The Effect of Threads Geometry on Insertion Torque (IT) and Periotest Implant Primary Stability: A High-Density Polyurethane Simulation for the Anterior Mandible
Abstract
1. Introduction
- (1)
- (2)
- (3)
- the macro and micro geometry of the implant screw.
2. Materials and Methods
2.1. Polyurethane Foam Blocks
2.2. Implants Characteristics
2.3. Preparation of Implant Insertion Site
2.4. Measurement of Insertion Torque
2.5. Periotest Analysis
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrektsson, T.; Lekholm, U. Osseointegration: Current state of the art. Dent. Clin. North Am. 1989, 33, 537–554. [Google Scholar]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.-L. The effect of thread pattern upon implant osseointegration. Clin. Oral. Implant. Res. 2010, 21, 129–136. [Google Scholar] [CrossRef]
- Branemark, P.-I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An Update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef]
- Gehrke, A.; Mazon, P.; Del Fabbro, M.; Tumedei, M.; Aramburù, J.; Perez-Diaz, L.; De Aza, P. Histological and His-tomorphometric Analyses of Two Bovine Bone Blocks Implanted in Rabbit Calvaria. Symmetry 2019, 11, 641. [Google Scholar] [CrossRef]
- Tumedei, M.; Savadori, P.; Del Fabbro, M.; Fabbro, D. Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4221. [Google Scholar] [CrossRef]
- Gao, S.-S.; Zhang, Y.-R.; Zhu, Z.-L.; Yu, H.-Y. Micromotions and combined damages at the dental implant/bone interface. Int. J. Oral. Sci. 2012, 4, 182–188. [Google Scholar] [CrossRef]
- Piattelli, A.; Scarano, A.; Piattelli, M. Microscopical aspects of failure in osseointegrated dental implants: A report of five cases. Biomaterials 1996, 17, 1235–1241. [Google Scholar] [CrossRef]
- Olate, S.; Lyrio, M.C.N.; De Moraes, M.; Mazzonetto, R.; Moreira, R.W.F. Influence of Diameter and Length of Implant on Early Dental Implant Failure. J. Oral. Maxillofac. Surg. 2010, 68, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Tumedei, M.; Júnior, J.A.; Treichel, T.L.E.; Kolerman, R.; Lepore, S.; Piattelli, A.; Iezzi, G. Histological and Histomorphometrical Evaluation of a New Implant Macrogeometry. A Sheep Study. Int. J. Environ. Res. Public Health 2020, 17, 3477. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Yasutake, M.; Tsuiki, K.; Nakano, T.; Sawase, T. Structural and Qualitative Bone Remodeling Around Repetitive Loaded Implants in Rabbits. Clin. Implant. Dent. Relat. Res. 2015, 17, e699–e710. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding Peri-Implant Endosseous Healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Albrektsson, T.; Eriksson, A.; Friberg, B.; Lekholm, U.; Lindahl, L.; Nevins, M.; Oikarinen, V.; Roos, J.; Sennerby, L.; Astrand, P. Histologic investigations on 33 retrieved Nobelpharma implants. Clin. Mater. 1993, 12, 1–9. [Google Scholar] [CrossRef]
- Amari, Y.; Piattelli, A.; Alccayhuaman, K.A.A.; Mesa, N.F.; Ferri, M.; Iezzi, G.; Botticelli, D. Bone healing at non-submerged implants installed with different insertion torques: A split-mouth histomorphometric randomized controlled trial. Int. J. Implant. Dent. 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Kato, S.; Bengazi, F.; Velez, J.U.; Tumedei, M.; Kotsu, M.; Botticelli, D. Healing at implants installed in osteotomies prepared either with a piezoelectric device or drills: An experimental study in dogs. Oral. Maxillofac. Surg. 2021, 25, 65–73. [Google Scholar] [CrossRef]
- Kotsu, M.; Velez, J.U.; Bengazi, F.; Tumedei, M.; Fujiwara, S.; Kato, S.; Botticelli, D. Healing at implants installed from ~ 70- to < 10-Ncm insertion torques: An experimental study in dogs. Oral. Maxillofac. Surg. 2021, 25, 55–64. [Google Scholar] [CrossRef]
- Huwais, S.; Meyer, E.G. A Novel Osseous Densification Approach in Implant Osteotomy Preparation to Increase Biomechanical Primary Stability, Bone Mineral Density, and Bone-to-Implant Contact. Int. J. Oral. Maxillofac. Implant. 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Kuroshima, S.; Kaku, M.; Ishimoto, T.; Sasaki, M.; Nakano, T.; Sawase, T. A paradigm shift for bone quality in dentistry: A literature review. J. Prosthodont. Res. 2017, 61, 353–362. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of Underpreparation on Primary Stability of Implants Inserted in Poor Quality Bone Sites: An In Vitro Study. J. Oral. Maxillofac. Surg. 2015, 73, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Judy, K.W. Classification of partially edentulous arches for implant dentistry. Int. J. Oral. Implant. Implant. 1987, 4, 7–13. [Google Scholar]
- Misch, C.E. Bone Density: A Key Determinant for Clinical Success. Contemp. Implant. Dent. 1999, 8, 109–118. [Google Scholar]
- Scarano, A.; Carinci, F.; Lorusso, F.; Festa, F.; Bevilacqua, L.; De Oliveira, P.S.; Maglione, M. Ultrasonic vs Drill Implant Site Preparation: Post-Operative Pain Measurement Through VAS, Swelling and Crestal Bone Remodeling: A Randomized Clinical Study. Materials 2018, 11, 2516. [Google Scholar] [CrossRef] [PubMed]
- Favero, V.; Sakuma, S.; Alccayhuaman, K.A.A.; Benedetto, G.A.; Bengazi, F.; Botticelli, D. Healing at sites prepared using different drilling protocols. An experimental study in the tibiae of sheep. PLoS ONE 2018, 13, e0202957. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. New Osseodensification Implant Site Preparation Method to Increase Bone Density in Low-Density Bone. Implant. Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lahens, B.; Neiva, R.; Tovar, N.; Alifarag, A.M.; Jimbo, R.; Bonfante, E.A.; Bowers, M.M.; Cuppini, M.; Freitas, H.; Witek, L.; et al. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone. An experimental study in sheep. J. Mech. Behav. Biomed. Mater. 2016, 63, 56–65. [Google Scholar] [CrossRef]
- Zizzari, V.L.; Berardi, D.; Congedi, F.; Tumedei, M.; Cataldi, A.; Perfetti, G. Morphological Aspect and iNOS and Bax Expression Modification in Bone Tissue Around Dental Implants Positioned Using Piezoelectric Bone Surgery Versus Conventional Drill Technique. J. Craniofacial Surg. 2015, 26, 741–744. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1230. [Google Scholar] [CrossRef]
- Scarano, A.; Noumbissi, S.; Gupta, S.; Inchingolo, F.; Stilla, P.; Lorusso, F. Scanning Electron Microscopy Analysis and Energy Dispersion X-ray Microanalysis to Evaluate the Effects of Decontamination Chemicals and Heat Sterilization on Implant Surgical Drills: Zirconia vs. Steel. Appl. Sci. 2019, 9, 2837. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary Stability Determination by Means of Insertion Torque and RFA in a Sample of 4,135 Implants. Clin. Implant. Dent. Relat. Res. 2010, 14, 501–507. [Google Scholar] [CrossRef]
- Valente, M.L.D.C.; De Castro, D.T.; Shimano, A.C.; Lepri, C.P.; Dos Reis, A.C. Analysis of the influence of implant shape on primary stability using the correlation of multiple methods. Clin. Oral. Investig. 2015, 19, 1861–1866. [Google Scholar] [CrossRef]
- Allen, R.F.; Baldini, N.C.; Donofrio, P.E.; Gutman, E.L.; Keefe, E.; Kramer, J.G. Standard Specification for Rigid Poly-urethane Foam for Use as a Standard Material for Testing Orthopedic Devices and Instruments (F1839-97). In Annual Book of ASTM Standards, Medical Devices and Services; ASTM: West Conshohocken, PA, USA, 1998. [Google Scholar]
- Cordioli, G.; Majzoub, Z.; Piattelli, A.; Scarano, A. Removal Torque and Histomorphometric Investigation of 4 Dif-ferent Titanium Surfaces: An Experimental Study in the Rabbit Tibia. Int. J. Oral. Maxillofac. Implants 2000, 15, 668–674. [Google Scholar] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Perrotti, V.; Iezzi, G.; Scarano, A.; Piattelli, A. Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dent. J. 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.R.; Marino, V.; Bartold, P.M. Human cadaveric histomorphological and metallurgical analysis of dental implants following 12.5 years of service. Clin. Oral. Implant. Res. 2012, 25, 266–271. [Google Scholar] [CrossRef]
- Comuzzi, L.; Iezzi, G.; Piattelli, A.; Tumedei, M. An In Vitro Evaluation, on Polyurethane Foam Sheets, of the Insertion Torque (IT) Values, Pull-Out Torque Values, and Resonance Frequency Analysis (RFA) of NanoShort Dental Implants. Polymer 2019, 11, 1020. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Pontes, A.E.; Piattelli, A.; Iezzi, G. Primary Stability of Dental Implants in Low-Density (10 and 20 pcf) Polyurethane Foam Blocks: Conical vs Cylindrical Implants. Int. J. Environ. Res. Public Health 2020, 17, 2617. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Piattelli, A.; Iezzi, G. Short vs. Standard Length Cone Morse Connection Implants: An In Vitro Pilot Study in Low Density Polyurethane Foam. Symmetry 2019, 11, 1349. [Google Scholar] [CrossRef]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A Narrative Review of the Histological and Histomorphometrical Evaluation of the Peri-Implant Bone in Loaded and Unloaded Dental Implants. A 30-Year Experience (1988–2018). Int. J. Environ. Res. Public Health 2020, 17, 2088. [Google Scholar] [CrossRef] [PubMed]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A 30-Year (1988–2018) Retrospective Microscopical Evaluation of Dental Implants Retrieved for Different Causes: A Narrative Review. Int. J. Periodontics Restor. Dent. 2020, 40, e211–e227. [Google Scholar] [CrossRef] [PubMed]
- ASTM. F 67-95: Standard Specification for Unalloyed Titanium for Surgical Implant Applications. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 1995. [Google Scholar]
- Devlin, H.; Horner, K.; Ledgerton, D. A comparison of maxillary and mandibular bone mineral densities. J. Prosthet. Dent. 1998, 79, 323–327. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Pagnutti, S.; Vinci, R.; Gherlone, E.F. Distribution of Trabecular Bone Density in the Maxilla and Mandible. Implant. Dent. 2019, 28, 340–348. [Google Scholar] [CrossRef]
- Katranji, A.; Misch, K.; Wang, H.-L. Cortical Bone Thickness in Dentate and Edentulous Human Cadavers. J. Periodontol. 2007, 78, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H.; Piñas, L.; Orive, G. Efficacy of biologically guided implant site preparation to obtain adequate primary implant stability. Ann. Anat.-Anat. Anz. 2015, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; De Oliveira, P.S.; Traini, T.; Lorusso, F. Sinus Membrane Elevation with Heterologous Cortical Lamina: A Randomized Study of a New Surgical Technique for Maxillary Sinus Floor Augmentation without Bone Graft. Materials 2018, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Murias-Freijo, A.; Alkhraisat, M.H. Implant Site Under-Preparation to Compensate the Remodeling of an Autologous Bone Block Graft. J. Craniofacial Surg. 2015, 26, e374–e377. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Vercellotti, T.; Torelli, L.; Furlan, F.; Di Lenarda, R. Changes in Implant Stability Using Different Site Preparation Techniques: Twist Drills versus Piezosurgery. A Single-Blinded, Randomized, Controlled Clinical Trial. Clin. Implant. Dent. Relat. Res. 2011, 15, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.-W.; Fu, E.; Lin, F.-G.; Chang, W.-J.; Hsieh, Y.-D.; Shen, E.-C. Correlation Between Resonance Frequency Analysis and Bone Quality Assessments at Dental Implant Recipient Sites. Int. J. Oral. Maxillofac. Implant. 2017, 32, 180–187. [Google Scholar] [CrossRef]
- Andruch, K.; Płachta, A. Evaluating Maxilla Bone Quality Through Clinical Investigation of Voxel Grey Scale Values from Cone-Beam Computed Tomography for Dental Use. Adv. Clin. Exp. Med. 2015, 24, 1071–1077. [Google Scholar] [CrossRef]
- Romanos, G.E.; Delgado-Ruiz, R.A.; Sacks, D.; Calvo-Guirado, J.L. Influence of the implant diameter and bone quality on the primary stability of porous tantalum trabecular metal dental implants: Anin vitrobiomechanical study. Clin. Oral. Implant. Res. 2016, 29, 649–655. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of Stepped Osteotomy on Primary Stability of Implants Inserted in Low-Density Bone Sites: An In Vitro Study. Int. J. Oral. Maxillofac. Implant. 2017, 32, 37–41. [Google Scholar] [CrossRef]
- Scarano, A.; Crocetta, E.; Quaranta, A.; Lorusso, F. Influence of the Thermal Treatment to Address a Better Osseointegration of Ti6Al4V Dental Implants: Histological and Histomorphometrical Study in a Rabbit Model. BioMed. Res. Int. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Scarano, A.; Assenza, B.; Inchingolo, F.; Mastrangelo, F.; Lorusso, F. New Implant Design with Midcrestal and Apical Wing Thread for Increased Implant Stability in Single Postextraction Maxillary Implant. Case Rep. Dent. 2019, 2019, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Bevilacqua, L.; Dotto, F.; Costantinides, F.; Lorusso, F.; Scarano, A. Observational Study on the Preparation of the Implant Site with Piezosurgery vs. Drill: Comparison between the Two Methods in terms of Postoperative Pain, Surgical Times, and Operational Advantages. BioMed. Res. Int. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Shibli, J.A.; Pires, J.T.; Luongo, G.; Piattelli, A.; Iezzi, G. Early Bone Formation around Immediately Loaded Transitional Implants Inserted in the Human Posterior Maxilla: The Effects of Fixture Design and Surface. BioMed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Mangano, F.G.; Pires, J.T.; Shibli, J.A.; Mijiritsky, E.; Iezzi, G.; Piattelli, A.; Mangano, C. Early Bone Response to Dual Acid-Etched and Machined Dental Implants Placed in the Posterior Maxilla. Implant. Dent. 2017, 26, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Steigenga, J.T.; Al-Shammari, K.F.; Nociti, F.H.; Misch, C.E.; Wang, H.-L. Dental Implant Design and Its Relationship to Long-Term Implant Success. Implant. Dent. 2003, 12, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, T.; Tanaka, H.; Ayukawa, Y.; Howashi, M.; Masuzaki, T.; Kiyosue, T.; Koyano, K.; Nakamura, S. Primary Stability of a Hybrid Implant Compared with Tapered and Cylindrical Implants in an Ex Vivo Model. Clin. Implant. Dent. Relat. Res. 2014, 17, 950–956. [Google Scholar] [CrossRef]
- Naves, M.M.; Menezes, H.H.M.; Magalhães, D.; Ferreira, J.A.; Ribeiro, S.F.; De Mello, J.D.B.; Costa, H.L. Effect of Macrogeometry on the Surface Topography of Dental Implants. Int. J. Oral. Maxillofac. Implant. 2015, 30, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.-S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Boring, G.J.; Cooper, R.C.; Gao, C.; Givaruangsawat, S.; Gilbert, J.A.; Misch, C.M.; Steflik, D.E. Preliminary Evaluation of a New Dental Implant Design in Canine Models. Implant. Dent. 2000, 9, 252–260. [Google Scholar] [CrossRef]
- Fanali, S.; Tumedei, M.; Pignatelli, P.; Inchingolo, F.; Pennacchietti, P.; Pace, G.; Piattelli, A. Implant primary stability with an osteocondensation drilling protocol in different density polyurethane blocks. Comput. Methods Biomech. Biomed. Eng. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Li, T.; Liu, Y.; Ding, Y.; Wu, G.; Hu, K.; Kong, L. Optimal design of thread height and width on an immediately loaded cylinder implant: A finite element analysis. Comput. Biol. Med. 2010, 40, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.-J.; Cheong, S.-Y.; Han, J.-H.; Heo, S.-J.; Chung, J.-P.; Rhyu, I.-C.; Choi, Y.-C.; Baik, H.-K.; Ku, Y.; Kim, M.-H. Evaluation of design parameters of osseointegrated dental implants using finite element analysis. J. Oral. Rehabil. 2002, 29, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, B.; Hu, K.-J.; Li, D.-H.; Song, Y.-L.; Ma, P.; Yang, J. [Optimized thread pitch design and stress analysis of the cylinder screwed dental implant]. Hua Xi Kou Qiang Yi Xue Za Zhi 2006, 24, 509–512. [Google Scholar] [PubMed]
- Calandriello, R.; Tomatis, M.; Rangert, B. Immediate functional loading of Brånemark System implants with enhanced initial stability: A prospective 1- to 2-year clinical and radiographic study. Clin. Implant. Dent. Relat. Res. 2003, 5, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, R.; Piattelli, A.; Grande, N.-M.; Cataneo, E.; Crispino, A.; Petrini, M. Implant insertion torque value in immediate loading: A retrospective study. Med. Oral. Patol. Oral. Cir. Bucal 2019, 24, e398–e403. [Google Scholar] [CrossRef] [PubMed]
- Akkocaoglu, M.; Uysal, S.; Tekdemir, I.; Akca, K.; Cehreli, M.C. Implant design and intraosseous stability of immediately placed implants: A human cadaver study. Clin. Oral. Implant. Res. 2005, 16, 202–209. [Google Scholar] [CrossRef]
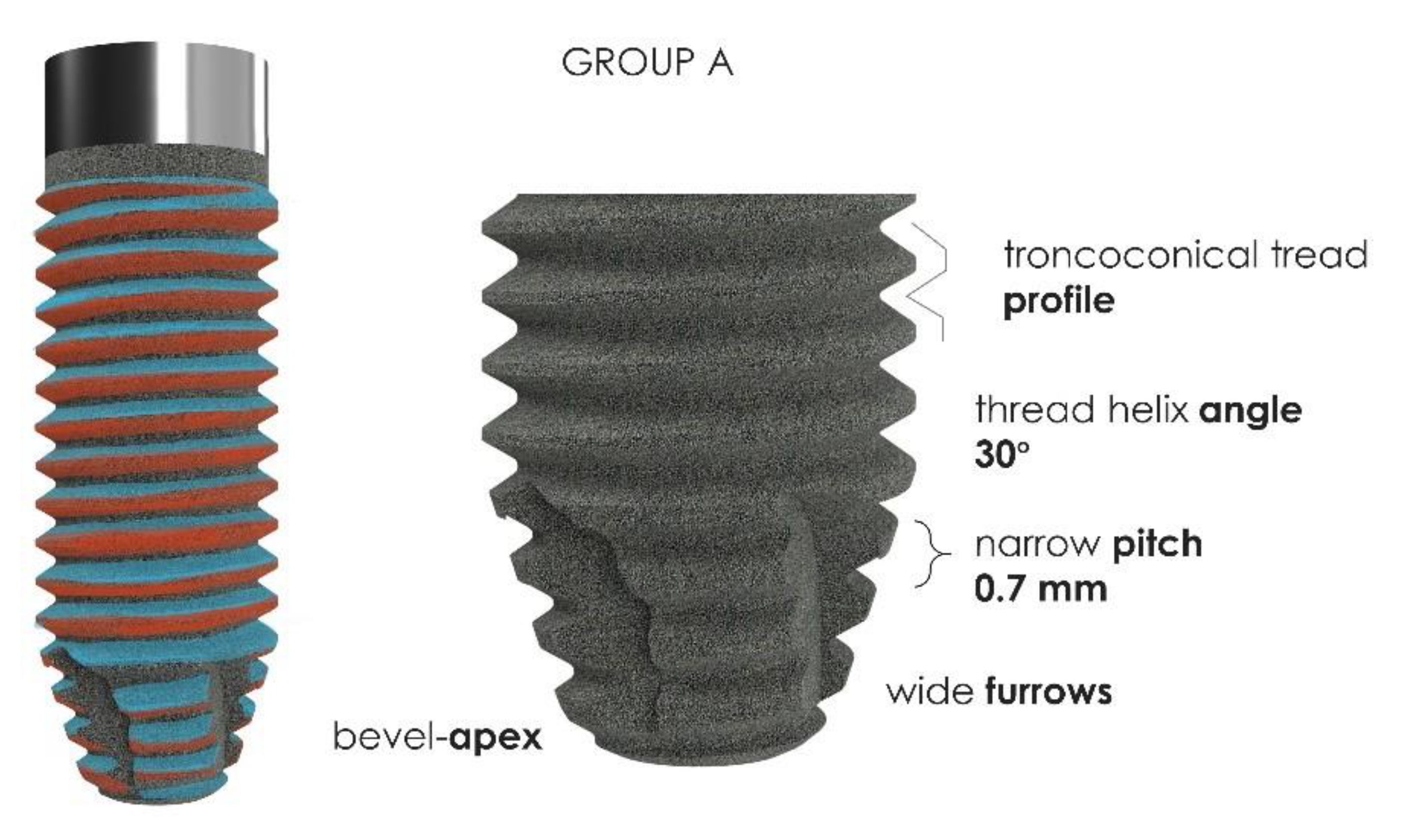

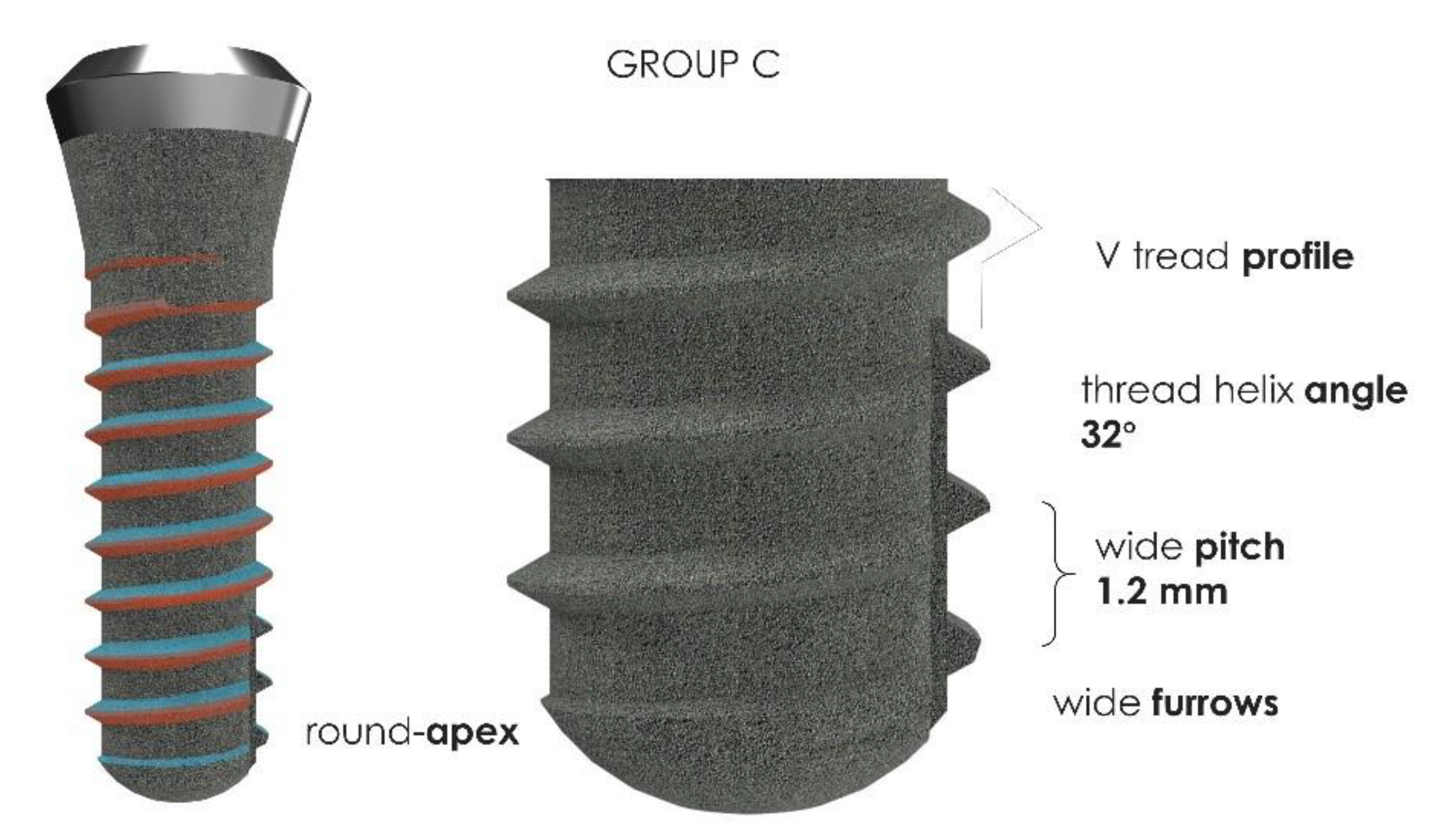




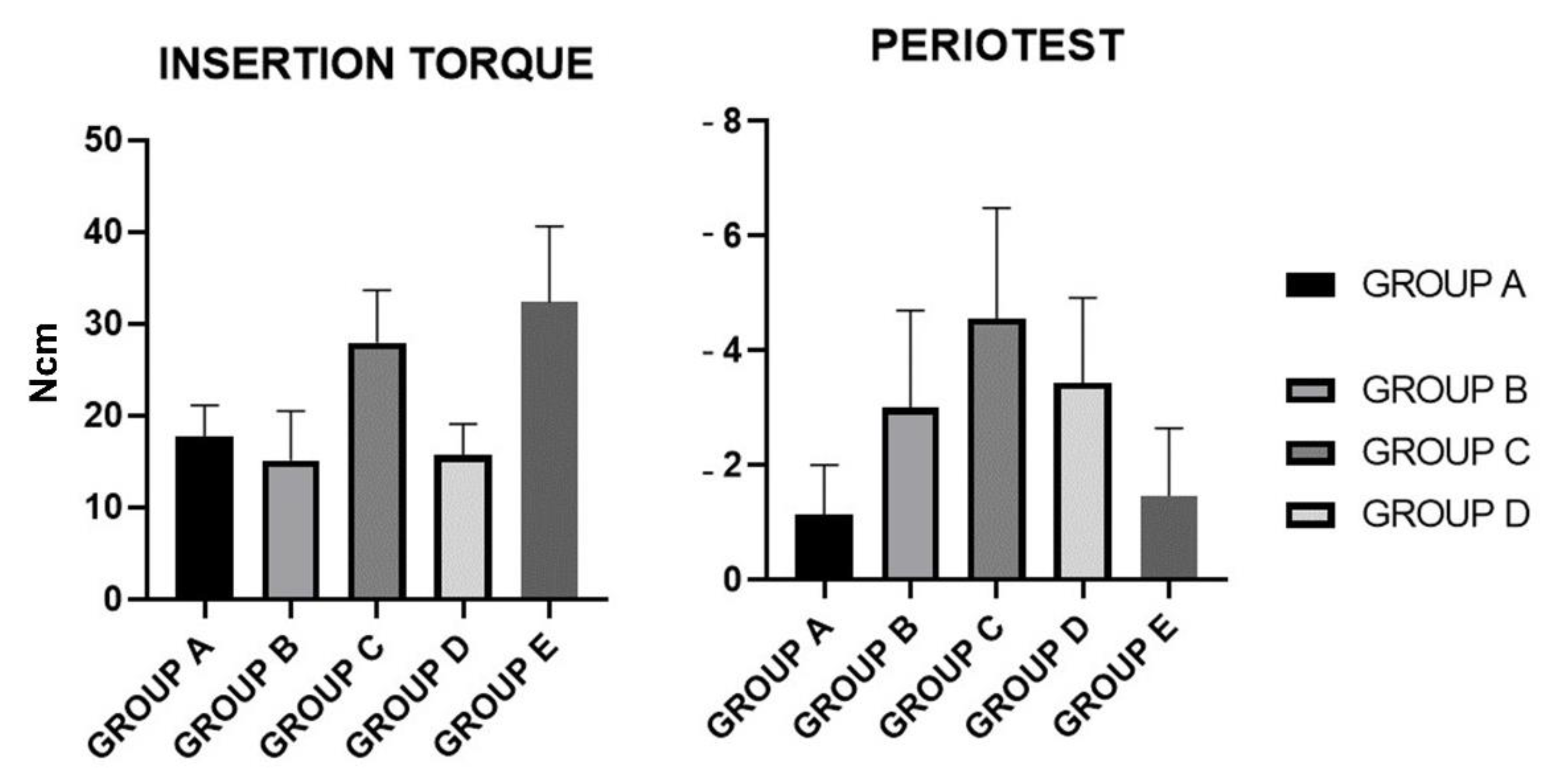
| Insertion Torque (Ncm2) | Mean | SD |
|---|---|---|
| GROUP A | 17.75 | 3.354 |
| GROUP B | 15.15 | 5.383 |
| GROUP C | 27.95 | 5.68 |
| GROUP D | 15.7 | 3.42 |
| GROUP E | 32.3 | 8.298 |
| Insertion Torque | Mean Difference | 95.00% CI | p-Value |
|---|---|---|---|
| GROUP A vs. GROUP B | 2.600 | −2.414 to 7.614 | 0.7801 |
| GROUP A vs. GROUP C | −10.20 | −15.21 to −5.186 | <0.0001 |
| GROUP A vs. GROUP D | 2.050 | −2.964 to 7.064 | 0.9392 |
| GROUP A vs. GROUP E | −14.55 | −19.56 to −9.536 | <0.0001 |
| GROUP B vs. GROUP C | −12.80 | −17.81 to −7.786 | <0.0001 |
| GROUP B vs. GROUP D | −0.5500 | −5.564 to 4.464 | >0.9999 |
| GROUP B vs. GROUP E | −17.15 | −22.16 to −12.14 | <0.0001 |
| GROUP C vs. GROUP D | 12.25 | 7.236 to 17.26 | <0.0001 |
| GROUP C vs. GROUP E | −4.350 | −9.364 to 0.6643 | 0.1372 |
| Periotest | Mean | SD |
|---|---|---|
| GROUP A | −1.143 | 0.8603 |
| GROUP B | −2.998 | 1.703 |
| GROUP C | −4.563 | 1.93 |
| GROUP D | −3.438 | 1.486 |
| GROUP E | −1.463 | 1.183 |
| Periotest | Mean Difference | 95.00% CI | p-Value |
|---|---|---|---|
| GROUP A vs. GROUP B | 1.855 | 3.158 to 0.5523 | 0.0013 |
| GROUP A vs. GROUP C | 3.420 | 4.723 to 2.117 | <0.0001 |
| GROUP A vs. GROUP D | 2.295 | 3.598 to 0.9923 | <0.0001 |
| GROUP A vs. GROUP E | 0.3200 | 1.623 to −0.9827 | 0.9597 |
| GROUP B vs. GROUP C | 1.565 | 2.868 to −0.2623 | 0.0103 |
| GROUP B vs. GROUP D | 0.4400 | 1.743 to −0.8627 | 0.8808 |
| GROUP B vs. GROUP E | −1.535 | −0.2323 to −2.838 | 0.0125 |
| GROUP C vs. GROUP D | −1.125 | 0.1777 to −2.428 | 0.1239 |
| GROUP C vs. GROUP E | −3.100 | −1.797 to −4.403 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanali, S.; Tumedei, M.; Pignatelli, P.; Petrini, M.; Piattelli, A.; Iezzi, G. The Effect of Threads Geometry on Insertion Torque (IT) and Periotest Implant Primary Stability: A High-Density Polyurethane Simulation for the Anterior Mandible. Crystals 2021, 11, 308. https://doi.org/10.3390/cryst11030308
Fanali S, Tumedei M, Pignatelli P, Petrini M, Piattelli A, Iezzi G. The Effect of Threads Geometry on Insertion Torque (IT) and Periotest Implant Primary Stability: A High-Density Polyurethane Simulation for the Anterior Mandible. Crystals. 2021; 11(3):308. https://doi.org/10.3390/cryst11030308
Chicago/Turabian StyleFanali, Stefano, Margherita Tumedei, Pamela Pignatelli, Morena Petrini, Adriano Piattelli, and Giovanna Iezzi. 2021. "The Effect of Threads Geometry on Insertion Torque (IT) and Periotest Implant Primary Stability: A High-Density Polyurethane Simulation for the Anterior Mandible" Crystals 11, no. 3: 308. https://doi.org/10.3390/cryst11030308
APA StyleFanali, S., Tumedei, M., Pignatelli, P., Petrini, M., Piattelli, A., & Iezzi, G. (2021). The Effect of Threads Geometry on Insertion Torque (IT) and Periotest Implant Primary Stability: A High-Density Polyurethane Simulation for the Anterior Mandible. Crystals, 11(3), 308. https://doi.org/10.3390/cryst11030308










