Application of PAT-Based Feedback Control Approaches in Pharmaceutical Crystallization
Abstract
:1. Introduction
2. Solution Crystallization Process Control Approaches
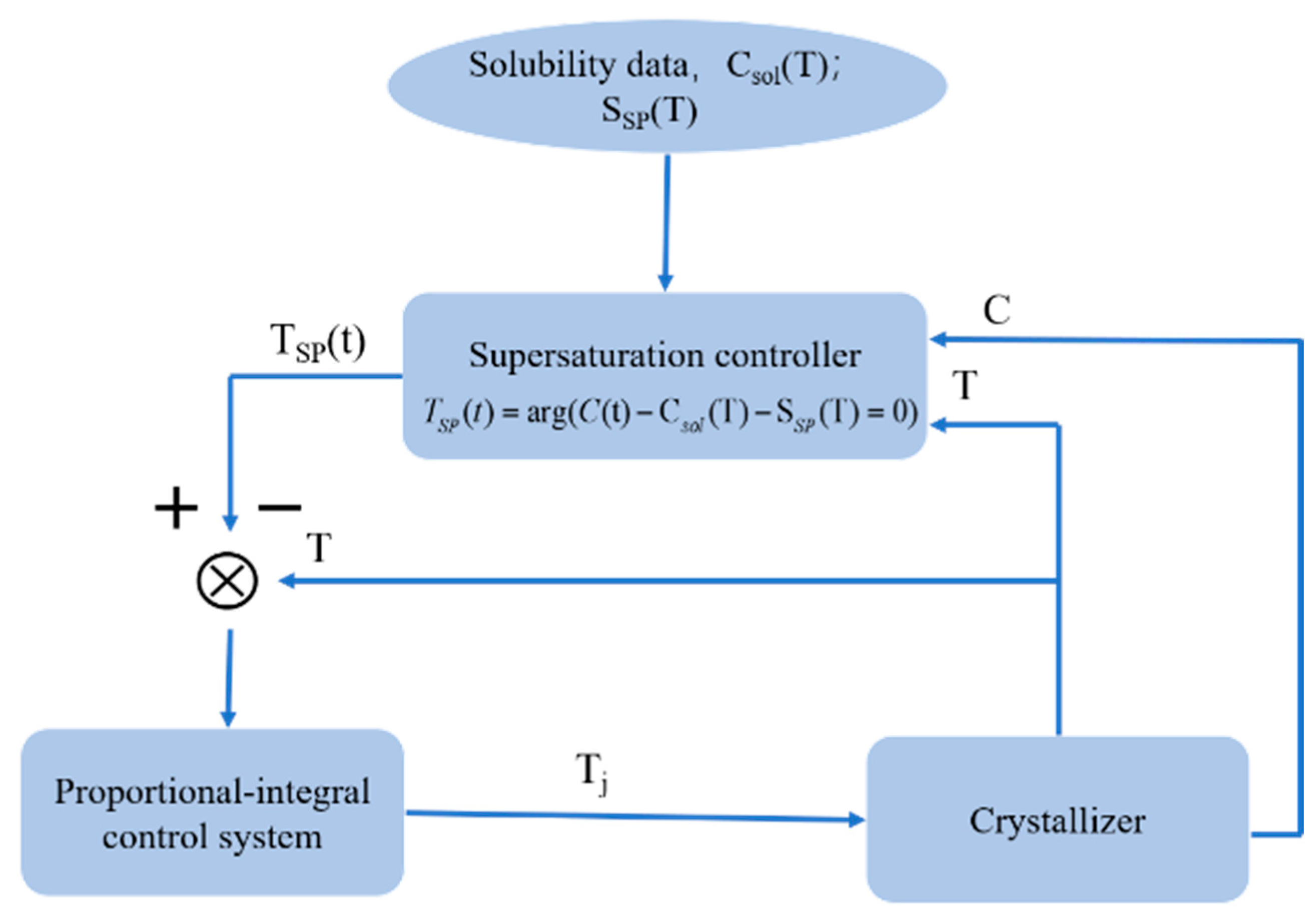
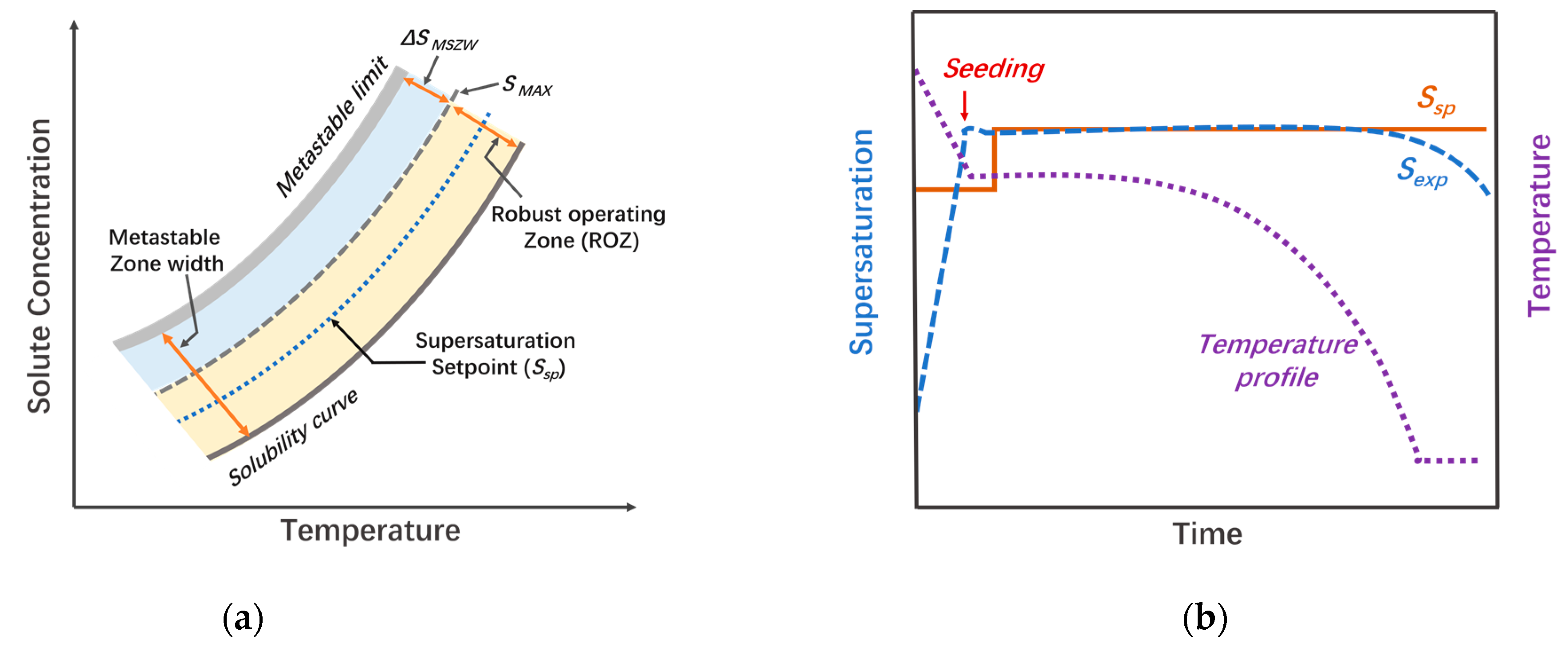
2.1. Supersaturation Control/Concentration Feedback Control
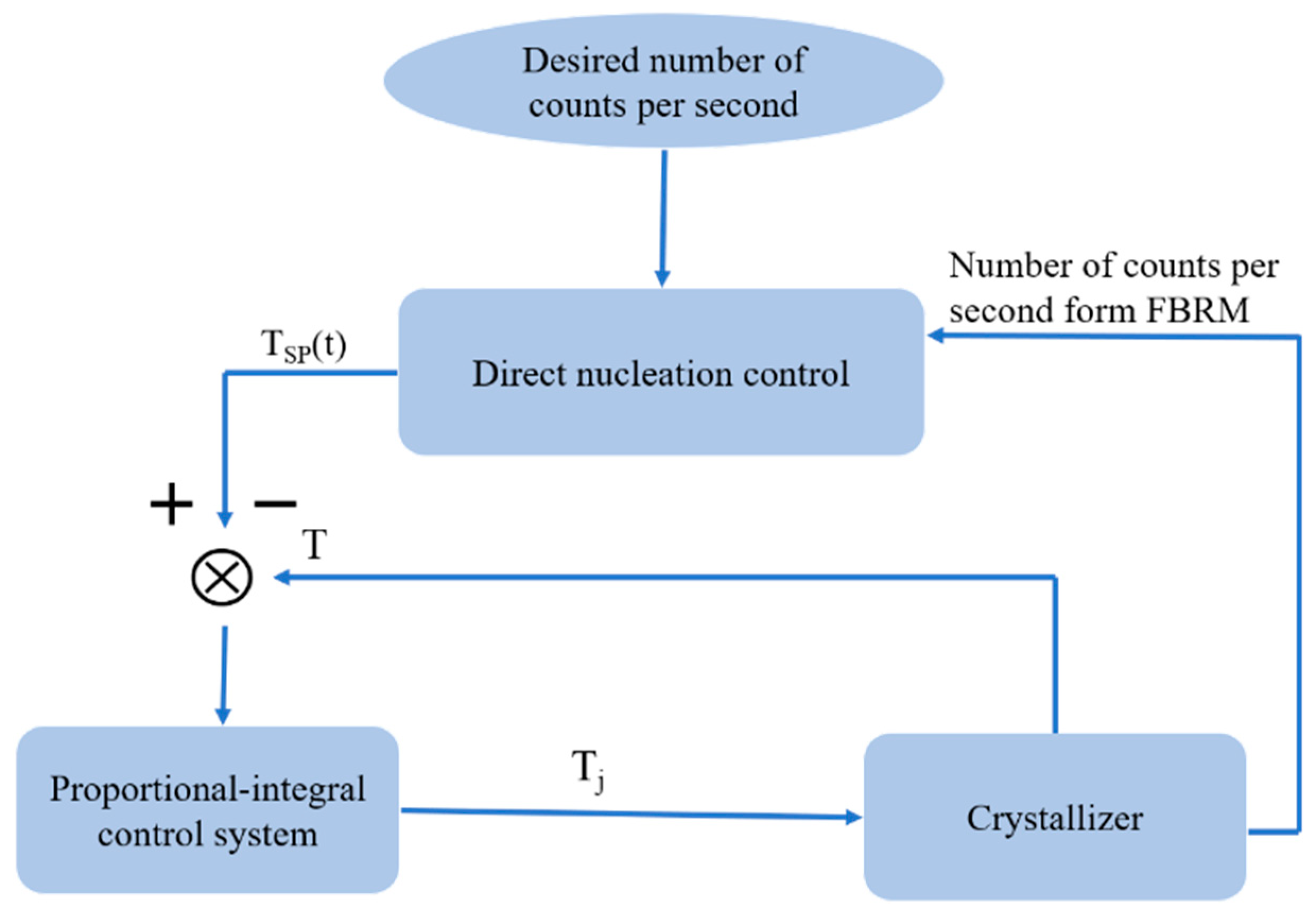
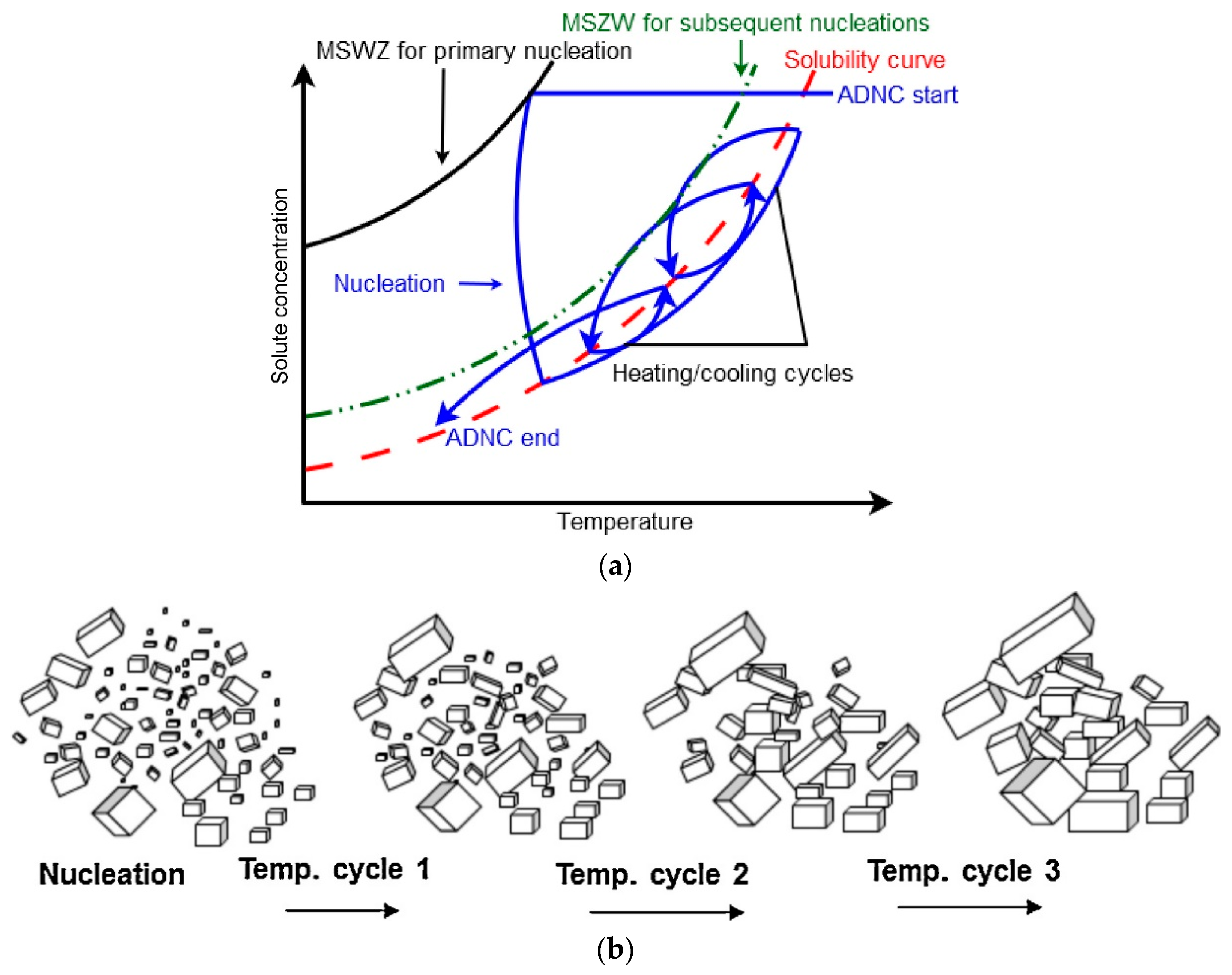
2.2. Direct Nucleation Control
2.3. Image Analysis Based Feedback Control
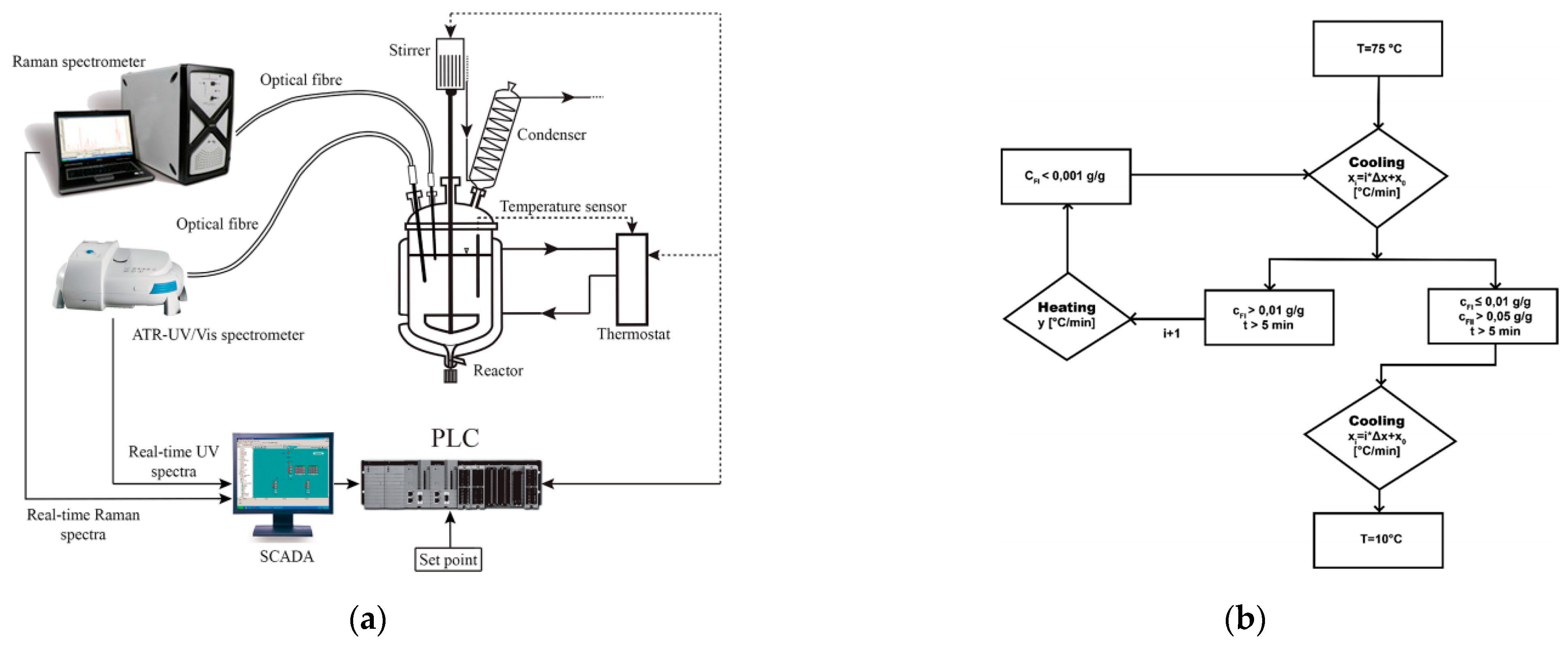
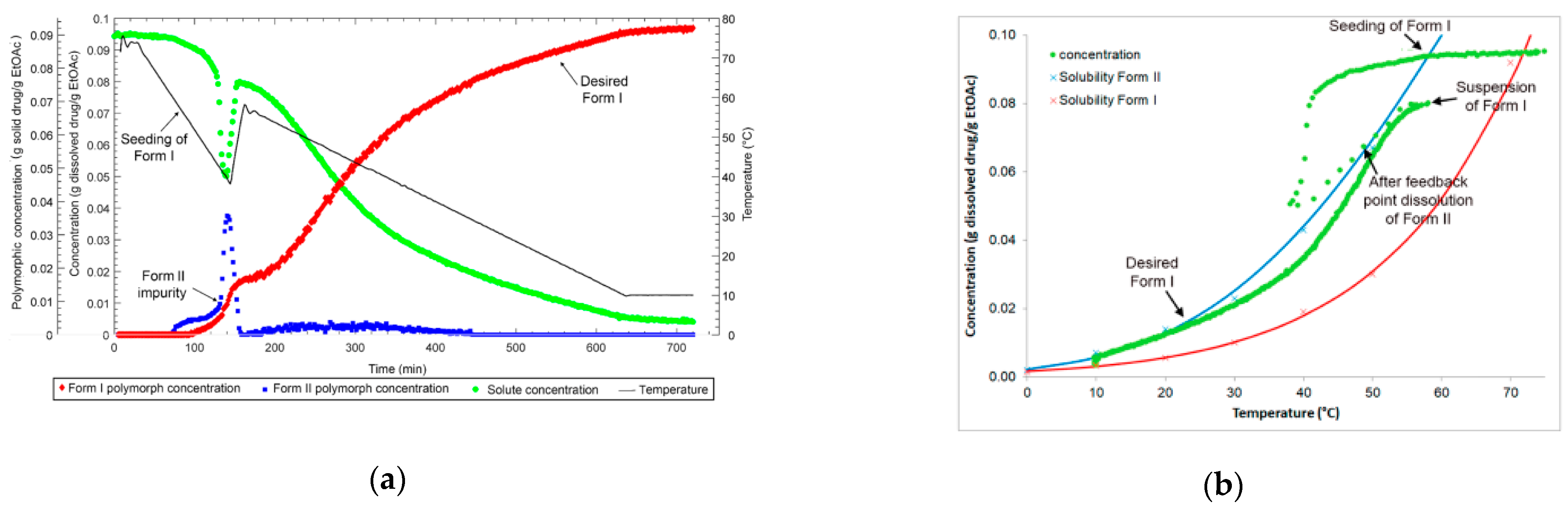
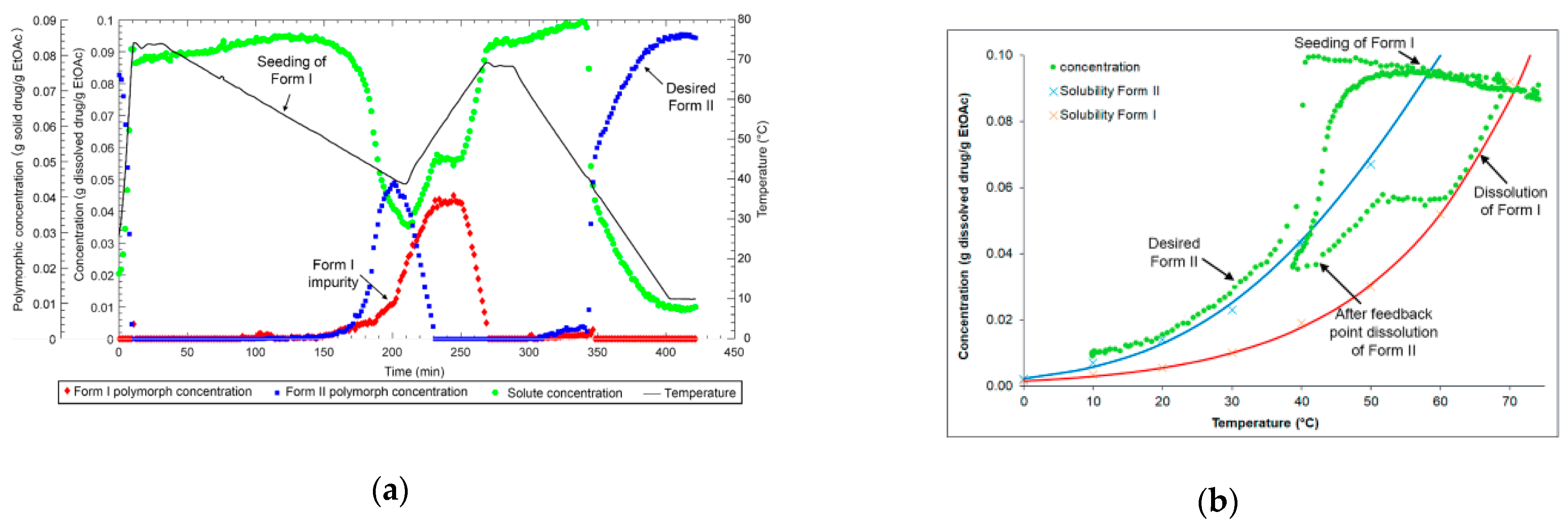
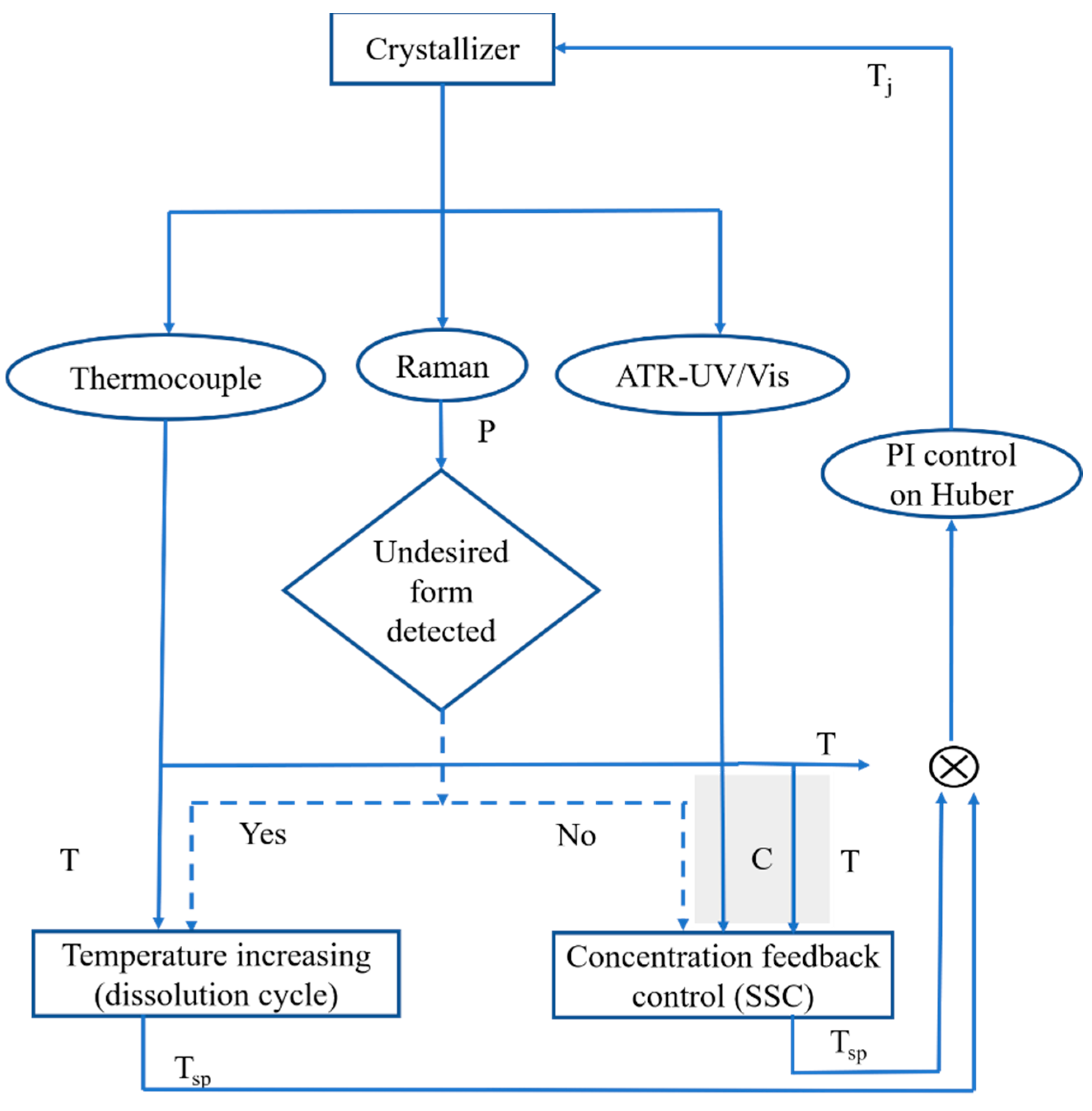
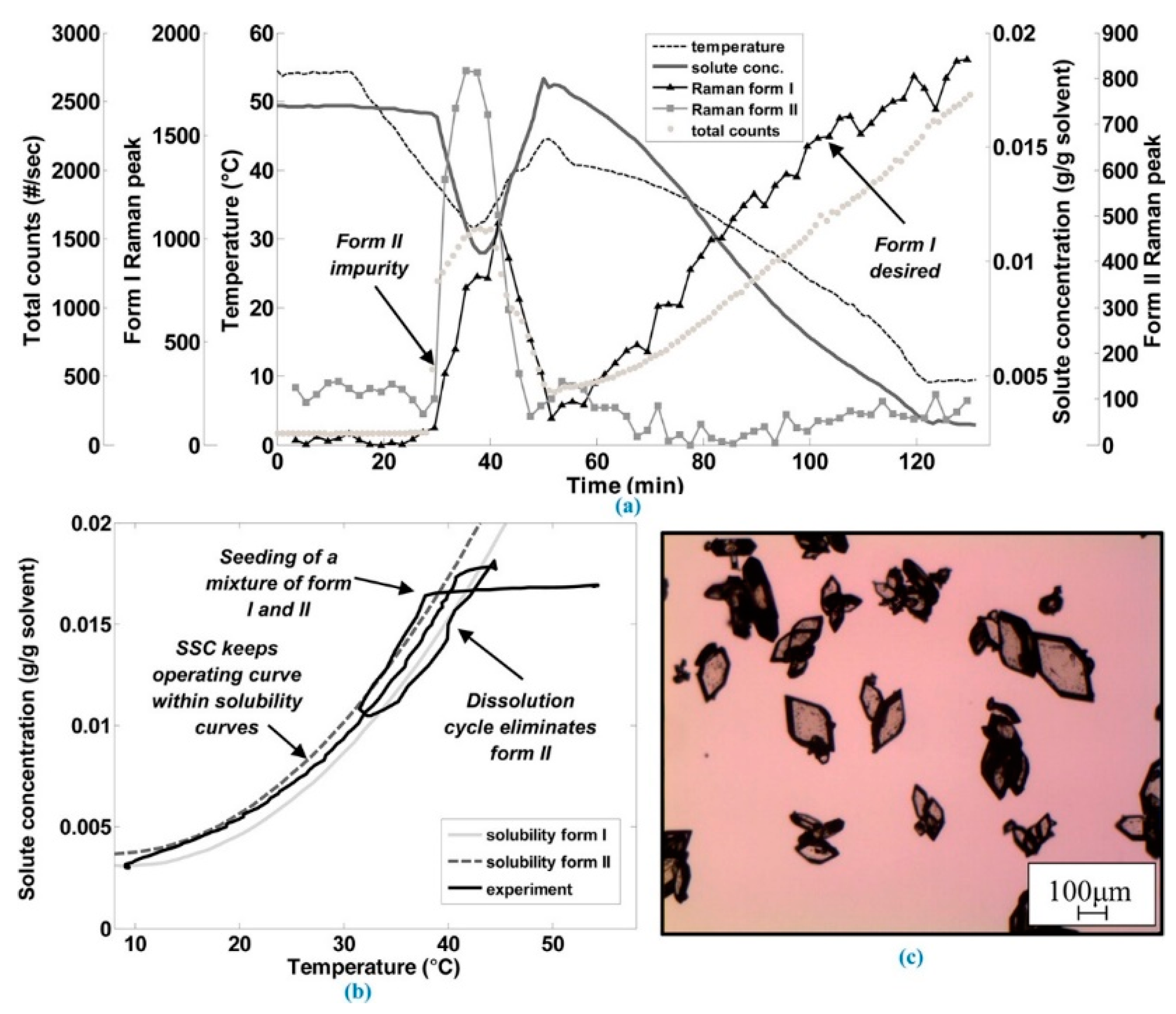
2.4. Polymorphic Feedback Control
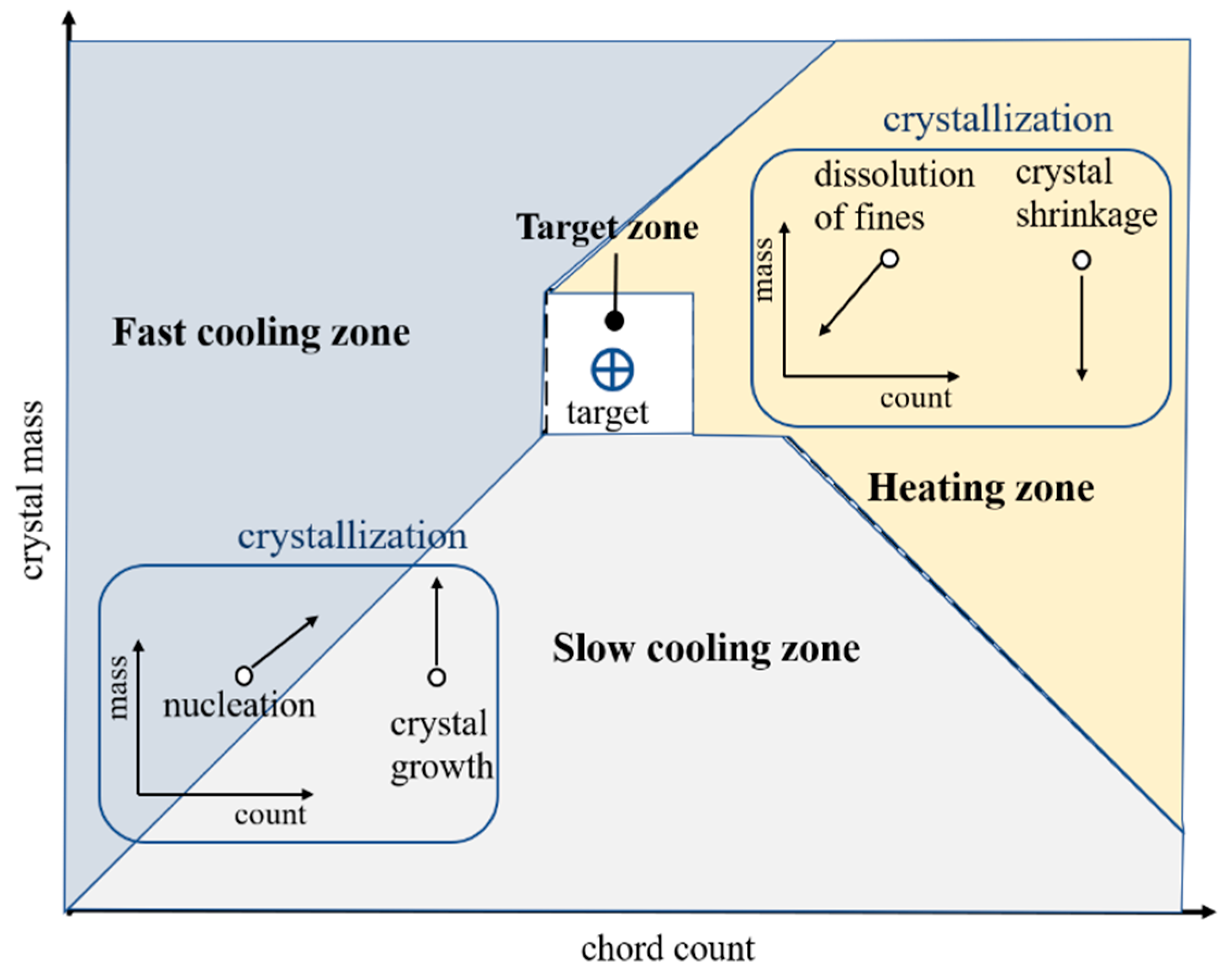
2.5. The Mass-Count Framework
3. Applications of Feedback Control in Crystallization
3.1. Polymorph Control
3.2. Purity Improvement
3.3. Crystal Morphology Control
3.4. Optimization of the Crystal Size Distribution
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAT | process analysis technology |
| CPPs | critical process parameters |
| QbD | quality-by-design |
| QbC | quality-by-control |
| ART-FTIR | Fourier transformed infrared spectroscopy |
| UV/Vis | ultraviolet-visible spectroscopy |
| PVM | particle vision and measurement |
| FBRM | focused beam reflectance measurement |
| PID | proportional-integral-derivative |
| ANN | artificial neural networks |
| RBF | radial basis function |
| SSC | supersaturation control |
| CFC | concentration feedback control |
| DNC | direct nucleation control |
| PCC | polymorph concentration control |
| IA-DNC | image analysis based direct nucleation control |
| MC | mass-count |
| APFC | active polymorphic feedback control |
| CSD | crystal size distribution |
| DIA | dynamic image analysis |
| AR | aspect ratio |
| OABA | o-aminobenzoic acid |
| ADNC | automated direct nucleation control |
| MSMPR | mixed-suspension mixed-product removal |
References
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent developments in the crystallization process: Toward the pharmaceutical industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Fevotte, G.; Kramer, H.; Simon, L.L. Recent advances in the monitoring, modelling and control of crystallization systems. Chem. Eng. Res. Des. 2013, 91, 1903–1922. [Google Scholar] [CrossRef]
- Braatz, R.D. Advanced control of crystallization processes. Annu. Rev. Control 2002, 26, 87–99. [Google Scholar] [CrossRef]
- Mangin, D.; Puel, F.; Veesler, S. Polymorphism in Processes of Crystallization in Solution: A Practical Review. Org. Process Res. Dev. 2009, 13, 1241–1253. [Google Scholar] [CrossRef]
- Kitamura, M.; Hara, T.; Takimoto-Kamimura, M. Solvent Effect on Polymorphism in Crystallization of BPT Propyl Ester. Cryst. Growth Des. 2006, 6, 1945–1950. [Google Scholar] [CrossRef]
- Chieng, N.; Rades, T.; Aaltonen, J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J. Pharm. Biomed. Anal. 2011, 55, 618–644. [Google Scholar] [CrossRef]
- Bernstein, J. Polymorphism—A perspective. Cryst. Growth Des. 2011, 11, 632–650. [Google Scholar] [CrossRef]
- Kadam, S.S.; Van Der Windt, E.; Daudey, P.J.; Kramer, H.J.M. A Comparative Study of ATR-FTIR and FT-NIR Spectroscopy for In-Situ Concentration Monitoring during Batch Cooling Crystallization Processes. Cryst. Growth Des. 2010, 10, 2629–2640. [Google Scholar] [CrossRef]
- Saleemi, A.; Rielly, C.; Nagy, Z.K. Automated direct nucleation control for in situ dynamic fines removal in batch cooling crystallization. CrystEngComm 2012, 14, 2196–2203. [Google Scholar] [CrossRef]
- Simone, E.; Saleemi, A.N.; Nagy, Z.K. In Situ Monitoring of Polymorphic Transformations Using a Composite Sensor Array of Raman, NIR, and ATR-UV/vis Spectroscopy, FBRM, and PVM for an Intelligent Decision Support System. Org. Process Res. Dev. 2015, 19, 167–177. [Google Scholar] [CrossRef]
- Zhou, G.; Moment, A.; Cuff, J.; Schafer, W.; Orella, C.; Sirota, E.; Gong, X.; Welch, C. Process Development and Control with Recent New FBRM, PVM, and IR. Org. Process Res. Dev. 2014, 19, 227–235. [Google Scholar] [CrossRef]
- Simone, E.; Saleemi, A.N.; Nagy, Z.K. Raman, UV, NIR, and Mid-IR Spectroscopy with Focused Beam Reflectance Measurement in Monitoring Polymorphic Transformations. Chem. Eng. Technol. 2014, 37, 1305–1313. [Google Scholar] [CrossRef]
- Simon, L.L.; Nagy, Z.K.; Hungerbuhler, K. Comparison of external bulk video imaging with focused beam reflectance measurement and ultra-violet visible spectroscopy for metastable zone identification in food and pharmaceutical crystallization processes. Chem. Eng. Sci. 2009, 64, 3344–3351. [Google Scholar] [CrossRef] [Green Version]
- Powell, K.A.; Croker, D.M.; Rielly, C.D.; Nagy, Z.K. Pat-based design of agrochemical co-crystallization processes: A case-study for the selective crystallization of 1:1 and 3:2 co-crystals of p-toluenesulfonamideitriphenylphosphine oxide. Chem. Eng. Sci. 2016, 152, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Peña, R.; Nagy, Z.K. Process Intensification through Continuous Spherical Crystallization Using a Two-Stage Mixed Suspension Mixed Product Removal (MSMPR) System. Cryst. Growth Des. 2015, 15, 4225–4236. [Google Scholar] [CrossRef]
- Liu, W.J.; Wei, H.Y.; Zhao, J.T.; Black, S.; Sun, C. Investigation into the cooling crystallization and transformations of carbamazepine using in situ fbrm and pvm. Org. Process Res. Dev. 2013, 17, 1406–1412. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Tu, Y.-R.; Ge, J.-L.; Sun, B.-W. Monitoring the Crystallization Process of Methylprednisolone Hemisuccinate (MPHS) from Ethanol Solution by Combined ATR-FTIR- FBRM- PVM. Sep. Sci. Technol. 2013, 48, 1881–1890. [Google Scholar] [CrossRef]
- Klapwijk, A.R.; Simone, E.; Nagy, Z.K.; Wilson, C.C. Tuning Crystal Morphology of Succinic Acid Using a Polymer Additive. Cryst. Growth Des. 2016, 16, 4349–4359. [Google Scholar] [CrossRef] [Green Version]
- Koswara, A.N.Z.K. On–off feedback control of plug-flow crystallization: A case of quality-by-control in continuous manufacturing. IEEE Life Sci. Let. 2017, 3, 1–4. [Google Scholar] [CrossRef]
- Simone, E.; Saleemi, A.; Nagy, Z. Application of quantitative Raman spectroscopy for the monitoring of polymorphic transformation in crystallization processes using a good calibration practice procedure. Chem. Eng. Res. Des. 2014, 92, 594–611. [Google Scholar] [CrossRef]
- Samad, N.A.F.A.; Sin, G.; Gernaey, K.V.; Gani, R. A systematic framework for design of process monitoring and control (PAT) systems for crystallization processes. Comput. Chem. Eng. 2013, 54, 8–23. [Google Scholar] [CrossRef]
- Damour, C.; Benne, M.; Grondin-Perez, B.; Chabriat, J.-P. Nonlinear predictive control based on artificial neural network model for industrial crystallization. J. Food Eng. 2010, 99, 225–231. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Braatz, R.D. Advances and New Directions in Crystallization Control. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 55–75. [Google Scholar] [CrossRef] [Green Version]
- Corriou, J.-P.; Rohani, S. A new look at optimal control of a batch crystallizer. AIChE J. 2008, 54, 3188–3206. [Google Scholar] [CrossRef]
- Lu, T.; Luo, F.; Mao, Z.; Wen, S. Parameters soft-sensing based on neural network in crystallizing process of cane sugar. In Proceedings of the 4th World Congress on Intelligent Control and Automation (Cat. No.02EX527), Shanghai, China, 10–14 June 2002. [Google Scholar] [CrossRef]
- Iciek, J.; Jezierski, A. Neural model of relative mass flows of water and sugar in an installation for crystallizing and centrifuging A massecuite. Sugar Ind. 2010, 506–514. [Google Scholar] [CrossRef]
- Pekalski, J.; Rzadkowski, W.; Panagiotopoulos, A.Z. Shear-induced ordering in systems with competing interactions: A ma-chine learning study. J. Chem. Phys. 2020, 152, 8. [Google Scholar] [CrossRef]
- Gomez-Peralta, J.I.; Bokhimi, X. Discovering new perovskites with artificial intelligence. J. Solid State Chem. 2020, 285, 6. [Google Scholar] [CrossRef]
- Braun, D.E.; Maas, S.G.; Zencirci, N.; Langes, C.; Urbanetz, N.A.; Griesser, U.J. Simultaneous quantitative analysis of ternary mixtures of d-mannitol polymorphs by FT-Raman spectroscopy and multivariate calibration models. Int. J. Pharm. 2010, 385, 29–36. [Google Scholar] [CrossRef]
- Szilagyi, B.; Nagy, Z.K. Aspect Ratio Distribution and Chord Length Distribution Driven Modeling of Crystallization of Two-Dimensional Crystals for Real-Time Model-Based Applications. Cryst. Growth Des. 2018, 18, 5311–5321. [Google Scholar] [CrossRef]
- Szilagyi, B.; Nagy, Z.K. Real-time feasible model-based crystal size and shape control of crystallization processes. Comput. Aided Chem. Eng. 2019, 46, 1273–1278. [Google Scholar] [CrossRef]
- Öner, M.; Montes, F.C.; Ståhlberg, T.; Stocks, S.M.; Bajtner, J.E.; Sin, G. Comprehensive evaluation of a data driven control strategy: Experimental application to a pharmaceutical crystallization process. Chem. Eng. Res. Des. 2020, 163, 248–261. [Google Scholar] [CrossRef]
- Smiljanic, R.; Lazic, D.; Gligoric, M.; Jotanovic, M.; Zivkovic, Z.; Mihajlovic, I. Modelling the process of Al(OH)3 crystallization from industrial sodium aluminate solutions using artificial neural networks. J. Serb. Chem. Soc. 2011, 76, 1163–1175. [Google Scholar] [CrossRef]
- Fujiwara, M.; Nagy, Z.K.; Chew, J.W.; Braatz, R.D. First-principles and direct design approaches for the control of phar-maceutical crystallization. J. Process Contr. 2005, 15, 493–504. [Google Scholar] [CrossRef]
- Saleemi, A.N.; Steele, G.; Pedge, N.I.; Freeman, A.; Nagy, Z.K. Enhancing crystalline properties of a cardiovascular active pharmaceutical ingredient using a process analytical technology based crystallization feedback control strategy. Int. J. Pharm. 2012, 430, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Tacsi, K.; Gyürkés, M.; Csontos, I.; Farkas, A.; Borbas, E.; Nagy, Z.K.; Marosi, G.; Pataki, H. Polymorphic Concentration Control for Crystallization Using Raman and Attenuated Total Reflectance Ultraviolet Visible Spectroscopy. Cryst. Growth Des. 2019, 20, 73–86. [Google Scholar] [CrossRef]
- Borsos, Á.; Szilágyi, B.; Agachi, P.Ş.; Nagy, Z.K. Real-Time Image Processing Based Online Feedback Control System for Cooling Batch Crystallization. Org. Process Res. Dev. 2017, 21, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Ostergaard, I.; Szilagyi, B.; De Diego, H.L.; Qu, H.; Nagy, Z.K. Polymorphic Control and Scale-Up Strategy for Antisolvent Crystallization Using a Sequential Supersaturation and Direct Nucleation Control Approach. Cryst. Growth Des. 2020, 20, 5538–5550. [Google Scholar] [CrossRef]
- Griffin, D.J.; Kawajiri, Y.; Rousseau, R.W.; Grover, M.A. Using MC plots for control of paracetamol crystallization. Chem. Eng. Sci. 2017, 164, 344–360. [Google Scholar] [CrossRef]
- Simone, E.; Saleemi, A.N.; Tonnon, N.; Nagy, Z.K. Active Polymorphic Feedback Control of Crystallization Processes Using a Combined Raman and ATR-UV/Vis Spectroscopy Approach. Cryst. Growth Des. 2014, 14, 1839–1850. [Google Scholar] [CrossRef]
- Gron, H.; Borissova, A.; Roberts, K.J. In-process atr-ftir spectroscopy for closed-loop supersaturation control of a batch crys-tallizer producing monosodium glutamate crystals of defined size. Ind. Eng. Chem. Res. 2003, 42, 198–206. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Chew, J.W.; Fujiwara, M.; Braatz, R.D. Comparative performance of concentration and temperature controlled batch crystallizations. J. Process Control 2008, 18, 399–407. [Google Scholar] [CrossRef]
- Zhou, G.X.; Fujiwara, M.; Woo, X.Y.; Rusli, E.; Tung, H.-H.; Starbuck, C.; Davidson, O.; Ge, Z.; Braatz, R.D. Direct Design of Pharmaceutical Antisolvent Crystallization through Concentration Control. Cryst. Growth Des. 2006, 6, 892–898. [Google Scholar] [CrossRef]
- Liotta, V.; Sabesan, V. Monitoring and Feedback Control of Supersaturation Using ATR-FTIR to Produce an Active Pharmaceutical Ingredient of a Desired Crystal Size. Org. Process Res. Dev. 2004, 8, 488–494. [Google Scholar] [CrossRef]
- Nonoyama, N.; Hanaki, K.; Yabuki, Y. Constant Supersaturation Control of Antisolvent-Addition Batch Crystallization. Org. Process Res. Dev. 2006, 10, 727–732. [Google Scholar] [CrossRef]
- Hermanto, M.W.; Braatz, R.D.; Chiu, M.-S. Integrated batch-to-batch and nonlinear model predictive control for polymorphic transformation in pharmaceutical crystallization. AIChE J. 2010, 57, 1008–1019. [Google Scholar] [CrossRef]
- Kalbasenka, A.N.; Spierings, L.C.P.; Huesman, A.E.M.; Kramer, H.J.M. Application of Seeding as a Process Actuator in a Model Predictive Control Framework for Fed-Batch Crystallization of Ammonium Sulphate. Part. Part. Syst. Charact. 2007, 24, 40–48. [Google Scholar] [CrossRef]
- Hatakka, H.; Alatalo, H.; Louhi-Kultanen, M.; Lassila, I.; Haeggström, E.; Hæggström, E. Closed-Loop Control of Reactive Crystallization PART II: Polymorphism Control of L-Glutamic Acid by Sonocrystallization and Seeding. Chem. Eng. Technol. 2010, 33, 751–756. [Google Scholar] [CrossRef]
- Alatalo, H.M.; Hatakka, H.; Louhi-Kultanen, M.; Kohonen, J.; Reinikainen, S.-P. Closed-Loop Control of Reactive Crystallization. Part I: Supersaturation-Controlled Crystallization of L-Glutamic Acid. Chem. Eng. Technol. 2010, 33, 743–750. [Google Scholar] [CrossRef]
- Ostergaard, I.; Szilagyi, B.; Nagy, Z.K.; De Diego, H.L.; Qu, H. Polymorphic Control and Scale-up Strategy for Crystallization from a Ternary Antisolvent System by Supersaturation Control. Cryst. Growth Des. 2019, 20, 1337–1346. [Google Scholar] [CrossRef]
- Ma, C.Y.; Wang, X.Z. Model identification of crystal facet growth kinetics in morphological population balance modeling of l-glutamic acid crystallization and experimental validation. Chem. Eng. Sci. 2012, 70, 22–30. [Google Scholar] [CrossRef]
- Saleemi, A.N.; Rielly, C.D.; Nagy, Z.K. Comparative Investigation of Supersaturation and Automated Direct Nucleation Control of Crystal Size Distributions using ATR-UV/vis Spectroscopy and FBRM. Cryst. Growth Des. 2012, 12, 1792–1807. [Google Scholar] [CrossRef]
- Simone, E.; Nagy, Z.K. A link between the ATR-UV/Vis and Raman spectra of zwitterionic solutions and the polymorphic outcome in cooling crystallization. CrystEngComm 2015, 17, 6538–6547. [Google Scholar] [CrossRef]
- Simone, E.; Zhang, W.; Nagy, Z.K. Application of Process Analytical Technology-Based Feedback Control Strategies To Improve Purity and Size Distribution in Biopharmaceutical Crystallization. Cryst. Growth Des. 2015, 15, 2908–2919. [Google Scholar] [CrossRef]
- Kee, N.C.S.; Arendt, P.D.; Tan, R.B.H.; Braatz, R.D. Selective crystallization of the metastable anhydrate form in the en-antiotropic pseudo-dimorph system of l-phenylalanine using concentration feedback control. Cryst. Growth Des. 2009, 9, 3052–3061. [Google Scholar] [CrossRef]
- Kee, N.C.S.; Tan, R.B.H.; Braatz, R.D. Selective crystallization of the metastable alpha-form of l-glutamic acid using con-centration feedback control. Cryst. Growth Des. 2009, 9, 3044–3051. [Google Scholar] [CrossRef]
- Zhou, G.; Moment, A.; Yaung, S.; Cote, A.; Hu, T.-E. Evolution and Application of an Automated Platform for the Development of Crystallization Processes. Org. Process Res. Dev. 2013, 17, 1320–1329. [Google Scholar] [CrossRef]
- Khan, S.; Ma, C.Y.; Mahmud, T.; Penchev, R.Y.; Roberts, K.J.; Morris, J.; Oezkan, L.; White, G.; Grieve, B.; Hall, A.; et al. In-process monitoring and control of supersaturation in seeded batch cooling crystallisation of l-glutamic acid: From laboratory to industrial pilot plant. Org. Process Res. Dev. 2011, 15, 540–555. [Google Scholar] [CrossRef]
- Kleinert, T.; Weickgenannt, M.; Judat, B.; Hagenmeyer, V. Cascaded two-degree-of-freedom control of seeded batch crystsallisations based on explicit system inversion. J. Process Control 2010, 20, 29–44. [Google Scholar] [CrossRef]
- Chen, Z.; Lovett, D.; Morris, J. Process analytical technologies and real time process control a review of some spectroscopic issues and challenges. J. Process Control 2011, 21, 1467–1482. [Google Scholar] [CrossRef]
- Duffy, D.; Barrett, M.; Glennon, B. Novel, Calibration-Free Strategies for Supersaturation Control in Antisolvent Crystallization Processes. Cryst. Growth Des. 2013, 13, 3321–3332. [Google Scholar] [CrossRef]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.-T.C.; Cornevin, J.; Ferguson, S.; Li, N.; Trout, B.L.; Myerson, A.S. Control of Polymorphism in Continuous Crystallization via Mixed Suspension Mixed Product Removal Systems Cascade Design. Cryst. Growth Des. 2015, 15, 3374–3382. [Google Scholar] [CrossRef]
- Abu Bakar, M.R.; Nagy, Z.K.; Saleemi, A.N.; Rielly, C.D. The Impact of Direct Nucleation Control on Crystal Size Distribution in Pharmaceutical Crystallization Processes. Cryst. Growth Des. 2009, 9, 1378–1384. [Google Scholar] [CrossRef]
- Simon, L.L.; Pataki, H.; Marosi, G.; Meemken, F.; Hungerbühler, K.; Baiker, A.; Tummala, S.; Glennon, B.; Kuentz, M.; Steele, G.; et al. Assessment of Recent Process Analytical Technology (PAT) Trends: A Multiauthor Review. Org. Process Res. Dev. 2015, 19, 3–62. [Google Scholar] [CrossRef]
- Woo, X.Y.; Nagy, Z.K.; Tan, R.B.H.; Braatz, R.D. Adaptive Concentration Control of Cooling and Antisolvent Crystallization with Laser Backscattering Measurement. Cryst. Growth Des. 2009, 9, 182–191. [Google Scholar] [CrossRef]
- Doki, N.; Seki, H.; Takano, K.; Asatani, H.; Yokota, A.M.; Kubota, N. Process Control of Seeded Batch Cooling Crystallization of the Metastable α-Form Glycine Using an In-Situ ATR-FTIR Spectrometer and an In-Situ FBRM Particle Counter. Cryst. Growth Des. 2004, 4, 949–953. [Google Scholar] [CrossRef]
- Abu Bakar, M.R.; Nagy, Z.K.; Rielly, C.D. Investigation of the Effect of Temperature Cycling on Surface Features of Sulfathiazole Crystals during Seeded Batch Cooling Crystallization. Cryst. Growth Des. 2010, 10, 3892–3900. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.K.; Kim, H.S.; Koo, K.K. Application of internal seeding and temperature cycling for reduction of liquid inclusion in the crystallization of rdx. Org. Process Res. Dev. 2011, 15, 602–609. [Google Scholar] [CrossRef]
- Ostergaard, I.; Szilagyi, B.; De Diego, H.L.; Qu, H.; Nagy, Z.K. Polymorphic Control and Scale-Up Strategy for Antisolvent Crystallization Using Direct Nucleation Control. Cryst. Growth Des. 2020, 20, 2683–2697. [Google Scholar] [CrossRef]
- Mangal, H.; Kleinebudde, P. Experimental determination of residence time distribution in continuous dry granulation. Int. J. Pharm. 2017, 524, 91–100. [Google Scholar] [CrossRef]
- Meier, R.; Thommes, M.; Rasenack, N.; Moll, K.-P.; Krumme, M.; Kleinebudde, P. Granule size distributions after twin-screw granulation—Do not forget the feeding systems. Eur. J. Pharm. Biopharm. 2016, 106, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Madarász, L.; Köte, Á.; Gyürkés, M.; Farkas, A.; Hambalkó, B.; Pataki, H.; Fülöp, G.; Marosi, G.; Lengyel, L.; Casian, T.; et al. Videometric mass flow control: A new method for real-time measurement and feedback control of powder micro-feeding based on image analysis. Int. J. Pharm. 2020, 580, 119223. [Google Scholar] [CrossRef]
- Sayin, R.; Martínez-Marcos, L.; Osorio, J.G.; Cruise, P.; Jones, I.; Halbert, G.W.; Lamprou, D.A.; Litster, J.D. Investigation of an 11 mm diameter twin screw granulator: Screw element performance and in-line monitoring via image analysis. Int. J. Pharm. 2015, 496, 24–32. [Google Scholar] [CrossRef] [Green Version]
- D’Ausilio, A. Arduino: A low-cost multipurpose lab equipment. Behav. Res. Methods 2011, 44, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Bötschi, S.; Rajagopalan, A.K.; Morari, M.; Mazzotti, M. Feedback Control for the Size and Shape Evolution of Needle-like Crystals in Suspension. I. Concepts and Simulation Studies. Cryst. Growth Des. 2018, 18, 4470–4483. [Google Scholar] [CrossRef]
- Rajagopalan, A.K.; Bötschi, S.; Morari, M.; Mazzotti, M. Feedback Control for the Size and Shape Evolution of Needle-like Crystals in Suspension. II. Cooling Crystallization Experiments. Cryst. Growth Des. 2018, 18, 6185–6196. [Google Scholar] [CrossRef]
- Bötschi, S.; Rajagopalan, A.K.; Morari, M.; Mazzotti, M. Feedback Control for the Size and Shape Evolution of Needle-like Crystals in Suspension. IV. Modeling and Control of Dissolution. Cryst. Growth Des. 2019, 19, 4029–4043. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, A.K.; Bötschi, S.; Morari, M.; Mazzotti, M. Feedback Control for the Size and Shape Evolution of Needle-like Crystals in Suspension. III. Wet Milling. Cryst. Growth Des. 2019, 19, 2845–2861. [Google Scholar] [CrossRef]
- Simon, L.L.; Simone, E.; Oucherif, K.A. Crystallization process monitoring and control using process analytical technology. Comput. Aided Chem. Eng. 2018, 215–242. [Google Scholar] [CrossRef]
- Hirsch, E.; Pataki, H.; Farkas, A.; Bata, H.; Vass, P.; Fehér, C.; Barta, Z.; Párta, L.; Csontos, I.; Ballagi, A.; et al. Raman-Based Feedback Control of the Enzymatic Hydrolysis of Lactose. Org. Process Res. Dev. 2016, 20, 1721–1727. [Google Scholar] [CrossRef]
- Pataki, H.; Csontos, I.; Nagy, Z.K.; Vajna, B.; Molnar, M.; Katona, L.; Marosi, G. Implementation of Raman Signal Feedback to Perform Controlled Crystallization of Carvedilol. Org. Process Res. Dev. 2012, 17, 493–499. [Google Scholar] [CrossRef]
- Simone, E.; Szilagyi, B.; Nagy, Z. Systematic model identification and optimization-based active polymorphic control of crystallization processes. Chem. Eng. Sci. 2017, 174, 374–386. [Google Scholar] [CrossRef] [Green Version]
- Hulburt, H.M.; Katz, S. Some problems in particle technology: A statistical mechanical formulation. Chem. Eng. Sci. 1964, 19, 555–574. [Google Scholar] [CrossRef]
- Randolph, A.D. A population balance for countable entities. Can. J. Chem. Eng. 1964, 42, 280–281. [Google Scholar] [CrossRef]
- Griffin, D.J.; Grover, M.A.; Kawajiri, Y.; Rousseau, R.W. Mass–count plots for crystal size control. Chem. Eng. Sci. 2015, 137, 338–351. [Google Scholar] [CrossRef]
- Griffin, D.J.; Grover, M.A.; Kawajiri, Y.; Rousseau, R.W. Data-Driven Modeling and Dynamic Programming Applied to Batch Cooling Crystallization. Ind. Eng. Chem. Res. 2016, 55, 1361–1372. [Google Scholar] [CrossRef]
- Grover, M.A.; Griffin, D.J.; Tang, X.; Kim, Y.; Rousseau, R.W. Optimal feedback control of batch self-assembly processes using dynamic programming. J. Process Control 2020, 88, 32–42. [Google Scholar] [CrossRef]
- Grover, M.A.; Griffin, D.J.; Tang, X. Control of Self-Assembly with Dynamic Programming. IFAC-PapersOnLine 2019, 52, 1–9. [Google Scholar] [CrossRef]
- Kovaleva, E.L.; Bagirova, V.L.; Shanazarov, K.S. Developing methodological approaches to standardization of pharmaceutical substances. Pharm. Chem. J. 2010, 44, 33–39. [Google Scholar] [CrossRef]
- Olefir, Y.V.; Sakanyan, E.I.; Shishova, L.I.; Senchenko, S.P. Pharmacopoeial Quality Requirements for Medicinal Products. Pharm. Chem. J. 2019, 52, 936–941. [Google Scholar] [CrossRef]
- Wu, H.; Dong, Z.; Li, H.; Khan, M. An integrated process analytical technology (pat) approach for pharmaceutical crystallization process understanding to ensure product quality and safety: Fda scientist’s perspective. Org. Process Res. Dev. 2015, 19, 89–101. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef] [PubMed]
- Singhal, D.; Curatolo, W. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug. Deliver. Rev. 2004, 56, 335–347. [Google Scholar] [CrossRef]
- Coté, A.; Zhou, G.; Stanik, M. A Novel Crystallization Methodology To Ensure Isolation of the Most Stable Crystal Form. Org. Process Res. Dev. 2009, 13, 1276–1283. [Google Scholar] [CrossRef]
- Zhang, T.; Szilagyi, B.; Gong, J.; Nagy, Z.K. Thermodynamic polymorph selection in enantiotropic systems using supersaturation-controlled batch and semibatch cooling crystallization. Cryst. Growth Des. 2019, 19, 6715–6726. [Google Scholar] [CrossRef]
- Ma, L.-J.; Guo, L.-J. Study of the phase transformation of TiO2 with in-situ XRD in different gas. Guang pu xue yu Guang pu fen xi = Guang pu 2011, 31, 1133–1137. [Google Scholar] [PubMed]
- Heinrich, J.; Elter, T.; Ulrich, J. Data Preprocessing of In Situ Laser-Backscattering Measurements. Chem. Eng. Technol. 2011, 34, 977–984. [Google Scholar] [CrossRef]
- Prediger, A.; Bluma, A.; Hoepfner, T.; Lindner, P.; Beutel, S.; Mueller, J.J.; Hilterhaus, L.; Liese, A.; Scheper, T. In Situ mi-croscopy for online monitoring of enzyme supports and two-phase systems. Chem. Ing. Tech. 2011, 83, 884–887. [Google Scholar] [CrossRef]
- Howard, K.S.; Nagy, Z.K.; Saha, B.; Robertson, A.L.; Steele, G.; Martin, D. A Process Analytical Technology Based Investigation of the Polymorphic Transformations during the Antisolvent Crystallization of Sodium Benzoate from IPA/Water Mixture. Cryst. Growth Des. 2009, 9, 3964–3975. [Google Scholar] [CrossRef]
- Veintemillas-Verdaguer, S.J.P.I.C.G. Chemical aspects of the effect of impurities in crystal growth. Prog. Cryst. Growth Charact. Mater. 1996, 32, 75–109. [Google Scholar] [CrossRef]
- Darmali, C.; Mansouri, S.; Yazdanpanah, N.; Woo, M.W. Mechanisms and Control of Impurities in Continuous Crystallization: A Review. Ind. Eng. Chem. Res. 2018, 58, 1463–1479. [Google Scholar] [CrossRef]
- Nagy, Z.; Aamir, E. Systematic design of supersaturation controlled crystallization processes for shaping the crystal size distribution using an analytical estimator. Chem. Eng. Sci. 2012, 84, 656–670. [Google Scholar] [CrossRef]
- Krishnaiah, C.; Reddy, A.R.; Kumar, R.; Mukkanti, K. Stability-indicating UPLC method for determination of Valsartan and their degradation products in active pharmaceutical ingredient and pharmaceutical dosage forms. J. Pharm. Biomed. Anal. 2010, 53, 483–489. [Google Scholar] [CrossRef]
- Ali, N.W.; Abbas, S.S.; Zaazaa, H.E.-S.; Abdelrahman, M.M.; Abdelkawy, M. Validated stability indicating methods for determination of nitazoxanide in presence of its degradation products. J. Pharm. Anal. 2012, 2, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Borsos, A.; Majumder, A.; Nagy, Z.K. Multi-Impurity Adsorption Model for Modeling Crystal Purity and Shape Evolution during Crystallization Processes in Impure Media. Cryst. Growth Des. 2015, 16, 555–568. [Google Scholar] [CrossRef] [Green Version]
- Pal, K.; Yang, Y.; Nagy, Z.K. Model-based optimization of cooling crystallization of active pharmaceutical ingredients undergoing thermal degradation. Cryst. Growth Des. 2019, 19, 3417–3429. [Google Scholar] [CrossRef]
- Amrani, F.; Secrétan, P.-H.; Sadou-Yayé, H.; Aymes-Chodur, C.; Bernard, M.; Solgadi, A.; Yagoubi, N.; Do, B. Identification of dabigatran etexilate major degradation pathways by liquid chromatography coupled to multi stage high-resolution mass spectrometry. RSC Adv. 2015, 5, 45068–45081. [Google Scholar] [CrossRef]
- Zhang, T.; Szilágyi, B.; Gong, J.; Nagy, Z.K. Novel semibatch supersaturation control approach for the cooling crystallization of heat-sensitive materials. AIChE J. 2020, 66. [Google Scholar] [CrossRef]
- Lovette, M.A.; Browning, A.R.; Griffin, D.W.; Sizemore, J.P.; Snyder, R.C.; Doherty, M.F. Crystal Shape Engineering. Ind. Eng. Chem. Res. 2008, 47, 9812–9833. [Google Scholar] [CrossRef] [Green Version]
- Variankaval, N.; Cote, A.S.; Doherty, M.F. From form to function: Crystallization of active pharmaceutical ingredients. AIChE J. 2008, 54, 1682–1688. [Google Scholar] [CrossRef]
- Yang, Y.; Pal, K.; Koswara, A.; Sun, Q.; Zhang, Y.; Quon, J.; McKeown, R.; Goss, C.; Nagy, Z.K. Application of feedback control and in situ milling to improve particle size and shape in the crystallization of a slow growing needle-like active pharmaceutical ingredient. Int. J. Pharm. 2017, 533, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Nagy, Z.K. Prediction and control of crystal shape distribution in the presence of crystal growth modifiers. Chem. Eng. Sci. 2013, 101, 593–602. [Google Scholar] [CrossRef]
- Eisenschmidt, H.; Voigt, A.; Sundmacher, K. Face-Specific Growth and Dissolution Kinetics of Potassium Dihydrogen Phosphate Crystals from Batch Crystallization Experiments. Cryst. Growth Des. 2014, 15, 219–227. [Google Scholar] [CrossRef]
- Eisenschmidt, H.; Bajcinca, N.; Sundmacher, K. Optimal Control of Crystal Shapes in Batch Crystallization Experiments by Growth-Dissolution Cycles. Cryst. Growth Des. 2016, 16, 3297–3306. [Google Scholar] [CrossRef]
- Nagy, Z.K. Model based robust control approach for batch crystallization product design. Comput. Chem. Eng. 2009, 33, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Larsen, P.A.; Patience, D.B.; Rawlings, J.B. Industrial crystallization process control. IEEE Control. Syst. 2006, 26, 70–80. [Google Scholar] [CrossRef]
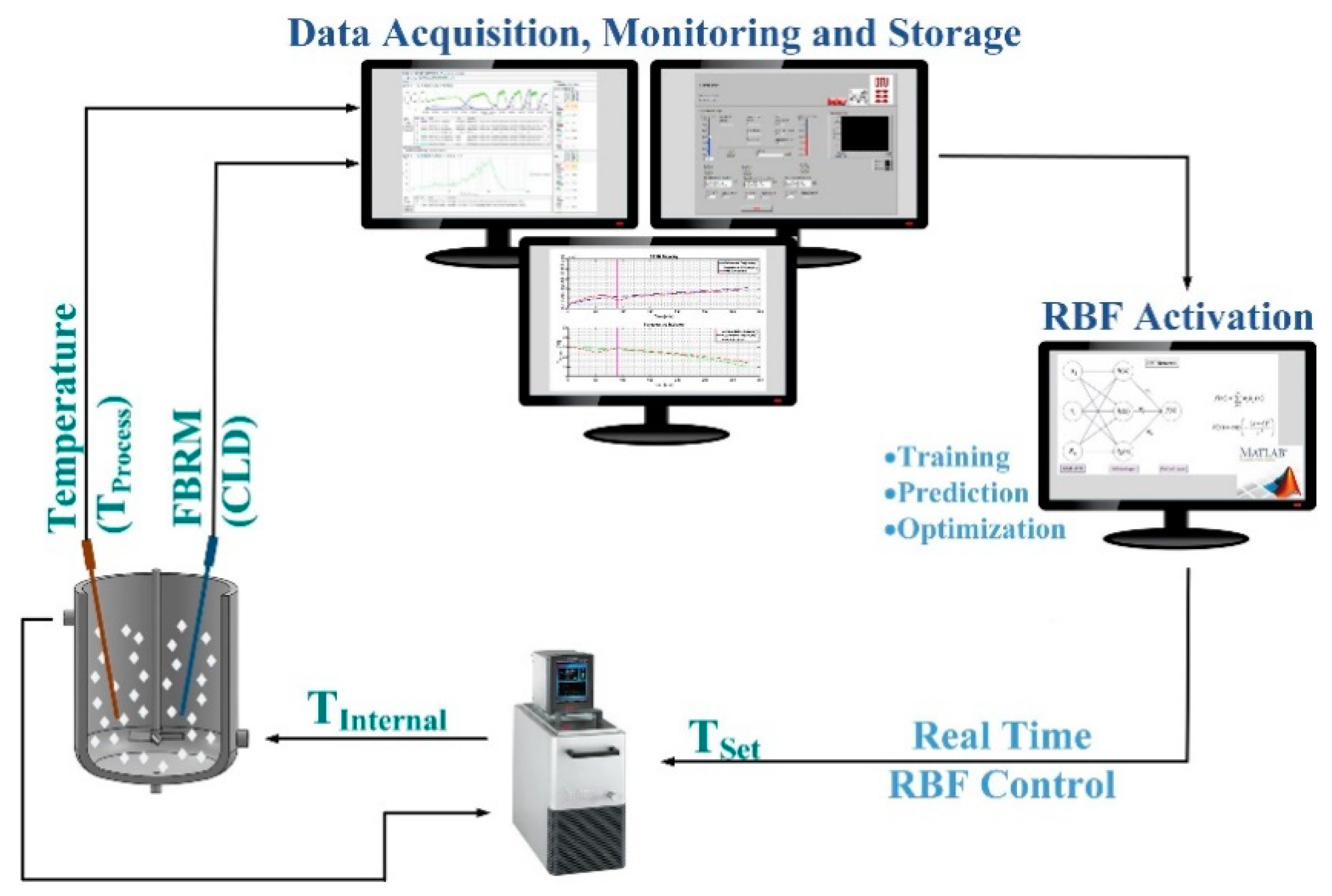


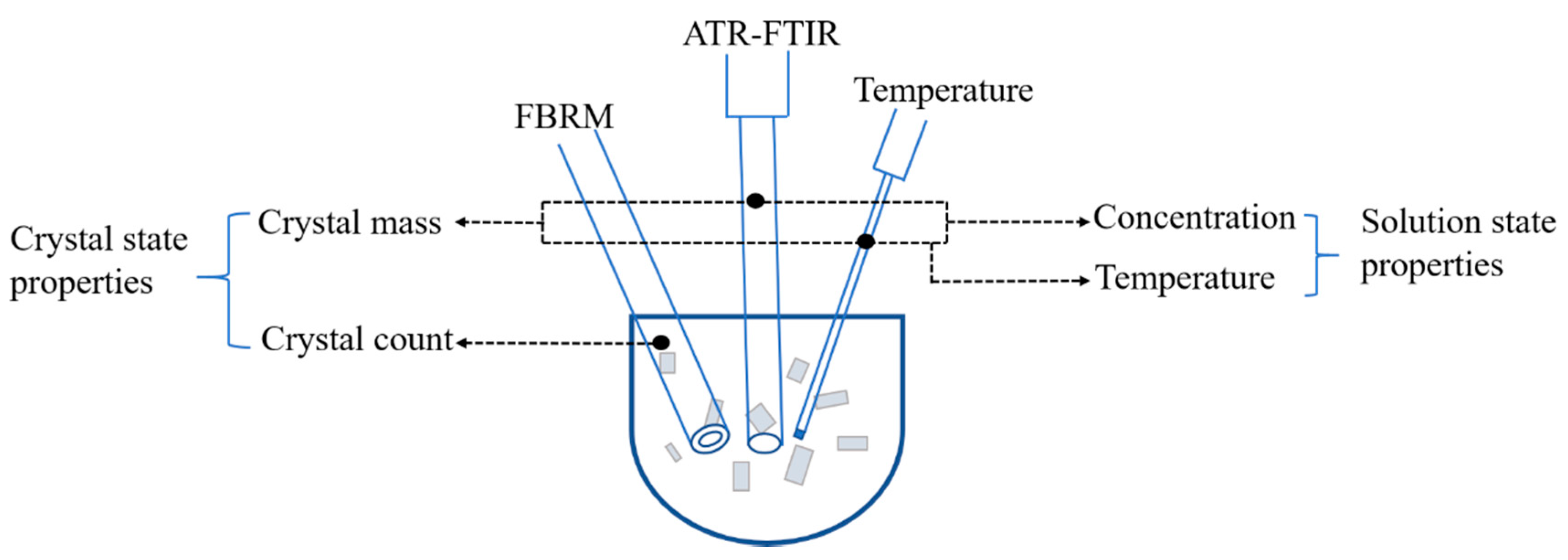

| PAT Tools | Working Principle | Application |
|---|---|---|
| FBRM | Receive the reflection signal generated by the laser beam through the particles, and calculate the time the laser beam passes through the particles to obtain the chord length distribution | Real-time monitoring of particle size and particle number changes as well as nucleation and growth processes [13,14,15] |
| PVM | The video microscope on the probe can intuitively display the particle status, continuously capture high-resolution images under various process conditions, without the need for sampling or offline analysis | Monitor nucleation, growth, coalescence, fragmentation, shape evolution, and polymorphic behavior of the crystallization process, endoscope probes can detect color-related changes [16,17,18] |
| ATR-FTIR | The crystal sample absorbs infrared light at the incident frequency, the reflected light intensity is reduced, and a spectrum similar to the transmission absorption is generated, thereby obtaining structural information of the chemical composition of the sample surface | Infrared measurement of solute concentration during crystallization [8] |
| ATR-UV/Vis | The ultraviolet-visible absorption spectrum is an electronic spectrum, which is produced by the transition of valence electrons. The composition, content, and structure of a substance can be analyzed, determined, and infer determined by using ultraviolet-visible absorption spectrum and the degree of absorption of ultraviolet-visible light by substance molecules or ions | In situ monitoring of nucleation [9], crystal form transformation and supersaturation changes [10] |
| Raman | Raman spectroscopy is a kind of scattering spectrum, which is analyzed with different incident light frequencies to obtain information on molecular vibration and rotation. Raman frequency offset is represented by the abscissa, and Raman intensity is represented by the ordinate, which is complementary to the infrared spectrum and available for analyzing information related to intermolecular bond energy | Identify differences in polymorphic forms and enable qualitative and quantitative studies on solvent-mediated transformation, solution concentration, and the ratio of different crystalline forms in solid mixtures [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, T.; Ma, Y.; Xue, F.; Gao, Z.; Hou, B.; Gong, J. Application of PAT-Based Feedback Control Approaches in Pharmaceutical Crystallization. Crystals 2021, 11, 221. https://doi.org/10.3390/cryst11030221
Gao Y, Zhang T, Ma Y, Xue F, Gao Z, Hou B, Gong J. Application of PAT-Based Feedback Control Approaches in Pharmaceutical Crystallization. Crystals. 2021; 11(3):221. https://doi.org/10.3390/cryst11030221
Chicago/Turabian StyleGao, Ye, Teng Zhang, Yiming Ma, Fumin Xue, Zhenguo Gao, Baohong Hou, and Junbo Gong. 2021. "Application of PAT-Based Feedback Control Approaches in Pharmaceutical Crystallization" Crystals 11, no. 3: 221. https://doi.org/10.3390/cryst11030221
APA StyleGao, Y., Zhang, T., Ma, Y., Xue, F., Gao, Z., Hou, B., & Gong, J. (2021). Application of PAT-Based Feedback Control Approaches in Pharmaceutical Crystallization. Crystals, 11(3), 221. https://doi.org/10.3390/cryst11030221









