A New Process of Extracting Titanium from Vanadium–Titanium Magnetite
Abstract
1. Introduction
2. Experimental Methods
2.1. Raw Materials
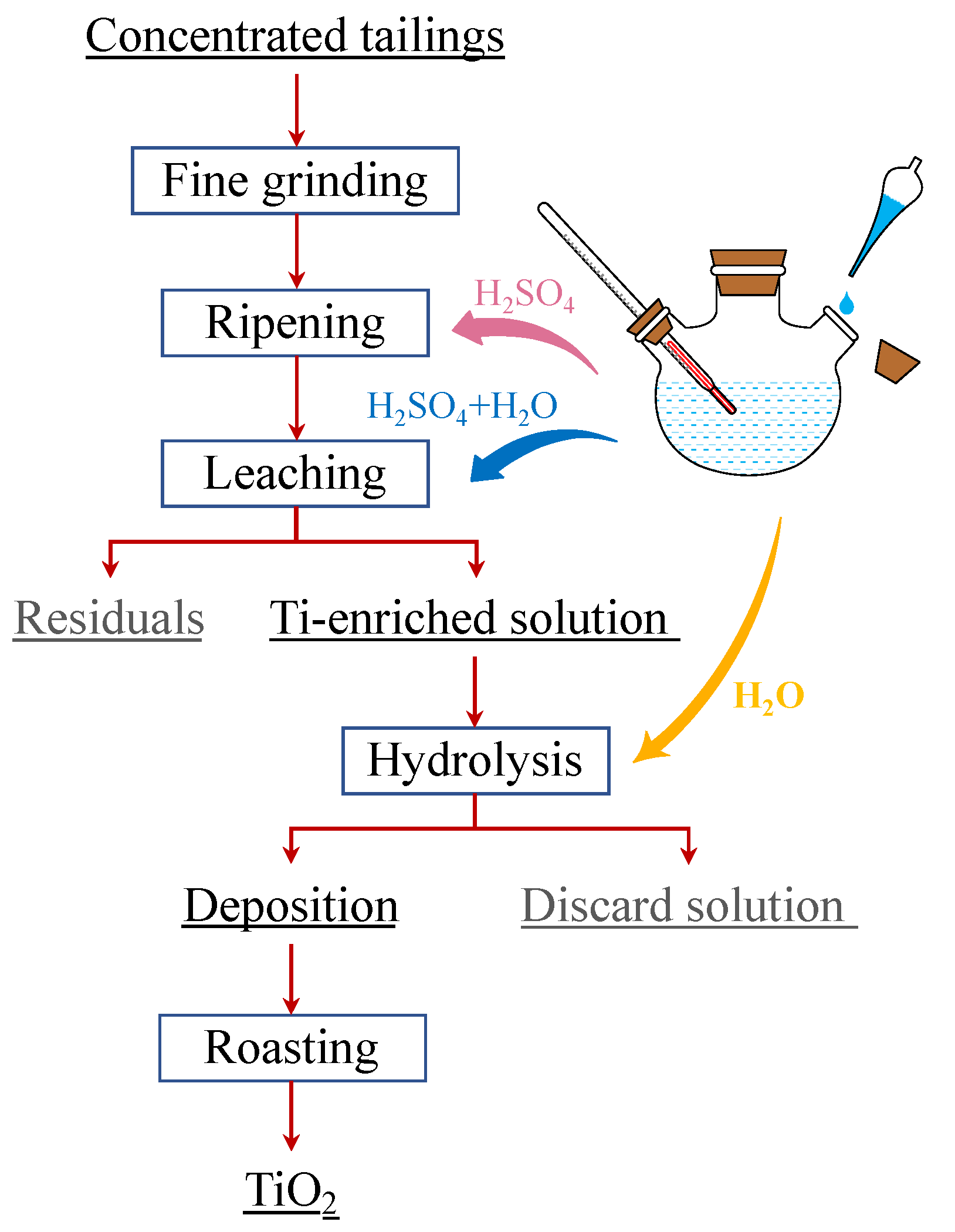
2.2. Experimental Procedure
3. Results and Discussions
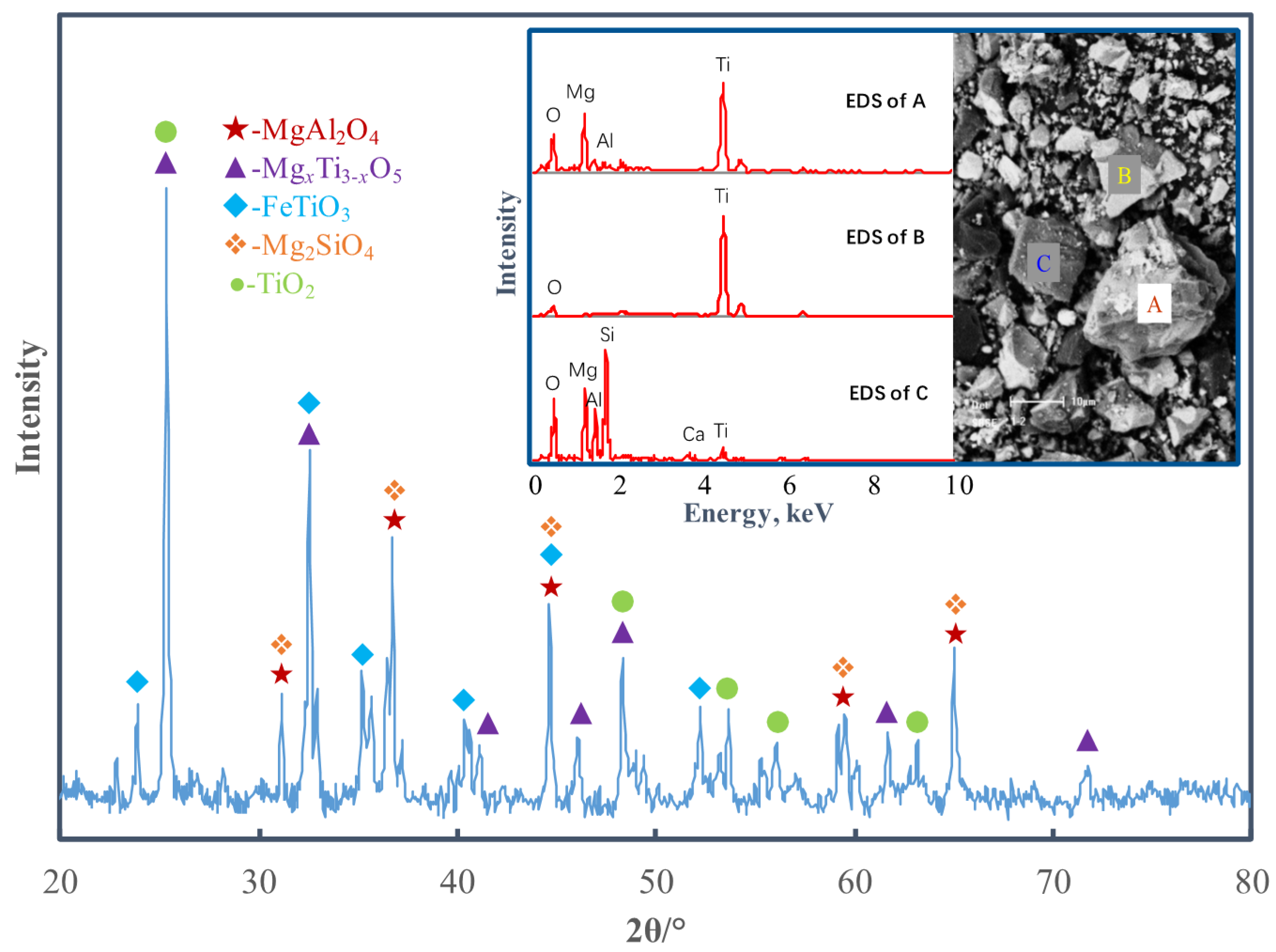
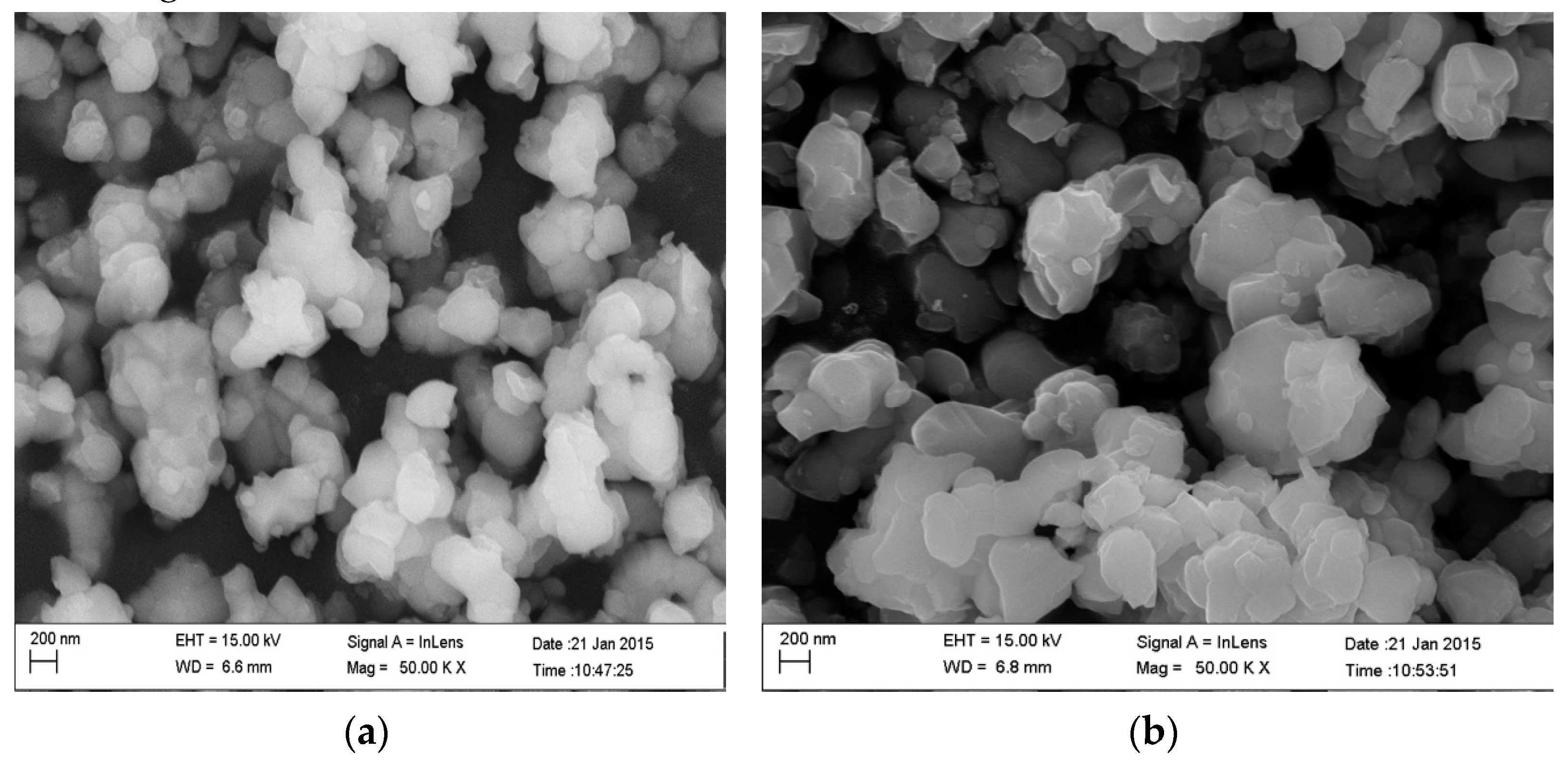
3.1. Preparation of Ti-Bearing Slag
3.2. Leaching of Ti-Bearing Slag by H2SO4
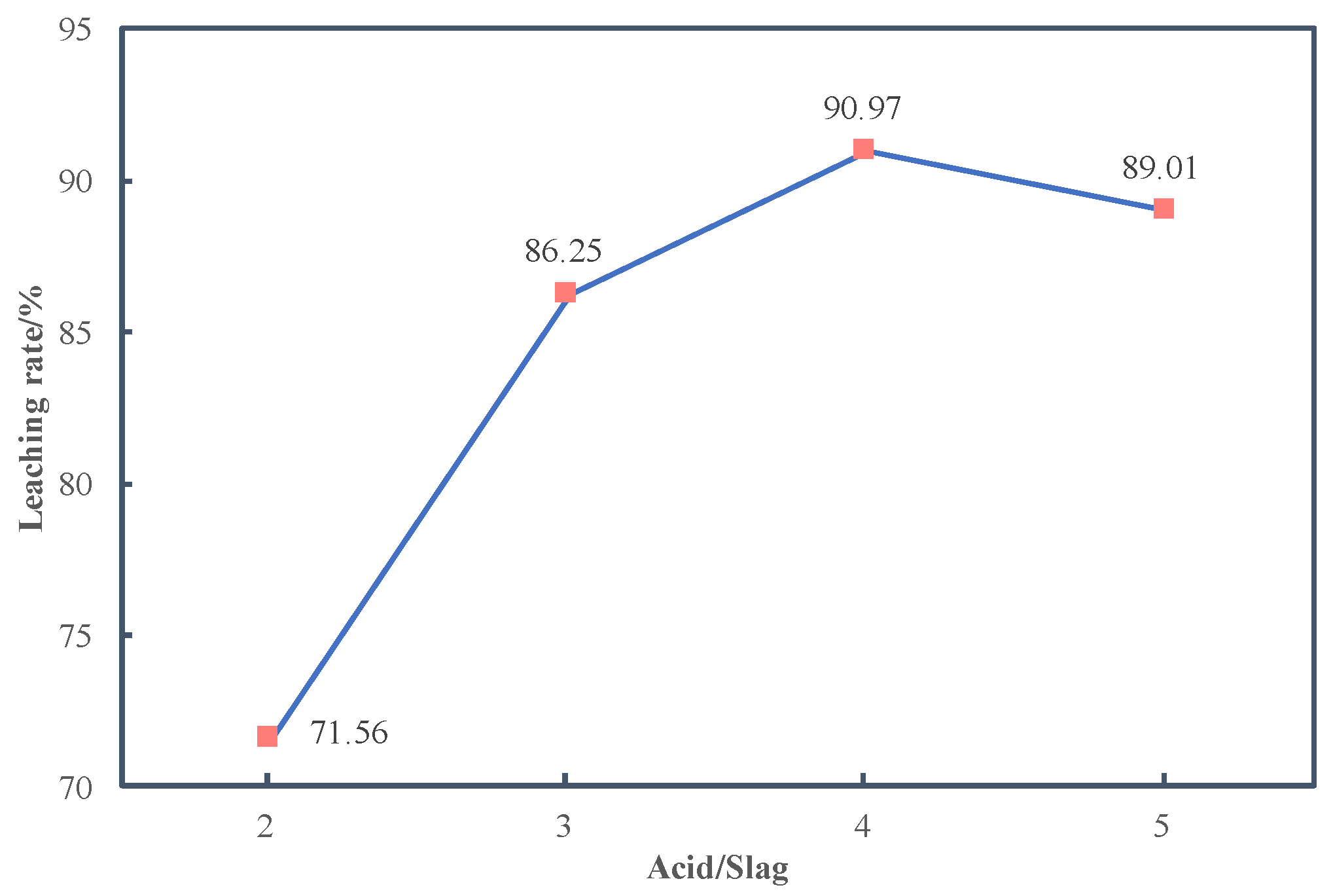
3.2.1. Effects of Acid/Slag Ratio on Leaching Rate
3.2.2. Effects of Temperature on Leaching Rate
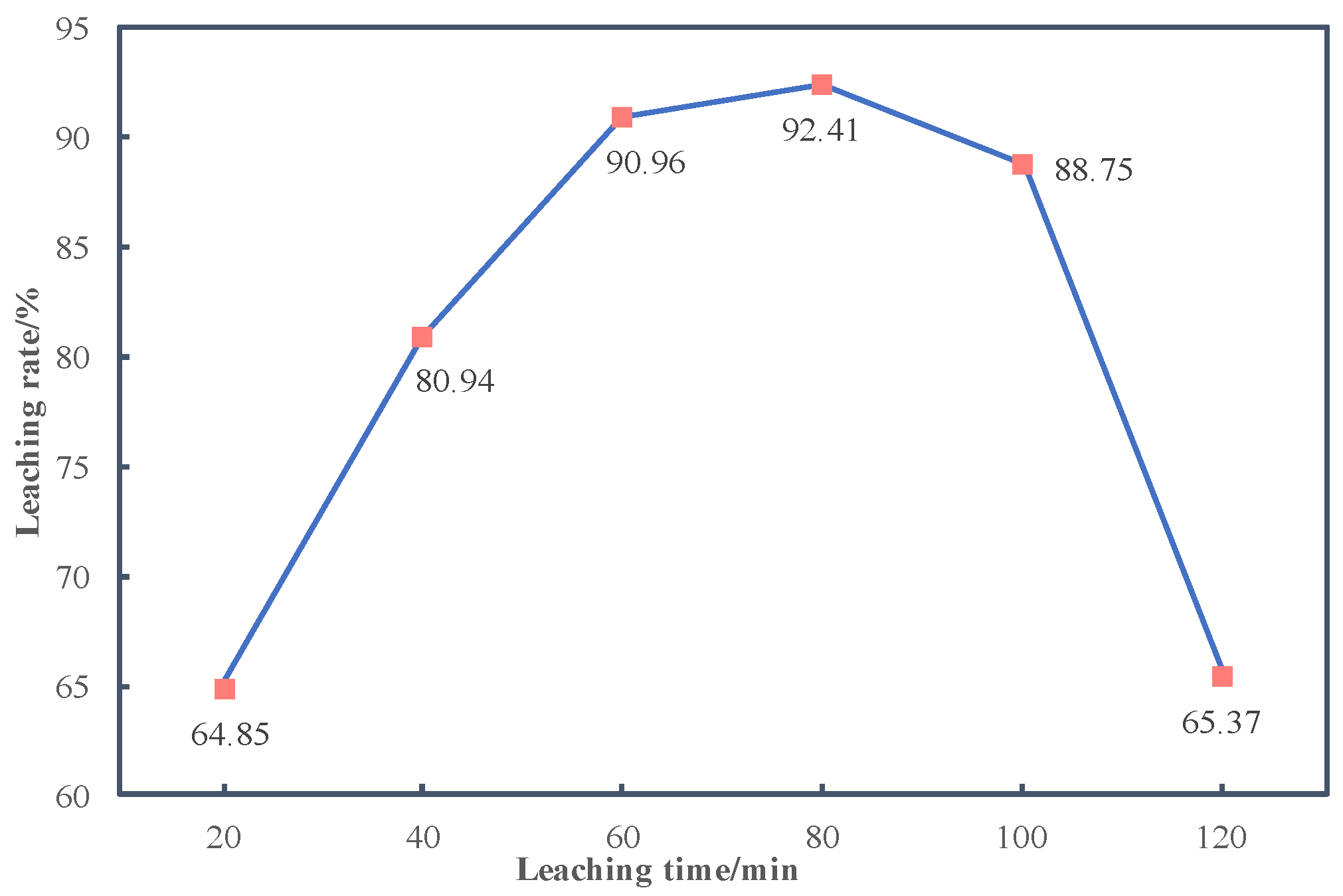
3.2.3. Effects of Reaction Time on Leaching Rate
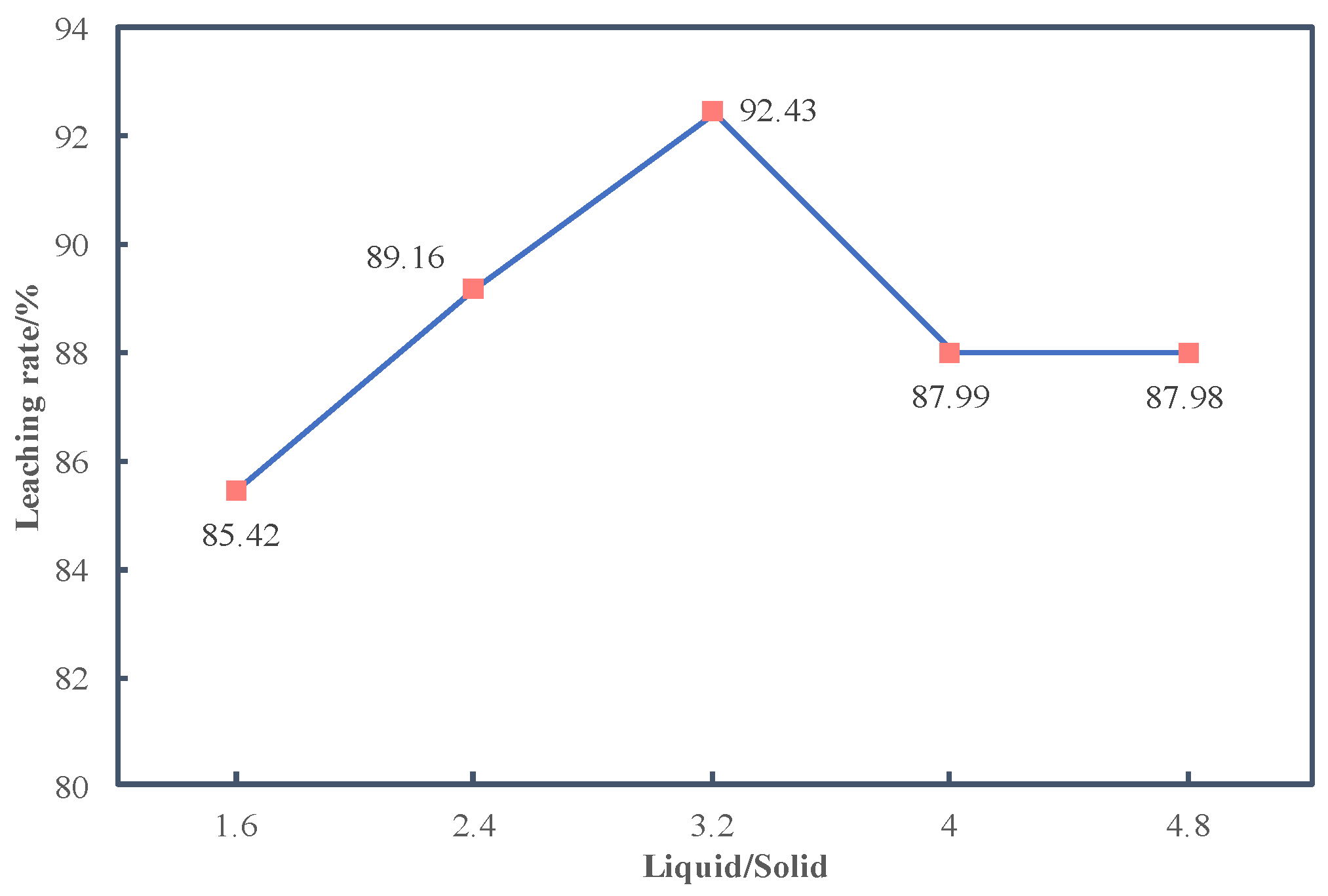
3.2.4. Effects of Liquid/Solid Ratio on Leaching Rate
3.3. Hydrolyzing of Acid Leaching Liquor
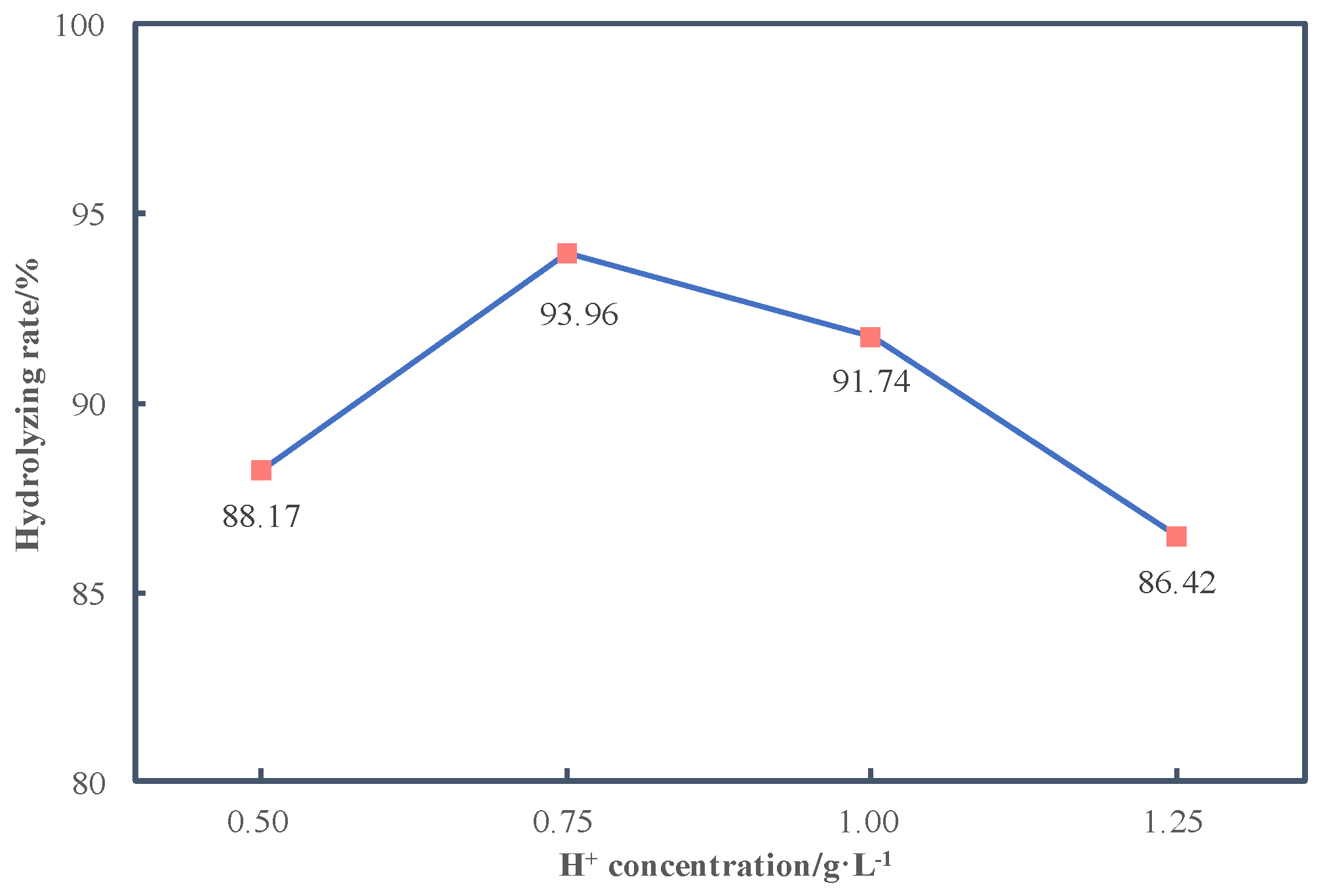
3.3.1. Effects of H+ Concentration on Hydrolyzing Rate
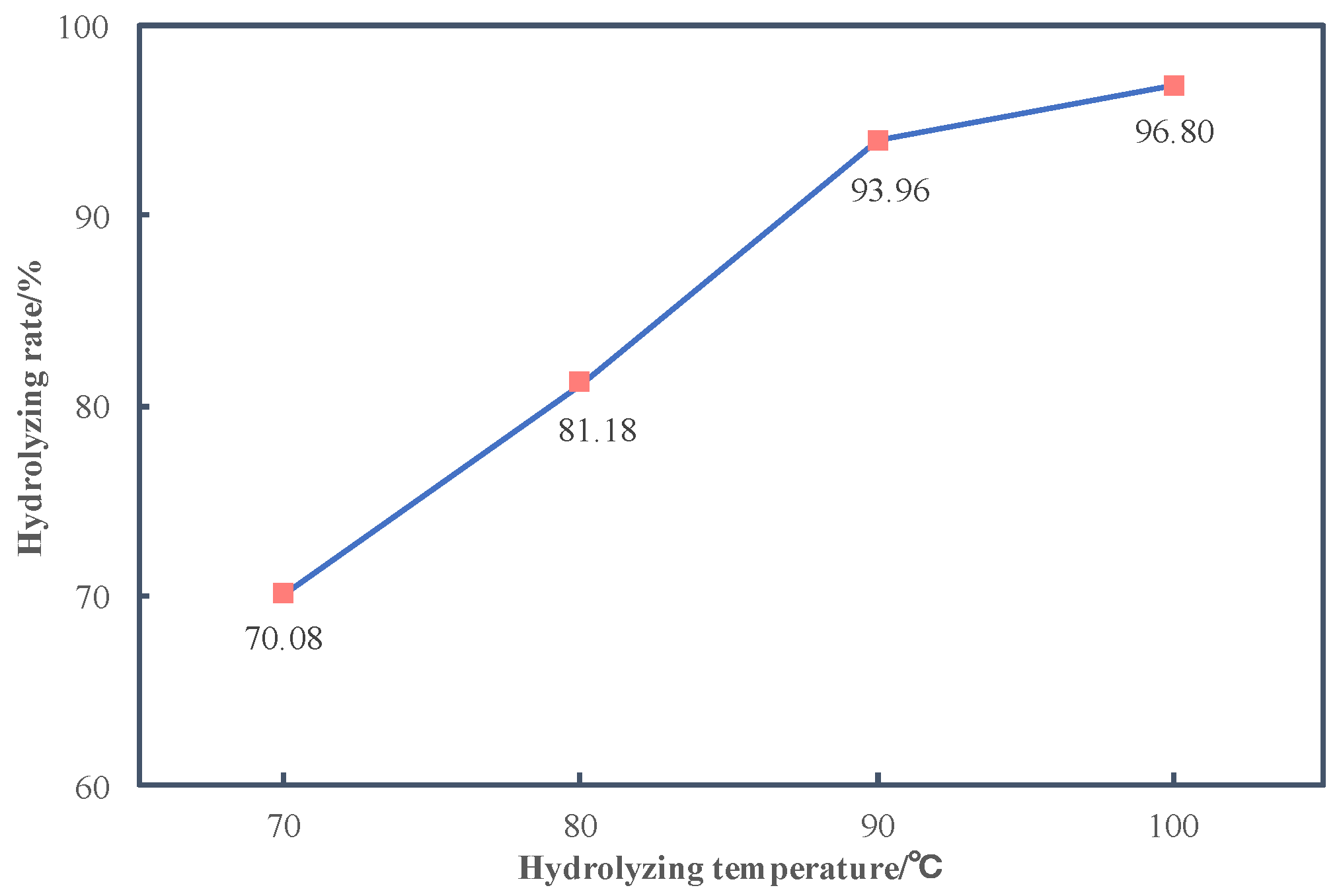
3.3.2. Effects of Temperature on Hydrolyzing Rate
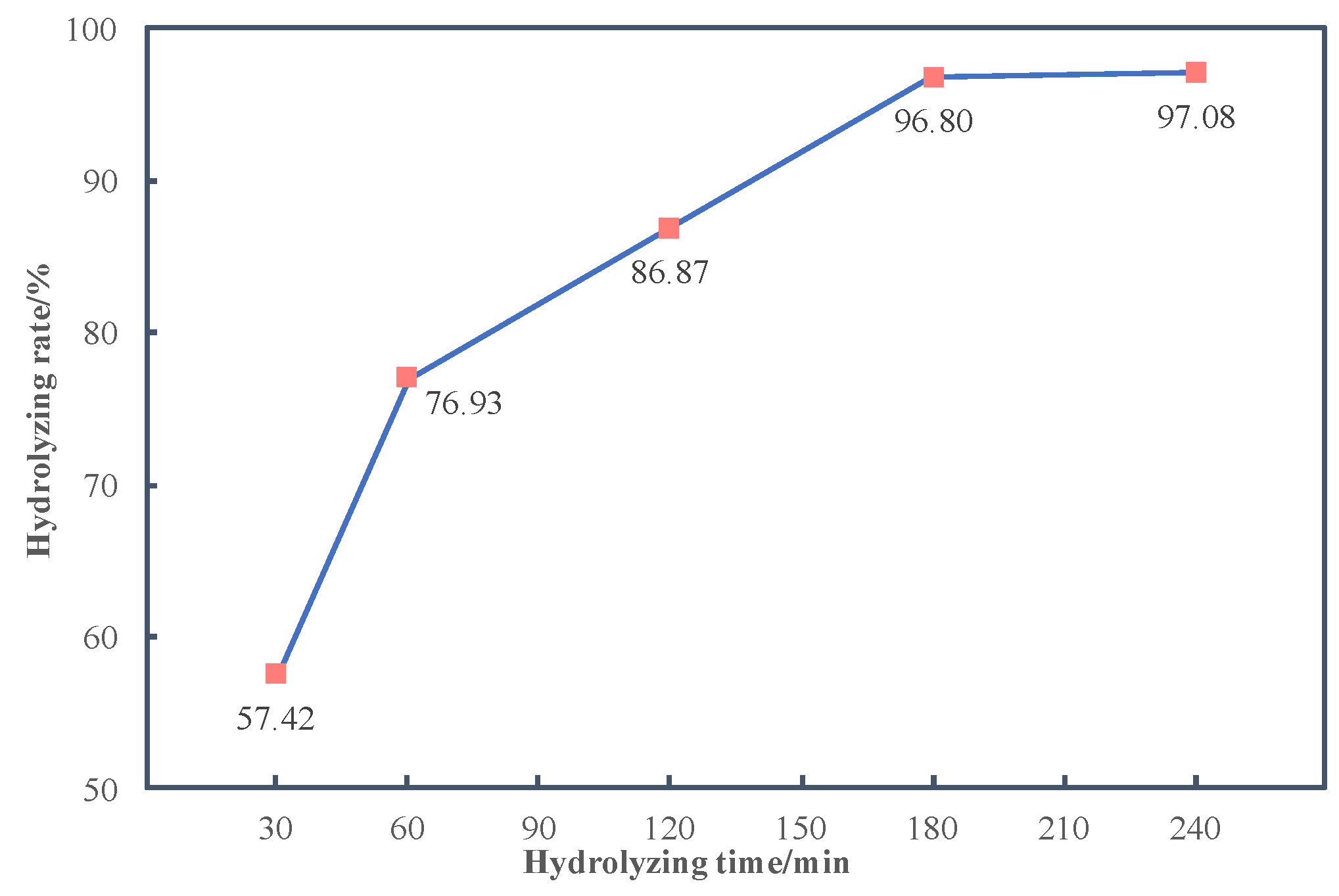
3.3.3. Effects of Time on Hydrolyzing Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Lu, M.; Zhou, Y.; Su, Z.; Liu, B.; Li, G.; Jiang, T. Interfacial Interaction between Humic Acid and Vanadium, Titanium-Bearing Magnetite (VTM) Particles. Miner. Process. Extr. Met. Rev. 2018, 41, 75–84. [Google Scholar] [CrossRef]
- Chen, D.S.; Song, B.; Wang, L.N.; Qi, T.; Wang, Y.; Wang, W.J. Solid state reduction of Panzhihua titanomagnetite concentrates with pulverized coal. Miner. Eng. 2011, 24, 864–869. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chu, M.-S. Metalizing reduction and magnetic separation of vanadium titano-magnetite based on hot briquetting. Int. J. Miner. Metall. Mater. 2014, 21, 225–233. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.Q.; Chu, M.S.; Zhu, M.Y. Oxidation induration process and kinetics of Hongge vanadium titanium-bearing magnetite pellets. Ironmak. Steelmak. 2017, 44, 294–303. [Google Scholar] [CrossRef]
- Chen, S.; Fu, X.; Chu, M.; Liu, Z.; Tang, J. Life cycle assessment of the comprehensive utilisation of vanadium titano-magnetite. J. Clean. Prod. 2015, 101, 122–128. [Google Scholar] [CrossRef]
- Chen, W.; Dong, Z.; Jiao, Y.; Liu, L.; Wang, X. Preparation, sintering behavior and consolidation mechanism of vanadium-titanium magnetite pellets. Crystals 2021, 11, 188. [Google Scholar] [CrossRef]
- Li, W.; Fu, G.; Chu, M.; Zhu, M. An effective and cleaner process to recovery iron, titanium, vanadium, and chromium from Hongge vanadium titanomagnetite with hydrogen-rich gases. Ironmak. Steelmak. 2020, 1–7. [Google Scholar] [CrossRef]
- Fu, G.; Li, W.; Chu, M.; Zhu, M. Influence mechanism of SiO2 on the oxidation behavior and induration process of hongge vanadium titanomagnetite pellets. Metall. Mater. Trans. B 2020, 51, 114–123. [Google Scholar] [CrossRef]
- Akhgar, B.N.; Pazouki, M.; Ranjbar, M.; Hosseinnia, A.; Keyanpour-Rad, M. Preparation of nanosized synthetic rutile from ilmenite concentrate. Miner. Eng. 2010, 23, 587–589. [Google Scholar] [CrossRef]
- Chernet, T. Applied mineralogical studies on Australian sand ilmenite concentrate with special reference to its behaviour in the sulphate process. Miner. Eng. 1999, 12, 485–495. [Google Scholar] [CrossRef]
- Han, K.N.; Rubcumintara, T.; Fuerstenau, M.C. Leaching behavior of ilmenite with sulfuric acid. Metall. Trans. B 1987, 18, 325–330. [Google Scholar] [CrossRef]
- Jabłoński, M.; Przepiera, A. Kinetic Model for the Reaction of Ilmenite with Sulphuric Acid. J. Therm. Anal. Calorim. 2001, 65, 583–590. [Google Scholar] [CrossRef]
- Lasheen, T.A.I. Chemical benefication of Rosetta ilmenite by direct reduction leaching. Hydrometallurgy 2005, 76, 123–129. [Google Scholar] [CrossRef]
- Li, C.; Liang, B.; Guo, L.-H. Dissolution of mechanically activated Panzhihua ilmenites in dilute solutions of sulphuric acid. Hydrometallurgy 2007, 89, 1–10. [Google Scholar] [CrossRef]
- Sasikumar, C.; Rao, D.S.; Srikanth, S.; Ravikumar, B.; Mukhopadhyay, N.K.; Mehrotra, S.P. Effect of mechanical activation on the kinetics of sulfuric acid leaching of beach sand ilmenite from Orissa, India. Hydrometallurgy 2004, 75, 189–204. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Puvvada, G.V.K.; Sridhar, R.; Lakshmanan, V.I. Chloride metallurgy: PGM recovery and titanium dioxide production. JOM 2003, 55, 38–41. [Google Scholar] [CrossRef]
- Rhee, K.I.; Sohn, H.Y. The selective chlorination of iron from llmenite ore by CO-Cl2 mixtures: Part I. intrinsic kinetics. Metall. Trans. B 1990, 21, 321–330. [Google Scholar] [CrossRef]
- Van Deventer, J.S.J. Kinetics of the selective chlorination of ilmenite. Thermochim. Acta 1988, 124, 205–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, T.; Zhang, Y. A novel preparation of titanium dioxide from titanium slag. Hydrometallurgy 2009, 96, 52–56. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, M.S. A new process for the recovery of iron, vanadium, and titanium from vanadium titanomagnetite. J. S. Afr. Inst. Min. Metall. 2014, 114, 481–488. [Google Scholar]

| VTM | Coal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFe | FeO | V2O5 | TiO2 | CaO | SiO2 | MgO | Al2O3 | Aad | Vad | Mad | FCad |
| 53.91 | 31.13 | 0.52 | 13.03 | 0.68 | 3.20 | 2.71 | 3.82 | 8.88 | 27.52 | 1.92 | 61.68 |
| TiO2 | CaO | SiO2 | MgO | Al2O3 | TFe |
|---|---|---|---|---|---|
| 40.91 | 1.47 | 14.04 | 12.95 | 12.34 | 8.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, S.; Duan, H. A New Process of Extracting Titanium from Vanadium–Titanium Magnetite. Crystals 2021, 11, 327. https://doi.org/10.3390/cryst11040327
Li Y, Chen S, Duan H. A New Process of Extracting Titanium from Vanadium–Titanium Magnetite. Crystals. 2021; 11(4):327. https://doi.org/10.3390/cryst11040327
Chicago/Turabian StyleLi, Yandong, Shuangyin Chen, and Huamei Duan. 2021. "A New Process of Extracting Titanium from Vanadium–Titanium Magnetite" Crystals 11, no. 4: 327. https://doi.org/10.3390/cryst11040327
APA StyleLi, Y., Chen, S., & Duan, H. (2021). A New Process of Extracting Titanium from Vanadium–Titanium Magnetite. Crystals, 11(4), 327. https://doi.org/10.3390/cryst11040327







