Tailoring the Morphology of Supraparticles by Primary Colloids with Different Shapes, Sizes and Dispersities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Superamphiphobic Soot Surface Fabrication
2.3. Particle Fabrication
2.4. Supraparticle fabrication
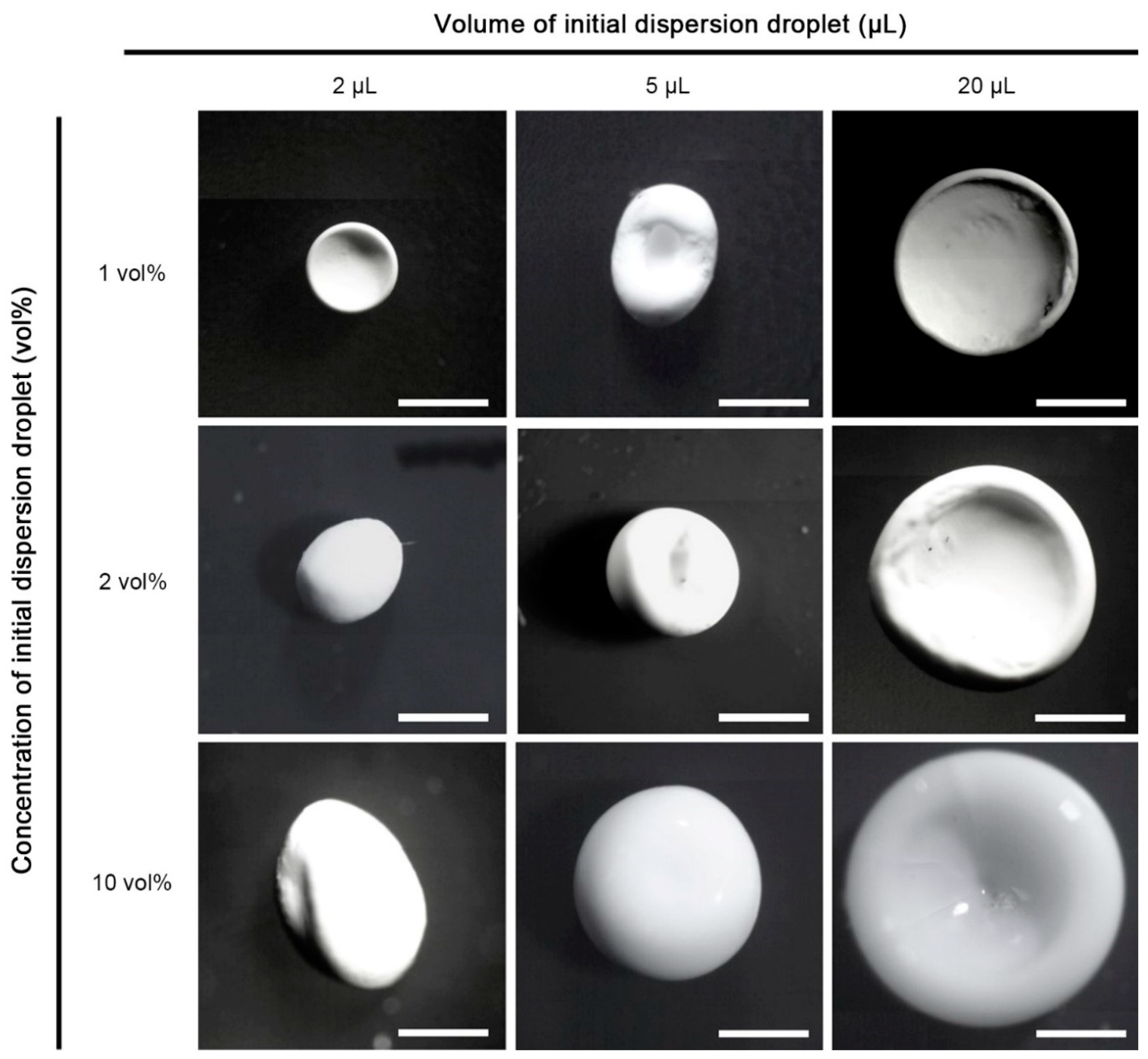
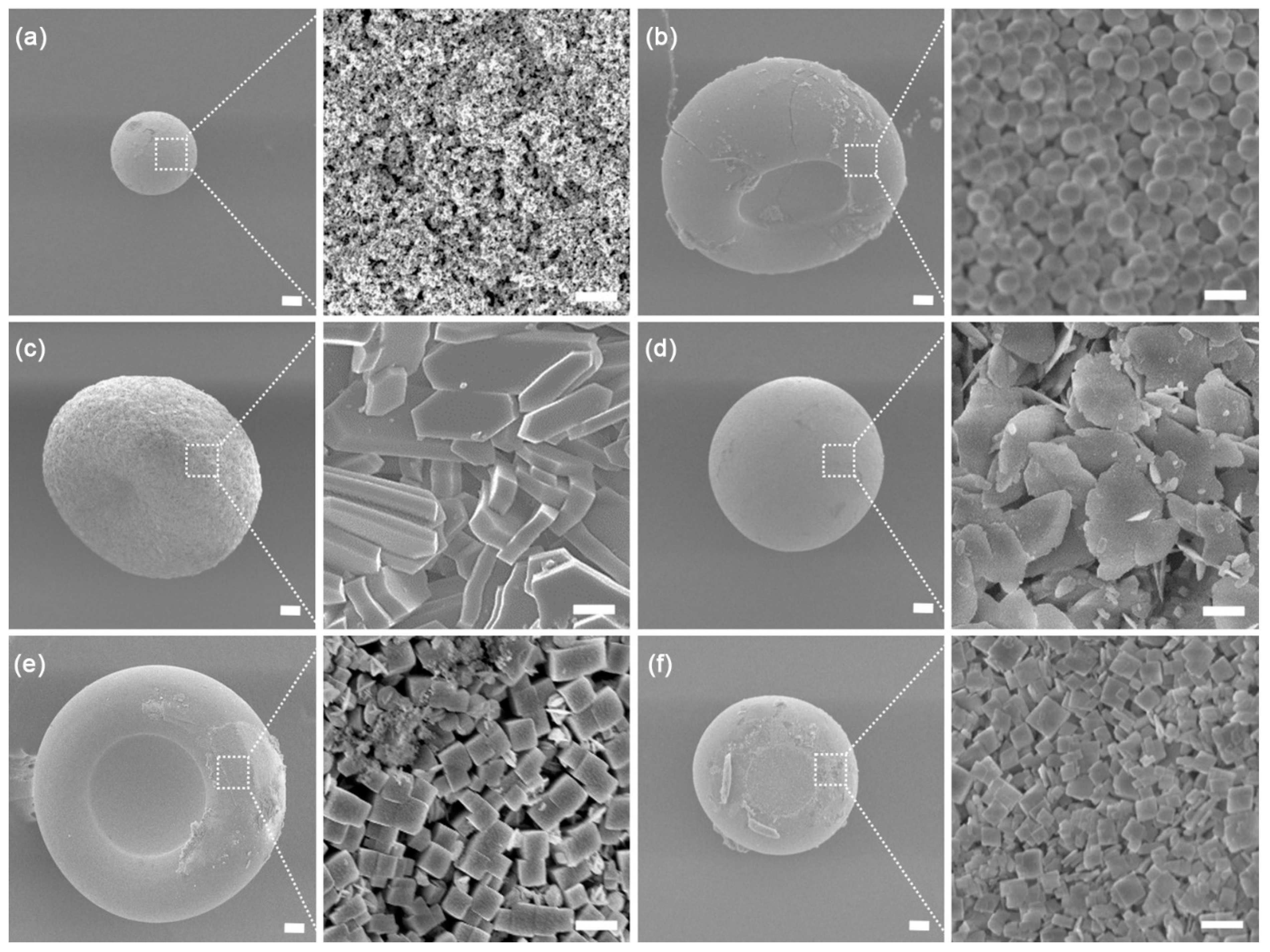
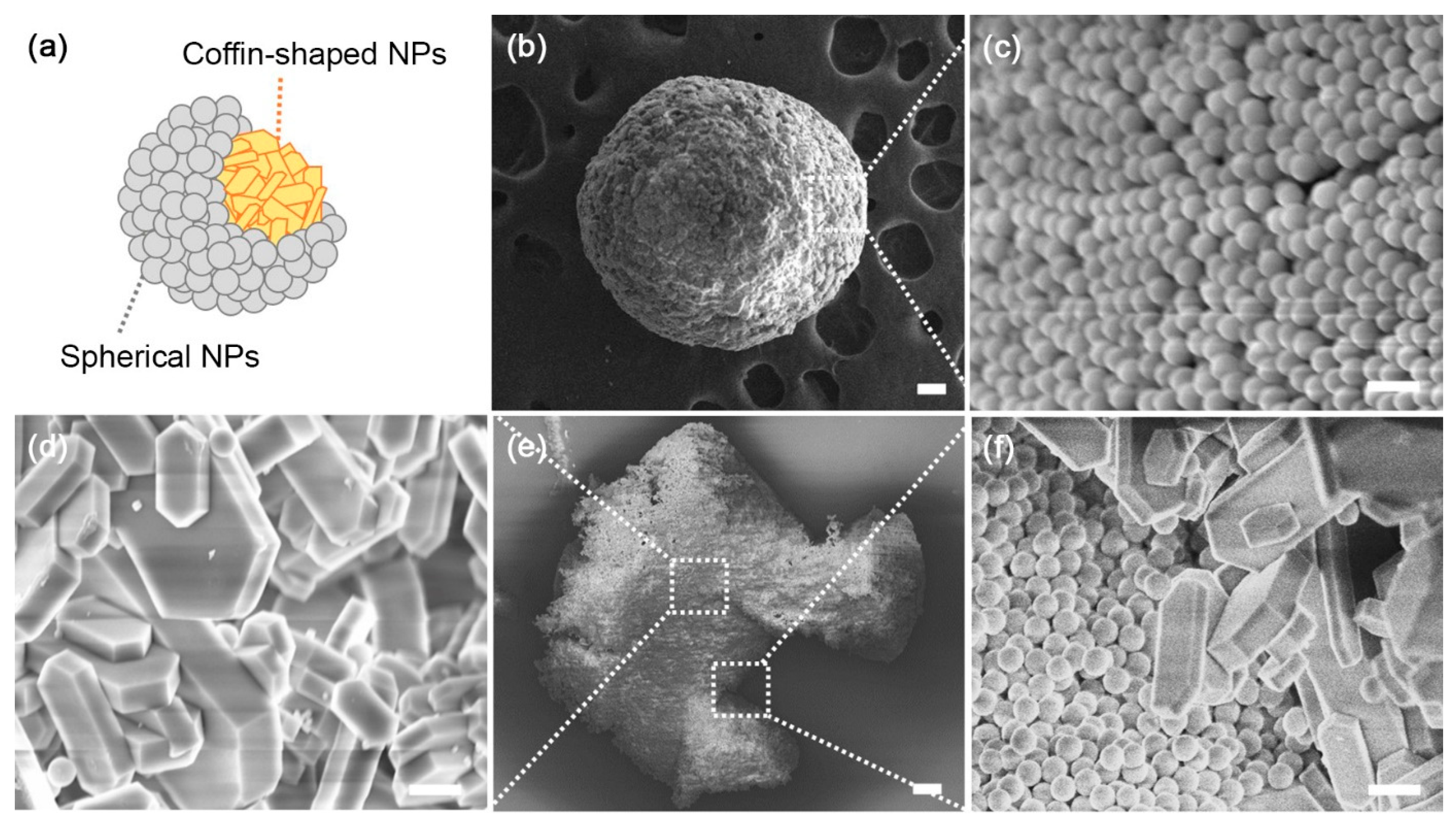
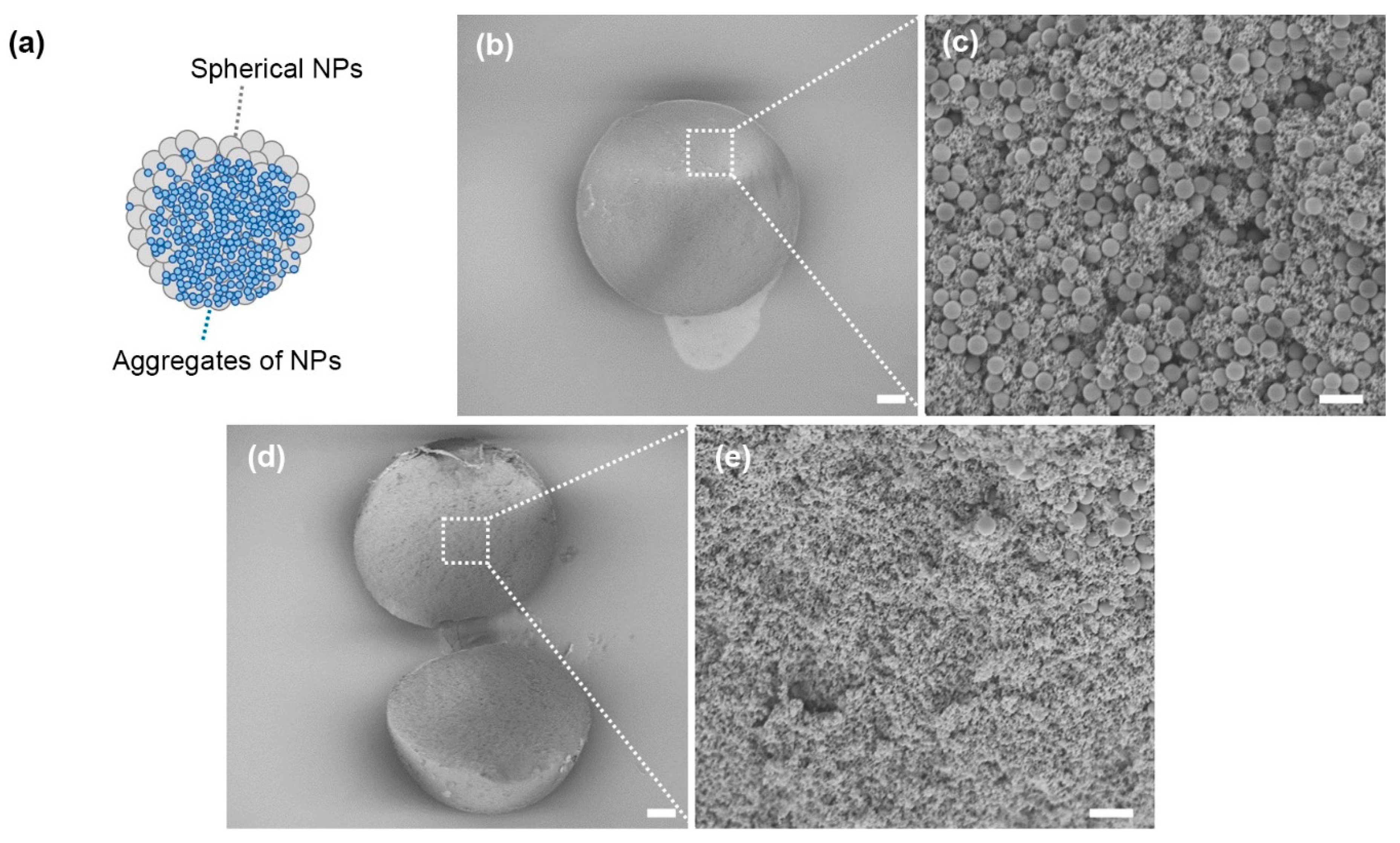
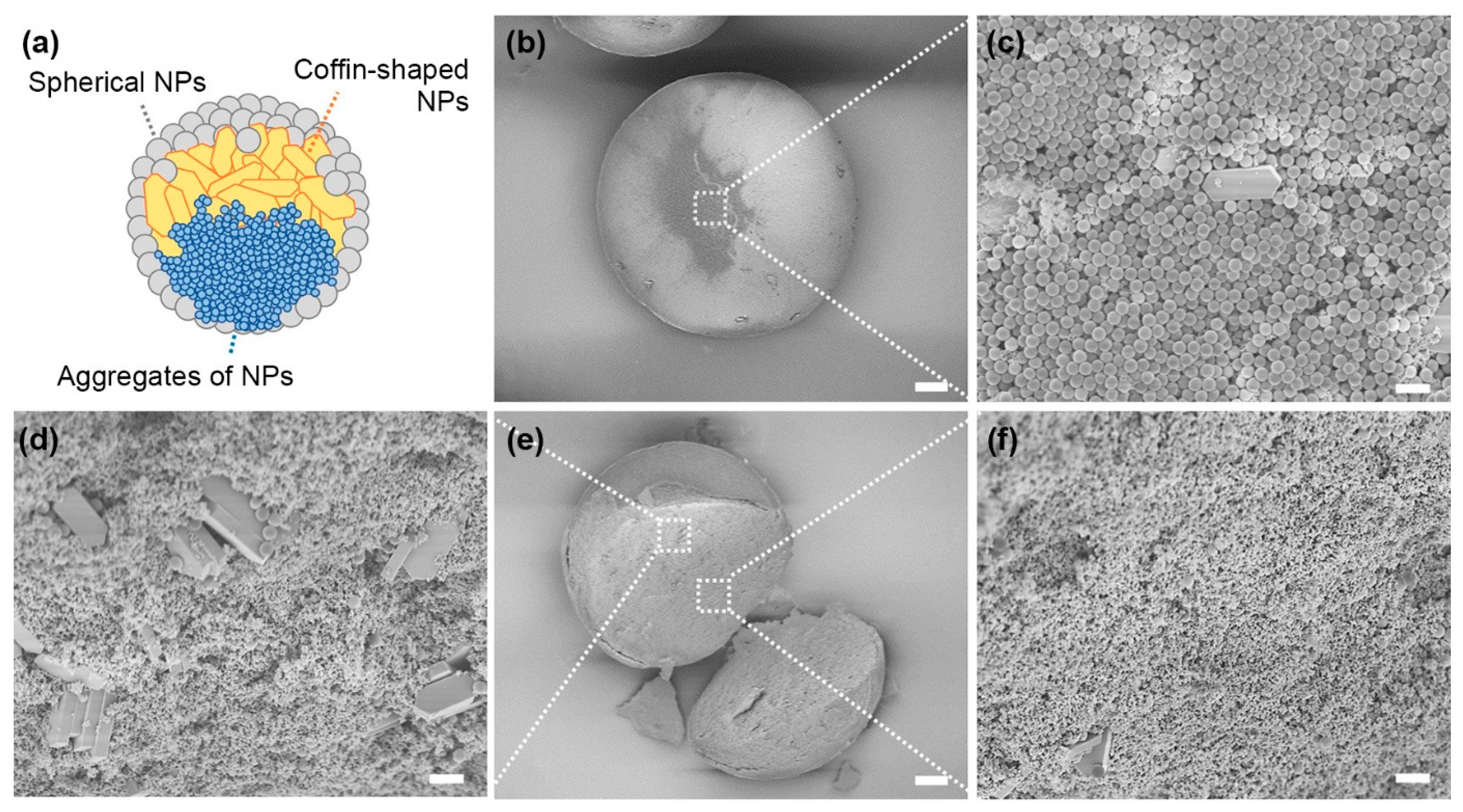
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wintzheimer, S.; Granath, T.; Oppmann, M.; Kister, T.; Thai, T.; Kraus, T.; Vogel, N.; Mandel, K. Supraparticles: Functionality from Uniform Structural Motifs. ACS Nano 2018, 12, 5093–5120. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, R.; Abd Hamid, S.B. Nanocatalysis: Mature science revisited or something really new? Angew. Chem. Int. Ed. 2004, 43, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Ramesar, N.S.; Heinz, H.; Xu, L.; Xu, C.; Kotov, N.A. Single-and multi-component chiral supraparticles as modular enantioselective catalysts. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Lee, W.-r.; Kim, M.; Kang, J.; Lee, J.S.; Thompson, L.T.; Kim, B.-S. Structure-tunable supraparticle assemblies of hollow cupric oxide sheathed with nanographenes. Nanoscale Adv. 2020, 2, 1236–1244. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Y.; Peng, Z.; Xu, Y.; Li, L.; Tan, L.; Yuan, K.; Chen, Y. Co3O4 supraparticle-based bubble nanofiber and bubble nanosheet with remarkable electrochemical performance. Adv. Sci. 2019, 6, 1900107. [Google Scholar] [CrossRef]
- Ma, Y.; Björnmalm, M.; Wise, A.K.; Cortez-Jugo, C.; Revalor, E.; Ju, Y.; Feeney, O.M.; Richardson, R.T.; Hanssen, E.; Shepherd, R.K. Gel-mediated electrospray assembly of silica supraparticles for sustained drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 31019–31031. [Google Scholar] [CrossRef]
- Ulrich, S.; Hirsch, C.; Diener, L.; Wick, P.; Rossi, R.M.; Bannwarth, M.B.; Boesel, L.F. Preparation of ellipsoid-shaped supraparticles with modular compositions and investigation of shape-dependent cell-uptake. RSC Adv. 2016, 6, 89028–89039. [Google Scholar] [CrossRef]
- Zhou, H.; Kim, J.P.; Bahng, J.H.; Kotov, N.A.; Lee, J. Self-Assembly Mechanism of Spiky Magnetoplasmonic Supraparticles. Adv. Funct. Mater. 2014, 24, 1439–1448. [Google Scholar] [CrossRef]
- Maas, M.; Silvério, C.C.; Laube, J.; Rezwan, K. Electrostatic assembly of zwitterionic and amphiphilic supraparticles. J. Colloid Interface Sci. 2017, 501, 256–266. [Google Scholar] [CrossRef]
- Egly, S.; Fröhlich, C.; Vogel, S.; Gruenewald, A.; Wang, J.; Detsch, R.; Boccaccini, A.R.; Vogel, N. Bottom-Up Assembly of Silica and Bioactive Glass Supraparticles with Tunable Hierarchical Porosity. Langmuir 2018, 34, 2063–2072. [Google Scholar] [CrossRef]
- Wang, W.-N.; Lenggoro, I.W.; Okuyama, K. Dispersion and aggregation of nanoparticles derived from colloidal droplets under low-pressure conditions. J. Colloid Interface Sci. 2005, 288, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef] [PubMed]
- Lintingre, E.; Lequeux, F.; Talini, L.; Tsapis, N. Control of particle morphology in the spray drying of colloidal suspensions. Soft Matter 2016, 12, 7435–7444. [Google Scholar] [CrossRef]
- Wooh, S.; Huesmann, H.; Tahir, M.N.; Paven, M.; Wichmann, K.; Vollmer, D.; Tremel, W.; Papadopoulos, P.; Butt, H.-J. Synthesis of Mesoporous Supraparticles on Superamphiphobic Surfaces. Adv. Mater. 2015, 27, 7338–7343. [Google Scholar] [CrossRef] [PubMed]
- Wooh, S.; Butt, H.-J. A Photocatalytically Active Lubricant-Impregnated Surface. Angew. Chem. Int. Ed. 2017, 56, 4965–4969. [Google Scholar] [CrossRef]
- Wang, L.; McCarthy, T.J. Covalently Attached Liquids: Instant Omniphobic Surfaces with Unprecedented Repellency. Angew. Chem. Int. Ed. 2016, 55, 244–248. [Google Scholar] [CrossRef]
- Chen, L.; Hong, J.; Butt, H.-J.; Wooh, S. Liquid-Repellent Metal Oxide Photocatalysts. Chem. A Eur. J. 2019, 25, 4535–4542. [Google Scholar] [CrossRef]
- Chen, L.; Park, S.; Yoo, J.; Hwang, H.; Kim, H.; Lee, J.; Hong, J.; Wooh, S. One-Step Fabrication of Universal Slippery Lubricated Surfaces. Adv. Mater. Interfaces 2020, 7, 2000305. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67. [Google Scholar] [CrossRef]
- Sekido, T.; Wooh, S.; Fuchs, R.; Kappl, M.; Nakamura, Y.; Butt, H.-J.; Fujii, S. Controlling the Structure of Supraballs by pH-Responsive Particle Assembly. Langmuir 2017, 33, 1995–2002. [Google Scholar] [CrossRef]
- Tan, H.; Wooh, S.; Butt, H.-J.; Zhang, X.; Lohse, D. Porous supraparticle assembly through self-lubricating evaporating colloidal ouzo drops. Nat. Commun. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kappl, M.; Butt, H.-J. Tuning the Porosity of Supraparticles. ACS Nano 2019, 13, 13949–13956. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Nguyen, T.D.; Yang, M.; Lee, B.; Santos, A.; Podsiadlo, P.; Tang, Z.; Glotzer, S.C.; Kotov, N.A. Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparticles. Nat. Nanotechnol. 2011, 6, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.; Velev, O.D.; Gradzielski, M. Controlling the Shape of Evaporating Droplets by Ionic Strength: Formation of Highly Anisometric Silica Supraparticles. Angew. Chem. Int. Ed. 2014, 53, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Butt, H.-J.; Landfester, K.; Bannwarth, M.B.; Wooh, S.; Thérien-Aubin, H. Shaping the Assembly of Superparamagnetic Nanoparticles. ACS Nano 2019, 13, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, L.; Song, Z.; Li, S.; Yu, M. Growth of high-quality, thickness-reduced zeolite membranes towards N2/CH4 separation using high-aspect-ratio seeds. Angew. Chem. 2015, 127, 10993–10997. [Google Scholar] [CrossRef]
- Yu, S.; Kwon, S.; Na, K. Synthesis of LTA zeolites with controlled crystal sizes by variation of synthetic parameters: Effect of Na+ concentration, aging time, and hydrothermal conditions. J. Sol Gel Sci. Technol. 2018, 1–11. [Google Scholar] [CrossRef]
- Jeon, M.Y.; Kim, D.; Kumar, P.; Lee, P.S.; Rangnekar, N.; Bai, P.; Shete, M.; Elyassi, B.; Lee, H.S.; Narasimharao, K. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature 2017, 543, 690–694. [Google Scholar] [CrossRef]
- Pham, T.C.T.; Kim, H.S.; Yoon, K.B. Growth of uniformly oriented silica MFI and BEA zeolite films on substrates. Science 2011, 334, 1533–1538. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Zikry, A.; Sharaf, M.A. Preparation of spherical silica nanoparticles: Stober silica. J. Am. Sci. 2010, 6, 985–989. [Google Scholar]
- Liu, W.; Midya, J.; Kappl, M.; Butt, H.-J.; Nikoubashman, A. Segregation in Drying Binary Colloidal Droplets. ACS Nano 2019, 13, 4972–4979. [Google Scholar] [CrossRef] [PubMed]
- Lotito, V.; Zambelli, T. Pattern detection in colloidal assembly: A mosaic of analysis techniques. Adv. Colloid Interface Sci. 2020, 284, 102252. [Google Scholar] [CrossRef] [PubMed]
- Lotito, V.; Zambelli, T. Pattern Formation in Binary Colloidal Assemblies: Hidden Symmetries in a Kaleidoscope of Structures. Langmuir 2018, 34, 7827–7843. [Google Scholar] [CrossRef] [PubMed]
- Lotito, V.; Zambelli, T. A Journey Through the Landscapes of Small Particles in Binary Colloidal Assemblies: Unveiling Structural Transitions from Isolated Particles to Clusters upon Variation in Composition. Nanomaterials 2019, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; García, A.A.; Marquez, M.; Velev, O.D. Anisotropic Particle Synthesis Inside Droplet Templates on Superhydrophobic Surfaces. Macromol. Rapid Commun. 2010, 31, 190–195. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, W.; Moon, C.S.; Kim, H.; Kim, H.S.; Zhang, H.; Kang, S.K.; Lee, P.S.; Wooh, S. Tailoring the Morphology of Supraparticles by Primary Colloids with Different Shapes, Sizes and Dispersities. Crystals 2021, 11, 79. https://doi.org/10.3390/cryst11020079
Shim W, Moon CS, Kim H, Kim HS, Zhang H, Kang SK, Lee PS, Wooh S. Tailoring the Morphology of Supraparticles by Primary Colloids with Different Shapes, Sizes and Dispersities. Crystals. 2021; 11(2):79. https://doi.org/10.3390/cryst11020079
Chicago/Turabian StyleShim, Wonmi, Chan Sik Moon, Hyeonjin Kim, Hyun Su Kim, Haoxiang Zhang, Su Kyung Kang, Pyung Soo Lee, and Sanghyuk Wooh. 2021. "Tailoring the Morphology of Supraparticles by Primary Colloids with Different Shapes, Sizes and Dispersities" Crystals 11, no. 2: 79. https://doi.org/10.3390/cryst11020079
APA StyleShim, W., Moon, C. S., Kim, H., Kim, H. S., Zhang, H., Kang, S. K., Lee, P. S., & Wooh, S. (2021). Tailoring the Morphology of Supraparticles by Primary Colloids with Different Shapes, Sizes and Dispersities. Crystals, 11(2), 79. https://doi.org/10.3390/cryst11020079





