore, the specimen should be crushed without oven-drying.
3.3. The Effect of Air-Exposure Time of the Specimen Powder on the pH Value
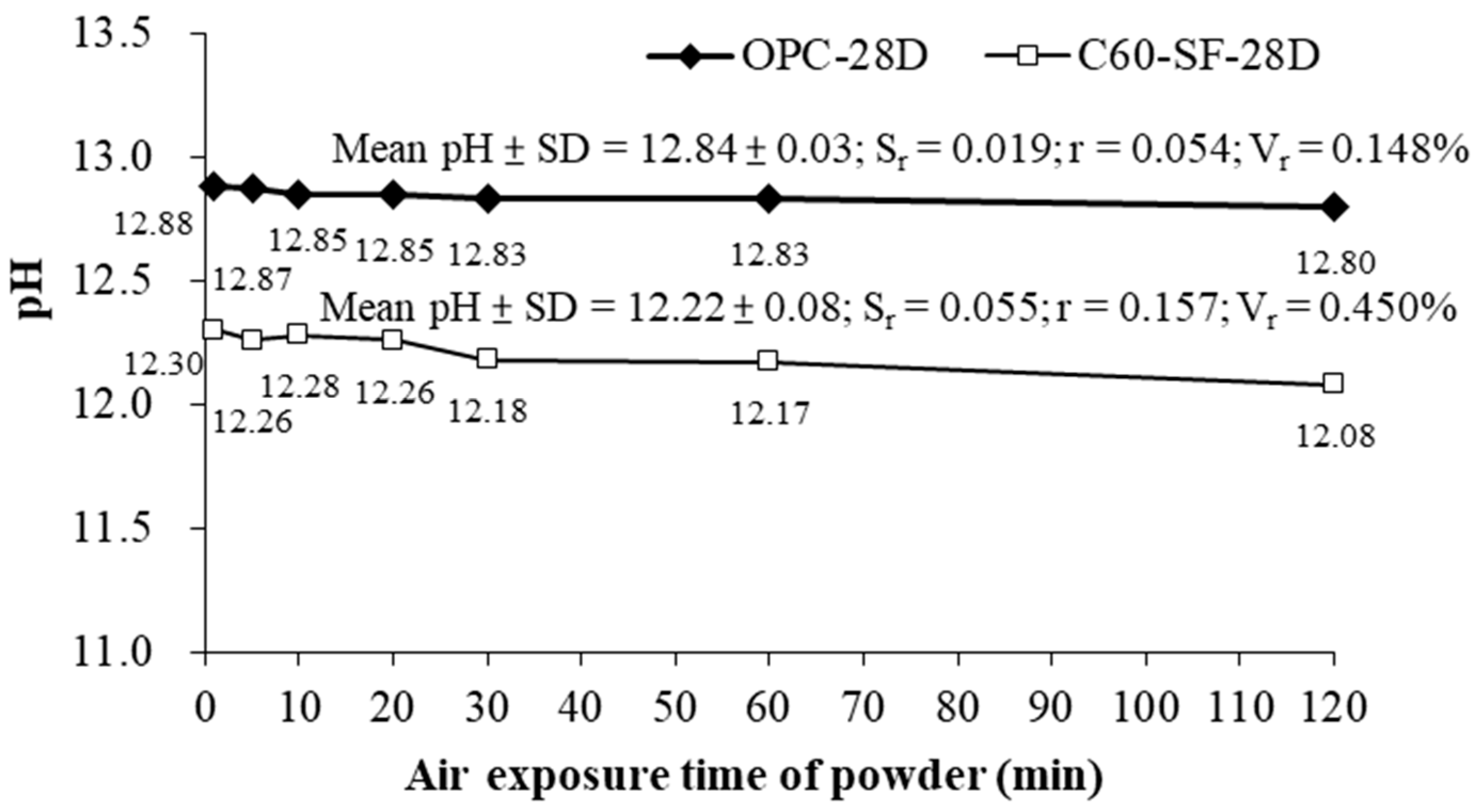
The experiment was conducted using OPC and C60-SF40 paste after 28 days of curing. According to the result shown in
Figure 5, the pH of the specimen decreased with the increase in air exposure duration. In addition, the pH value of the C60-SF40 reduced faster than that of the OPC paste. The pH reduction of the C60-SF40 was approximately 0.11 per hour. In contrast, the pH reduction rate of the OPC specimen was smaller and the rate was approximately 0.04 per hour. The reason for the pH value reduction is continuous hydration. For C60-SF40 paste, the hydration degree of curing for 28 days was lower than that of OPC. Therefore, the contact time between cement powder and the air has a significant impact on the reduction in C60-SF40 pH. Thus, after the specimen reaches the curing age, the subsequent procedure of pH testing should be carried out immediately after grinding, so as not to underestimate the pH of the concrete.
Using Equations (1)–(3), the result is shown in
Figure 5, when the exposure time of the OPC-28D’s powder is under 120 min, the mean and standard deviation of the measured pH value is 12.84 ± 0.03, the repeatability error S
r = 0.019, the value of repeatability r = 0.054, and repeatability variability V
r = 0.148%. For C60-SF-28D, the mean and standard deviation of the measured pH value is 12.22 ± 0.08, the repeatability error S
r = 0.055, the value of repeatability r = 0.157, and repeatability variability V
r = 0.450%. The result shows that the repeatability (r) of the OPC-28D is satisfied with the error of the pH electrode (±0.1). Besides, the repeatability (r) of the C60-SF-28D is acceptable, the values obtained are close to the instrument’s error of the pH electrode (±0.1). Although the statistical analysis results of the experiment showed that the specimen powder contacting with air under 120 min did not have a significant effect on the results, the S-ESL procedure is still required to mix with deionized water within 5 min for safety issues.
3.4. The Effect of Air-Exposed Duration of the Solution on pH
The pH value in the first stage of the air exposure is shown in
Figure 6, the one-day OPC specimen curing has the mean and standard deviation of the measured pH value is 12.84 ± 0.03, the repeatability error S
r = 0.019, the value of repeatability r = 0.054, and repeatability variability V
r = 0.148%. The numerical variation was small, which indicates that in the context of typical operating procedures, the cement paste solution which is air-exposed for 60 min, has an insignificant pH deviation.
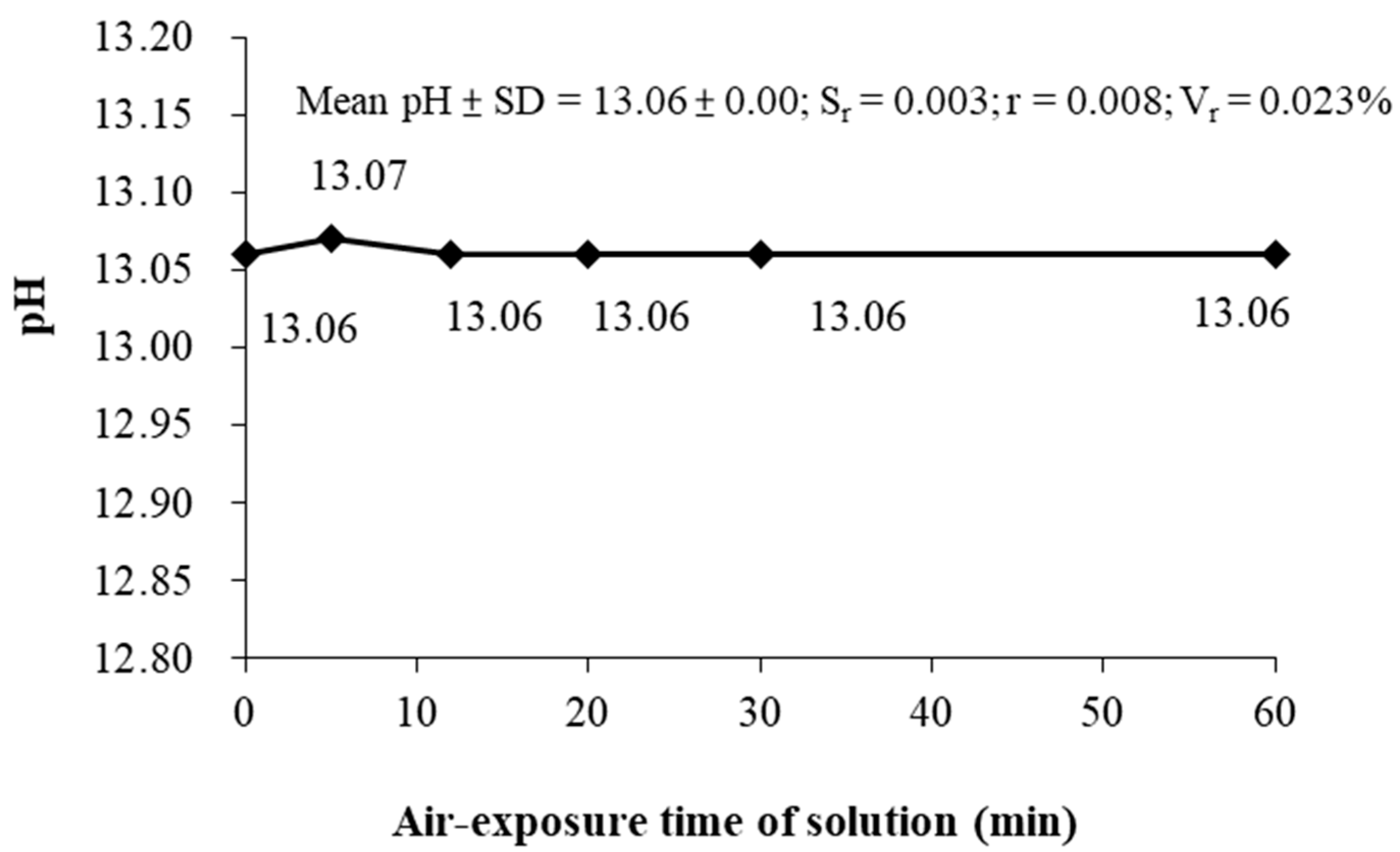
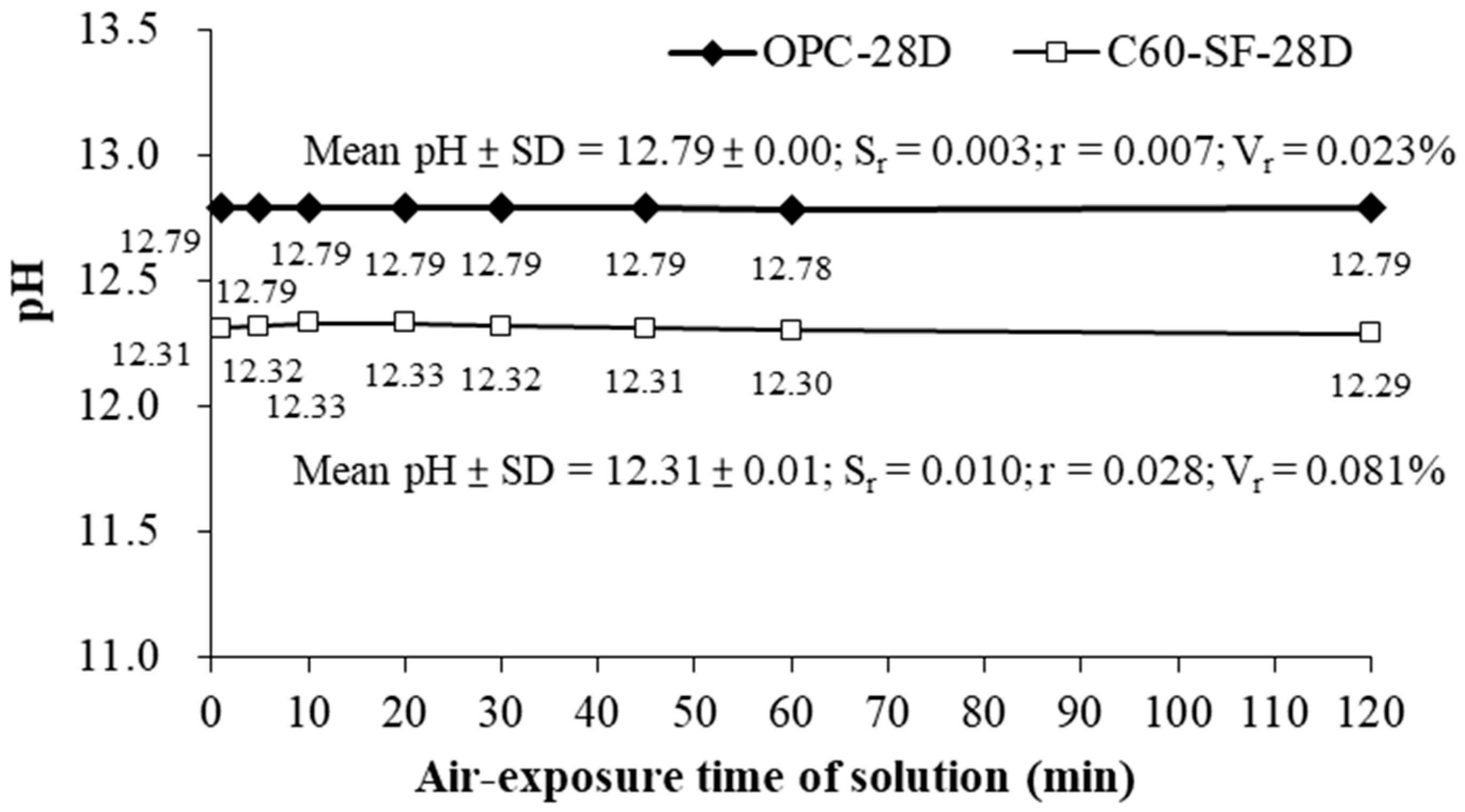
In the second stage of air exposure, pH measurement of 28-days curing of OPC and C60-SF40 was conducted, and the results are mentioned in
Figure 7, which showed that the 28-day curing OPC had the mean and standard deviation of the measured pH value is 12.79 ± 0.00, the repeatability error S
r = 0.003, the value of repeatability r = 0.007, and repeatability variability V
r = 0.023%. The result showed that the repeatability (r) of the pH measurement is satisfied with the error of the pH electrode (±0.1). The 28-day curing C60-SF40 had the mean and standard deviation of the measured pH value is 12.31 ± 0.01, the repeatability error S
r = 0.010, the value of repeatability r = 0.028, and repeatability variability V
r = 0.081%. The result shows that the repeatability (r) of the pH measurement is satisfied with the error of the pH electrode (±0.1). Thus, these results prove that in the cement paste operating procedures, within 120 min of air-exposure, the cement paste solution has no significant deviation in pH of the samples measured.
In conclusion, this stage of experiment is aimed to investigate whether the pH of the cement paste solution is affected by environmental components such as CO2 in the air and found that the effect of the environment on the cement paste solution is not significant.
3.5. Comparison of the pH Value of Multiple Beaker Solutions from the Same Source
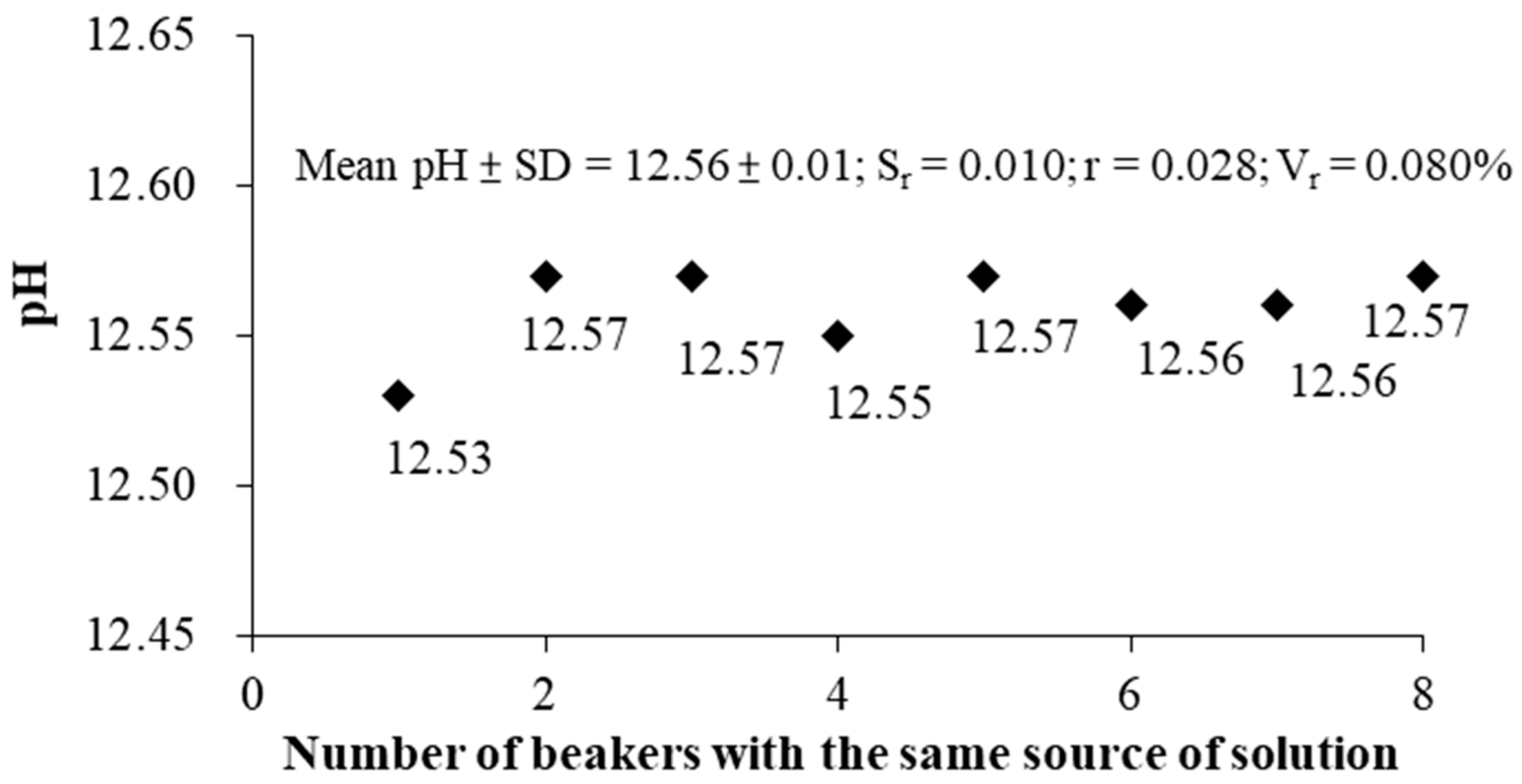
According to the results in
Figure 8, the mean and standard deviation of the measured pH value is 12.56 ± 0.01, the repeatability error S
r = 0.010, the value of repeatability r = 0.028, and repeatability variability V
r = 0.080%. The result shows that the repeatability (r) of the pH measurement is satisfied to the error of the pH electrode (±0.1). Measuring the pH value of eight beaker’s solutions from the same source, using the S-ESL method to measure pH value achieved acceptable repeatability results (SD < 0.1).
3.6. The Effect of pH Measurement in the Same Cup of Solution at Equal Time Interval
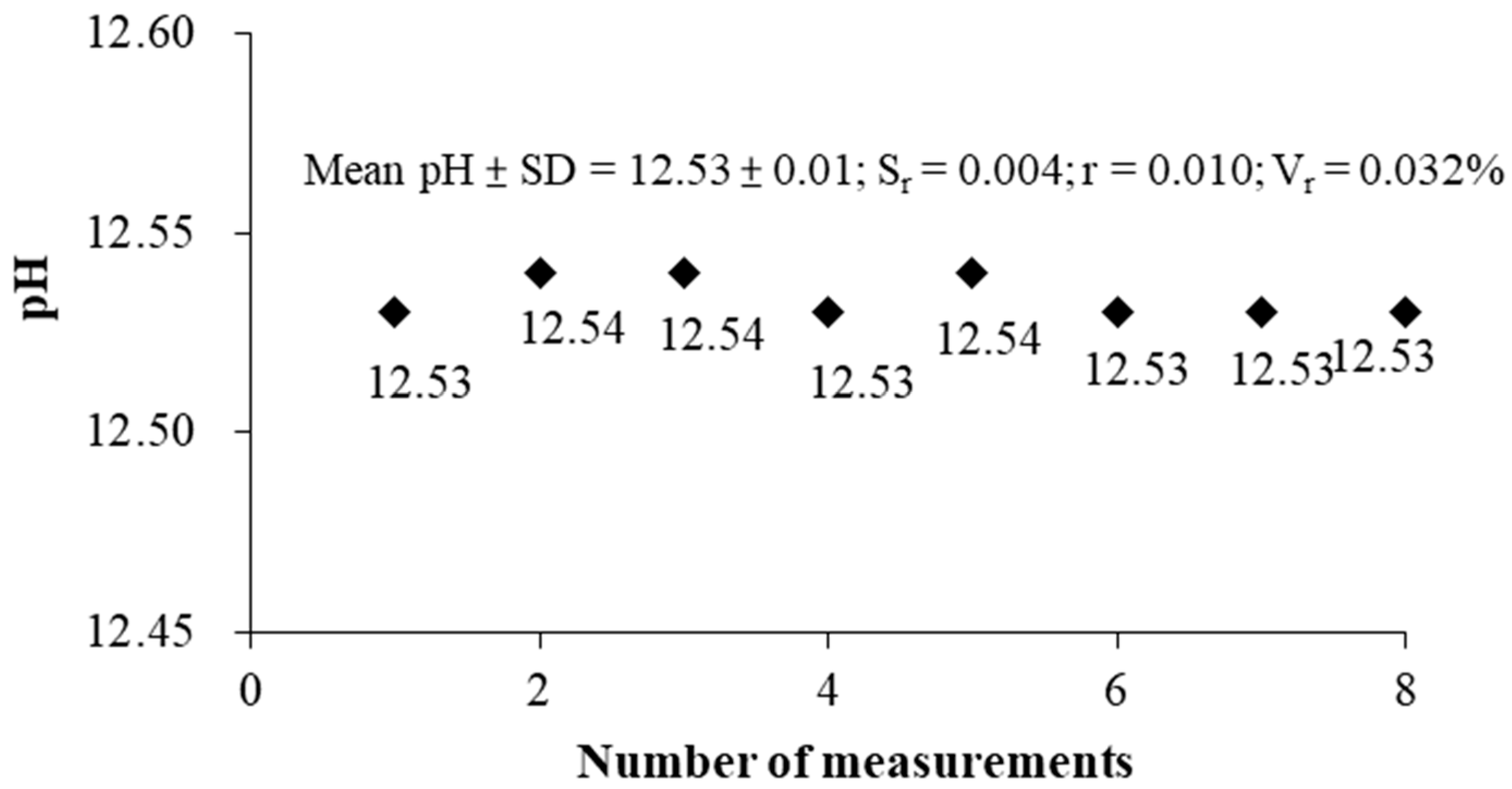
According to the results shown in
Figure 9, the mean and standard deviation of the measured pH value is 12.53 ± 0.01, the repeatability error S
r = 0.004, the value of repeatability r = 0.010, and repeatability variability V
r = 0.032%. The result shows that the repeatability (r) of the pH measurement is satisfied with the error of the pH electrode (±0.1). The pH measurement of the same cup of the solution at an equal time interval, using the S-ESL method to measure pH value has good repeatability results (SD < 0.1).
3.7. The Influence of Particle Sizes on the pH
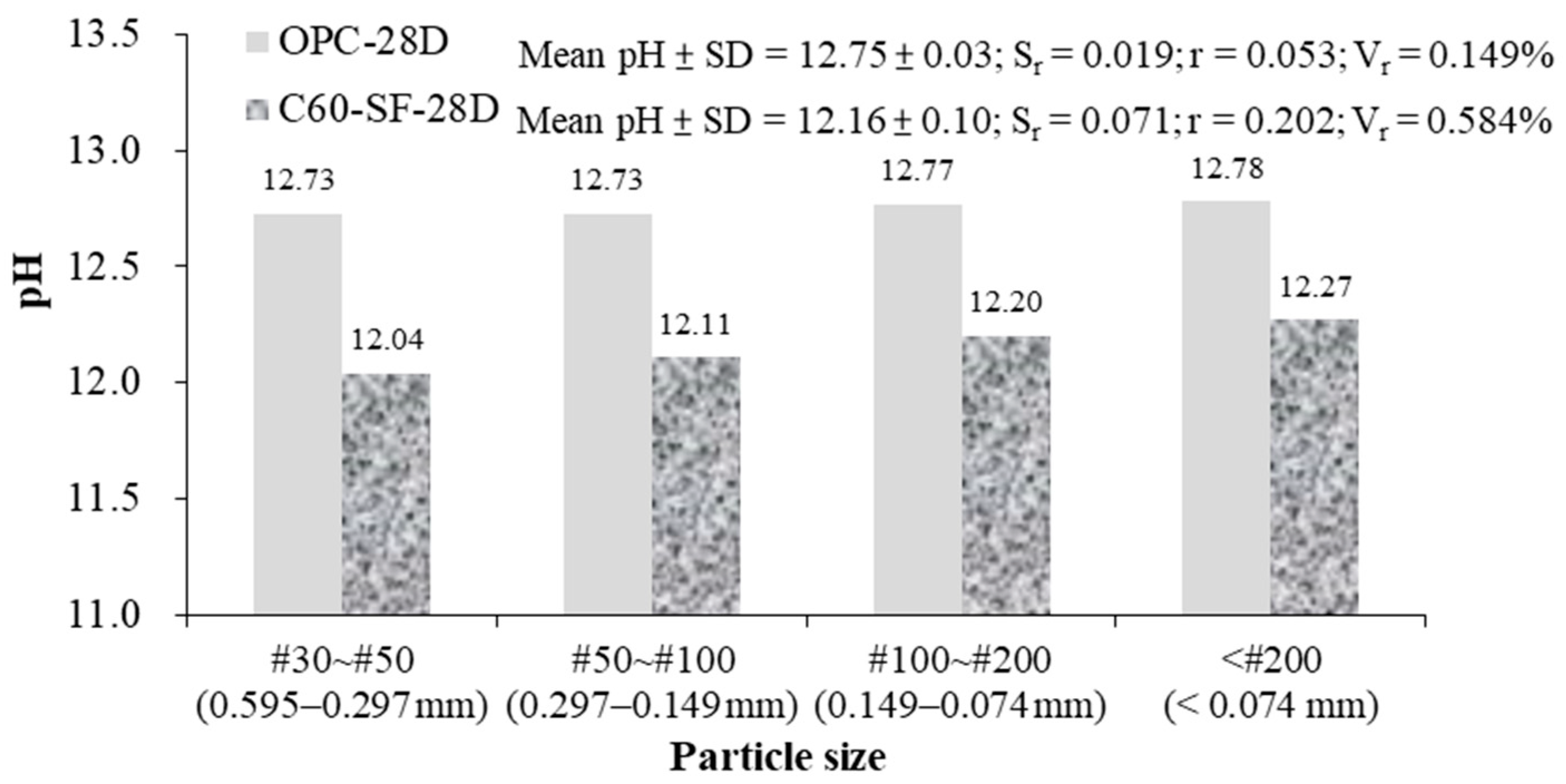
The effect of particle sizes on pH was as shown in
Figure 10. For the OPC-28D, the mean and standard deviation of the measured pH value is 12.75 ± 0.03, the repeatability error S
r = 0.019, the value of repeatability r = 0.05, and repeatability variability V
r = 0.149%. The result shows that the repeatability (r) of the pH measurement is satisfied with the error of the pH electrode (±0.1). However, for the C60-SF-28D, the mean and standard deviation of the measured pH value is 12.16 ± 0.10, the repeatability error S
r = 0.071, value of repeatability r = 0.202, and repeatability variability V
r = 0.584%. The results show that the repeatability (r) of the pH measurement is unacceptable. The repeatability (r) is two times higher than the error of the pH electrode (±0.1).
If the concrete is used as a tank for the final disposal site of high-level radioactive nuclear waste, concrete is required to use low-alkaline cement. The results in
Figure 10 show that for the C60-SF-28D, the larger the specimen size, the measured pH value will be lower. Therefore, using the S-ESL method with the particle size smaller than the #200 sieve (0.074 mm) can measure the pH value of concrete more accurate.
3.8. The Effect of the Solution’s Temperature on the pH of the Cement Paste Solution
The effect of the temperature on the solution’s pH is shown in
Figure 11. The pH of 28-days cured OPC solution at 25 °C has the maximum pH value. The mean and standard deviation of the measured pH value is 12.76 ± 0.03, the repeatability error S
r = 0.050, the value of repeatability r = 0.140, and repeatability variability V
r = 0.392%. The result shows that the repeatability (r) of the pH measurement is satisfied with the error of the pH electrode (±0.1). However, for the C60-SF-28D, the pH at 25 °C has the maximum value, the mean and standard deviation of the measured pH value is 12.18 ± 0.10, the repeatability error S
r = 0.071, value of repeatability r = 0.200, and repeatability variability V
r = 0.583%. The results show that the repeatability (r) of the pH measurement is unacceptable. The repeatability (r) is two times higher than the error of the pH electrode (±0.1). The results above suggest that the suitable curing temperature should be set to room temperature (25 °C). Besides, while using air conditioning, the temperature should be controlled at 25 °C ± 2°C.
3.9. The S-ESL Method Goals
The S-ESL procedure proposed in this research hopes that the entire procedure can be operated under normal environmental condition instead of under N2 requirement condition, the protocol becomes simpler and using more popular instruments, which will benefit a variety of laboratories lacking N2 equipment. On the other hand, by researching the variation of results produced by each different operation step, more reasonable operation steps are proposed, and the requirements of accuracy and repeatability can be achieved.
Variations explored in this study include: air-drying time and oven-drying time before specimen crushing, air exposure time of powder or solution, the multiple beaker solutions from the same source, measuring the same cup of solution at equal time intervals, the particle size of the specimen, and the solution’s temperature. According to the repeatability statistical analysis results, it is found that if the test conditions are not restricted properly, factors such as air-drying time and oven-drying time before specimen crushing, the particle size of the specimen, and the solution’s temperature can make the measurement result become inaccurate. Therefore, these test conditions need to be strictly controlled in the S-ESL procedure.
Increasing the air-drying time and oven-drying time before crushing will increase the value of repeatability (r) of the test. The reason can be related to the continuous hydration of cement during the pH measurement process. Therefore, in the S-ESL procedure, after grinding, the specimen’s powder needs to be placed in a zipper bag with soda lime to stop hydration.
Increasing the particle size of the specimen will increase the value of repeatability (r) of the test. The reason can because the particle size increases, which lead to the decreasing of the amount of alkali dissolution in the specimen during the mixing and stirring process. Therefore, in the S-ESL procedure, the specimen needs to pass the #200 sieve (0.074 mm), so the alkali in the specimen can be dissolved into the deionized water.
In addition, increasing the temperature of the test solution will reduce the measured pH value. Therefore, the temperature of the solution should be controlled during the measurement process, at least the same as room temperature, and this study suggest that it is best to be able to control it at 25 °C ± 2 °C.