Bis (Diamines) Cu and Zn Complexes of Flurbiprofen as Potential Cholinesterase Inhibitors: In Vitro Studies and Docking Simulations
Abstract
1. Introduction
2. Materials and Methods
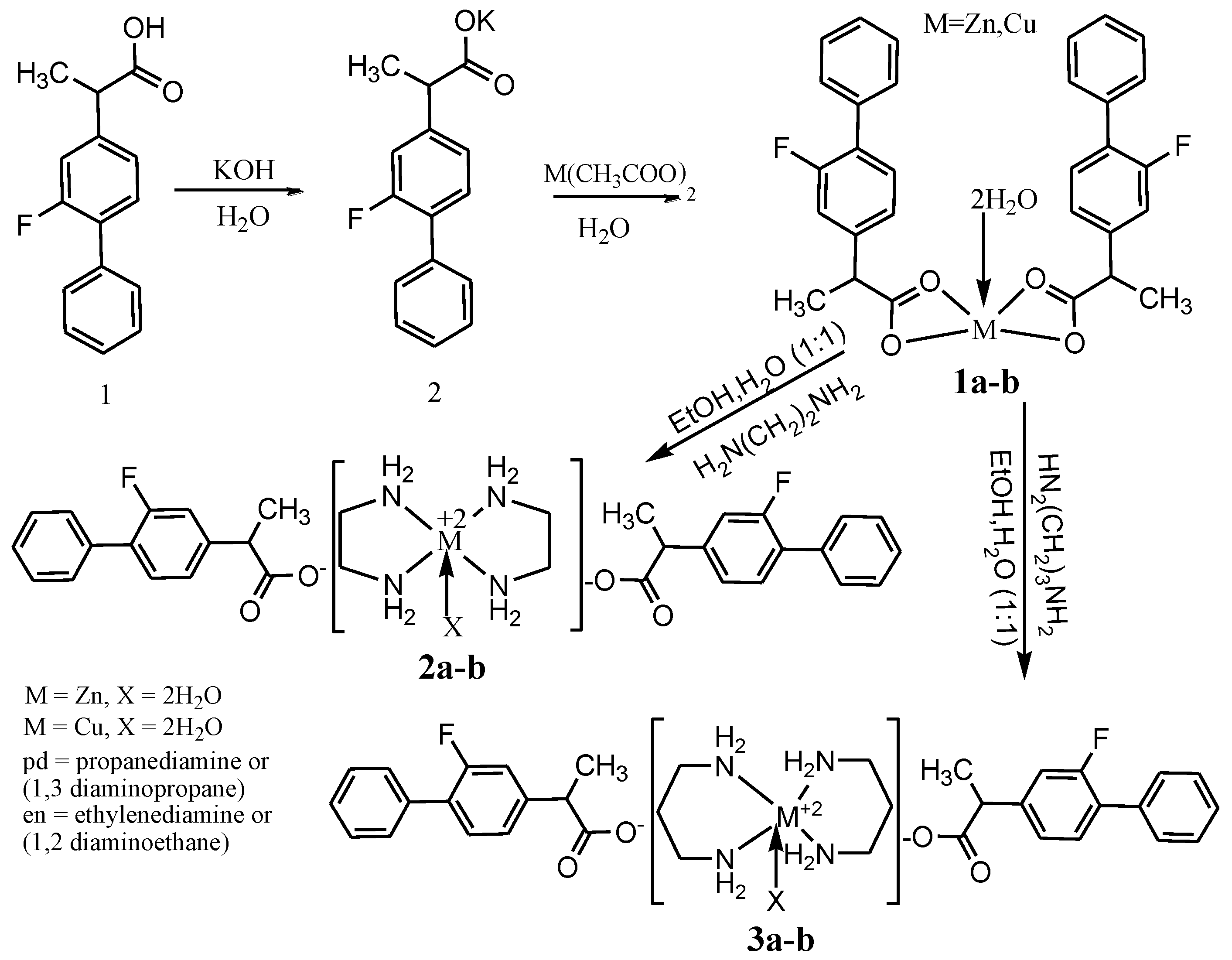
2.1. Synthesis of Flurbiprofen Metal Complexes (1a-b)
2.1.1. Zn(H2O)2(L)2 (1a)
2.1.2. Cu(H2O)2(L)2 (1b)
2.2. Synthesis of Bis (1,2-diaminoethane) Metal Flurbiprofen Complexes (2a-b)
2.2.1. Zn(H2O)2 (C2H8N2)2 (C15H12FO2)2 (2a)
2.2.2. Cu(H2O)2 (L1)2 (L)2 (2b)
2.3. Synthesis of Bis (1,3-diaminopropane) Metal Flurbiprofen Complexes (3a-b)
2.3.1. Zn(H2O)2 (L1)2 (L)2 (3a)
2.3.2. Cu(H2O)2 (L1)2 (CL)2 (3b)
2.4. Molecular Docking Simulations
2.5. Determination of AChE and BChE Inhibitory Activity
3. Results
3.1. Synthesis
3.2. X-ray Crystallography
3.3. NMR Analysis
3.4. FT-IR Analysis
3.5. UV/vis and Magnetic Susceptibility
3.6. Elemental Analysis
3.7. Conductivity Measurements
3.8. Anticholinesterase Activity
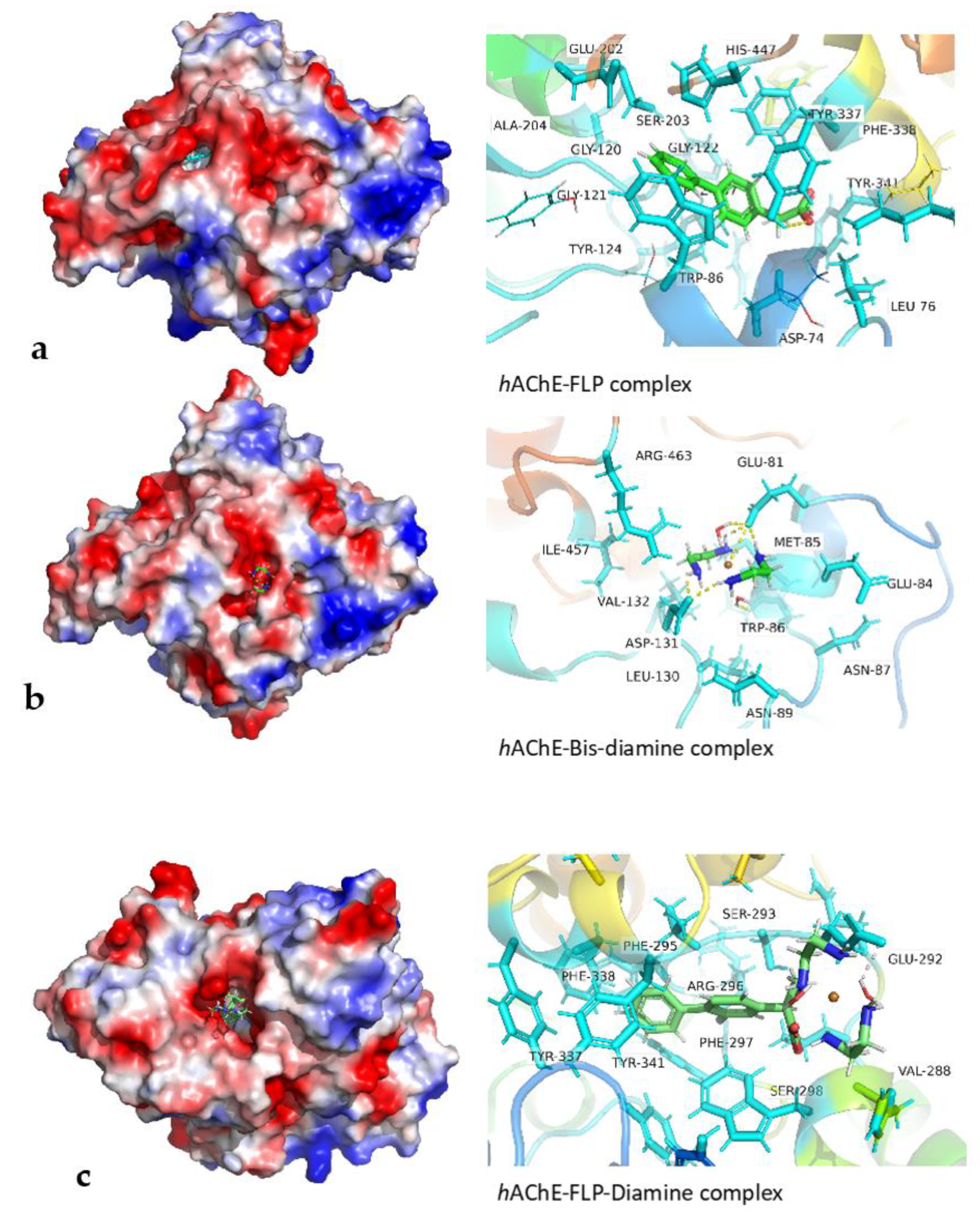
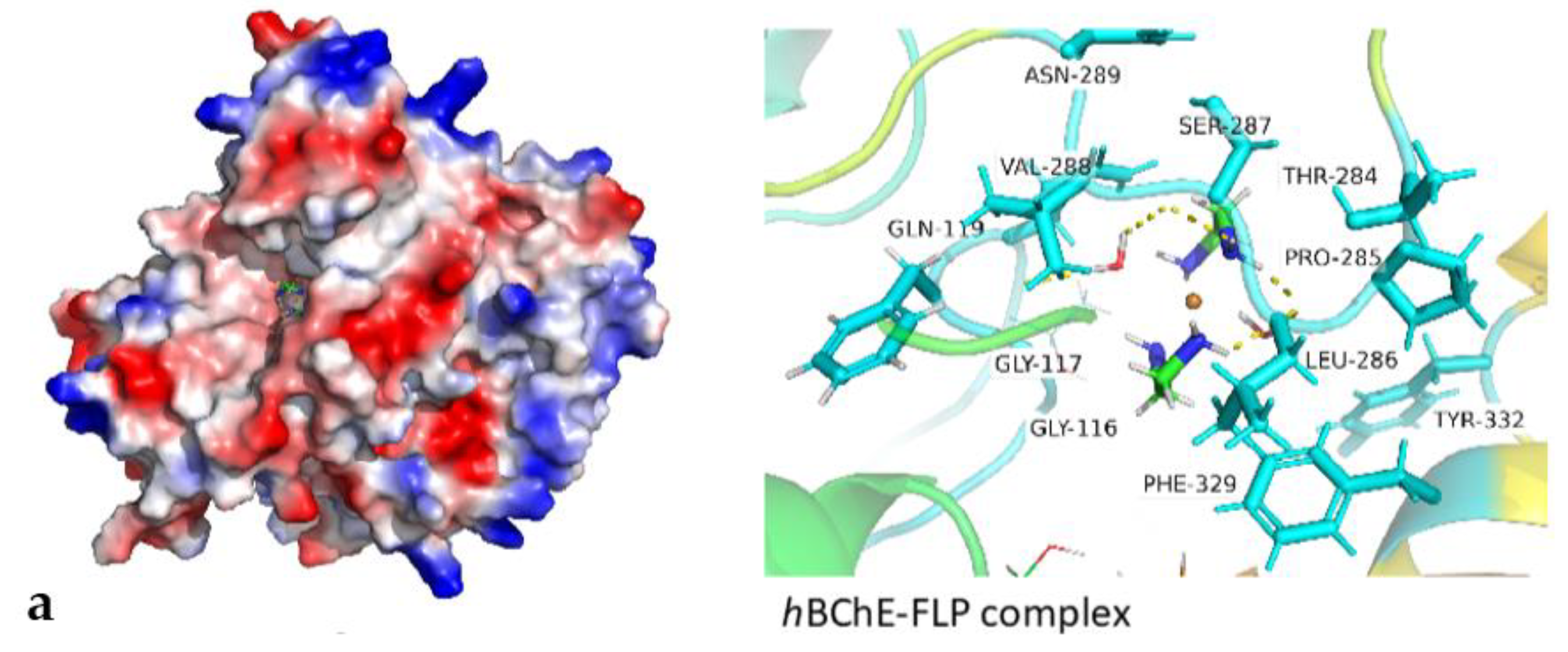
3.9. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bakkour, A.; Morris, J.C.; Dickerson, B.C. The cortical signature of prodromal AD: Regional thinning predicts mild AD dementia. Neurology 2009, 72, 1048–1055. [Google Scholar] [CrossRef]
- Liu, Y.; Patricelli, M.P.; Cravatt, B.F. Activity-based protein profiling: The serine hydrolases. Proc. Natl. Acad. Sci. USA 1999, 96, 14694–14699. [Google Scholar] [CrossRef]
- Simon, G.M.; Cravatt, B.F. Activity-based proteomics of enzyme superfamilies: Serine hydrolases as a case study. J. Biol. Chem. 2010, 285, 11051–11055. [Google Scholar] [CrossRef]
- Rosenbek, J.C.; Troche, M.S. Progressive neurologic disease and dysphagia (including Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, myasthenia gravis, post-polio syndrome). In Principles of Deglutition; Springer: Berlin/Heidelberg, Germany, 2013; pp. 395–409. [Google Scholar]
- Withiam-Leitch, S.; Pullicino, P. Ataxic hemiparesis with bilateral leg ataxia from pontine infarct. J. Neurol. Neurosurg. Psychiatry 1995, 59, 557. [Google Scholar] [CrossRef][Green Version]
- Shaw, K.; Aracava, Y.; Akaike, A.; Daly, J.; Rickett, D.; Albuquerque, E. The reversible cholinesterase inhibitor physostigmine has channel-blocking and agonist effects on the acetylcholine receptor-ion channel complex. Mol. Pharmacol. 1985, 28, 527–538. [Google Scholar] [PubMed]
- Gallucci, M.; Spagnolo, P.; Aricò, M.; Grossi, E. Predictors of response to cholinesterase inhibitors treatment of Alzheimer’s disease: Date mining from the TREDEM registry. J. Alzheimer’s Dis. 2016, 50, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Rossor, M.; Hecker, J.; Gauthier, S.; Petit, H.; Möller, H.-J.; Rogers, S.; Friedhoff, L. The Effects of Donepezil in Alzheimer’s Disease–Results from a Multinational Trial1. Dement. Geriatr. Cogn. Disord. 1999, 10, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.S.; Evans, J.G. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Cyrus, P.A.; Orazem, J.; Mas, J.; Bieber, F.; Ruzicka, B.B.; Gulanski, B. Metrifonate benefits cognitive, behavioral, and global function in patients with Alzheimer’s disease. Neurology 1998, 50, 1222–1230. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.C.; De Sarno, P.; Sugaya, K.; Giacobini, E. Effect of huperzine A, a new cholinesterase inhibitor, on the central cholinergic system of the rat. J. Neurosci. Res. 1989, 24, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Fabio, K.; Guillon, C.; Mohanta, P.; Halton, T.A.; Heck, D.E.; Flowers II, R.A.; Laskin, J.D.; Heindel, N.D. Peripheral site acetylcholinesterase inhibitors targeting both inflammation and cholinergic dysfunction. Bioorganic Med. Chem. Lett. 2010, 20, 2987–2990. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Wang, J.D.; Hahn, R.A.; Gordon, M.K.; Joseph, L.B.; Heck, D.E.; Heindel, N.D.; Young, S.C.; Sinko, P.J.; Casillas, R.P. Therapeutic potential of a non-steroidal bifunctional anti-inflammatory and anti-cholinergic agent against skin injury induced by sulfur mustard. Toxicol. Appl. Pharmacol. 2014, 280, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Young, S.C.; Fabio, K.M.; Huang, M.T.; Saxena, J.; Harman, M.P.; Guillon, C.D.; Vetrano, A.M.; Heck, D.E.; Flowers, R.A.; Heindel, N.D. Investigation of anticholinergic and non-steroidal anti-inflammatory prodrugs which reduce chemically induced skin inflammation. J. Appl. Toxicol. 2012, 32, 135–141. [Google Scholar] [CrossRef]
- Jamil, M.; Sultana, N.; Ashraf, R.; Sarfraz, M.; Tariq, M.I.; Mustaqeem, M. Tris-diamine-derived transition metal complexes of flurbiprofen as cholinesterase inhibitors. Trop. J. Pharm. Res. 2018, 17, 451–459. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Louie, A.Y.; Meade, T.J. Metal complexes as enzyme inhibitors. Chem. Rev. 1999, 99, 2711–2734. [Google Scholar] [CrossRef]
- Eremina, J.A.; Lider, E.V.; Samsonenko, D.G.; Sheludyakova, L.A.; Berezin, A.S.; Klyushova, L.S.; Ostrovskii, V.A.; Trifonov, R.E. Mixed-ligand copper (II) complexes with tetrazole derivatives and 2, 2′-bipyridine, 1, 10-phenanthroline: Synthesis, structure and cytotoxic activity. Inorg. Chim. Acta 2019, 487, 138–144. [Google Scholar] [CrossRef]
- Matesanz, A.I.; Caballero, A.B.; Lorenzo, C.; Espargaró, A.; Sabaté, R.; Quiroga, A.N.G.; Gamez, P. Thiosemicarbazone Derivatives as Inhibitors of Amyloid-β Aggregation: Effect of Metal Coordination. Inorg. Chem. 2020, 59, 6978. [Google Scholar] [CrossRef]
- Gomes, L.M.; Vieira, R.P.; Jones, M.R.; Wang, M.C.; Dyrager, C.; Souza-Fagundes, E.M.; Da Silva, J.G.; Storr, T.; Beraldo, H. 8-Hydroxyquinoline Schiff-base compounds as antioxidants and modulators of copper-mediated Aβ peptide aggregation. J. Inorg. Biochem. 2014, 139, 106–116. [Google Scholar] [CrossRef]
- Deraeve, C.; Pitié, M.; Mazarguil, H.; Meunier, B. Bis-8-hydroxyquinoline ligands as potential anti-Alzheimer agents. New J. Chem. 2007, 31, 193–195. [Google Scholar] [CrossRef]
- Ellman, J.; Mendel, D.; Anthony-Cahill, S.; Noren, C.J.; Schultz, P.G. Biosynthetic method for introducing unnatural amino acids site-specifically into proteins. Methods Enzymol. 1991, 202, 301–336. [Google Scholar] [PubMed]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Figiel, P.J.; Kirillov, A.M.; Guedes da Silva, M.F.C.; Lasri, J.; Pombeiro, A.J.L. Self-assembled dicopper(ii) diethanolaminate cores for mild aerobic and peroxidative oxidation of alcohols. Dalton Trans. 2010, 39, 9879–9888. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.S.P.; Kirillova, M.V.; André, V.; Kłak, J.; Kirillov, A.M. New tricopper(ii) cores self-assembled from aminoalcohol biobuffers and homophthalic acid: Synthesis, structural and topological features, magnetic properties and mild catalytic oxidation of cyclic and linear C5–C8 alkanes. Inorg. Chem. Front. 2015, 2, 525–537. [Google Scholar] [CrossRef]
- Eckert, P.K.; Vieira, I.d.S.; Gessner, V.H.; Börner, J.; Strohmann, C.; Herres-Pawlis, S. Simple is best: Diamine zinc complexes as unexpected catalysts in lactide polymerisation. Polyhedron 2013, 49, 151–157. [Google Scholar] [CrossRef]
- Girek, M.; Szymański, P. Tacrine hybrids as multi-target-directed ligands in Alzheimer’s disease: Influence of chemical structures on biological activities. Chem. Pap. 2019, 73, 269–289. [Google Scholar] [CrossRef]
- Jamil, M.; Sultana, N.; Sarfraz, M.; Tahir, M.N.; Tariq, M.I. Diamines Derived Transition Metal Complexes of Naproxen: Synthesis, Characterization and Urease Inhibition Studies. Iran. J. Chem. Chem. Eng. (Ijcce) 2020, 39, 45–57. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA View—Molecular graphics for all devices—From smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef]
- Bilal, S.; Mudassir Hassan, M.; Fayyaz ur Rehman, M.; Nasir, M.; Jamil Sami, A.; Hayat, A. An insect acetylcholinesterase biosensor utilizing WO3/g-C3N4 nanocomposite modified pencil graphite electrode for phosmet detection in stored grains. Food Chem. 2021, 346, 128894. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sami, A.J.; Bilal, S.; Khalid, M.; Shakoori, F.R.; Shakoori, A. Effect of crude neem (Azadirachta indica) powder and azadirachtin on the growth and acetylcholinesterase activity of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Pak. J. Zool 2016, 48, 881–886. [Google Scholar]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept®): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef]
- Bai, F.; Xu, Y.; Chen, J.; Liu, Q.; Gu, J.; Wang, X.; Ma, J.; Li, H.; Onuchic, J.N.; Jiang, H. Free energy landscape for the binding process of Huperzine A to acetylcholinesterase. Proc. Natl. Acad. Sci. USA 2013, 110, 4273–4278. [Google Scholar] [CrossRef] [PubMed]
- Berg, L.; Andersson, C.D.; Artursson, E.; Hörnberg, A.; Tunemalm, A.-K.; Linusson, A.; Ekström, F. Targeting acetylcholinesterase: Identification of chemical leads by high throughput screening, structure determination and molecular modeling. PLoS ONE 2011, 6, e26039. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.K.; Sasi, M.V.; Gupta, S.K.; Krishnamurthy, S.; Ayyannan, S.R. Design, synthesis, and pharmacological evaluation of 2-amino-5-nitrothiazole derived semicarbazones as dual inhibitors of monoamine oxidase and cholinesterase: Effect of the size of aryl binding site. J. Enzym. Inhib. Med. Chem. 2018, 33, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, A.K. Transition metal complexes with bioactive ligands: Mechanisms for selective ligand release and applications for drug delivery. Metallomics 2014, 6, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Jaros, S.W.; Guedes da Silva, M.F.C.; Florek, M.; Smoleński, P.; Pombeiro, A.J.L.; Kirillov, A.M. Silver(I) 1,3,5-Triaza-7-phosphaadamantane Coordination Polymers Driven by Substituted Glutarate and Malonate Building Blocks: Self-Assembly Synthesis, Structural Features, and Antimicrobial Properties. Inorg. Chem. 2016, 55, 5886–5894. [Google Scholar] [CrossRef] [PubMed]
- Dabrowiak, J.C. Metals in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]


| Identification Code | Shelx |
|---|---|
| Empirical formula | C34 H44 Cu F2 N4 O6 |
| Formula weight | 706.27 |
| Temperature | 296(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P 21/n |
| Unit cell dimensions | |
| a = 6.6291(8) Å | α = 90° |
| b = 40.392(5) Å | β = 112.243(6)° |
| c = 6.8061(9) Å | γ = 90° |
| Volume | 1686.8(4) Å3 |
| Z | 2 |
| Density (calculated) | 1.391 Mg/m3 |
| Absorption coefficient | 0.708 mm−1 |
| F(000) | 742 |
| Crystal size | 0.420 × 0.280 × 0.200 mm3 |
| Theta range for data collection | 1.008 to 27.356°. |
| Index ranges | −8 ≤ h≤ 7, −51 ≤ k ≤ 49, −5 ≤ l ≤ 8 |
| Reflections collected | 10,124 |
| Independent reflections | 3611 [R(int) = 0.0400] |
| Completeness to theta = 25.242° | 98.4% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3611/0/221 |
| Goodness-of-fit on F2 | 1.172 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0884, wR2 = 0.2301 |
| R indices (all data) | R1 = 0.1181, wR2 = 0.2532 |
| Extinction coefficient | n/a |
| Largest diff. peak and hole | 1.684 and −0.713 e.Å−3 |
| (1a-b, 2a-b and 3a-b) | ||||
|---|---|---|---|---|
| Sample Code | Compounds | Anticholinesterase Activity * (IC50 µM) | ||
| eeAChE | eqBChE | SI | ||
| 1a | Zn(H2O)2(L)2 | 32.3 ± 1.4 | 43.2 ± 1.23 | 1.3 |
| 2a | Zn(H2O)2(L1)2(L)2 | 3.5 ± 0.14 | 13.6 ± 0.38 | 5.4 |
| 3a | Zn(H2O)2(L2)2(L)2 | 9.3 ± 0.22 | 21.3 ± 2.31 | 2.2 |
| 1b | Cu(H2O)2(L)2 | 22.5 ± 0.62 | 45.4 ± 1.45 | 2.0 |
| 2b | Cu(H2O)2(L1)2(L)2 | 3.0 ± 0.24 | 12.3 ± 0.21 | 4.1 |
| 3b | Cu(H2O)2(L2)2(L)2 | 3.4 ± 0.17 | 14.5 ± 0.27 | 4.2 |
| A * | Galantamine | 4.0 ± 0.10 | 15.0 ± 0.67 | 3.7 |
| L | Flurbiprofen | 45 ± 0.13 | 443 ± 30.73 | 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, M.; Sultana, N.; Ashraf, R.; Bashir, M.; Rehman, M.F.u.; Kanwal, F.; Ellahi, H.; Lu, C.; Zhang, W.X.; Tariq, M.I. Bis (Diamines) Cu and Zn Complexes of Flurbiprofen as Potential Cholinesterase Inhibitors: In Vitro Studies and Docking Simulations. Crystals 2021, 11, 208. https://doi.org/10.3390/cryst11020208
Jamil M, Sultana N, Ashraf R, Bashir M, Rehman MFu, Kanwal F, Ellahi H, Lu C, Zhang WX, Tariq MI. Bis (Diamines) Cu and Zn Complexes of Flurbiprofen as Potential Cholinesterase Inhibitors: In Vitro Studies and Docking Simulations. Crystals. 2021; 11(2):208. https://doi.org/10.3390/cryst11020208
Chicago/Turabian StyleJamil, Muhammad, Nargis Sultana, Rizwan Ashraf, Maryam Bashir, Muhammad Fayyaz ur Rehman, Fariha Kanwal, Humna Ellahi, Changrui Lu, Wei Xing Zhang, and Muhammad Ilyas Tariq. 2021. "Bis (Diamines) Cu and Zn Complexes of Flurbiprofen as Potential Cholinesterase Inhibitors: In Vitro Studies and Docking Simulations" Crystals 11, no. 2: 208. https://doi.org/10.3390/cryst11020208
APA StyleJamil, M., Sultana, N., Ashraf, R., Bashir, M., Rehman, M. F. u., Kanwal, F., Ellahi, H., Lu, C., Zhang, W. X., & Tariq, M. I. (2021). Bis (Diamines) Cu and Zn Complexes of Flurbiprofen as Potential Cholinesterase Inhibitors: In Vitro Studies and Docking Simulations. Crystals, 11(2), 208. https://doi.org/10.3390/cryst11020208







