Abstract
A new sodium manganese-nickel phosphate of alluaudite supergroup with the general formula NaMnNi2(H2/3PO4)3 was synthesized by a hydrothermal method. The synthesis was carried out in the temperature range from 540 to 660 K and at the general pressure of 80 atm from the oxides mixture in the molar ratio MnCl2: 2NiCl2: 2Na3PO4: H3BO3: 10H2O. The crystal structure was studied by a single-crystal X-ray diffraction analysis: space group C2/c (No. 15), a = 16.8913(4), b = 5.6406(1), c = 8.3591(3) Å, β = 93.919(3), V = 794.57(4) Å3. The compound belongs to the alluaudite structure type based upon a mixed hetero-polyhedral framework formed by MX6-octahedra and TX4-tetrahedra. The characteristic feature of the title compound is the absence of cations or H2O molecules in channel II, while the negative charge of the framework is balanced by the partial protonation of PO4 tetrahedra. The presence of the transition metals at the A-type sites results in the changes of stoichiometry and the local topological features. Topological analysis of the hetero-polyhedral alluaudite-type frameworks and its derivatives (johillerite-, KCd4(VO4)3-, and keyite-type) and quantitative characterization of their differences was performed by means of natural tilings.
1. Introduction
Inorganic nickel phosphates attract interest because of their magnetic [1,2,3,4] and electrical [5,6,7] properties. Among them, compounds containing heteropolyhedral frameworks based upon the condensation of PO4 tetrahedra and NiO6 octahedra are related to zeolites and zeolite-type materials [8,9,10,11] and, owing to the presence of wide channels and cavities, possess interesting sorption and ion-exchange properties [12,13,14,15].
The alluaudite structure type (named after the mineral alluaudite, NaMnFe3+2(PO4)3 [16]) corresponds to natural oxysalts of the alluaudite supergroup [17] and synthetic compounds [18,19] with the general formula [A2A2′A2″2][A1A1′A1″2]{M1M22(TO4)3}, where M and T are octahedral and tetrahedral cations A1, A1′, and A1″ are cation sites located in the channel I A2, A2′, and A2″ are cation sites in channel II. Figure and square brackets denote the compositions of the framework and extra framework cations, respectively [19,20,21]. Because of the wide isomorphism in the cationic and anionic parts of the alluaudite-type structures, they attract interest as advanced materials for Li-ion batteries [22,23,24,25,26,27,28]. Despite the observed chemical diversity, Ni-containing alluaudite-type compounds are represented only by anhydrous phosphates Na2MNi2(PO4)3 (M = Fe, Al) [29,30] and CaFeNi2(PO4)3 [31], mixed phosphate-hydrogen phosphate AgNi3(PO4)(HPO4)2 [32], sulfate Na2Ni2(SO4)3 [33,34], and mixed sulfate-selenate Na3Ni1.5(SO4)3-1.5x(SeO4)1.5x [35].
The present work continues our systematic study of topologic features of compounds with hetero-polyhedral frameworks [36,37,38,39,40] and their comparison with zeolites. Here we report the results of hydrothermal synthesis of novel mixed nickel-manganese phosphate with an alluaudite-type structure and its characterization by the means of single-crystal X-ray diffraction analysis.
2. Materials and Methods
2.1. Synthesis and Sample Characterization
Single crystals of NaMnNi2(H2/3PO4)3 (1) were synthesized by a hydrothermal method. The synthesis was carried out in the temperature range from 540 to 660 K and at the general pressure of 80 atm from the mixture of oxides taken in the molar ratio MnCl2: 2NiCl2: 2Na3PO4: H3BO3: 10H2O. A standard Cu-lined stainless steel autoclave of 5 mL capacity was used. The coefficient of the autoclave filling was selected so that the pressure was constant. The experimental duration was 20 days and corresponds to the full completion of the chemical reaction. Final cooling after the synthesis experiment to room temperature was done over 24 h. The precipitate was separated by filtration and washed several times with hot distilled water and finally dried at room temperature for 12 h. The reaction products were small green crystals of the new phase in the estimated yield of 5%, which have been selected manually for the further studies.
The elemental contents of the selected crystals were determined by a Jeol JSM6480LV scanning electron microscope equipped with an INCA Wave 500 wave length spectrometer. The conditions of analysis were: accelerating voltage of 20 kV, a current of 20 nA, and a beam diameter 3 of μm. Chemical composition of 1 is (at.%): Na 5.86, Mn 6.59, Ni 9.58, P 16.56, O 61.41.
2.2. Single Crystal X-ray Diffraction Analysis
A yellow, round-shaped grain of 1 (0.04 × 0.05 × 0.11 mm3) was selected carefully under a polarizing microscope and used for single-crystal X-ray diffraction data collection. The single-crystal X-ray diffraction data were collected at room temperature on an Xcalibur S Oxford Diffraction diffractometer with graphite monochromatized MoKα radiation (λ = 0.71073 Å) and a CCD detector using the ω scanning mode. The raw data were integrated and then scaled, merged, and corrected for Lorentz-polarization effects using the CrysAlis package [41]. The C2/c (No. 15) space group was chosen based on the reflection statistics and confirmed by the successful refinement of the crystal structure. The experimental details of the data collection and refinement are listed in Table 1.

Table 1.
Crystal data, data collection and refinement procedural results.
The structural determinations and refinements were carried out using the Jana2006 program package [42]. Atomic scattering factors for neutral atoms together with anomalous dispersion corrections were taken from International Tables for Crystallography [43]. Illustrations were produced with the Jana2006 program package in combination with the program DIAMOND 3 [44]. The distribution of cations on the structural A-, M1, and M2-sites was proposed taking into account site-scattering factors [45], interatomic distances and ionic radii of the cations. Table 2 lists the fractional atomic coordinates, occupancies, site symmetries and equivalent atomic displacement parameters (Ueq). Selected interatomic distances are given in Table 3. CDS 2042360 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, accessed on 24 February 2021, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK, fax: +44 1223 336033.

Table 2.
Fractional site coordinates, equivalent displacement parameters (Ueq, Å2), site multiplicities (Mult.), the number of electrons associated with the atoms at the site (ecalc), and site composition for NaMnNi2(H2/3PO4)3.

Table 3.
Selected interatomic distances (Å) for NaMnNi2(H2/3PO4)3.
Bond-valence sums (BVS, Table 4) can be used for the indirect verification of mixed oxygen/hydroxyl site presence in the structure. BVS calculations were calculated using the bond valence parameters for the Na+–O, Mn2+–O, Ni2+–O, and P5+–O bonds [46].

Table 4.
Bond-valence analysis (v.u. = valence units) for NaMnNi2(H2/3PO4)3.
2.3. Topological Analysis and Complexity Calculations
Topological analysis of the frameworks was performed by means of natural tilings (the smallest polyhedral cationic clusters forming a framework) analysis of the 3D cation nets [47]. The complexity parameters were calculated as the Shannon information amounts per atom (IG) and per reduced unit cell (IG,total) [48,49]. The topological and complexity analysis was done using the ToposPro software [50].
3. Results
3.1. Crystal Structure
The crystal structure of NaMnNi2(H2/3PO4)3 (Figure 1) is similar to other members of the alluaudite supergroup [16,17] and is based upon zig-zag chains of edge-sharing M1O6 and M2O6 octahedra formed by Mn and Ni atoms, respectively. The mean <M-O> distances in the octahedra (<M1–O> = 2.219 Å; <M2–O> = 2.093 Å) are in good agreement with the ionic radii of these elements (r(Mn2+) = 0.83 Å, r(Ni2+) = 0.69 Å [51]). The chains are linked by P1Ø4- and P2Ø4-tetrahedra (Ø = O2–, OH–) forming a hetero-polyhedral framework that contains two types of channels with hexagonal cross-sections. Sodium atoms fill channel I at the A1′-site with the coordinates (0 ~½ ~¼) at the center of a large eight-vertex polyhedron with the mean distance <A1′–O> = 2.640 Å. The refined detailed crystal chemical formula can be written as (Z = 4): □NaMnNi2(HPO4)(H0.5PO4)2 (where ‘□’ means vacancy at A2-site).
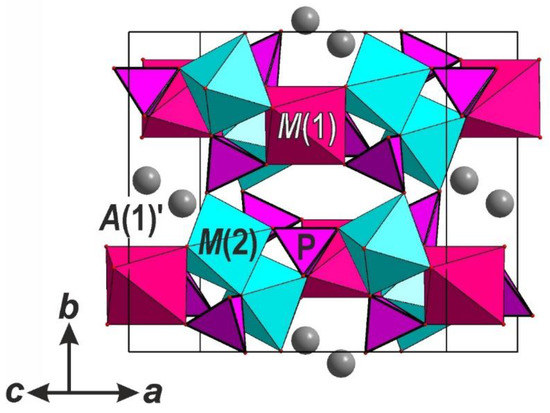
Figure 1.
General view of the crystal structure of 1.
The systematic of the alluaudite-type compounds has previously been reported on the basis of the distribution of extra-framework cations within the channels [19]. The studied compounds belongs to the “KCd4(VO4)3” subgroup [52] with the general formula A2A1′{M1M22(TO4)} [19]. The main characteristic feature of 1 is the absence of any cations or H2O molecules in the channel II [A(2) = □] with the negative charge of the framework balanced by the partial protonation of PO4 tetrahedra (Table 3). The compound Na2FeNi2(PO4)3 reported in Reference [29] belongs to the same family, but differs from 1 by the presence of Na in both types of channels (<Na(A1′)–O> = 2.625 Å, <Na(A2)–O> = 2.525 Å) and by the absence of hydroxyl groups. The M1O6- and M2O6-octahedra of the heteropolyhedral framework in the compound Na2FeNi2(PO4)3 are occupied by Fe3+ and Ni atoms, respectively, with the following mean <M-O> distances: <M1–O> = 2.137 Å, <M2–O> = 2.043 Å [29].
3.2. Hydrogen Bonding
In the crystal structure of 1, channel II is filled only by statistically disordered hydrogen atoms of P1Ø4- and P2Ø4-tetrahedra (Figure 2a). The strong hydrogen bonds are formed between O2-oxygens and O4-oxygens with the distance O2⋯O4 = 2.482(7) Å and possible local distributions of hydrogens are shown in Figure 2b–e. Based on the Libowitzky equations [53]:
and
the following vibrations should be observed in the IR spectrum: ~1488 cm−1 (O⋯O), ~2385 cm−1 (H⋯O), and ~2.129 cm−1 (H⋯O).
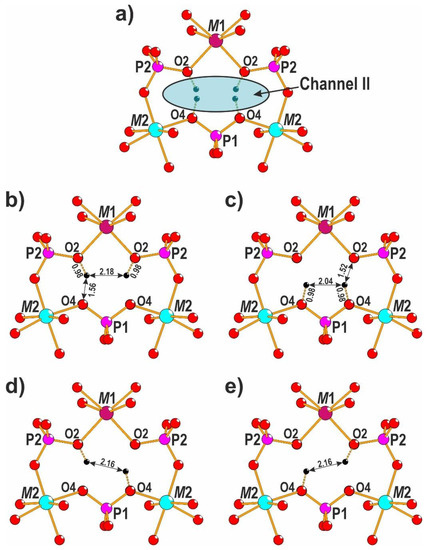
Figure 2.
The statistical arrangement of hydrogen atoms in the crystal structure of 1 (a) and possible models of local hydrogen bonds (b–e).
The mixed phosphates-hydrous phosphates are well-known [54,55,56,57,58]. In the crystal structures of NaMn3(PO4)(HPO4)2 [54] and NaMg3(PO4)(HPO4)2 [58] with the localized position of hydrogens the similar scheme of hydrogen bonds is reported. Moreover, for mixed arsenate-hydrous arsenate, a good agreement between observed and calculated stretching vibrations in the IR spectrum was shown [59].
4. Discussion
4.1. General Formula and Stoichiometry of the Alluaudite-type Heteropolyhedral Framework
The crystal structures of minerals and synthetic compounds with the alluaudite structure type are based upon a mixed hetero-polyhedral MT-framework of MX6-octahedra and TX4-tetrahedra [60,61,62] containing two types of symmetrically non-equivalent M1X6-octahedra and M2X6-octahedra and two types of symmetrically non-equivalent T1X4- tetrahedra and T2X4-tetrahedra. The anionic X-ligands of the framework are represented by bridging O atoms shared between two (pII-ligands [60,61]) as well as three M and T cations (pIII-ligands). The general crystal chemical formula of the framework (taking into account the degree of sharing of X-ligands) can be written as:
or
which the charge (W) determined by the oxidation states of the M-cations and T-cations.
As it was mentioned above, in the majority of the alluaudite-type structures, the anionic X-ligands are represented by the oxygen atoms. However, in the case of the charge deficiency of bridging pII-ligands, they could be partially protonated and Equation (4) can be rewritten as:
If X = O2– and X′ = (OH)–, Formula (5) can also be modified as:
where x = 0–1. The charge of the alluaudite-type framework can be defined using the following equation:
where VM and VT are the valences of the M and T cations, respectively.
The simplified formula is:
Formulas (5) and (6) indicate that the electroneutral framework (W = 0) is possible in the case of M = Me2+ (e.g., Me = Mn2+, Ni2+, etc.), T = T6+ (S6+, Se6+, Mo6+, etc.), and x = 0. However, such alluaudite-type compounds with the neutral frameworks have not been found so far and, typically, the framework has a negative charge (W < 0) balanced by the extra-framework A-type cations. Large A-cations as well as H2O molecules may occupy channels of two types characterized by the different topologies: [32.42.62/2] (channel I) and [38.44.62/2] (channel II) (Figure 3). The alluaudite-type framework density [8] is 27.57 (M+T)/1000 Å and the information-based complexity parameters [48,49] are: v = 36 atoms, IG = 3.281 bit/atom, and IG,total = 118.117 bit/unit cell.
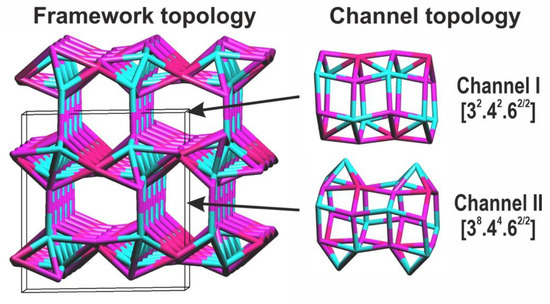
Figure 3.
General view of the topology of the alluaudite-type framework and channels I and II.
In accordance with the rules for the description of ordered microporous and mesoporous materials with inorganic hosts approved by the International Zeolite Association, the crystal chemical formula for such a material has to be written in the following order: |guest composition| [host composition] h{dimensionality of the host Dh} p{dimensionality of the pore system Dp–shape of the pore –direction of the channel [uvw]} (symmetry) [44,45]. Consequently, the general crystal chemical formula of the studied compounds and related materials (Table 3) can be written as follows (Z = 1):
That indicates that (1) the guests are A-cations (with the coordination numbers ranging from 4 to 8) and H2O molecules, and (2) the 3D host structure is formed by octahedral M-cations and tetrahedral T-cations and consists of two different, unidimensional channels extending along [001] with the topologies [324262/2] and [384462/2] [both channels have a 6-membered ring (6R) pore opening], respectively.
4.2. The Influence of the Hetero-Polyhedral Substitutions at Extraframework A-Sites on the Stoichiometry of the Alluaudite-Type Framework
In the alluaudite-type structures, extra-framework alkaline and alkaline-earth A-cations may possess high mobility and migrate along the channels. Moreover, transition metal cations can also fill the channels through incorporation into the A(1)′-site (the 4e Wyckoff site with the coordinates (0 y ¼), y ~ 0.25) or A(2)-site (the 4a site with the coordinates (0 0 0)) in the centers of flat squares. The incorporation of these elements changes the ratio between the pII- and pIII-type X-ligands as well as the topology of the framework. As a result, Formula (4) can be rewritten as:
or
where x = 0–1. The derivative of the alluaudite-type framework containing occupied A(1)′ sites is described by the formula (10) and can be denoted as the johillerite-type (by the analogy with the mineral johillerite, NaCuMgMg2(AsO4)3 [63,64]), while the derivative with the occupied A(2) sites and the formula (11) is denoted as the KCd3(VO4)3-type [52,65]. Taking into account that both derivatives also contain bridging pII-type X-ligands that can be partially protonated, Formulas (10) and (11) can be modified as:
or
If X = O2– and X′= OH–, Formulas (12) and (13) can be simplified by the following.
The keyite-type derivative of the alluaudite-type framework is characterized by the presence of transition metals in both A(1)′ and A(2) sites. Its general formula can be written as:
Because of the absence of the pII-type X-ligands, the protonation is no longer possible (X = O2-) and the simplified formula of the keyite-type derivative (named after the mineral keyite, (□0.5Cu0.5)CuCdZn2(AsO4)3·H2O [66]) is:
4.3. Topological Features of Alluaudite-Type Framework and Its Derivatives
The presence of transition metals at the A sites of the alluaudite-type framework results in the changes of stoichiometry and the local topological features. The topological analysis using a ToposPro software [50] makes it possible to find common tilings in generally similar heteropolyhedral frameworks of aluaudite-type and its derivatives (the johillerite, KCd4(VO4)3, and keyite types) and to investigate their differences.
An ‘empty’ parent alluaudite-type framework consists of four natural tilings: (2T3M)-[43] (formed by two T-cations and three M-cations), (2T3M)-[32.42], (4T5M)-[32.42.62], and (6T8M)-[38.44.62] (Figure 4a). The occupancy of the A sites by additional transition metal cations changes the topology of its derivatives significantly. The johillerite-type derivative (with the occupied A(1)′-site in channel I) is formed by five types of natural tiles. Among them, the (2T3M)-[43], (2T3M)-[32.42], and (4T5M)-[32.42.62] tilings are identical to those in the parent alluaudite-type framework, while the (6T8M2A)-[320.44] and (2T4M)-[34.42] tilings are unique (Figure 4b). The KCd4(VO4)3-type derivative (the A(2)-site is occupied in channel II) is also characterized by the presence of five types of tilings: the (2T3M)-[32.42] and (4T5M)-[32.42.62] tilings are identical to those of the parent framework, while the (3T5M2A)-[310.43], (3T2M1A)-[32.43], and (2T4M)-[34.42] tilings are unique (Figure 4c). In the keyite-type derivative (both channels I and II are filled by cations at the A(1)′- and A(2)-sites). There are five types of natural tilings (Figure 4d): (2T3M)-[43] is identical to that in the parent structure, while the (6T8M2A)-[320.44] tiling is identical to that in johillerite. The (1T2M1A)-[34], (2T3M1A)-[34.42] (topologically identical to other [34.42] tilings, but chemically different) and (3T3M2A)-[36.43] tilings are unique and appear due to the presence of cations in both types of channels. Comparative data on the general features of the alluaudite-type frameworks and its derivatives are summarized in Table 5.
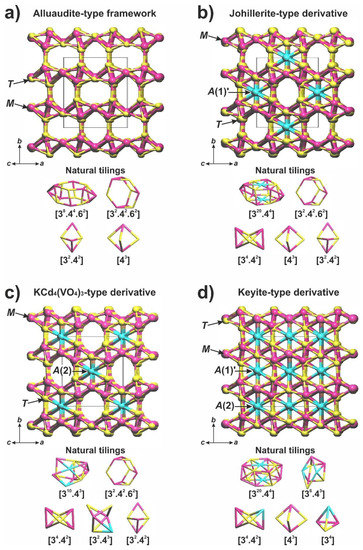
Figure 4.
Alluaudite type framework (a) and its derivatives: johillerite-type (b), KCd4(VO4)3-type (c), and keyite-type (d). General view and natural tilings.

Table 5.
Crystal data, data collection and refinement procedural results.
5. Conclusions
The occupancy of the A sites by additional transition metal cations significantly changes the topology of its derivatives. Detailed analysis of the alluaudite-type framework as well as its derivatives shows the influence of the different types of the cation arrangement within extra-framework sites on the stoichiometry (the possible amount of OH groups), complexity parameters of the frameworks, and types of natural tiling.
Author Contributions
Conceptualization, S.M.A., N.A.Y. and S.V.K. Synthesis, A.S.V. and O.V.D. Formal analysis, N.A.K., O.A.G., and D.V.D. Writing—original draft preparation, S.M.A., N.A.Y., and S.V.K. Supervision, S.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant numbers 20-77-10065 (Topological and modular analysis; complexity calculations) and 19-77-10013 (Single-crystal X-ray analysis).
Acknowledgments
The authors are grateful to three reviewers and the Special issue editors Professor Igor V. Pekov and Natalia V. Zubkova for their useful comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanz, F.; Parada, C.; Rojo, J.M.; Ruíz-Valero, C. Synthesis, structural characterization, magnetic properties, and ionic conductivity of Na4MII3(PO4)2(P2O7) (M II = Mn, Co, Ni). Chem. Mater. 2001, 13, 1334–1340. [Google Scholar] [CrossRef]
- De Pedro, I.; Rojo, J.M.; Jubera, V.; Fernández, J.R.; Marcos, J.S.; Lezama, L.; Rojo, T. Effect of Ni2+ (S = 1) and Cu2+ (S = ½) substitution on the antiferromagnetic ordered phase Co2(OH)4 with spin glass behaviour. J. Mater. Chem. 2004, 14, 1157–1163. [Google Scholar] [CrossRef]
- Yamnova, N.A.; Aksenov, S.M.; Mironov, V.S.; Volkov, A.S.; Borovikova, E.Y.; Gurbanova, O.A.; Dimitrova, O.V.; Deyneko, D.V. The first layer potassium–bismuth-nickel oxophosphate KBi4Ni2(PO4)3O4: Synthesis, crystal structure, and expected magnetic properties. Crystallogr. Rep. 2017, 62. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Mironov, V.S.; Borovikova, E.Y.; Yamnova, N.A.; Gurbanova, O.A.; Volkov, A.S.; Dimitrova, O.V.; Deyneko, D.V. Synthesis, crystal structure, vibrational spectroscopy and expected magnetic properties of a new bismuth nickel phosphate Ni(BiO)2(PO4)(OH) with a namibite-type structure. Solid State Sci. 2017, 63. [Google Scholar] [CrossRef]
- Li, X.; Xiao, X.; Li, Q.; Wei, J.; Xue, H.; Pang, H. Metal (M = Co, Ni) phosphate based materials for high-performance supercapacitors. Inorg. Chem. Front. 2018, 5, 11–28. [Google Scholar] [CrossRef]
- Rommel, S.M.; Schall, N.; Brünig, C.; Weihrich, R. Challenges in the synthesis of high voltage electrode materials for lithium-ion batteries: A review on LiNiPO4. Mon. Chem. Chem. Mon. 2014, 145, 385–404. [Google Scholar] [CrossRef]
- Herle, P.S.; Ellis, B.; Coombs, N.; Nazar, L.F. Nano-network electronic conduction in iron and nickel olivine phosphates. Nat. Mater. 2004, 3, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K. Crystal chemistry, properties and synthesis of microporous silicates containing transition elements. Russ. Chem. Rev. 2004, 73, 205–223. [Google Scholar] [CrossRef]
- Rocha, J.; Lin, Z. Microporous Mixed Octahedral-Pentahedral-Tetrahedral Framework Silicates. Rev. Mineral. Geochem. 2005, 57, 173–201. [Google Scholar] [CrossRef]
- Rocha, J.; Anderson, M.W. Microporous titanosilicates and other novel mixed octahedral-tetrahedral framework oxides. Eur. J. Inorg. Chem. 2000, 2000, 801–818. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef]
- Guillou, N.; Gao, Q.; Nogues, M.; Morris, R.E.; Hervieu, M.; Férey, G.; Cheetham, A.K. Zeolitic and magnetic properties of a 24-membered ring porous nickel(II) phosphate, VSB-1. Comptes Rendus lAcadémie Sci. Ser. IIC Chem. 1999, 2, 387–392. [Google Scholar] [CrossRef]
- Garcia, R.; Philp, E.F.; Slawin, A.M.Z.; Wright, P.A.; Cox, P.A. Nickel complexed within an azamacrocycle as a structure directing agent in the crystallization of the framework metalloaluminophosphates STA-6 and STA-7. J. Mater. Chem. 2001, 11, 1421–1427. [Google Scholar] [CrossRef]
- Guillou, N.; Gao, Q.; Forster, P.M.; Chang, J.-S.; Noguès, M.; Park, S.-E.; Férey, G.; Cheetham, A.K. Nickel(II) Phosphate VSB-5: A magnetic nanoporous hydrogenation catalyst with 24-ring tunnels. Angew. Chemie Int. Ed. 2001, 40, 2831–2834. [Google Scholar] [CrossRef]
- Dechambenoit, P.; Long, J.R. Microporous magnets. Chem. Soc. Rev. 2011, 40, 3249. [Google Scholar] [CrossRef]
- Moore, P.Β. Crystal chemistry of the alluaudite structure type: Contribution to the paragenesis of pegmatite phosphate giant crystals. Am. Mineral. 1971, 56, 1955–1975. [Google Scholar]
- Hatert, F. A new nomenclature scheme for the alluaudite supergroup. Eur. J. Mineral. 2019, 31, 807–822. [Google Scholar] [CrossRef]
- Ðorðević, T.; Wittwer, A.; Krivovichev, S.V. Three new alluaudite-like protonated arsenates: NaMg3(AsO4)(AsO3OH)2, NaZn3(AsO4)(AsO3OH)2 and Na(Na0.6Zn0.4)Zn2(H0.6AsO4)(AsO3OH)2. Eur. J. Mineral. 2015, 27, 559–573. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Kiryukhina, G.V.; Dimitrova, O.V. Crystal chemistry of KCuMn3(VO4)3 in the context of detailed systematics of the alluaudite family. Crystallogr. Rep. 2016, 61, 566–575. [Google Scholar] [CrossRef]
- Hatert, F.; Keller, P.; Lissner, F.; Antenucci, D.; Fransolet, A.-M. First experimental evidence of alluaudite-like phosphates with high Li-content: The (Na1-xLix)MnFe2(PO4)3 series (x = 0 to 1). Eur. J. Mineral. 2000, 12, 847–857. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Vergasova, L.P.; Filatov, S.K.; Rybin, D.S.; Britvin, S.N.; Ananiev, V.V. Hatertite, Na2(Ca,Na) (Fe3+,Cu)2(AsO4)3, a new alluaudite-group mineral from Tolbachik fumaroles, Kamchatka peninsula, Russia. Eur. J. Mineral. 2013, 25, 683–691. [Google Scholar] [CrossRef]
- Trad, K.; Carlier, D.; Croguennec, L.; Wattiaux, A.; Ben Amara, M.; Delmas, C. NaMnFe2(PO4)3 Alluaudite phase: Synthesis, structure, and electrochemical properties as positive electrode in lithium and sodium batteries. Chem. Mater. 2010, 22, 5554–5562. [Google Scholar] [CrossRef]
- Barpanda, P.; Oyama, G.; Nishimura, S.; Chung, S.-C.; Yamada, A. A 3.8-V earth-abundant sodium battery electrode. Nat. Commun. 2014, 5, 4358. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-Ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Dwibedi, D.; Araujo, R.B.; Chakraborty, S.; Shanbogh, P.P.; Sundaram, N.G.; Ahuja, R.; Barpanda, P. Na2.44Mn1.79(SO4)3: A new member of the alluaudite family of insertion compounds for sodium ion batteries. J. Mater. Chem. A 2015, 3, 18564–18571. [Google Scholar] [CrossRef]
- Wong, L.L.; Chen, H.M.; Adams, S. Sodium-ion diffusion mechanisms in the low cost high voltage cathode material Na2+δ Fe2−δ/2(SO4)3. Phys. Chem. Chem. Phys. 2015, 17, 9186–9193. [Google Scholar] [CrossRef]
- Meng, Y.; Yu, T.; Zhang, S.; Deng, C. Top-down synthesis of muscle-inspired alluaudite Na2+2x Fe2−x (SO4)3/SWNT spindle as a high-rate and high-potential cathode for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 1624–1631. [Google Scholar] [CrossRef]
- Lu, J.; Nishimura, S.; Yamada, A. Polyanionic solid-solution cathodes for rechargeable batteries. Chem. Mater. 2017, 29, 3597–3602. [Google Scholar] [CrossRef]
- Essehli, R.; Bali, B.E.; Benmokhtar, S.; Bouziane, K.; Manoun, B.; Abdalslam, M.A.; Ehrenberg, H. Crystal structures and magnetic properties of iron (III)-based phosphates: Na4NiFe(PO4)3 and Na2Ni2Fe(PO4)3. J. Alloys Compd. 2011, 509, 1163–1171. [Google Scholar] [CrossRef]
- Harbaoui, D.; Sanad, M.M.S.; Rossignol, C.; Hlil, E.K.; Amdouni, N.; Obbade, S. Synthesis and structural, electrical, and magnetic properties of new Iron–Aluminum alluaudite phases β-Na2Ni2M(PO4)3 (M = Fe and Al). Inorg. Chem. 2017, 56, 13051–13061. [Google Scholar] [CrossRef]
- Ouaatta, S.; Assani, A.; Saadi, M.; El Ammari, L. Crystal structure of calcium dinickel(II) iron(III) tris(orthophosphate): CaNi2Fe(PO4)3. Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 893–895. [Google Scholar] [CrossRef]
- Ben Smail, R.; Jouini, T. AgNi3(PO4)(HPO4)2: An alluaudite-like structure. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2002, 58, i61–i62. [Google Scholar] [CrossRef]
- Dwibedi, D.; Barpanda, P. Solution-assisted Energy-savvy Synthesis of High-voltage Na2M2(SO4)3 (M = 3d metals) alluaudite family of sodium insertion materials. Mater. Today Proc. 2018, 5, 23439–23442. [Google Scholar] [CrossRef]
- Marinova, D.M.; Kukeva, R.R.; Zhecheva, E.N.; Stoyanova, R.K. Selective sodium intercalation into sodium nickel–manganese sulfate for dual Na–Li-ion batteries. Phys. Chem. Chem. Phys. 2018, 20, 12755–12766. [Google Scholar] [CrossRef]
- Driscoll, L.L.; Kendrick, E.; Knight, K.S.; Wright, A.J.; Slater, P.R. Investigation into the dehydration of selenate doped Na2M(SO4)2·2H2O (M = Mn, Fe, Co and Ni): Stabilisation of the high Na content alluaudite phases Na3M1.5(SO4)3-1.5x(SeO4)1.5x (M = Mn, Co and Ni) through selenate incorporation. J. Solid State Chem. 2018, 258, 64–71. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Mackley, S.A.; Deyneko, D.V.; Taroev, V.K.; Tauson, V.L.; Rastsvetaeva, R.K.; Burns, P.C. Crystal chemistry of compounds with lanthanide based microporous heteropolyhedral frameworks: Synthesis, crystal structures, and luminescence properties of novel potassium cerium and erbium silicates. Microporous Mesoporous Mater. 2019, 284, 25–35. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Chukanov, N.V.; Pekov, I.V.; Rastsvetaeva, R.K.; Hixon, A.E. Crystal structure and topological features of manganonaujakasite, a mineral with microporous heteropolyhedral framework related to AlPO-25 (ATV). Microporous Mesoporous Mater. 2019, 279, 128–132. [Google Scholar] [CrossRef]
- Dal Bo, F.; Aksenov, S.M.; Burns, P.C. A novel family of microporous uranyl germanates: Framework topology and complexity of the crystal structures. J. Solid State Chem. 2019, 271, 126–134. [Google Scholar] [CrossRef]
- Chong, S.; Aksenov, S.M.; Dal Bo, F.; Perry, S.N.; Dimakopoulou, F.; Burns, P.C. Framework polymorphism and modular crystal structures of uranyl vanadates of divalent cations: Synthesis and characterization of M(UO2)(V2O7) (M = Ca, Sr) and Sr3(UO2)(V2O7)2. Z. Anorg. Allg. Chem. 2019, 645, 981–987. [Google Scholar] [CrossRef]
- Zhang, L.; Aksenov, S.M.; Kokot, A.M.; Perry, S.N.; Olds, T.A.; Burns, P.C. Crystal chemistry and structural complexity of Uranium(IV) sulfates: Synthesis of U3H2(SO4)7(H2O)5·3H2O and U3(UO2)0.2(SO4)6(OH)0.4·2.3H2O with Framework structures by the photochemical reduction of uranyl. Inorg. Chem. 2020, 59, 5813–5817. [Google Scholar] [CrossRef] [PubMed]
- Oxford Diffraction. CrysAlisPro; Oxford Diffraction Ltd.: Abingdon, UK, 2009. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Krist. 2014, 229, 345–352. [Google Scholar]
- Prince, E. (Ed.) International Tables for Crystallography; International Union of Crystallography: Chester, UK, 2006; Volume C, ISBN 978-1-4020-1900-5. [Google Scholar]
- Brandenburg, K.; Putz, H. DIAMOND, Version 3; Crystal Impact GbR: Bonn, Germany, 2005. [Google Scholar]
- Hawthorne, F.C.; Ungaretti, L.; Oberti, R. Site populations in minerals; terminology and presentation of results of crystal-structure refinement. Can. Mineral. 1995, 33, 907–911. [Google Scholar]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Blatov, V.A.; O’Keeffe, M.; Proserpio, D.M. Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: Recommended terminology. CrystEngComm 2010, 12, 44–48. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Mineral. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Eddahby, L.; Berrada, A.; Boukhari, A.; Holt, E.M. Potassium cadmium orthovanadate and potassium tetracadmium trisorthovanadate. Eur. J. Inorg. Chem. 1997, 34, 527–551. [Google Scholar]
- Libowitzky, E. Korrelation von O*H-Streckfrequenzen und O*H{ctdot};O-Wasserstoffbrückenlängenin Mineralen. Mon. Chem. Chem. Mon. 1999, 130, 1047. [Google Scholar] [CrossRef]
- Leroux, F.; Mar, A.; Payen, C.; Guyomard, D.; Verbaere, A.; Piffard, Y. Synthesis and Structure of NaMn3(PO4)(HPO4)2, an unoxidized variant of the alluaudite structure type. J. Solid State Chem. 1995, 115, 240–246. [Google Scholar] [CrossRef]
- Leroux, F.; Mar, A.; Guyomard, D.; Piffard, Y. Cation substitution in the alluaudite structure type: Synthesis and structure of AgMn3(PO4)(HPO4)2. J. Solid State Chem. 1995, 117, 206–212. [Google Scholar] [CrossRef]
- Cooper, M.A.; Hawthorne, F.C.; Ball, N.A.; Ramik, R.A.; Roberts, A.C. Groatite, Na Ca Mn2+2(PO4) [PO3(OH)]2, a new mineral species of the alluaudite group from the tanco pegmatite, bernic lake, manitoba, Canada: Description and crystal structure. Can. Mineral. 2009, 47, 1225–1235. [Google Scholar] [CrossRef]
- Assani, A.; El Ammari, L.; Zriouil, M.; Saadi, M. Disilver(I) trinickel(II) hydrogenphosphate bis(phosphate), Ag2Ni3(HPO4)(PO4)2. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, i40. [Google Scholar] [CrossRef]
- Ould Saleck, A.; Assani, A.; Saadi, M.; Mercier, C.; Follet, C.; El Ammari, L. Crystal structure of alluaudite-type NaMg3(HPO4)2(PO4). Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, 813–815. [Google Scholar] [CrossRef]
- Stojanović, J.; Đorđević, T.; Karanović, L. Structural features of two novel alluaudite-like arsenates Cd1.16Zn2.34(AsO4)1.5(HAsO4)(H2AsO4)0.5 and Cd0.74Mg2.76(AsO4)1.5(HAsO4)(H2AsO4)0.5. J. Alloys Compd. 2012, 520, 180–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Voronkov, A.A.; Ilyukhin, V.V.; Belov, N.V. Crystal chemistry of mixed frameworks—Principles of their formation. Kristallografiya 1975, 20, 556–566. [Google Scholar]
- Sandomirskiy, P.A.; Belov, N.V. Crystal Chemistry of Mixed Anionic Radicals; Nauka: Moscow, Russia, 1984. [Google Scholar]
- Ilyushin, G.D.; Blatov, V.A. Crystal chemistry of zirconosilicates and their analogs: Topological classification of MT frameworks and suprapolyhedral invariants. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 198–218. [Google Scholar] [CrossRef] [PubMed]
- Keller, P.; Hess, H.; Dunn, P.J. Johillerit, Na(Mg,Zn)3Cu(AsO4)3 ein neues mineral aus tsumeb, namibia. Tschermaks Mineral. Petrogr. Mitt. 1982, 29, 169–175. [Google Scholar] [CrossRef]
- Koshlyakova, N.N.; Zubkova, N.V.; Pekov, I.V.; Giester, G.; Sidorov, E.G. Crystal chemistry of johillerite. Can. Mineral. 2018, 56, 189–201. [Google Scholar] [CrossRef]
- Holt, E.; Drai, S.; Olazcuaga, R.; Vlasse, M. The crystal structure of cadmium potassium orthovanadate KCd4(VO4)3. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 95–98. [Google Scholar] [CrossRef]
- Malcherek, T.; Schlüter, J. The keyite crystal structure, revisited. Z. Krist. Cryst. Mater. 2013, 228. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).