Physiological Metals Can Induce Conformational Changes in Transthyretin Structure: Neuroprotection or Misfolding Induction?
Abstract
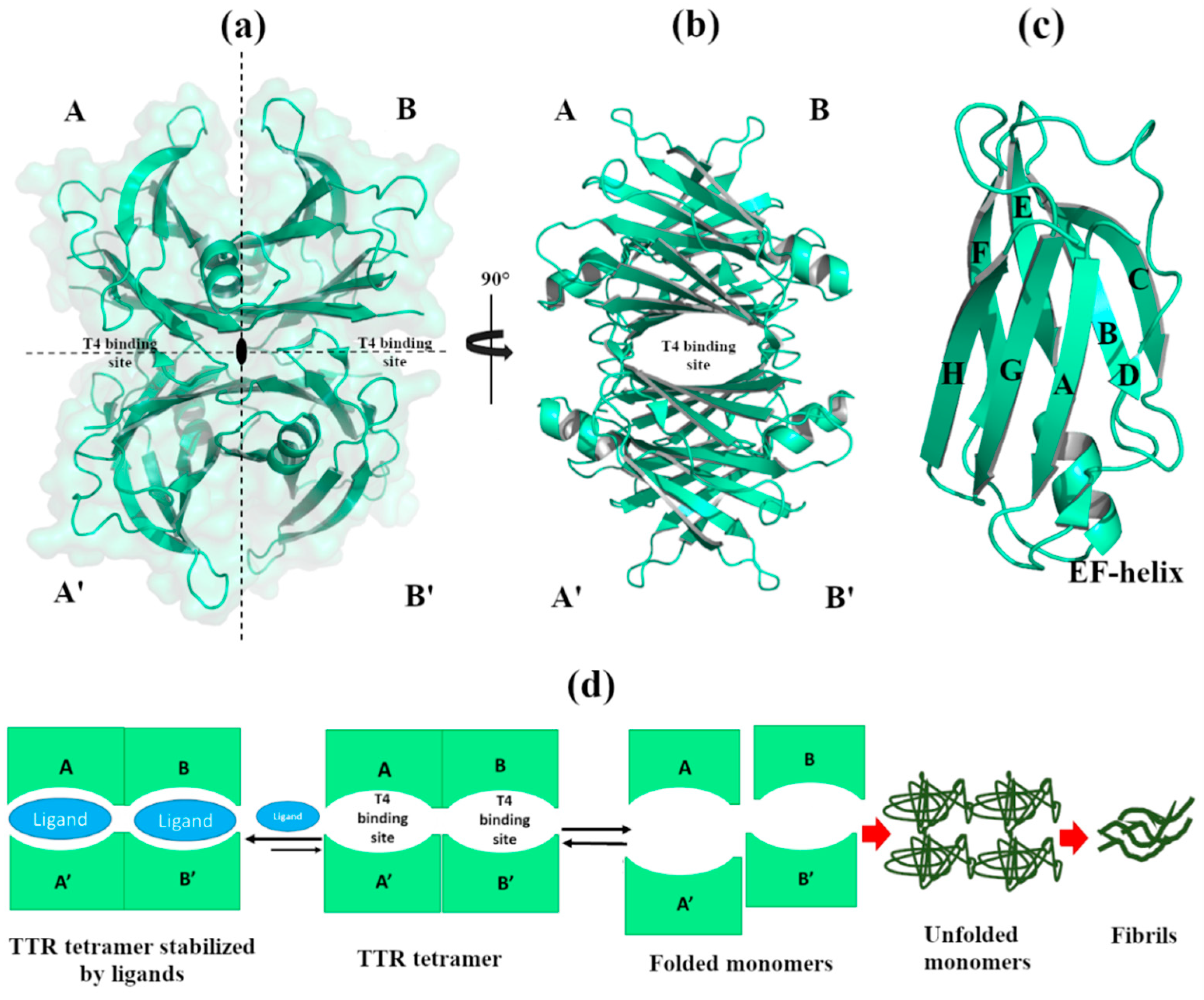
1. Introduction
2. Effect of Metals in TTR Structure and Function
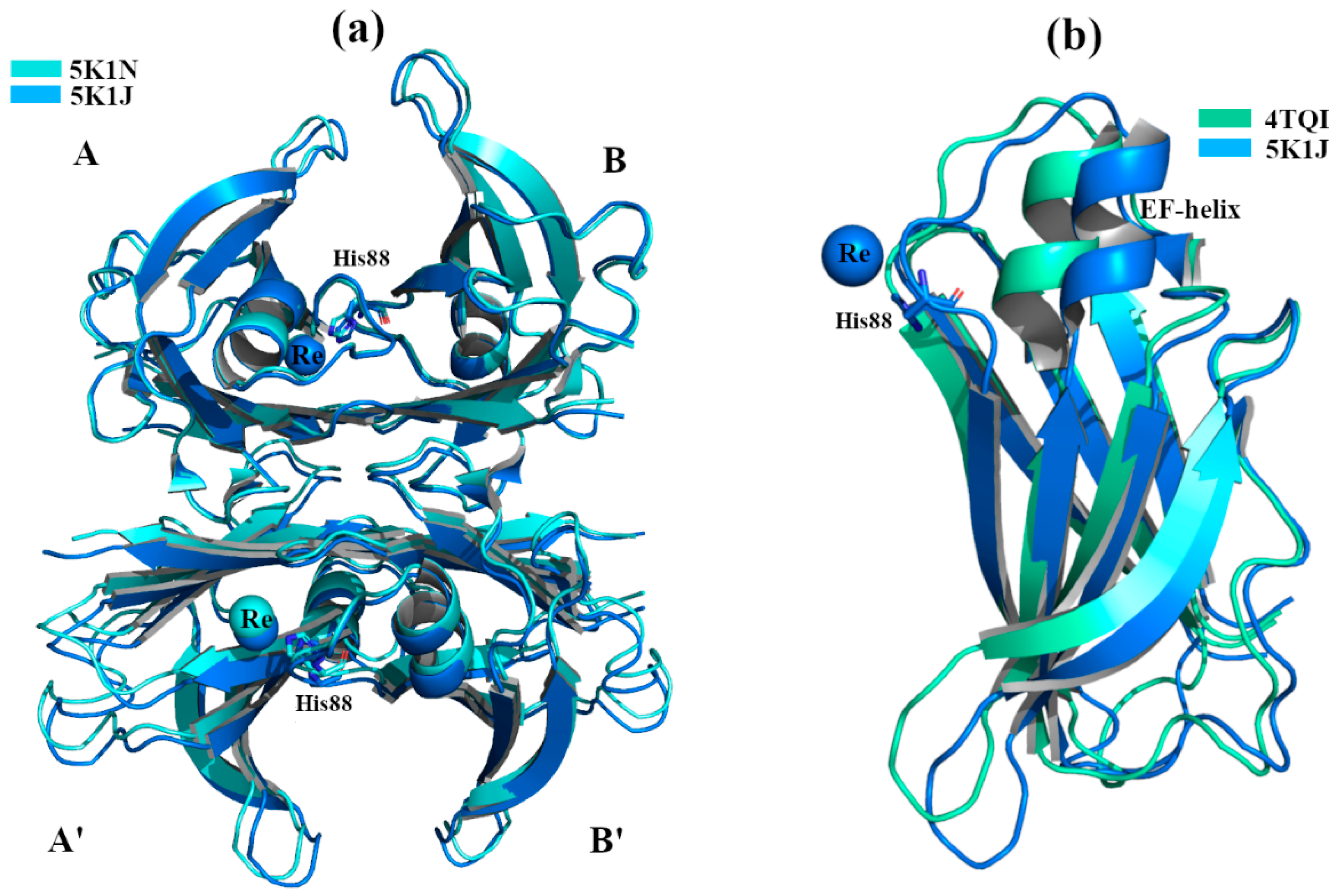
2.1. Non-Physiological Metals: Cr3+ and Re2+
2.2. Physiological Metals
2.2.1. Zn2+
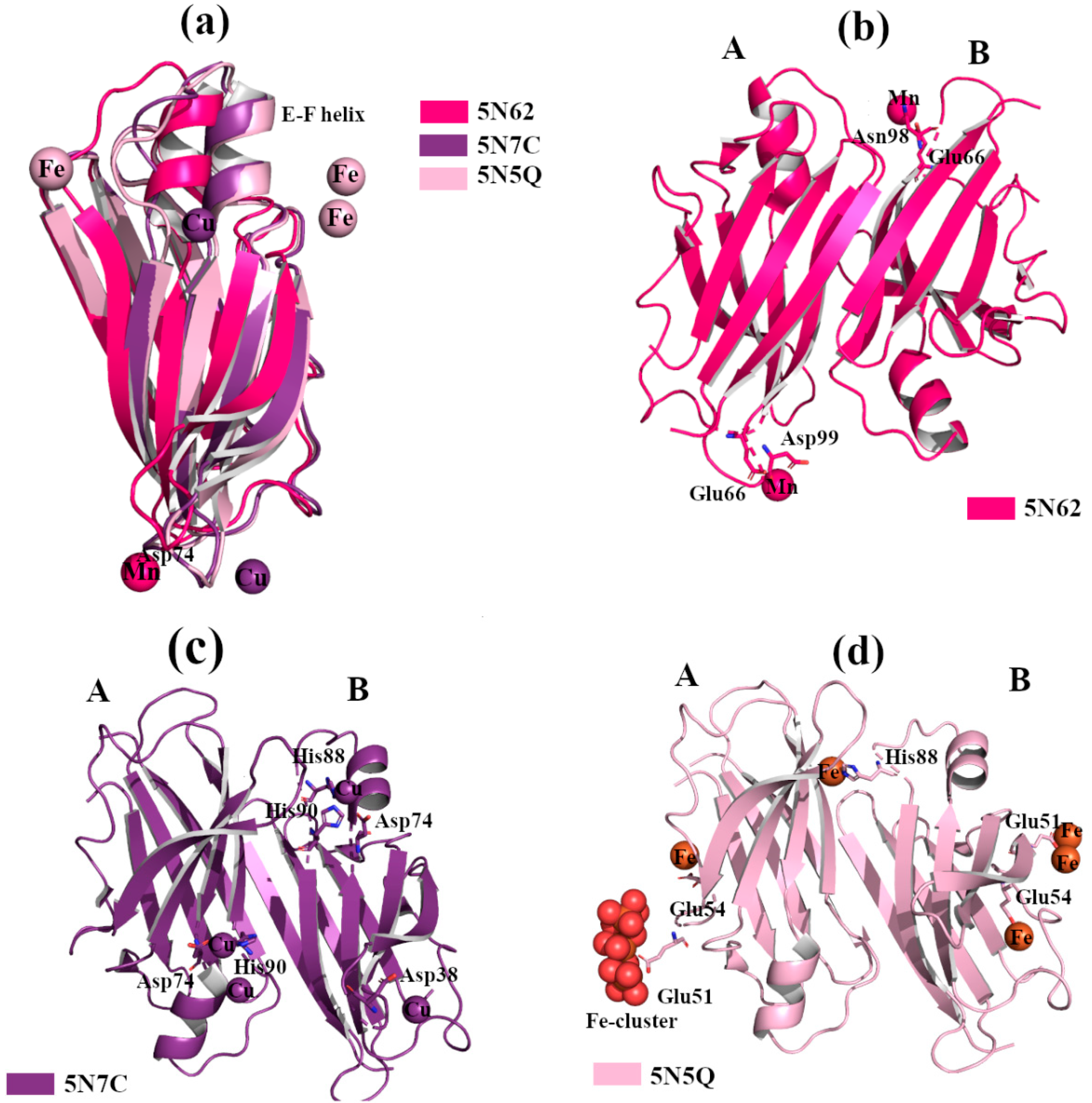
2.2.2. Cu2+, Fe2+ and Mn2+
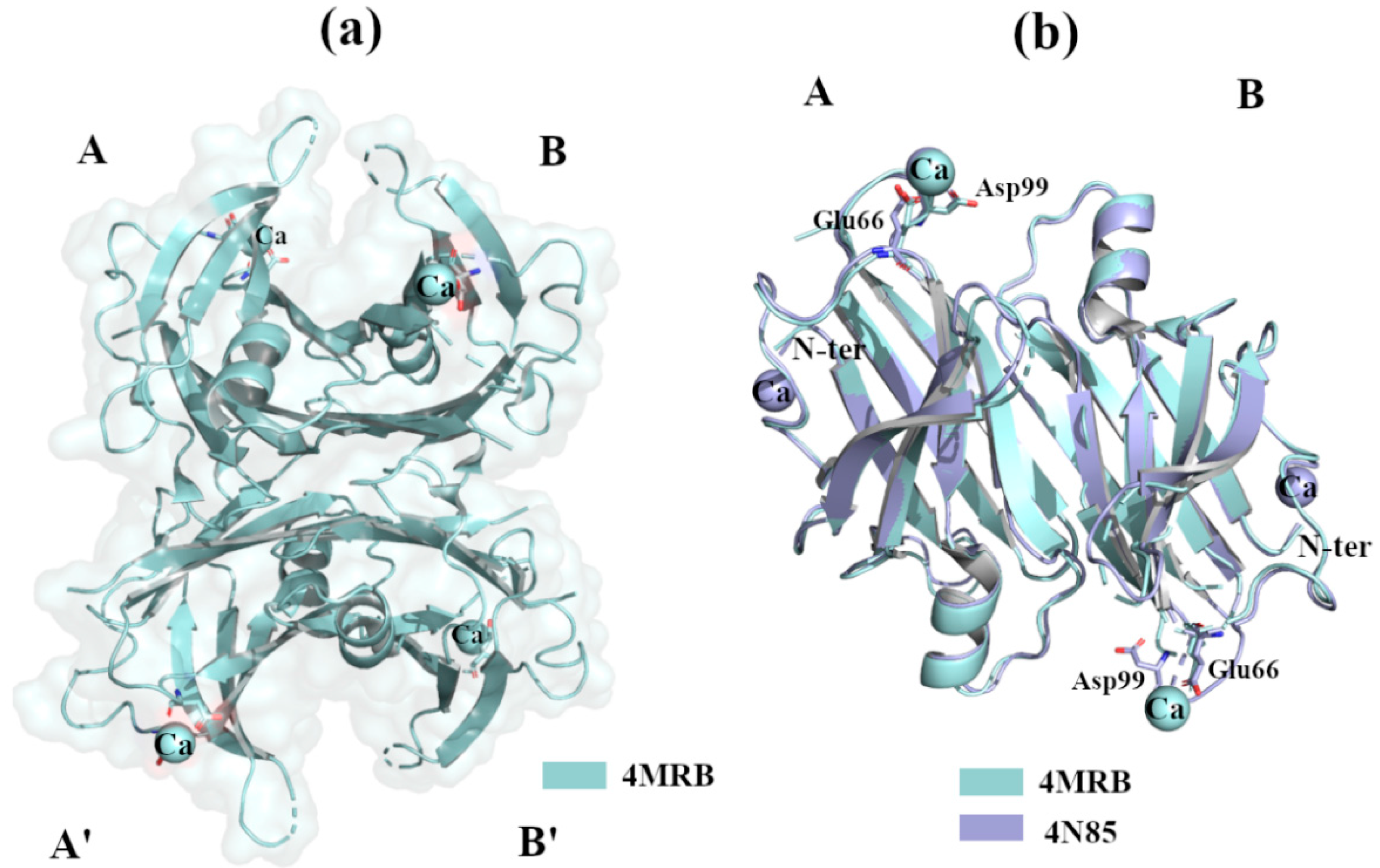
2.2.3. Ca2+
3. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aldred, A.R.; Brack, C.M.; Schreiber, G. The Cerebral Expression of Plasma Protein Genes in Different Species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 111, 1–15. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Benson, M.D. Transthyretin: A Review from a Structural Perspective. Cell. Mol. Life Sci. CMLS 2001, 58, 1491–1521. [Google Scholar] [CrossRef]
- Wojtczak, A.; Neumann, P.; Cody, V. Structure of a New Polymorphic Monoclinic Form of Human Transthyretin at 3 Å Resolution Reveals a Mixed Complex between Unliganded and T4-Bound Tetramers of TTR. Acta Crystallogr. Sect. D Biol. Crystallogr. 2001, 57, 957–967. [Google Scholar] [CrossRef]
- Ciccone, L.; Nencetti, S.; Rossello, A.; Tepshi, L.; Stura, E.A.; Orlandini, E. X-ray Crystal Structure and Activity of Fluorenyl-Based Compounds as Transthyretin Fibrillogenesis Inhibitors. J. Enzym. Inhib. Med. Chem. 2016, 31, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Gonçalves, A.; Saraiva, M.J.; Cardoso, I. Transthyretin Binding to A-Beta Peptide—Impact on A-Beta Fibrillogenesis and Toxicity. FEBS Lett. 2008, 582, 936–942. [Google Scholar] [CrossRef]
- Alemi, M.; Gaiteiro, C.; Ribeiro, C.A.; Santos, L.M.; Gomes, J.R.; Oliveira, S.M.; Couraud, P.-O.; Weksler, B.; Romero, I.; Saraiva, M.J.; et al. Transthyretin Participates in Beta-Amyloid Transport from the Brain to the Liver- Involvement of the Low-Density Lipoprotein Receptor-Related Protein 1? Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Ciccone, L.; Shi, C.; di Lorenzo, D.; Van Baelen, A.-C.; Tonali, N. The Positive Side of the Alzheimer’s Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1. Molecules 2020, 25, 2439. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Lai, Z.; Kelly, J.W. Characterization of the Transthyretin Acid Denaturation Pathways by Analytical Ultracentrifugation: Implications for Wild-Type, V30M, and L55P Amyloid Fibril Formation. Biochemistry 1998, 37, 17851–17864. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, G.G.; Murdoch, W.L.; Kyle, R.A.; Westermark, P.; Pitkänen, P. Frequency and Distribution of Senile Cardiovascular Amyloid: A Clinicopathologic Correlation. Am. J. Med. 1983, 75, 618–623. [Google Scholar] [CrossRef]
- Connors, L.H.; Lim, A.; Prokaeva, T.; Roskens, V.A.; Costello, C.E. Tabulation of Human Transthyretin (TTR) Variants, 2003. Amyloid 2003, 10, 160–184. [Google Scholar] [CrossRef]
- Saraiva, M.J.; Birken, S.; Costa, P.P.; Goodman, D.S. Amyloid Fibril Protein in Familial Amyloidotic Polyneuropathy, Portuguese Type. Definition of Molecular Abnormality in Transthyretin (Prealbumin). J. Clin. Investig. 1984, 74, 104–119. [Google Scholar] [CrossRef]
- Westermark, P.; Sletten, K.; Johansson, B.; Cornwell, G.G. Fibril in Senile Systemic Amyloidosis Is Derived from Normal Transthyretin. Proc. Natl. Acad. Sci. USA 1990, 87, 2843–2845. [Google Scholar] [CrossRef]
- Benson, M.D. Leptomeningeal Amyloid and Variant Transthyretins. Am. J. Pathol. 1996, 148, 351–354. [Google Scholar]
- Schneider, F.; Hammarström, P.; Kelly, J.W. Transthyretin Slowly Exchanges Subunits under Physiological Conditions: A Convenient Chromatographic Method to Study Subunit Exchange in Oligomeric Proteins. Protein Sci. 2001, 10, 1606–1613. [Google Scholar] [CrossRef]
- Sousa, M.M.; Saraiva, M.J. Neurodegeneration in Familial Amyloid Polyneuropathy: From Pathology to Molecular Signaling. Prog. Neurobiol. 2003, 71, 385–400. [Google Scholar] [CrossRef]
- Foss, T.R.; Wiseman, R.L.; Kelly, J.W. The Pathway by Which the Tetrameric Protein Transthyretin Dissociates. Biochemistry 2005, 44, 15525–15533. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.W.; Aldeghi, M.; Blakeley, M.P.; Ostermann, A.; Mas, P.J.; Moulin, M.; de Sanctis, D.; Bowler, M.W.; Mueller-Dieckmann, C.; Mitchell, E.P.; et al. A Molecular Mechanism for Transthyretin Amyloidogenesis. Nat. Commun. 2019, 10, 925. [Google Scholar] [CrossRef]
- Colon, W.; Kelly, J.W. Partial Denaturation of Transthyretin Is Sufficient for Amyloid Fibril Formation in Vitro. Biochemistry 1992, 31, 8654–8660. [Google Scholar] [CrossRef]
- Merlini, G.; Coelho, T.; Waddington Cruz, M.; Li, H.; Stewart, M.; Ebede, B. Evaluation of Mortality During Long-Term Treatment with Tafamidis for Transthyretin Amyloidosis with Polyneuropathy: Clinical Trial Results up to 8.5 Years. Neurol. Ther. 2020, 9, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, Z.; Zheng, Y.; Li, Y.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Review on the Structures and Activities of Transthyretin Amyloidogenesis Inhibitors. Drug Des. Dev. Ther. 2020, 14, 1057–1081. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, L.; Tonali, N.; Nencetti, S.; Orlandini, E. Natural Compounds as Inhibitors of Transthyretin Amyloidosis and Neuroprotective Agents: Analysis of Structural Data for Future Drug Design. J. Enzym. Inhib. Med. Chem. 2020, 35, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Ortore, G.; Orlandini, E.; Braca, A.; Ciccone, L.; Rossello, A.; Martinelli, A.; Nencetti, S. Targeting Different Transthyretin Binding Sites with Unusual Natural Compounds. ChemMedChem 2016, 11, 1865–1874. [Google Scholar] [CrossRef]
- Atwood, C.S.; Moir, R.D.; Huang, X.; Scarpa, R.C.; Bacarra, N.M.E.; Romano, D.M.; Hartshorn, M.A.; Tanzi, R.E.; Bush, A.I. Dramatic Aggregation of Alzheimer Aβ by Cu(II) Is Induced by Conditions Representing Physiological Acidosis. J. Biol. Chem. 1998, 273, 12817–12826. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, Iron and Zinc in Alzheimer’s Disease Senile Plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Acc. Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef]
- Bisaglia, M.; Bubacco, L. Copper Ions and Parkinson’s Disease: Why Is Homeostasis So Relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Dales, J.-P.; Desplat-Jégo, S. Metal Imbalance in Neurodegenerative Diseases with a Specific Concern to the Brain of Multiple Sclerosis Patients. Int. J. Mol. Sci. 2020, 21, 9105. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-White, L.E.; Easterbrook-Smith, S.B. Characterization of the Binding of Cu(II) and Zn(II) to Transthyretin: Effects on Amyloid Formation. Biochemistry 2007, 46, 9123–9132. [Google Scholar] [CrossRef]
- Hörnberg, A.; Hultdin, U.W.; Olofsson, A.; Sauer-Eriksson, A.E. The Effect of Iodide and Chloride on Transthyretin Structure and Stability. Biochemistry 2005, 44, 9290–9299. [Google Scholar] [CrossRef]
- Sato, T.; Ando, Y.; Susuki, S.; Mikami, F.; Ikemizu, S.; Nakamura, M.; Suhr, O.; Anraku, M.; Kai, T.; Suico, M.A.; et al. Chromium(III) Ion and Thyroxine Cooperate to Stabilize the Transthyretin Tetramer and Suppress in Vitro Amyloid Fibril Formation. FEBS Lett. 2006, 580, 491–496. [Google Scholar] [CrossRef]
- Ciccone, L.; Policar, C.; Stura, E.A.; Shepard, W. Human TTR Conformation Altered by Rhenium Tris-Carbonyl Derivatives. J. Struct. Biol. 2016, 195, 353–364. [Google Scholar] [CrossRef]
- Ciccone, L.; Vera, L.; Tepshi, L.; Rosalia, L.; Rossello, A.; Stura, E.A. Multicomponent Mixtures for Cryoprotection and Ligand Solubilization. Biotechnol. Rep. 2015, 7, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Cody, V.; Wojtczak, A. Structural Basis of Negative Cooperativity in Transthyretin. Acta Biochim. Pol. 2001, 48, 867–875. [Google Scholar] [CrossRef]
- Polsinelli, I.; Nencetti, S.; Shepard, W.; Ciccone, L.; Orlandini, E.; Stura, E.A. A New Crystal Form of Human Transthyretin Obtained with a Curcumin Derived Ligand. J. Struct. Biol. 2016, 194, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cho, P.Y.; Yang, D.T.; Murphy, R.M. Identification of Beta-Amyloid-Binding Sites on Transthyretin. Protein Eng. Des. Sel. 2012, 25, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.T.; Joshi, G.; Cho, P.Y.; Johnson, J.A.; Murphy, R.M. Transthyretin as Both a Sensor and a Scavenger of β-Amyloid Oligomers. Biochemistry 2013, 52, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Susuki, S.; Ando, Y.; Sato, T.; Nishiyama, M.; Miyata, M.; Suico, M.A.; Shuto, T.; Kai, H. Multi-Elemental Analysis of Serum and Amyloid Fibrils in Familial Amyloid Polyneuropathy Patients. Amyloid 2008, 15, 108–116. [Google Scholar] [CrossRef]
- Liz, M.A.; Leite, S.C.; Juliano, L.; Saraiva, M.J.; Damas, A.M.; Bur, D.; Sousa, M.M. Transthyretin Is a Metallopeptidase with an Inducible Active Site. Biochem. J. 2012, 443, 769–778. [Google Scholar] [CrossRef]
- Jiang, X.; Smith, C.S.; Petrassi, H.M.; Hammarström, P.; White, J.T.; Sacchettini, J.C.; Kelly, J.W. An Engineered Transthyretin Monomer That Is Nonamyloidogenic, Unless It Is Partially Denatured. Biochemistry 2001, 40, 11442–11452. [Google Scholar] [CrossRef]
- Palmieri, L.d.C.; Lima, L.M.T.R.; Freire, J.B.B.; Bleicher, L.; Polikarpov, I.; Almeida, F.C.L.; Foguel, D. Novel Zn2+-Binding Sites in Human Transthyretin: Implications for Amyloidogenesis and Retinol-Binding Protein Recognition. J. Biol. Chem. 2010, 285, 31731–31741. [Google Scholar] [CrossRef]
- Naylor, H.M.; Newcomer, M.E. The Structure of Human Retinol-Binding Protein (RBP) with Its Carrier Protein Transthyretin Reveals an Interaction with the Carboxy Terminus of RBP. Biochemistry 1999, 38, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Palaninathan, S.K.; Mohamedmohaideen, N.N.; Snee, W.C.; Kelly, J.W.; Sacchettini, J.C. Structural Insight into PH-Induced Conformational Changes within the Native Human Transthyretin Tetramer. J. Mol. Biol. 2008, 382, 1157–1167. [Google Scholar] [CrossRef]
- Morais-de-Sá, E.; Neto-Silva, R.M.; Pereira, P.J.; Saraiva, M.J.; Damas, A.M. The Binding of 2, 4-Dinitrophenol to Wild-Type and Amyloidogenic Transthyretin. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 62, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Cendron, L.; Trovato, A.; Seno, F.; Folli, C.; Alfieri, B.; Zanotti, G.; Berni, R. Amyloidogenic Potential of Transthyretin Variants. J. Biol. Chem. 2009, 284, 25832–25841. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodrigues, A.F.; Gales, L.; Saraiva, M.J.; Damas, A.M. Structural Insights into a Zinc-Dependent Pathway Leading to Leu55Pro Transthyretin Amyloid Fibrils. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 1035–1044. [Google Scholar] [CrossRef]
- Gouvea, I.E.; Kondo, M.Y.; Assis, D.M.; Alves, F.M.; Liz, M.A.; Juliano, M.A.; Juliano, L. Studies on the Peptidase Activity of Transthyretin (TTR). Biochimie 2013, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Ferreira-da-Silva, F.; Saraiva, M.J.; Cardoso, I. Transthyretin Protects against A-Beta Peptide Toxicity by Proteolytic Cleavage of the Peptide: A Mechanism Sensitive to the Kunitz Protease Inhibitor. PLoS ONE 2008, 3, e2899. [Google Scholar] [CrossRef]
- Liz, M.A.; Gomes, C.M.; Saraiva, M.J.; Sousa, M.M. ApoA-I Cleaved by Transthyretin Has Reduced Ability to Promote Cholesterol Efflux and Increased Amyloidogenicity. J. Lipid Res. 2007, 48, 2385–2395. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Pages, R.A.; Saroff, H.A.; Edelhoch, H.; Robbins, J. Analysis of Thyroid Hormone Binding to Human Serum Prealbumin by 8-Anilinonaphthalene-1-Sulfonate Fluorescence. Biochemistry 1977, 16, 3707–3713. [Google Scholar] [CrossRef]
- Lima, L.M.T.R.; Silva, V.d.A.; Palmieri, L.d.C.; Oliveira, M.C.B.R.; Foguel, D.; Polikarpov, I. Identification of a Novel Ligand Binding Motif in the Transthyretin Channel. Bioorg. Med. Chem. 2010, 18, 100–110. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Ladiwala, A.R.A.; Du, D.; Yadav, J.K.; Tessier, P.M.; Wright, P.E.; Kelly, J.W.; Buxbaum, J.N. Mechanisms of Transthyretin Inhibition of -Amyloid Aggregation In Vitro. J. Neurosci. 2013, 33, 19423–19433. [Google Scholar] [CrossRef]
- Ciccone, L.; Fruchart-Gaillard, C.; Mourier, G.; Savko, M.; Nencetti, S.; Orlandini, E.; Servent, D.; Stura, E.A.; Shepard, W. Copper Mediated Amyloid-β Binding to Transthyretin. Sci. Rep. 2018, 8, 13744. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Pettifer, R.F. CHOOCH: A Program for Deriving Anomalous-Scattering Factors from X-Ray Fluorescence Spectra. J. Appl. Cryst. 2001, 34, 82–86. [Google Scholar] [CrossRef]
- Polsinelli, I.; Savko, M.; Rouanet-Mehouas, C.; Ciccone, L.; Nencetti, S.; Orlandini, E.; Stura, E.A.; Shepard, W. Comparison of Helical Scan and Standard Rotation Methods in Single-Crystal X-Ray Data Collection Strategies. J. Synchrotron Radiat. 2017, 24, 42–52. [Google Scholar] [CrossRef]
- Du, J.; Murphy, R.M. Characterization of the Interaction of β-Amyloid with Transthyretin Monomers and Tetramers. Biochemistry 2010, 49, 8276–8289. [Google Scholar] [CrossRef] [PubMed]
- Tonali, N.; Nencetti, S.; Orlandini, E.; Ciccone, L. Application of PROTAC Strategy to TTR-Aβ Protein-Protein Interaction for the Development of Alzheimer’s Disease Drugs. Neural Regen. Res. 2021, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Alvarez-Illera, P.; García-Casas, P.; Fonteriz, R.I.; Montero, M. The Role of Ca2+ Signaling in Aging and Neurodegeneration: Insights from Caenorhabditis Elegans Models. Cells 2020, 9, 204. [Google Scholar] [CrossRef]
- Scott, B.J.; Bradwell, A.R. Identification of the Serum Binding Proteins for Iron, Zinc, Cadmium, Nickel, and Calcium. Clin. Chem. 1983, 29, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Mangione, P.P.; Porcari, R.; Gillmore, J.D.; Pucci, P.; Monti, M.; Porcari, M.; Giorgetti, S.; Marchese, L.; Raimondi, S.; Serpell, L.C.; et al. Proteolytic Cleavage of Ser52Pro Variant Transthyretin Triggers Its Amyloid Fibrillogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 1539–1544. [Google Scholar] [CrossRef]
- Yokoyama, T.; Kosaka, Y.; Mizuguchi, M. Crystal Structures of Human Transthyretin Complexed with Glabridin. J. Med. Chem. 2014, 57, 1090–1096. [Google Scholar] [CrossRef]
- Yokoyama, T.; Kosaka, Y.; Mizuguchi, M. Inhibitory Activities of Propolis and Its Promising Component, Caffeic Acid Phenethyl Ester, against Amyloidogenesis of Human Transthyretin. J. Med. Chem. 2014, 57, 8928–8935. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yokoyama, T.; Mizuguchi, M.; Toné, S.; Takaku, S.; Sango, K.; Nishimura, H.; Watabe, K.; Sunada, Y. A Low Amyloidogenic E61K Transthyretin Mutation May Cause Familial Amyloid Polyneuropathy. J. Neurochem. 2020. [Google Scholar] [CrossRef]
- Wieczorek, E.; Kędracka-Krok, S.; Bystranowska, D.; Ptak, M.; Wiak, K.; Wygralak, Z.; Jankowska, U.; Ożyhar, A. Destabilisation of the Structure of Transthyretin Is Driven by Ca2+. Int. J. Biol. Macromol. 2021, 166, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Li, J.; Fink, A.L. Metal-Triggered Structural Transformations, Aggregation, and Fibrillation of Human α-Synuclein. J. Biol. Chem. 2001, 276, 44284–44296. [Google Scholar] [CrossRef] [PubMed]
- Rasia, R.M.; Bertoncini, C.W.; Marsh, D.; Hoyer, W.; Cherny, D.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernandez, C.O. Structural Characterization of Copper(II) Binding to -Synuclein: Insights into the Bioinorganic Chemistry of Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2005, 102, 4294–4299. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciccone, L.; Tonali, N.; Shepard, W.; Nencetti, S.; Orlandini, E. Physiological Metals Can Induce Conformational Changes in Transthyretin Structure: Neuroprotection or Misfolding Induction? Crystals 2021, 11, 354. https://doi.org/10.3390/cryst11040354
Ciccone L, Tonali N, Shepard W, Nencetti S, Orlandini E. Physiological Metals Can Induce Conformational Changes in Transthyretin Structure: Neuroprotection or Misfolding Induction? Crystals. 2021; 11(4):354. https://doi.org/10.3390/cryst11040354
Chicago/Turabian StyleCiccone, Lidia, Nicolò Tonali, William Shepard, Susanna Nencetti, and Elisabetta Orlandini. 2021. "Physiological Metals Can Induce Conformational Changes in Transthyretin Structure: Neuroprotection or Misfolding Induction?" Crystals 11, no. 4: 354. https://doi.org/10.3390/cryst11040354
APA StyleCiccone, L., Tonali, N., Shepard, W., Nencetti, S., & Orlandini, E. (2021). Physiological Metals Can Induce Conformational Changes in Transthyretin Structure: Neuroprotection or Misfolding Induction? Crystals, 11(4), 354. https://doi.org/10.3390/cryst11040354