Long Rod-Like Liquid Crystal Containing Azobenzene and the Applications in Phase-Transition Regulation and Orientation of Nematic Liquid Crystal
Abstract
:1. Introduction
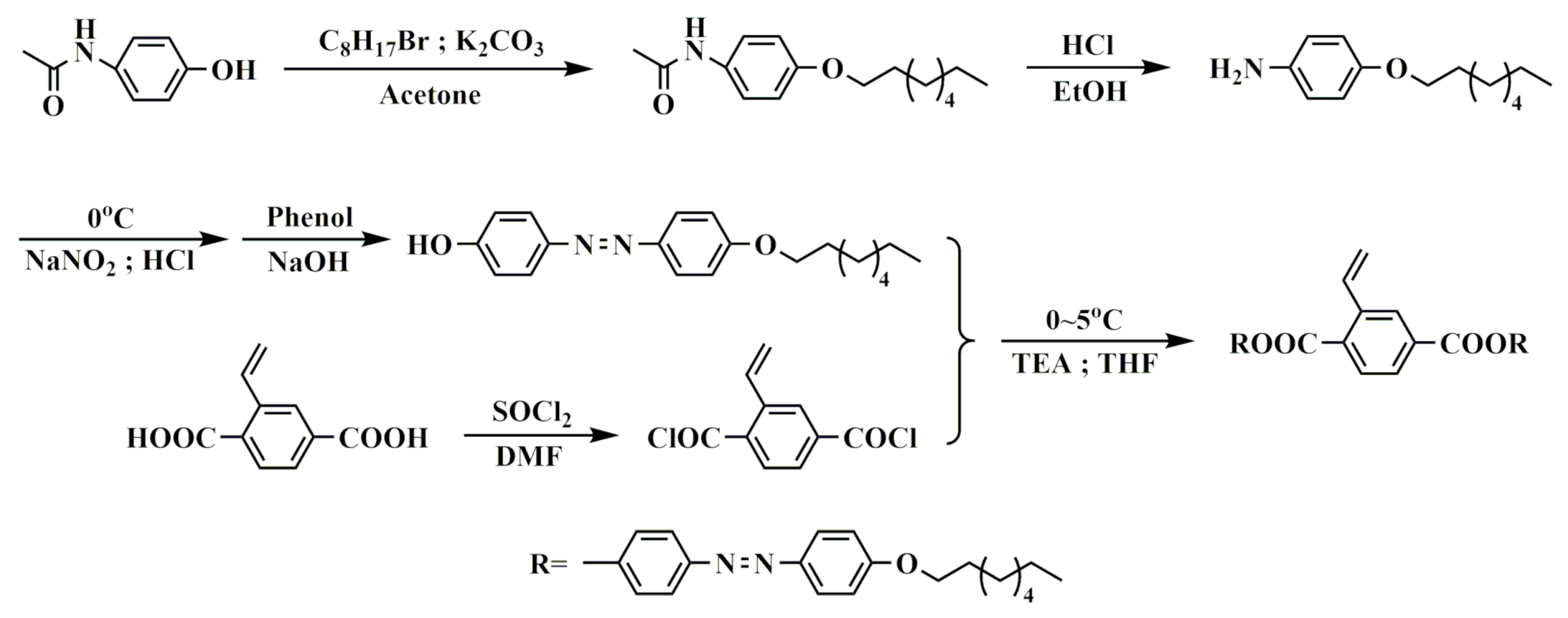
2. Experimental Section
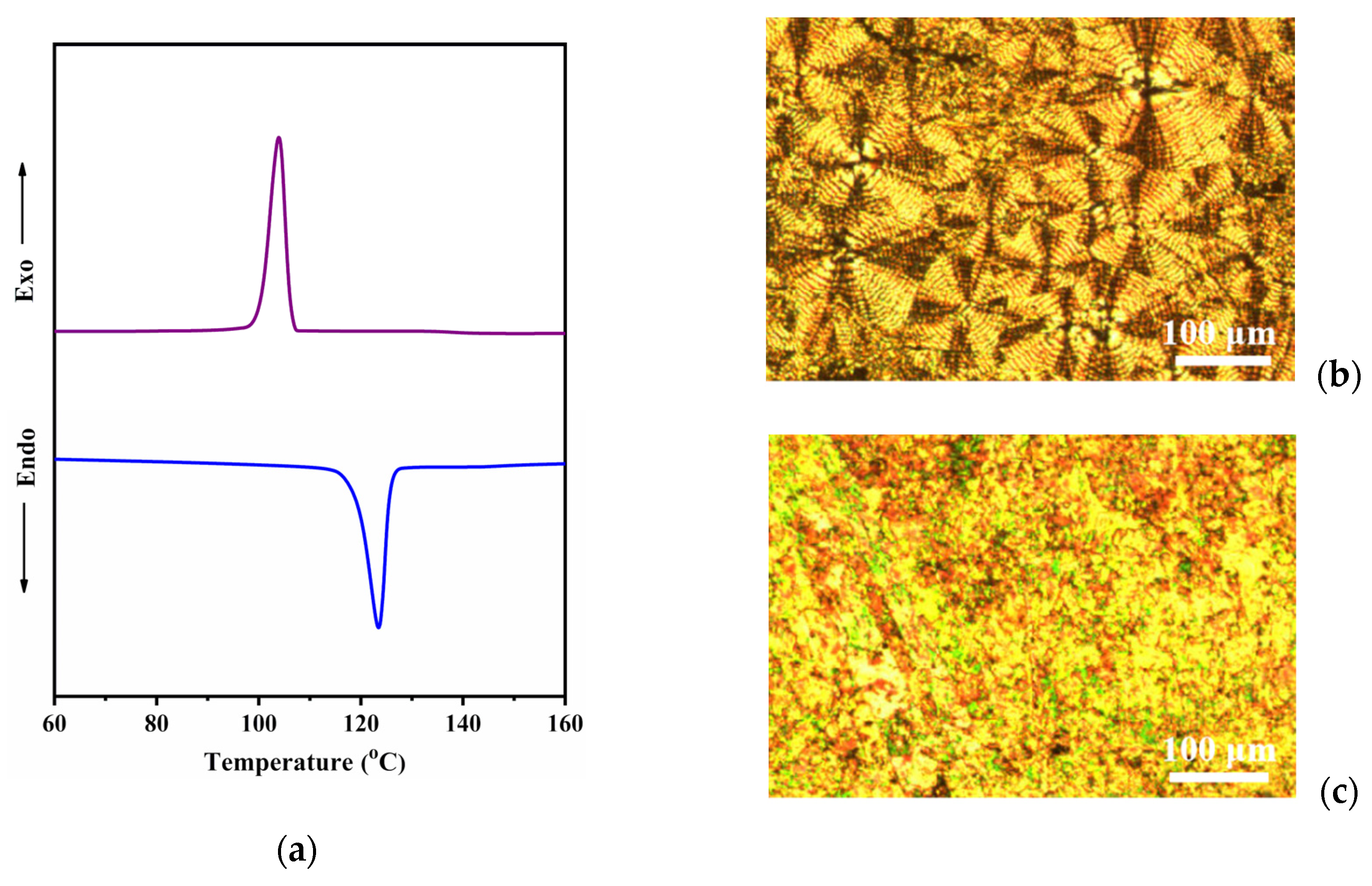
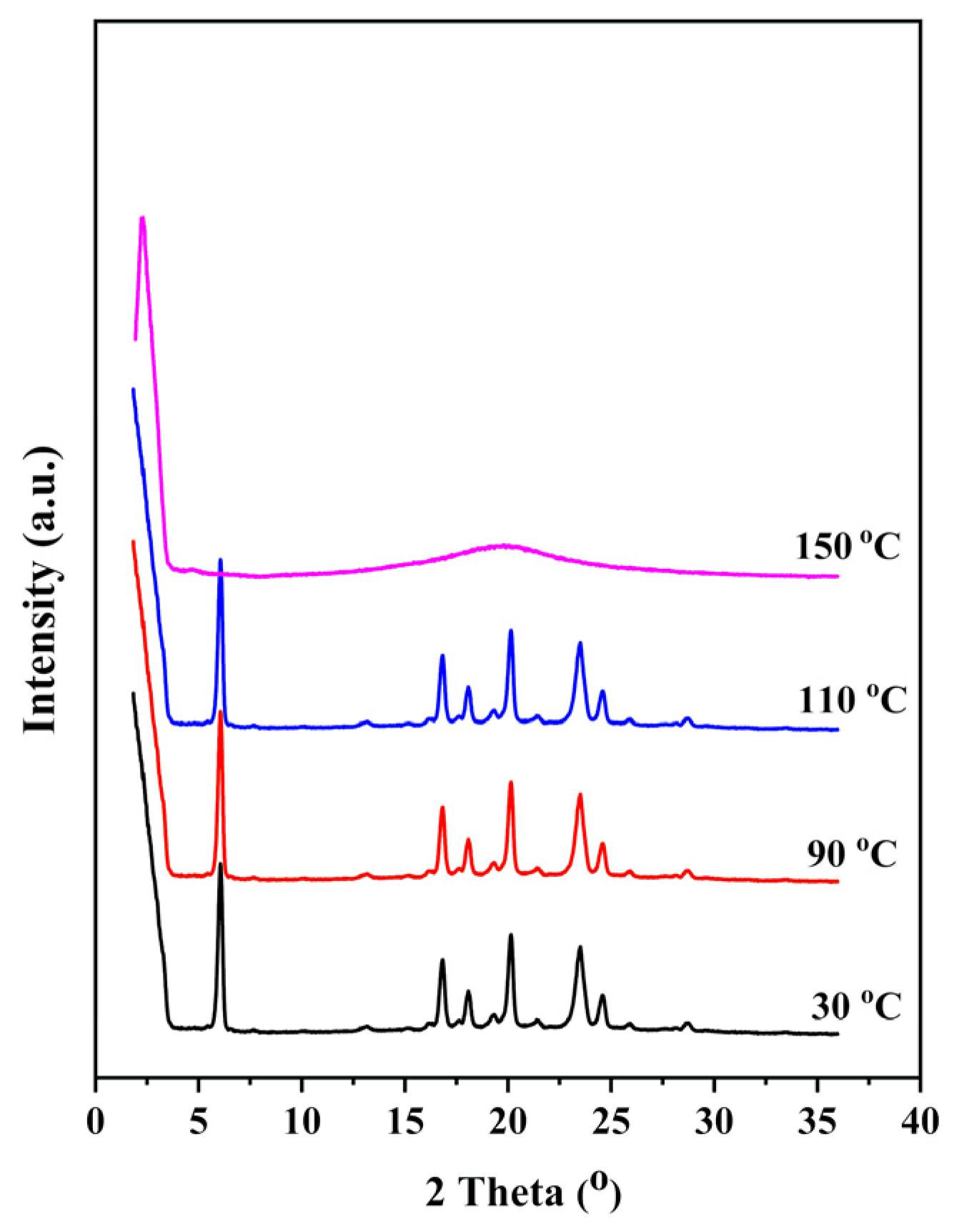
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bisoyi, H.K.; Bunning, T.J.; Li, Q. Stimuli-Driven Control of the Helical Axis of Self-Organized Soft Helical Superstructures. Adv. Mater. 2018, 30, 1706512. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef]
- Cha, Y.J.; Gim, M.-J.; Oh, K.; Yoon, D.K. In-Plane Switching Mode for Liquid Crystal Displays Using a DNA Alignment Layer. ACS Appl. Mater. Interface 2015, 7, 13627–13632. [Google Scholar] [CrossRef]
- Fernández, R.; Gallego, S.; Márquez, A.; Francés, J.; Martínez, F.J.; Pascual, I.; Beléndez, A. Analysis of holographic polymer-dispersed liquid crystals (HPDLCs) for tunable low frequency diffractive optical elements recording. Opt. Mater. 2018, 76, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Kendhale, A.M.; Schenning, A.P.H.J.; Debije, M.G. Superior alignment of multi-chromophoric perylenebisimides in nematic liquid crystals and their application in switchable optical waveguides. J. Mater. Chem. A 2013, 1, 229–232. [Google Scholar] [CrossRef]
- Rushnova, I.I.; Melnikova, E.A.; Tolstik, A.L.; Muravsky, A.A. Electrically switchable photonic liquid crystal devices for routing of a polarized light wave. Opt. Commun. 2018, 413, 179–183. [Google Scholar] [CrossRef]
- Kostko, A.F.; Cipriano, B.H.; Pinchuk, O.A.; Ziserman, L.; Anisimov, M.A.; Danino, D.; Raghavan, S.R. Salt Effects on the Phase Behavior, Structure, and Rheology of Chromonic Liquid Crystals. J. Phys. Chem. B 2005, 109, 19126–19133. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, A.; Schweizer, K.S. Effective Interactions, Structure, and Phase Behavior of Lightly Tethered Nanoparticles in Polymer Melts. Macromolecules 2008, 41, 9430–9438. [Google Scholar] [CrossRef]
- Jiang, R.; Jin, Q.; Li, B.; Ding, D.; Wickham, R.A.; Shi, A.-C. Phase Behavior of Gradient Copolymers. Macromolecules 2008, 41, 5457–5465. [Google Scholar] [CrossRef]
- Jiao-jiao, Y.; Yao-jian, F.; Lei, T.; He-lou, X.; Hai-liang, Z. Phase Behavior and Phase Structure of Polymerized Ionic Liquid Crystals with Different Alkyl Tail Length Based on Jacketing Effect. Acta Polym. Sin. 2017, 10, 1616–1623. [Google Scholar]
- Herrmann-Schönherr, O.; Wendorff, J.H.; Ringsdorf, H.; Tschirner, P. Structure of an aromatic polyamide with disc-like mesogens in the main chain. Die Makromol. Chem. Rapid Commun. 1986, 7, 791–796. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Sigaud, G.; Achard, M.F.; Hardouin, F.; Twieg, R.J.; Betterton, K. Rod-like mesogens with antipathetic fluorocarbon and hydrocarbon tails. Liq. Cryst. 1991, 10, 389–396. [Google Scholar] [CrossRef]
- Okano, K.; Mikami, Y.; Yamashita, T. Liquid-Crystalline Polymer with a Block Mesogenic Side Group: Photoinduced Manipulation of Nanophase-Separated Structures. Adv. Funct. Mater. 2009, 19, 3804–3808. [Google Scholar] [CrossRef]
- Sekine, T.; Niori, T.; Sone, M.; Watanabe, J.; Choi, S.-W.; Takanishi, Y.; Takezoe, H. Origin of Helix in Achiral Banana-Shaped Molecular Systems. Jpn. J. Appl. Phys. 1997, 36, 6455–6463. [Google Scholar] [CrossRef]
- Weissflog, W.; Demus, D.; Diele, S.; Nitschke, P.; Wedler, W. From laterally branched mesogens to novel twin molecules. Liq. Cryst. 1989, 5, 111–122. [Google Scholar] [CrossRef]
- Guillevic, M.-A.; Bruce, D.W. Mesomorphic imines and their complexes with rhenium (I): A cubic mesophase in a rod-like mesogen with perfluorinated terminal chains. Liq. Cryst. 2000, 27, 153–156. [Google Scholar] [CrossRef]
- Lee, M.; Yoo, Y.-S. Supramolecular organization of block oligomers based on rod-shaped mesogen into liquid crystalline assembly. J. Mater. Chem. 2002, 12, 2161–2168. [Google Scholar] [CrossRef]
- Zheng, J.-F.; Yu, Z.-Q.; Liu, X.; Chen, X.-F.; Yang, S.; Chen, E.-Q. Side-chain liquid-crystalline polymers based on flexible rod-like mesogen directly attached to backbone. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 5023–5031. [Google Scholar] [CrossRef]
- Tripathi, C.S.P.; Losada-Pérez, P.; Glorieux, C.; Kohlmeier, A.; Tamba, M.-G.; Mehl, G.H.; Leys, J. Nematic-nematic phase transition in the liquid crystal dimer CBC9CB and its mixtures with 5CB: A high-resolution adiabatic scanning calorimetric study. Phys. Rev. E 2011, 84, 041707. [Google Scholar] [CrossRef] [Green Version]
- Marinov, Y.G.; Hadjichristov, G.B.; Petrov, A.G.; Prasad, S.K. Thin films of silica nanoparticle doped nematic liquid crystal 7CB for electro-optic modulation. Photonics Lett. Pol. 2015, 7, 94–96. [Google Scholar]
- Jayaprada, P.; Rao, M.; Pardhasaradhi, P.; Prasad, P.D.; Manepalli, R.; Pisipati, V. Optical studies of n-octyloxy-cyanobiphenyl (8ocb) with dispersed ZnO nanoparticles for display device application. Optik 2019, 185, 1226–1237. [Google Scholar] [CrossRef]
- Singh, L.P.; Singh, N.P.; Srivastava, S.K. Terbium doped SnO2 nanoparticles as white emitters and SnO2: 5Tb/Fe3O4 magnetic luminescent nanohybrids for hyperthermia application and biocompatibility with HeLa cancer cells. Dalton Trans. 2015, 44, 6457–6465. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ji, C.; Zhao, X.; Han, Y.; Müllen, K.; Pan, K.; Yin, M. Green-Light-Triggered Phase Transition of Azobenzene Derivatives toward Reversible Adhesives. J. Am. Chem. Soc. 2019, 141, 7385–7390. [Google Scholar] [CrossRef]
- Yao, Y.; Waters, J.T.; Shneidman, A.V.; Cui, J.; Wang, X.; Mandsberg, N.K.; Li, S.; Balazs, A.C.; Aizenberg, J. Multiresponsive polymeric microstructures with encoded predetermined and self-regulated deformability. Proc. Natl. Acad. Sci. USA 2018, 115, 12950–12955. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, W.; Skupin, H.; Tolksdorf, C.; Gebhard, E.; Zentel, R.; Krüger, P.; Lösche, M.; Kremer, F. Giant lateral electrostriction in ferroelectric liquid-crystalline elastomers. Nature 2001, 410, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Guo, S.; Tong, X.; Xia, H.; Zhao, Y. Tunable Photocontrolled Motions Using Stored Strain Energy in Malleable Azobenzene Liquid Crystalline Polymer Actuators. Adv. Mater. 2017, 29, 1606467. [Google Scholar] [CrossRef]
- Mahimwalla, Z.; Yager, K.G.; Mamiya, J.-I.; Shishido, A.; Priimagi, A.; Barrett, C.J. Azobenzene photomechanics: Prospects and potential applications. Polym. Bull. 2012, 69, 967–1006. [Google Scholar] [CrossRef]
- Ni, B.; Xie, H.-L.; Tang, J.; Zhang, H.-L.; Chen, E.-Q. A self-healing photoinduced-deformable material fabricated by liquid crystalline elastomers using multivalent hydrogen bonds as cross-linkers. Chem. Commun. 2016, 52, 10257–10260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xue, C.; Weis, P.; Suzuki, Y.; Huang, S.; Koynov, K.; Auernhammer, G.K.; Berger, R.; Butt, H.-J.; Wu, S. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef]
- Lu, H.-F.; Wang, M.; Chen, X.-M.; Lin, B.-P.; Yang, H. Interpenetrating liquid-crystal polyurethane/polyacrylate elastomer with ultrastrong mechanical property. J. Am. Chem. Soc. 2019, 141, 14364–14369. [Google Scholar] [CrossRef] [PubMed]
- Urayama, K.; Honda, S.; Takigawa, T. Deformation Coupled to Director Rotation in Swollen Nematic Elastomers under Electric Fields. Macromolecules 2006, 39, 1943–1949. [Google Scholar] [CrossRef]
- Schuhladen, S.; Preller, F.; Rix, R.; Petsch, S.; Zentel, R.; Zappe, H. Iris-Like Tunable Aperture Employing Liquid-Crystal Elastomers. Adv. Mater. 2014, 26, 7247–7251. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Sakamoto, K.; Arafune, R.; Ushioda, S. Relation between the molecular orientations of a very thin liquid crystal layer and an underlying rubbed polyimide film. J. Appl. Phys. 2000, 88, 3235–3241. [Google Scholar] [CrossRef]
- Van der Vegt, N.F.A.; Müller-Plathe, F.; Geleßus, A.; Johannsmann, D. Orientation of liquid crystal monolayers on polyimide alignment layers: A molecular dynamics simulation study. J. Chem. Phys. 2001, 115, 9935–9946. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Chen, M.; Wang, Q.; Guo, S.; Zhang, L.; Yang, H. Active and passive modulation of solar light transmittance in a hybrid thermochromic soft-matter system for energy-saving smart window applications. J. Mater. Chem. C 2018, 6, 7054–7062. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Chen, M.; Guo, S.; Zhang, L.; Li, F.; Guo, S.; Yang, H. A roll-to-roll process for multi-responsive soft-matter composite films containing Cs x WO 3 nanorods for energy-efficient smart window applications. Nanoscale Horiz. 2017, 2, 319–325. [Google Scholar] [CrossRef]
- Pan, S.; Ho, J.Y.; Chigrinov, V.G.; Kwok, H.S. Novel Photoalignment Method Based on Low-Molecular-Weight Azobenzene Dyes and Its Application for High-Dichroic-Ratio Polarizers. ACS Appl. Mater. Interfaces 2018, 10, 9032–9037. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q. Photochromism into nanosystems: Towards lighting up the future nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097. [Google Scholar] [CrossRef]
- Zheng, Z.-G.; Yuan, C.-L.; Hu, W.; Bisoyi, H.K.; Tang, M.-J.; Liu, Z.; Sun, P.-Z.; Yang, W.-Q.; Wang, X.-Q.; Shen, D.; et al. Light-Patterned Crystallographic Direction of a Self-Organized 3D Soft Photonic Crystal. Adv. Mater. 2017, 29, 1703165. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Nagano, S.; Hara, M.; Seki, T. Free-surface molecular command systems for photoalignment of liquid crystalline materials. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matsumori, M.; Takahashi, A.; Tomioka, Y.; Hikima, T.; Takata, M.; Kajitani, T.; Fukushima, T. Photoalignment of an Azobenzene-Based Chromonic Liquid Crystal Dispersed in Triacetyl Cellulose: Single-Layer Alignment Films with an Exceptionally High Order Parameter. ACS Appl. Mater. Interfaces 2015, 7, 11074–11078. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Tao, L.; Zhong, C.-L.; Li, Z.-X.; Lan, K.; Feng, Y.; Wang, P.; Xie, H.-L. High-Efficiency Circularly Polarized Luminescence from Chiral Luminescent Liquid Crystalline Polymers with Aggregation-Induced Emission Properties. Macromolecules 2020, 53, 9758–9768. [Google Scholar] [CrossRef]
- Zhang, P.; Kragt, A.J.J.; Schenning, A.P.H.J.; de Haan, L.T.; Zhou, G. An easily coatable temperature responsive cholesteric liquid crystal oligomer for making structural colour patterns. J. Mater. Chem. C 2018, 6, 7184–7187. [Google Scholar] [CrossRef]
- Donovan, B.R.; Matavulj, V.M.; Ahn, S.k.; Guin, T.; White, T.J. All-Optical Control of Shape. Adv. Mater. 2019, 31, 1805750. [Google Scholar] [CrossRef] [PubMed]
- Gelebart, A.H.; Mulder, D.J.; Vantomme, G.; Schenning, A.P.H.J.; Broer, D.J. A Rewritable, Reprogrammable, Dual Light-Responsive Polymer Actuator. Angew. Chem. Int. Edit. 2017, 56, 13436–13439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, B.; Sun, S.; Wei, J.; Wu, L.; Yu, Y. Humidity- and Photo-Induced Mechanical Actuation of Cross-Linked Liquid Crystal Polymers. Adv. Mater. 2017, 29, 1604792. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.-Y.; Fan, Y.-J.; Tao, L.; Li, M.-L.; Zhao, N.; Wang, P.; Chen, E.-Q.; Fan, F.; Xie, H.-L. Alignment Control of Nematic Liquid Crystal using Gold Nanoparticles Grafted by the Liquid Crystalline Polymer with Azobenzene Mesogens as the Side Chains. ACS Appl. Mater. Interfaces 2018, 10, 27269–27277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.-X.; Wan, X.-H.; Zhou, Q.-F. Synthesis and characterization of a new series of “mesogen-jacketed liquid crystal polymers” based on the newly synthesized vinylterephthalic acid. Macromolecules 1999, 32, 5183–5185. [Google Scholar] [CrossRef]
- Zhu, J.-C.; Han, T.; Guo, Y.; Wang, P.; Xie, H.-L.; Meng, Z.G.; Yu, Z.-Q.; Tang, B.Z. Design and Synthesis of Luminescent Liquid Crystalline Polymers with “Jacketing” Effect andLuminescent Patterning Applications. Macromolecules 2019, 52, 3668–3679. [Google Scholar] [CrossRef]
- Qi, H.; Hegmann, T. Multiple Alignment Modes for Nematic Liquid Crystals Doped with Alkylthiol-Capped Gold Nanoparticles. ACS Appl. Mater. Interfaces 2009, 1, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nakano, M.; Yu, Y.; Tsutsumi, O.; Kanazawa, A. Anisotropic Bending and Unbending Behavior of Azobenzene Liquid-Crystalline Gels by Light Exposure. Adv. Mater. 2003, 15, 201–205. [Google Scholar] [CrossRef]

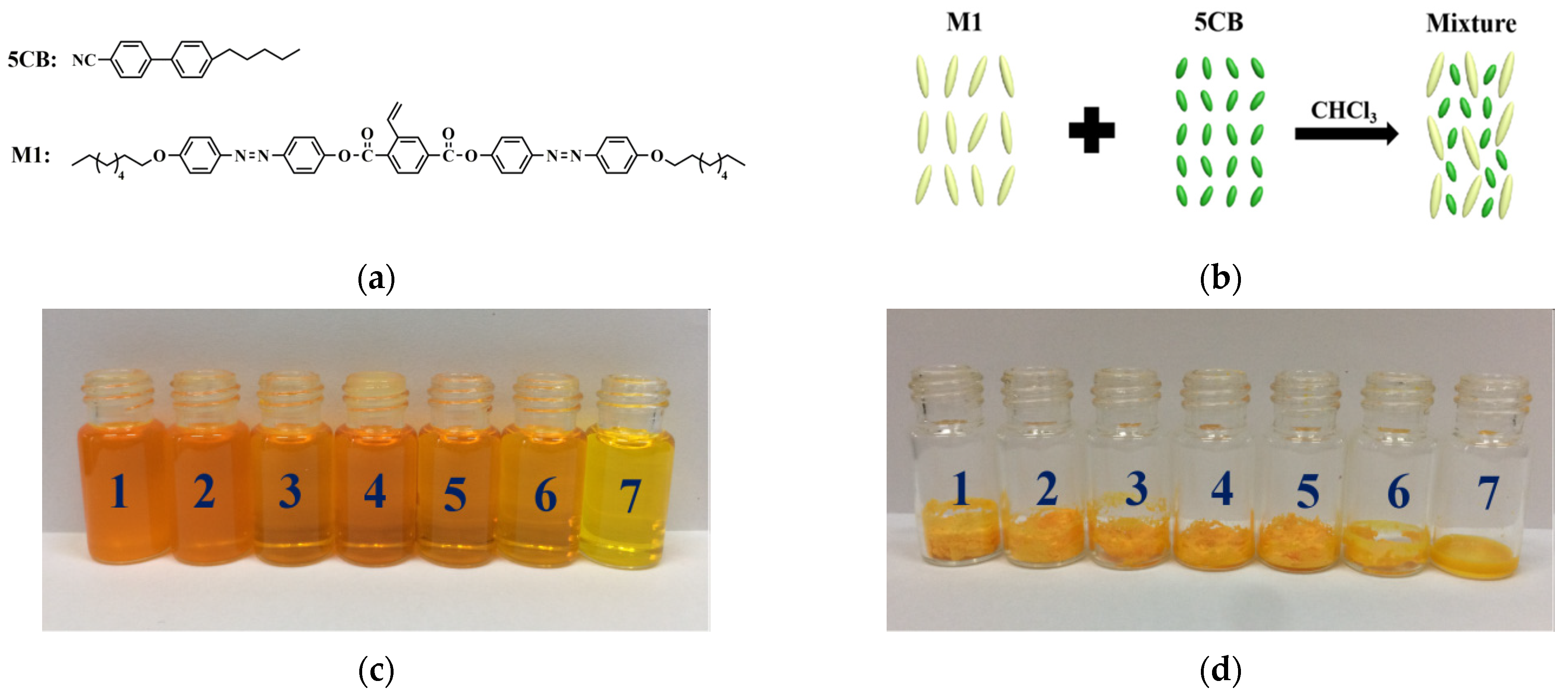
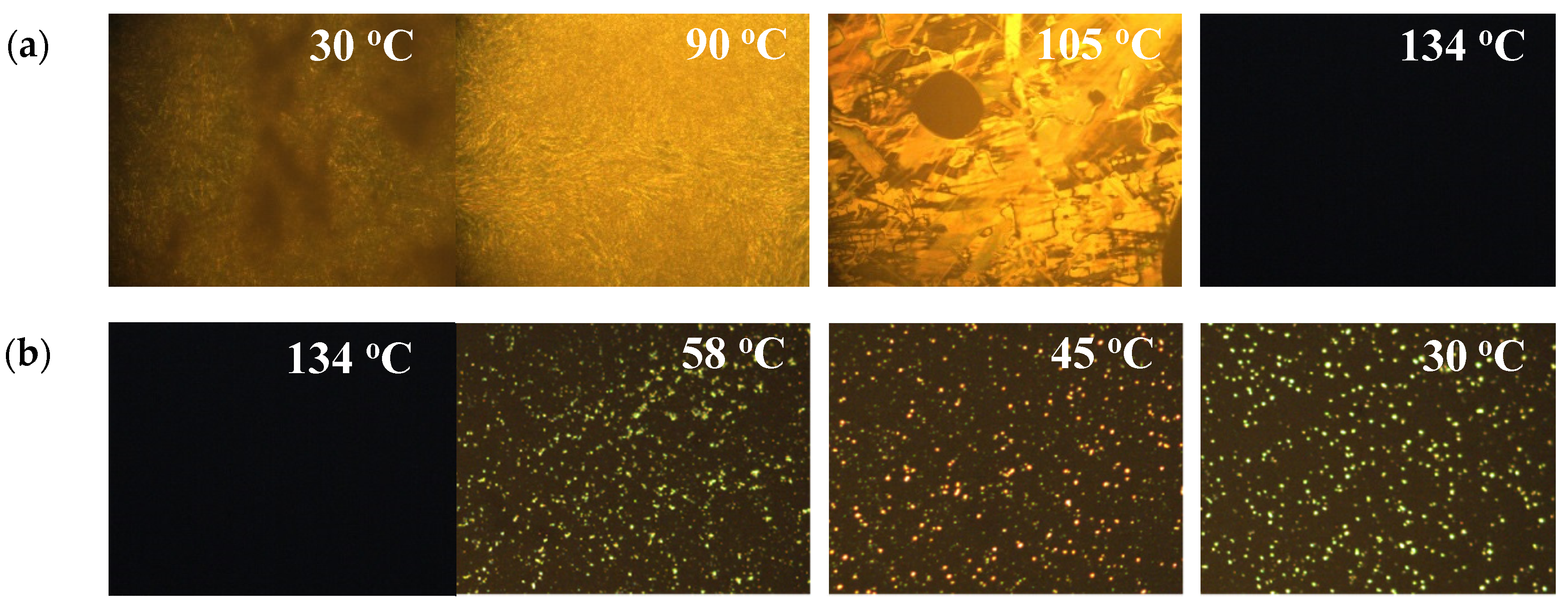
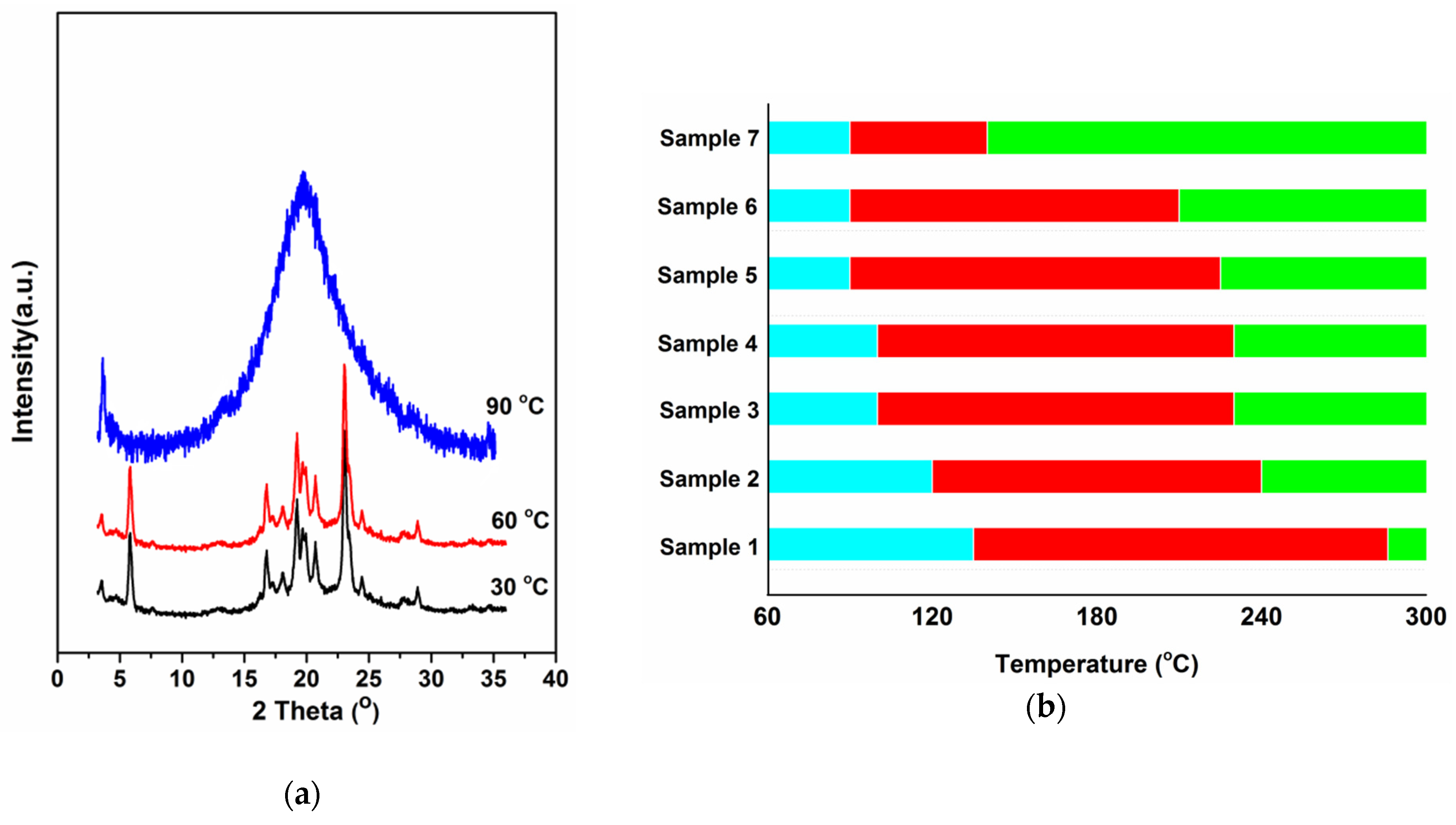
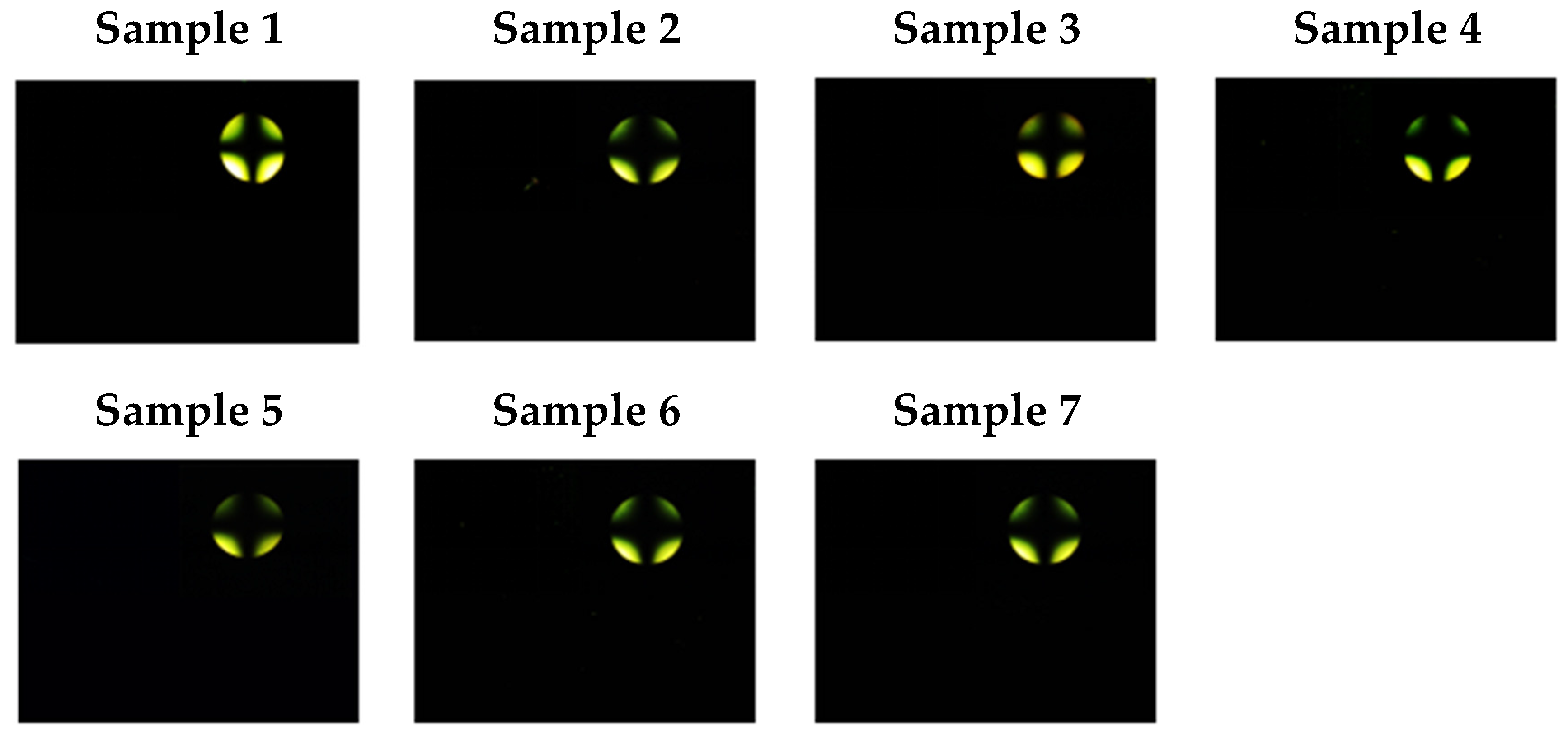

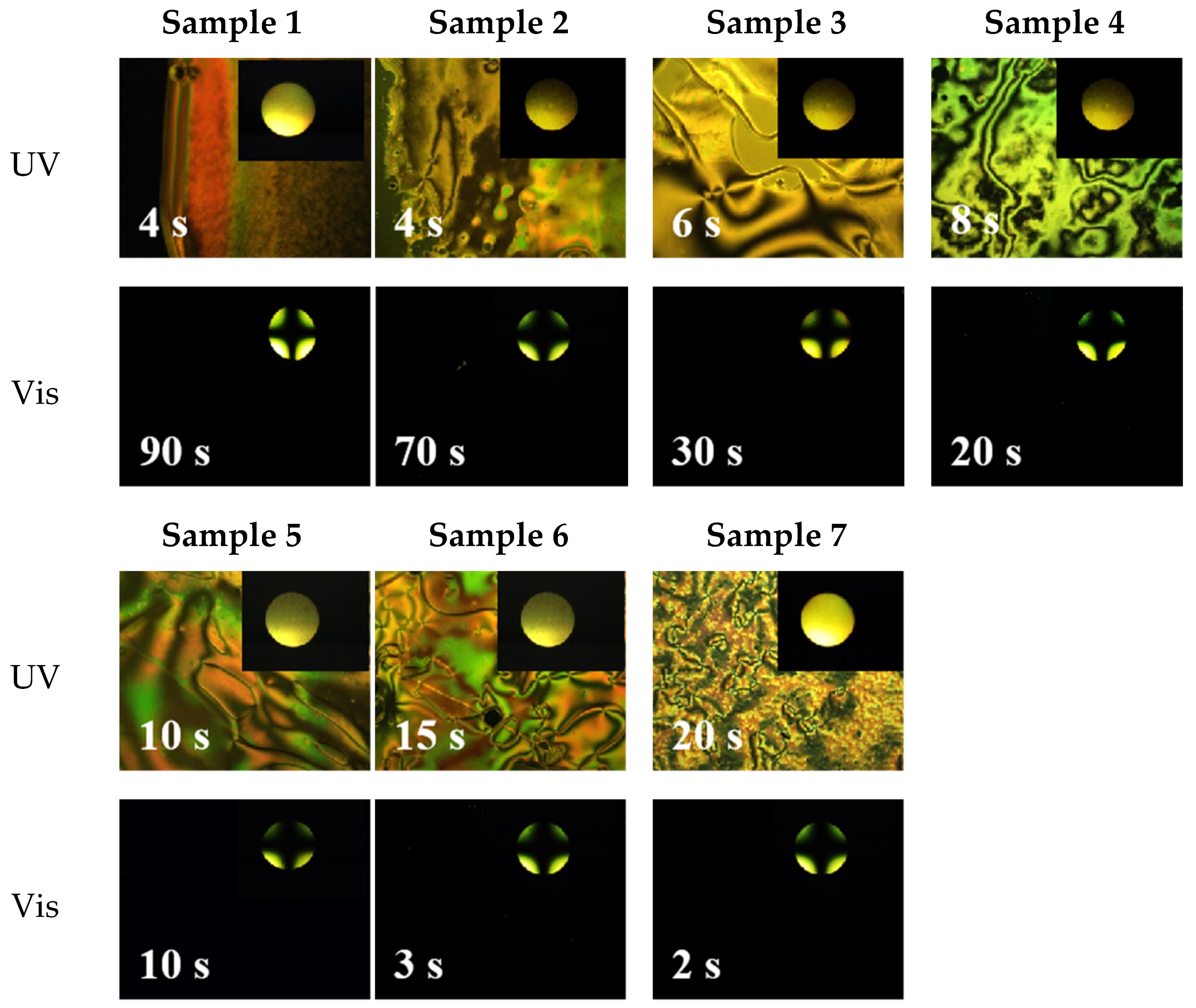

| Sample | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| 5CB (wt%) | 0 | 20 | 40 | 60 | 80 | 90 | 95 | 97.5 | 100 |
| M1 (wt%) | 100 | 80 | 60 | 40 | 20 | 10 | 5 | 2.5 | 0 |
| Tiso (°C) | / | 286 | 240 | 230 | 230 | 225 | 210 | 134 | 35 |
| Tm (°C) | 150 | 135 | 120 | 100 | 100 | 90 | 90 | 90 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Chen, H.; Xing, H.; Deng, Y.; Luo, Z.-W.; Xie, H.-L. Long Rod-Like Liquid Crystal Containing Azobenzene and the Applications in Phase-Transition Regulation and Orientation of Nematic Liquid Crystal. Crystals 2021, 11, 418. https://doi.org/10.3390/cryst11040418
Wang Q, Chen H, Xing H, Deng Y, Luo Z-W, Xie H-L. Long Rod-Like Liquid Crystal Containing Azobenzene and the Applications in Phase-Transition Regulation and Orientation of Nematic Liquid Crystal. Crystals. 2021; 11(4):418. https://doi.org/10.3390/cryst11040418
Chicago/Turabian StyleWang, Qing, Huang Chen, Hao Xing, Yuan Deng, Zhi-Wang Luo, and He-Lou Xie. 2021. "Long Rod-Like Liquid Crystal Containing Azobenzene and the Applications in Phase-Transition Regulation and Orientation of Nematic Liquid Crystal" Crystals 11, no. 4: 418. https://doi.org/10.3390/cryst11040418
APA StyleWang, Q., Chen, H., Xing, H., Deng, Y., Luo, Z.-W., & Xie, H.-L. (2021). Long Rod-Like Liquid Crystal Containing Azobenzene and the Applications in Phase-Transition Regulation and Orientation of Nematic Liquid Crystal. Crystals, 11(4), 418. https://doi.org/10.3390/cryst11040418





