Low-Temperature Co-Precipitation Synthesis of HoFeO3 Nanoparticles
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rempel, A.A. Nanotechnologies, Properties and applications of nanostructured materials. Russ. Chem. Rev. 2007, 76, 435–461. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Ads, E.H. Perovskite nanomaterials—Synthesis, characterization, and applications. In Perovskite Materials—Synthesis, Characterisation, Properties, and Applications; Pan, L., Zhu, G., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Mounkachi, O.; Masrour, R.; Hamedoun, M.; Hlil, E.K.; Benyoussef, A. Synthesis and super-paramagnetic properties of neodymium ferrites nanorods. J. Alloys Compd. 2013, 581, 776–781. [Google Scholar]
- Tien, N.A.; Almjasheva, O.V.; Mittova, I.Y.; Stognei, O.V.; Soldatenko, S.A. Synthesis and magnetic properties of YFeO3 nanocrystals. Inorg. Mater. 2009, 45, 1304–1308. [Google Scholar] [CrossRef]
- Haron, W.; Thaweechai, T.; Wattanathana, W.; Laobuthee, A.; Manaspiya, H.; Veranitisagul, C.; Koonsaeng, N. Structural characteristics and dielectric properties of La1-xCoxFeO3 and LaFe1-xCoxO3 synthesized via metal organic complexes. Energy Procedia 2013, 34, 791–800. [Google Scholar] [CrossRef]
- Bhat, M.; Kaur, B.; Kumar, R.; Joy, P.A.; Kulkarni, S.D.; Bamzai, K.K.; Kotru, P.N.; Wanklyn, B.M. Swift heavy ion irradiation effect on structural and magnetic characteristics of RFeO3 (R = Er, Ho and Y) crystals. Nucl. Instrum. Methods Phys. Res., Sect. B 2006, 243, 134–142. [Google Scholar] [CrossRef]
- Sasikala, C.; Durairaj, N.; Baskaran, I.; Sathyaseelan, B.; Henini, M.; Manikandan, E. Transition metal titanium (Ti) doped LaFeO3 nanoparticles for enhanced optical structural and magnetic properties. J. Alloys Compd. 2017, 712, 870–877. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Chau, H.D.; Nguyen, T.T.L.; Mittova, V.O.; Do, T.H.; Mittova, I.Y. Structural and magnetic properties of YFe1-xCoxO3 (0.1 ≤ x ≤ 0.5) perovskite nanomaterials synthesized by co-precipitation method. Nanosyst. Phys. Chem. Math. 2018, 9, 424–429. [Google Scholar] [CrossRef]
- Cheon, C.I.; Kim, J.S.; Jang, P.W. Ferroelectric and magnetic properties of PrFeO3-PbTiO3 and PrFeO3-BiFeO3-PbTiO3 thin films. Jpn. J. Appl. Phys. 2002, 41, 67–77. [Google Scholar] [CrossRef]
- Tien, N.A.; Mittova, I.Y.; Al’myasheva, O.V. Influence of the synthesis conditions on the particle size and morphology of yttrium orthoferrite obtained from aqueous solutions. Russ. J. Appl. Chem. 2009, 82, 1915–1918. [Google Scholar] [CrossRef]
- Habib, Z.; Majid, K.; Ikram, M.; Sultan, K.; Mir, S.A.; Asokan, K. Influence of Ni substitution at B-site for Fe3+ ions on morphological, optical and magnetic properties of HoFeO3 ceramics. Appl. Phys. A 2016, 122, 550. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, L.; Yang, H.; Liu, Q.; Ye, F. Hydrothermal synthesis and magnetic properties of multiferroic rare-earth orthoferrites. J. Alloys Compd. 2014, 583, 21–31. [Google Scholar] [CrossRef]
- Martinson, K.D.; Kondrashkova, I.S.; Omarov, S.O.; Sladkovskiy, D.A.; Kiselev, A.S.; Kiseleva, T.Y.; Popkov, V.I. Magnetically recoverable catalyst based on porous nanocrystalline HoFeO3 for processes of n-hexane conversion. Adv. Powder Technol. 2019, 31, 402–408. [Google Scholar] [CrossRef]
- Kondrashkova, I.S.; Martinson, K.D.; Zakharova, N.V.; Popkov, V.I. Synthesis of nanocrystalline HoFeO3 photocatalyst via heat treatment of products of glycine-nitrate combustion. Russ. J. Gen. Chem. 2018, 88, 2465–2471. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, W.; Wu, A.; Xu, J.; Liu, Q.; Qian, G.; Zhang, H. Low-temperature combustion synthesis of nanocrystalline HoFeO3 powders via a sol-gel method using glycine. Ceram. Int. 2012, 38, 3667–3672. [Google Scholar] [CrossRef]
- Popkov, V.I.; Almjasheva, O.V.; Semenova, A.S.; Kellerman, D.G.; Nevedomskiy, V.N.; Gusarov, V.V. Magnetic properties of YFeO3 nanocrystals obtained by different soft-chemical methods. J. Mater. Sci. Mater. Electron. 2017, 28, 7163–7170. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen, L.T.T.; Bui, V.X.; Nguyen, D.H.; Lieu, H.D.; Le, L.M.; Pham, V. Optical and magnetic properties of HoFeO3 nanocrystals prepared by a simple co-precipitation method using ethanol. J. Alloys Compd. 2020, 834, 155098. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Pham, V.N.; Le, H.T.; Chau, D.H.; Mittova, V.O.; Nguyen, L.T.T.; Dinh, D.A.; Hao, T.N.; Mittova, I.Y. Crystal structure and magnetic properties of LaFe1-xNixO3 nanomaterials prepared via a simple co-precipitation method. Ceram. Int. 2019, 45, 21768–21772. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Pham, V.N.; Nguyen, T.T.L.; Mittova, V.O.; Vo, Q.M.; Berezhnaya, M.V.; Mittova, I.Y.; Do, T.H.; Chau, H.D. Crystal structure and magnetic properties of perovskite YFe1-xMnxO3 nanopowders synthesized by co-precipitation method. Solid State Sci. 2019, 96, 105922. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Pham, V.; Chau, D.H.; Mittova, V.O.; Mittova, I.Y.; Kopeychenko, E.I.; Nguyen, L.T.T.; Bui, V.X.; Nguyen, A.T. Effect of Ni substitution on phase transition, crystal structure and magnetic properties of nanostructured YFeO3 perovskite. J. Mol. Struct. 2020, 1215, 128293. [Google Scholar] [CrossRef]
- Belous, A.G.; Pashkova, E.V.; Elshanskii, V.A.; Ivanitskii, V.P. Effect of precipitation conditions on the phase composition, particle morphology and properties of iron (III, II) hydroxide precipitates. Inorg. Mater. 2000, 36, 343–351. [Google Scholar] [CrossRef]
- Imanaka, N. Physical and Chemical Properties of Rare Earth Oxides. In Binary Rare Earth Oxides; Adachi, G., Imanaka, N., Kang, Z.C., Eds.; Springer: Dordrecht, The Netherland; Berlin, Germany, 2004; pp. 111–113. [Google Scholar]
- Gupta, H.C.; Kumar Singh, M.; Tiwari, L.M. Lattice dynamic investigation of Raman and infrared wavenumbers at the zone center of orthorhombic RFeO3 (R = Tb, Dy, Ho, Tm) perovskite. J. Raman Spectrosc. 2002, 33, 67–70. [Google Scholar] [CrossRef]
- Habib, Z.; Ikram, M.; Majid, K.; Asokan, K. Structural, dielectric and ac conductivity properties of Ni-doped HoFeO3 before and after gamma irradiation. Appl. Phys. A 2014, 116, 1327–1335. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Wiley-IEEE Press: Hoboken, NJ, USA, 2009; ISBN 978-0-471-47741-9. [Google Scholar]
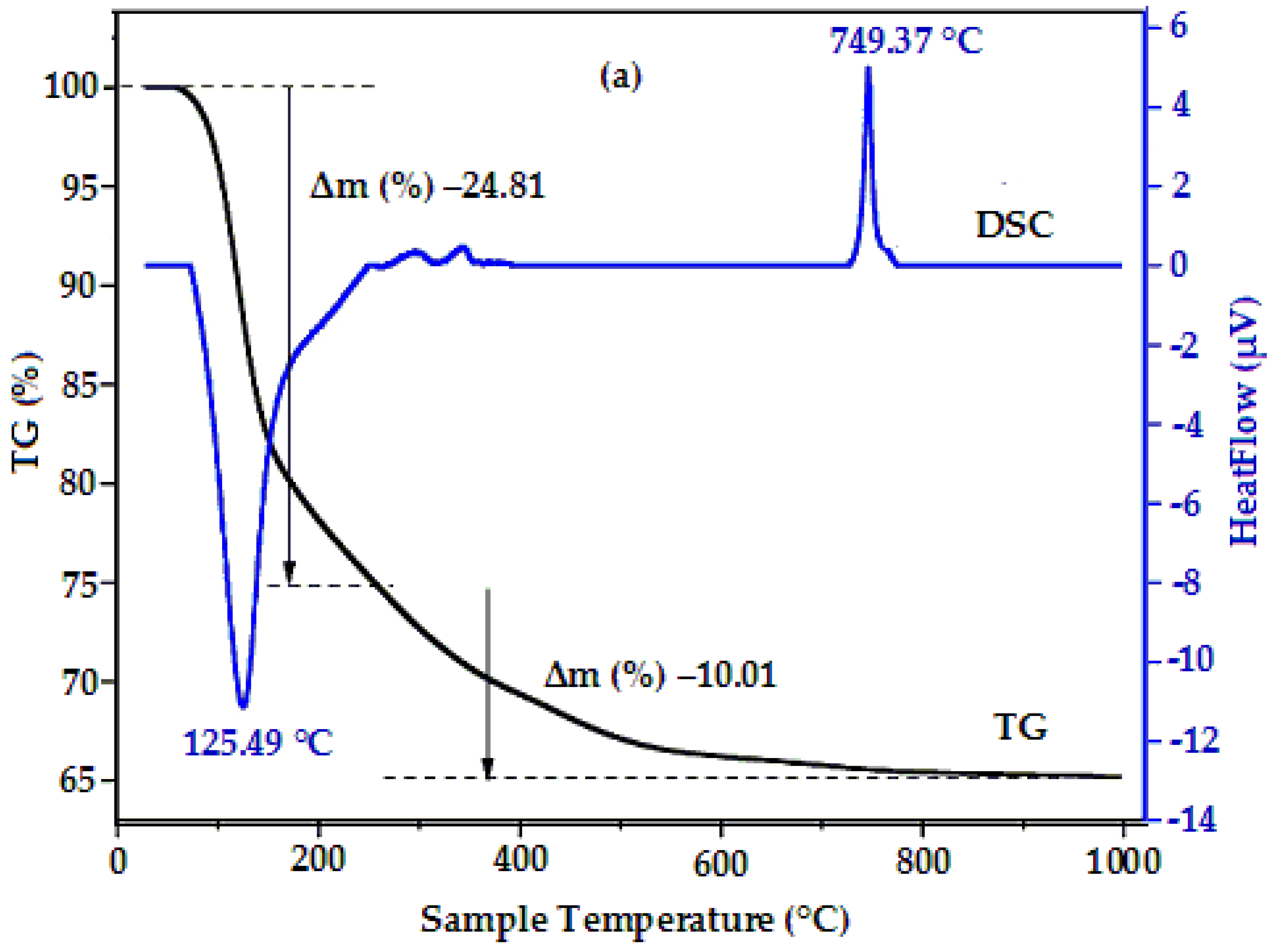
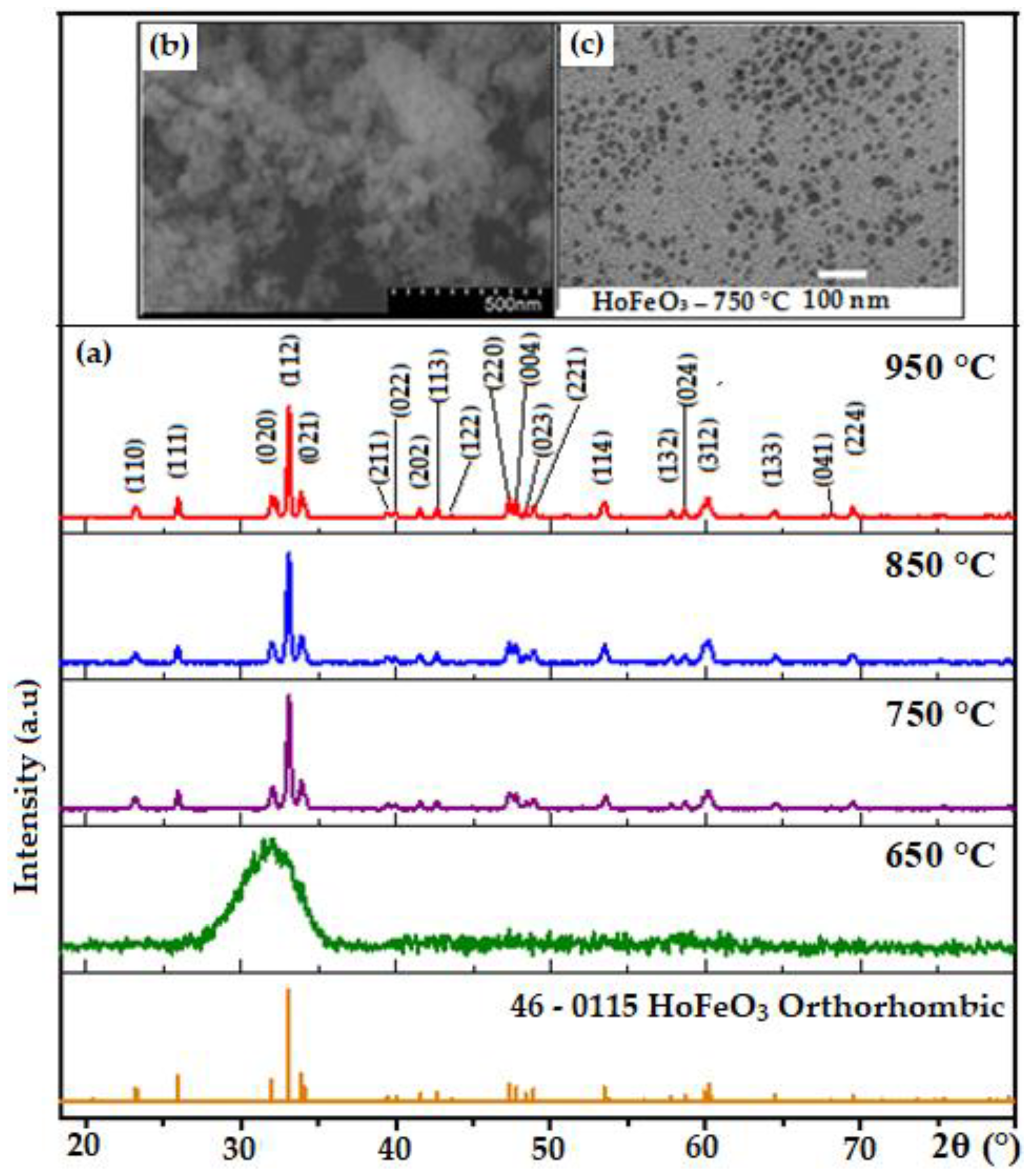

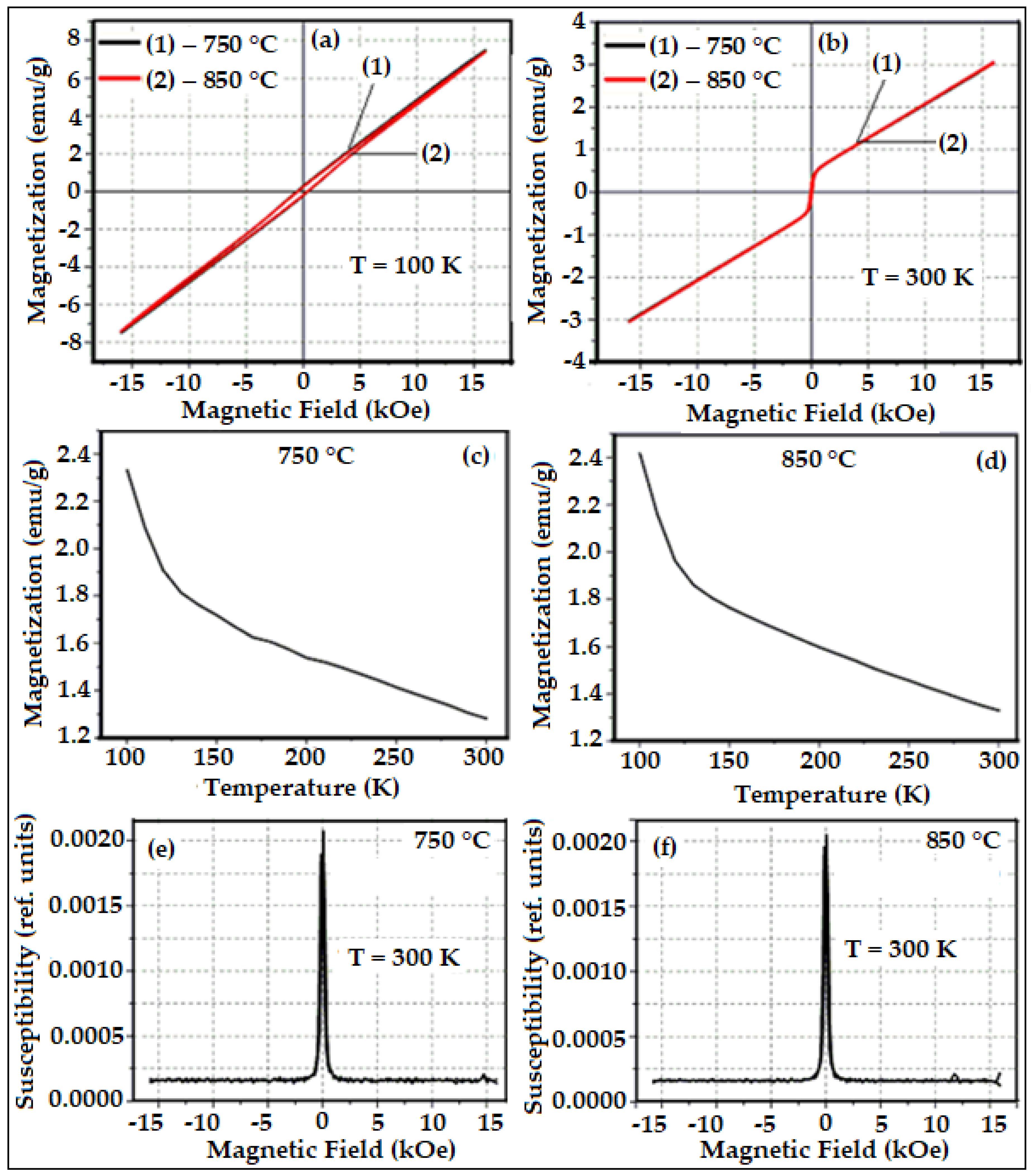
| HoFeO3 | Particle Size, nm | Eg, eV | Ms, emu/g | χ | ||
|---|---|---|---|---|---|---|
| 100 K | 300 K | 100 K | 300 K | |||
| 750 °C | 10–20 | 1.56 | ~7.5 | ~3.2 | ~0.53× 10−3 | ~2.1× 10−3 |
| 850 °C | – | – | ~0.51× 10−3 | |||
| [9] | 149.3 | 3.39 | – | 2.55 | – | – |
| [10] | 10–20 μm | – | – | <0.1 | – | – |
| [11,12] | 40–100 | 2.12–2.14 | – | – | – | – |
| [13] | 100–300 | – | 0.6 | 0.2 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, V.Q.; Tien, N.A. Low-Temperature Co-Precipitation Synthesis of HoFeO3 Nanoparticles. Crystals 2021, 11, 238. https://doi.org/10.3390/cryst11030238
Mai VQ, Tien NA. Low-Temperature Co-Precipitation Synthesis of HoFeO3 Nanoparticles. Crystals. 2021; 11(3):238. https://doi.org/10.3390/cryst11030238
Chicago/Turabian StyleMai, Vo Quang, and Nguyen Anh Tien. 2021. "Low-Temperature Co-Precipitation Synthesis of HoFeO3 Nanoparticles" Crystals 11, no. 3: 238. https://doi.org/10.3390/cryst11030238
APA StyleMai, V. Q., & Tien, N. A. (2021). Low-Temperature Co-Precipitation Synthesis of HoFeO3 Nanoparticles. Crystals, 11(3), 238. https://doi.org/10.3390/cryst11030238







