Application of Magnetic and Dielectric Nanofluids for Electromagnetic-Assistance Enhanced Oil Recovery: A Review
Abstract
:1. Introduction
2. Oil Displacement Mechanisms Using Nanoparticles
2.1. Mobility Ratio Improvement
2.2. Interfacial Tension (IFT) Reduction
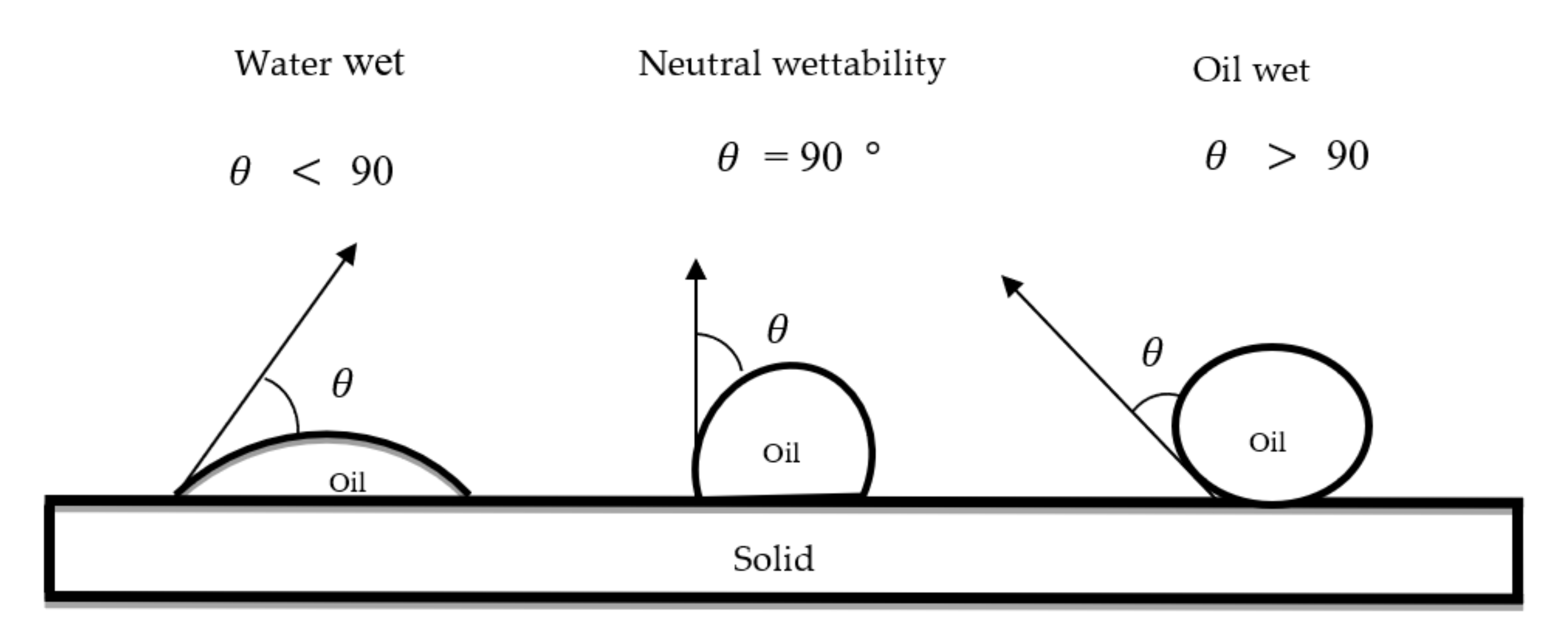
2.3. Wettability Alteration
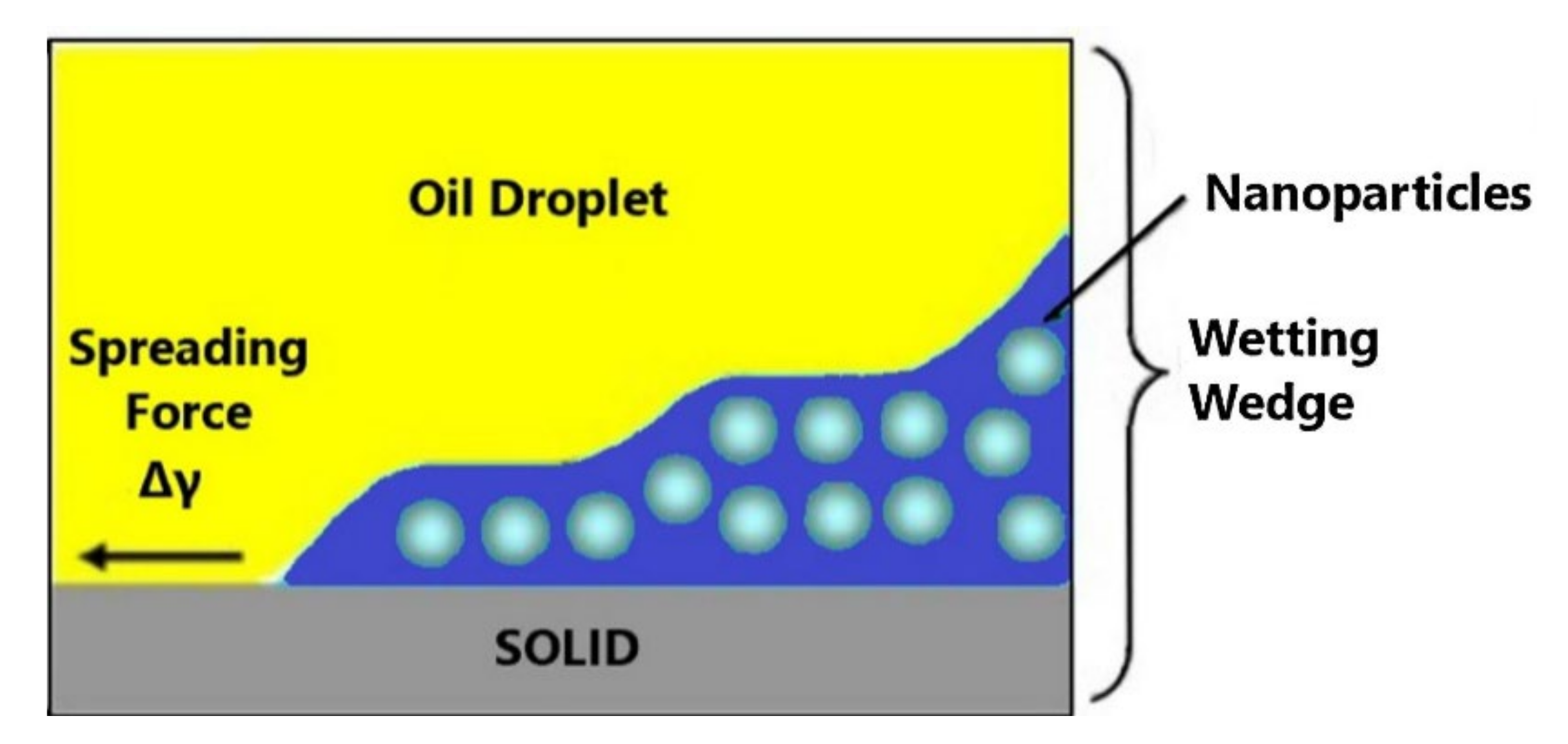
2.4. Disjoining Pressure
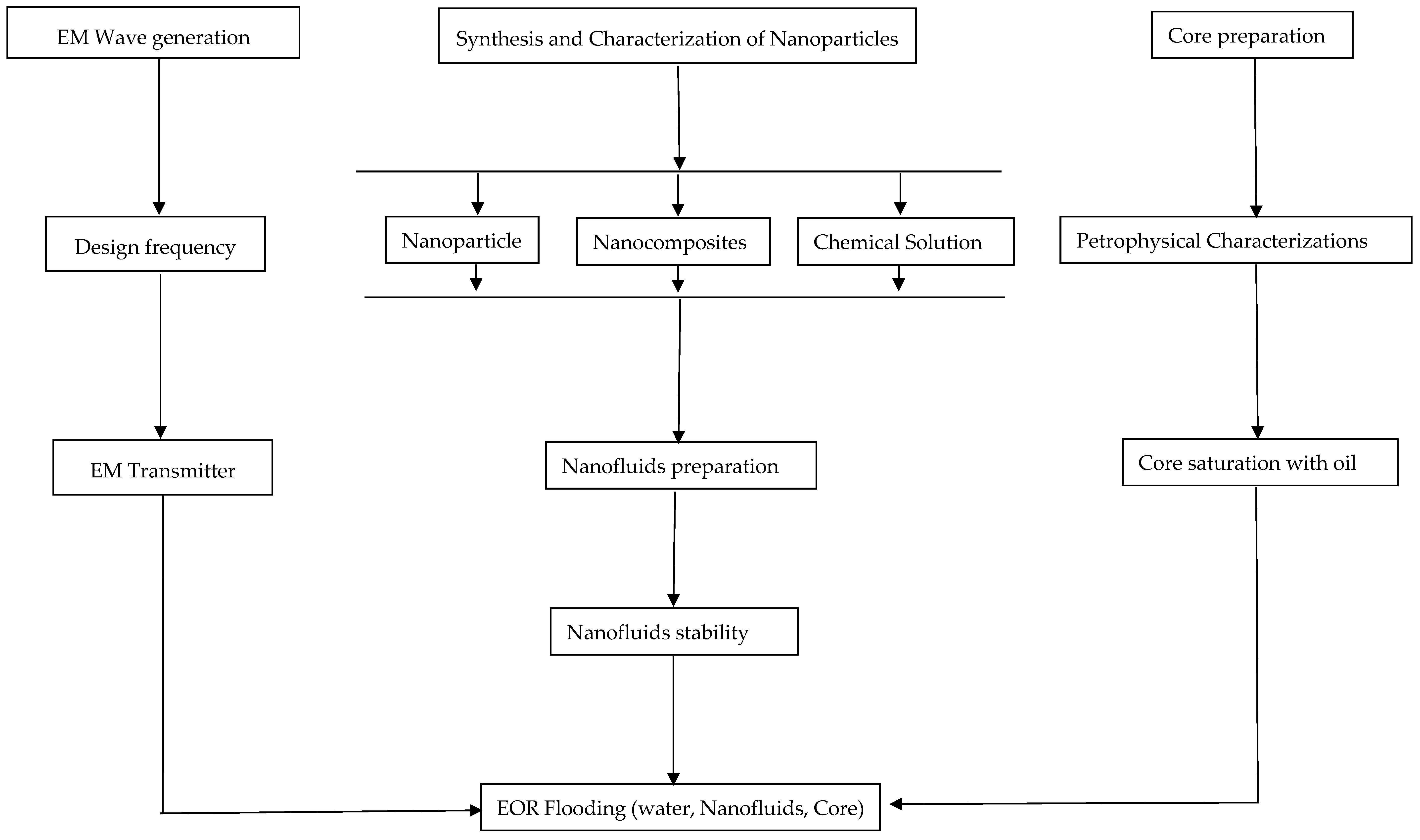
3. Influence of NPs’ Surface Modification for Nanofluids Stability
4. Metal Oxide NPs for EOR Application
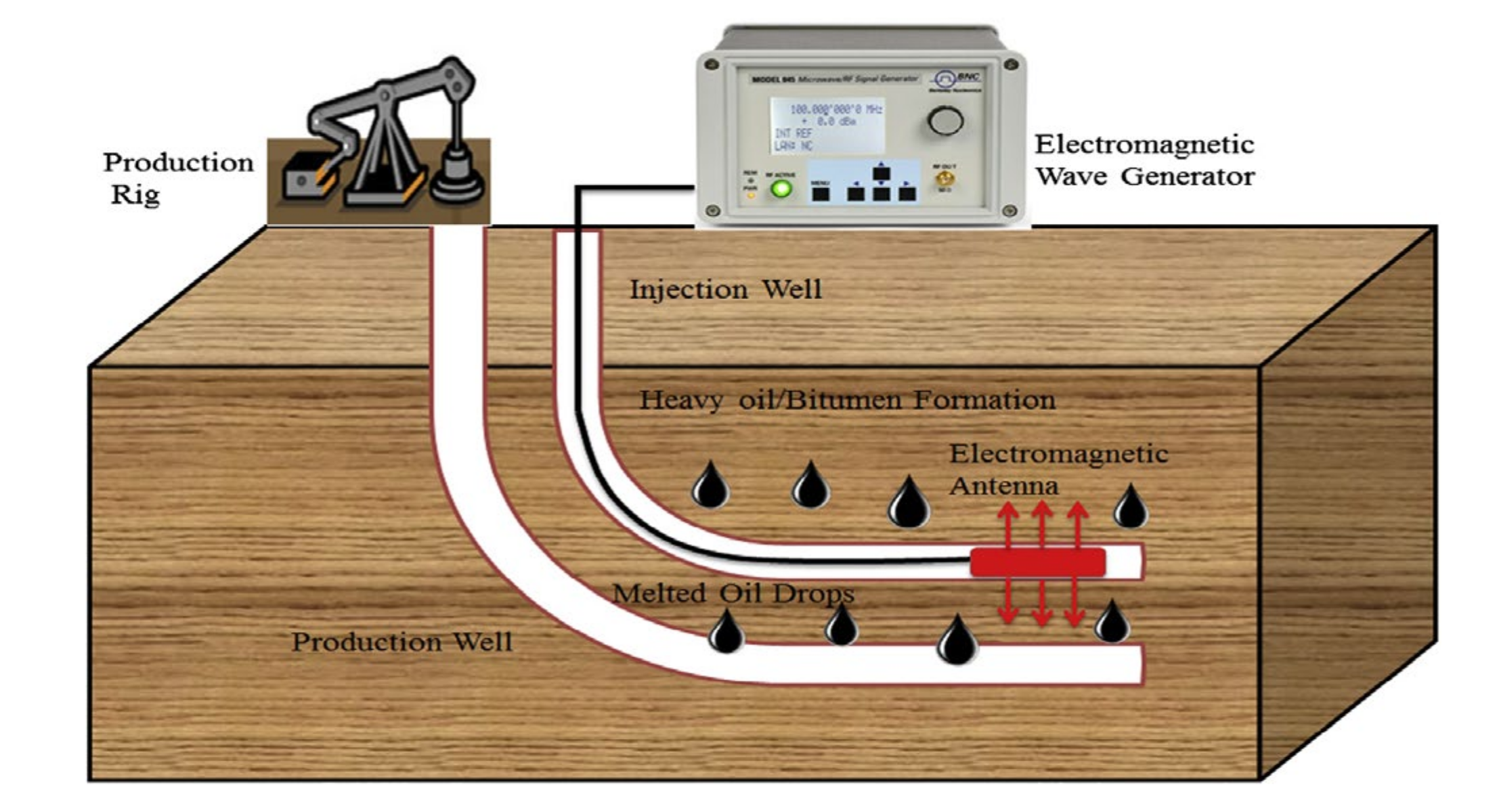
5. Role of Electromagnetic Waves in EOR
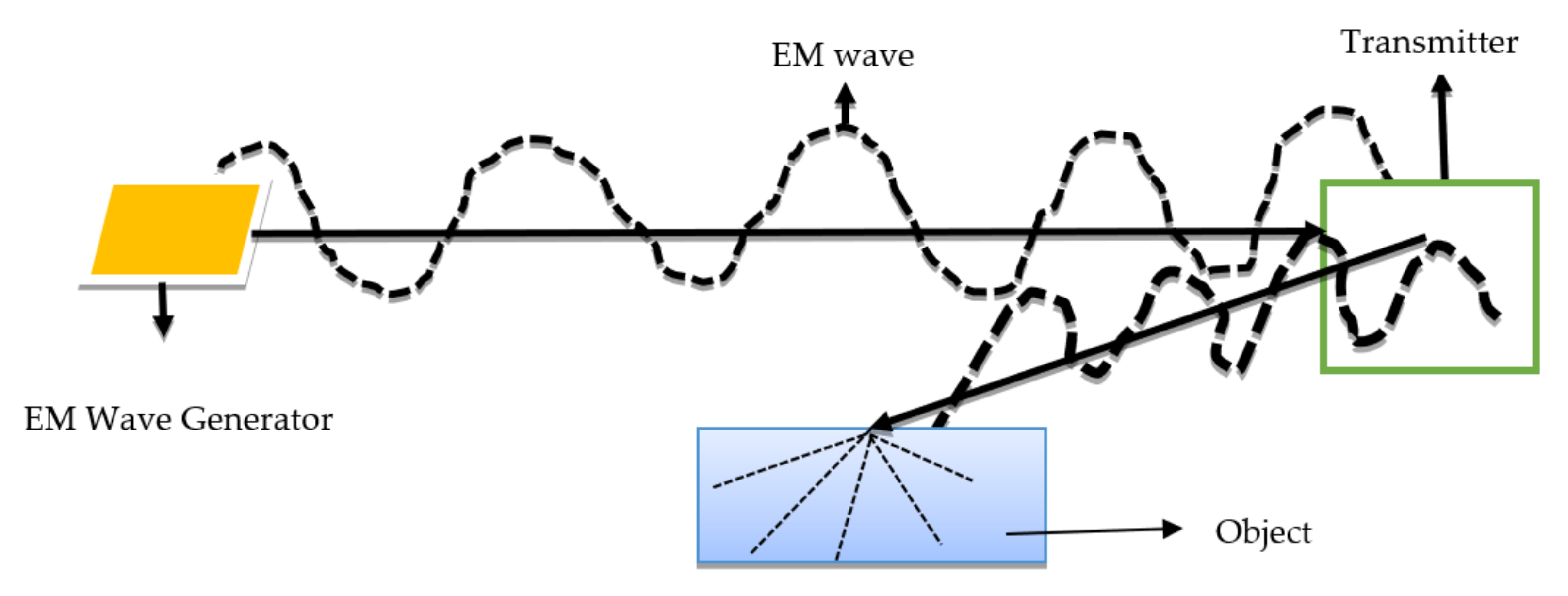
5.1. Background on EM Waves Radiation
5.2. Electromagnetic Heating for EOR
5.3. Influence of Nanofluids for EM-Assisted EOR
6. Nanoparticles for EM-Assisted EOR
6.1. Magnetic and Dielectric Nanofluids for EM-Assisted EOR
6.1.1. Ferro-Nanofluids
6.1.2. Cobalt Ferrite Nanofluids
6.1.3. Nickel-Zinc Ferrite Nanofluids (Ni1-xZnxFe2O3)
6.1.4. Lanthanum-Zinc Ferrite (MnZnLaxFe2-xO4)
6.1.5. Yttrium Iron Garnet (YIG) (Y3Fe5O12)
6.1.6. Fe2O3-Al2O3 Composite Nanofluids
6.1.7. Fe3O4/SiO2 and TiO2/SiO2 Composites Nanofluids
6.1.8. Coating Fe3O4 NPs
6.1.9. ZnO and Al2O3 Nanofluids
7. EM-Assisted Oil Recovery Mechanisms
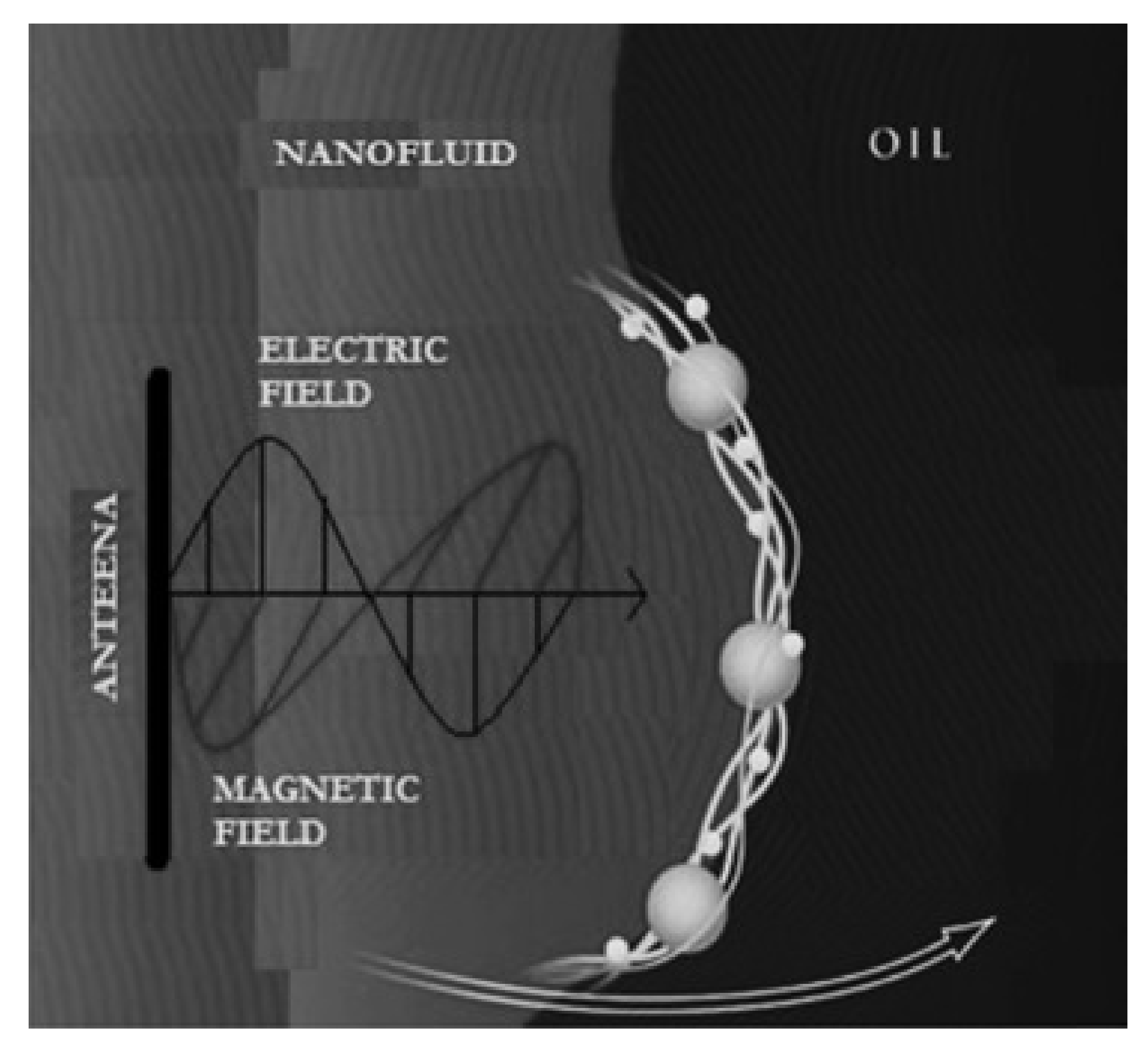
7.1. Electrorheological Effect
7.2. Oil Droplet Deformation
8. Challenges
- Most of the experimental analysis using dielectric and magnetic nanofluids were laboratory-scale analysis, in the sense that they were conducted under ambient temperature and had no practical applicability.
- Field application has shown that direct heating of the reservoir via radio frequency EM heating has been executed with an essential outcome by improving the heavy oils. Such fieldwork was conducted for the first time in 1992 in the USA, Canada, and Russia, despite the limited work in that regard. However, EM-assisted nanofluids remain a great challenge in field application because most of the NPs studied so far cannot withstand the harsh situation of the reservoir. This will lead to a situation whereby EM wave propagation in nanofluids will get disturbed and be worthless.
- Another great challenge has to do with nanofluids responses under the influence of EM waves, which required potential computational techniques that demand numerical simulators that will be used to examine essential and analytical modeling that will provide accurate and perfect calculations concerning the heat disaffection and distribution to the reservoir. However, the success of the work is also accredited to the optimum selection and application of the required frequency and power needed by the experiment.
- The high cost of nanoparticles remains problematic, because a huge amount of NPs is required for oil and gas industrial operation.
9. Future Outlook
- Reservoir conditions need to be taken into consideration while conducting experimental analysis in such a way that the laboratory experiment will comply with the field-scale applications. Consequently, proper selection of NPs that can withstand high temperature and high pressure is highly recommended for future analysis while conducting nanofluids flooding at reservoir temperatures, because most of the NPs are temperature sensitive. In addition to that, more nanofluids flooding experiments are required in an advanced manner, because the laboratory experiment is an output manifestation of the fieldwork.
- One of the significant ways to improve ahead of the existing experiment of the NPs on EOR is by modifying the surface of the particles, as a result of which the NPs’ properties can be altered towards the worthy and eligible standard for a particular analysis. This can be achieved by attaching an appropriate polymer/surfactant to the surface of the NPs. However, there is very limited work on that, despite the reasonable outcome displayed, which was why the influential effect of NPs surface modification concerning EOR is not well known and not fully understood. Moreover, polymers or surfactant coating on the surface of the NPs can provide an advanced level of improvement in the reservoir in different ways, such as improving sweep efficiency, solubility, and stability of the nanofluids, smoothness mobility of the fluids in a porous medium, temperature tolerance, etc., which in turn improve the EOR. The NPs type, size, and concentrations played a significant role in this regard. Furthermore, the proper selection of polymer/surfactant is the most important factor by considering some environmental features like temperature, pressure, salinity, etc. Otherwise, the wrong selection of an appropriate polymer/surfactant can lead to low recovery, and indeed it can be disadvantageous and extremely detrimental to the reservoir rocks, as they can block the reservoir rock pores.
- It is recommended to make further investigations with regards to the theoretical and analytical modeling to make accurate calculations concerning the required heat and frequency that favors the reservoir situation. Moreover, creating proper nanosensors that will be injected in the reservoir is also required which could help in creating optimum, significant, and effective signals to be applied during the exercise for the attainment of appropriate goals of improving oil productivity. It is also a logical and good idea to investigate the possible situation in which the sensors embedded with NPs can be used in that regard.
- The high cost of the NPs issue can be tackled by improving the major sources of forming the particles, thereby creating innovative cheaper raw materials that are cost-effective, electromagnetic field responsive, and environmentally feasible.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adam, A.A.; Dennis, J.O.; Al-Hadeethi, Y.; Mkawi, E.M.; Abdulkadir, B.A.; Usman, F.; Hassan, Y.M.; Wadi, I.A.; Sani, M. State of the Art and New Directions on Electrospun Lignin/Cellulose Nanofibers for Supercapacitor Application: A Systematic Literature Review. Polymers 2020, 12, 2884. [Google Scholar] [CrossRef] [PubMed]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Abidin, A.; Puspasari, T.; Nugroho, W. Polymers for Enhanced Oil Recovery Technology. Procedia Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of Nanoparticles in Enhanced Oil Recovery: A Critical Review of Recent Progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Zhang, Z.; Wang, Q.; Xu, Z.; Guo, Z.; Sun, H.; Cao, X.; Qiao, Q. Advances in Polymer Flooding and Alkaline/Surfactant/Polymer Processes as Developed and Applied in the People’s Republic of China. J. Pet. Technol. 2006, 58, 84–89. [Google Scholar] [CrossRef]
- Vargo, J.; Turner, J.; Bob, V.; Pitts, M.J.; Wyatt, K.; Surkalo, H.; Patterson, D. Alkaline-Surfactant-Polymer Flooding of the Cambridge Minnelusa Field. Soc. Pet. Eng. 1999. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Eshraghi, S.E.; Bahrami, P.; Hasanbeygi, M.; Kazemzadeh, Y.; Vahedian, A. Comprehensive Water–Alternating-Gas (WAG) injection study to evaluate the most effective method based on heavy oil recovery and asphaltene precipitation tests. J. Pet. Sci. Eng. 2015, 133, 123–129. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Zhang, Y.; Maini, B.B. Enhanced heavy oil recovery in thin reservoirs using foamy oil-assisted methane huff-n-puff method. Fuel 2015, 159, 962–973. [Google Scholar] [CrossRef]
- AlHomadhi, E.; Amro, M.; Almobarky, M. Experimental application of ultrasound waves to improved oil recovery during waterflooding. J. King Saud Univ. Eng. Sci. 2014, 26, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Sahni, A.; Kumar, M.; Knapp, R.B. Electromagnetic heating methods for heavy oil reservoirs. Soc. Pet. Eng. 2000. [Google Scholar] [CrossRef]
- Ovalles, C.; Fonseca, A.; Lara, A.; Alvarado, V.; Urrecheaga, K.; Ranson, A.; Mendoza, H. Opportunities of downhole dielectric heating in venezuela: Three case studies involving medium, heavy and extra-heavy crude oil reservoirs. Soc. Pet. Eng. 2002. [Google Scholar] [CrossRef]
- Fanchi, J.R. Feasibility of reservoir heating by electromagnetic irradiation. Soc. Pet. Eng. 1990. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Mohammadi, A.H. Recent advances in application of nanotechnology in chemical enhanced oil recovery: Effects of nanoparticles on wettability alteration, interfacial tension reduction, and flooding. Egypt. J. Pet. 2018, 27, 1371–1383. [Google Scholar] [CrossRef]
- Firozjaii, A.M.; Saghafi, H.R. Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation. Petroleum 2020, 6, 115–122. [Google Scholar] [CrossRef]
- Suleimanov, B.A.; Ismailov, F.; Veliyev, E. Nanofluid for enhanced oil recovery. J. Pet. Sci. Eng. 2011, 78, 431–437. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced Oil Recovery Using Nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar] [CrossRef] [Green Version]
- Zanganeh, P.; Ayatollahi, S.; Alamdari, A.; Zolghadr, A.; Dashti, H.; Kord, S. Asphaltene Deposition during CO2 Injection and Pressure Depletion: A Visual Study. Energy Fuels 2012, 26, 1412–1419. [Google Scholar] [CrossRef]
- Borton, D.; Pinkston, D.S.; Hurt, M.R.; Tan, X.; Azyat, K.; Scherer, A.; Tykwinski, R.; Gray, M.; Qian, K.; Kenttämaa, H.I. Molecular Structures of Asphaltenes Based on the Dissociation Reactions of Their Ions in Mass Spectrometry. Energy Fuels 2010, 24, 5548–5559. [Google Scholar] [CrossRef]
- Joonaki, E.; Buckman, J.; Burgass, R.; Tohidi, B. Water versus Asphaltenes; Liquid–Liquid and Solid–Liquid Molecular Interactions Unravel the Mechanisms behind an Improved Oil Recovery Methodology. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sheu, E.Y.; Mullins, O.C. Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Kazemzadeh, Y.; Parsaei, R.; Riazi, M. Experimental study of asphaltene precipitation prediction during gas injection to oil reservoirs by interfacial tension measurement. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 466, 138–146. [Google Scholar] [CrossRef]
- Tharanivasan, A.K.; Yarranton, H.W.; Taylor, S.D. Asphaltene Precipitation from Crude Oils in the Presence of Emulsified Water. Energy Fuels 2012, 26, 6869–6875. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Chen, A.; Sung, C.-A.; Kidder, K.M.; Lee, J.J.; Alhassan, S.M.; Vargas, F.M. Effect of Emulsified Water on Asphaltene Instability in Crude Oils. Energy Fuels 2016, 30, 3676–3686. [Google Scholar] [CrossRef]
- Aslan, S.; Firoozabadi, A. Effect of Water on Deposition, Aggregate Size, and Viscosity of Asphaltenes. Langmuir 2014, 30, 3658–3664. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Sabio, J.C.; Yen, A.; Joshi, N.; Hartman, R.L. Role of Water on the Precipitation and Deposition of Asphaltenes in Packed-Bed Microreactors. Ind. Eng. Chem. Res. 2015, 54, 4103–4112. [Google Scholar] [CrossRef]
- Fakoya, M.F.; Shah, S.N. Emergence of nanotechnology in the oil and gas industry: Emphasis on the application of silica nanoparticles. Petroleum 2017, 3, 391–405. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Aqueous dispersions of metallic nanoparticles: Preparation, stabilization and application. In Nanoscience: Colloidal and Interfacial Aspects; CRC Press: Boca Raton, FL, USA, 2010; pp. 747–778. [Google Scholar]
- Sekoai, P.T.; Ouma, C.N.M.; Du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Riley, M.; Young, S.; Stamatakis, E.; Guo, Q.; Ji, L.; De Stefano, G.; Price, K.; Friedheim, J. Wellbore Stability in Unconventional Shales—The Design of a Nano-Particle Fluid. In Proceedings of the SPE Oil and Gas India Conference and Exhibition, Mumbai, India, 28–30 March 2012. [Google Scholar]
- Wilson, M.; Kannangara, K.; Smith, G.; Simmons, M.; Raguse, B. Nanotechnology: Basic Science and Emerging Technologies; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Application of nanotechnology for enhancing oil recovery—A review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Engeset, B. The Potential of Hydrophilic Silica Nanoparticles for EOR Purposes: A Literateur Review and an Experimental Study. Master’s Thesis, Department of Petroleum Engineering and Applied Geophysics, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2012. [Google Scholar]
- Kazemzadeh, Y.; Shojaei, S.; Riazi, M.; Sharifi, M. Review on application of nanoparticles for EOR purposes: A critical review of the opportunities and challenges. Chin. J. Chem. Eng. 2019, 27, 237–246. [Google Scholar] [CrossRef]
- Zhang, T.; Davidson, D.; Bryant, S.L.; Huh, C. Nanoparticle-stabilized emulsions for applications in enhanced oil recovery. Soc. Pet. Eng. 2010. [Google Scholar] [CrossRef]
- Suleimanov, B.A.; Abbasov, H.F. Effect of copper nanoparticle aggregation on the thermal conductivity of nanofluids. Russ. J. Phys. Chem. A 2016, 90, 420–428. [Google Scholar] [CrossRef]
- Ko, S.; Huh, C. Use of nanoparticles for oil production applications. J. Pet. Sci. Eng. 2019, 172, 97–114. [Google Scholar] [CrossRef]
- Kumar, H.; Venkatesh, N.; Bhowmik, H.; Kuila, A. Metallic nanoparticle: A review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Effects of Nano-Sized Metals on Viscosity Reduction of Heavy Oil/Bitumen During Thermal Applications. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Rodríguez, J.A.; Fernández-García, M. Synthesis, Properties, and Applications of Oxide Nanomaterials; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Agista, M.N.; Guo, K.; Yu, Z. A State-of-the-Art Review of Nanoparticles Application in Petroleum with a Focus on Enhanced Oil Recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef] [Green Version]
- Bayat, A.E.; Junin, R.; Samsuri, A.; Piroozian, A.; Hokmabadi, M. Impact of Metal Oxide Nanoparticles on Enhanced Oil Recovery from Limestone Media at Several Temperatures. Energy Fuels 2014, 28, 6255–6266. [Google Scholar] [CrossRef]
- Zaid, H.M.; Latiff, N.R.A.; Yahya, N.; Soleimani, H.; Shafie, A. Application of Electromagnetic Waves and Dielectric Nanoparticles in Enhanced Oil Recovery. J. Nano Res. 2013, 26, 135–142. [Google Scholar] [CrossRef]
- Lee, K.C.; Bin Saipolbahri, Z.A.; Soleimani, H.; Zaid, H.M.; Guan, B.H.; Ching, D.L.C. Effect of Zinc Oxide Nanoparticle Sizes on Viscosity of Nanofluid for Application in Enhanced Oil Recovery. J. Nano Res. 2016, 38, 36–39. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y.; Goddard, W.A. New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. J. Pet. Sci. Eng. 2010, 71, 23–29. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Li, S.; Torsaeter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Pet. Sci. Eng. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Adil, M.; Lee, K.; Zaid, H.M.; Latiff, N.R.A.; Alnarabiji, M.S. Experimental study on electromagnetic-assisted ZnO nanofluid flooding for enhanced oil recovery (EOR). PLoS ONE 2018, 13, e0193518. [Google Scholar] [CrossRef]
- Torsater, O.; Engeset, B.; Hendraningrat, L.; Suwarno, S. Improved Oil Recovery by Nanofluids Flooding: An Experimental Study. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Society of Petroleum Engineers (SPE), Kuwait City, Kuwait, 10–12 December 2012. [Google Scholar]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2015, 5, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Almahfood, M.; Bai, B. The synergistic effects of nanoparticle-surfactant nanofluids in EOR applications. J. Pet. Sci. Eng. 2018, 171, 196–210. [Google Scholar] [CrossRef]
- Arashiro, E.Y.; Demarquette, N.R. Use of the pendant drop method to measure interfacial tension between molten polymers. Mater. Res. 1999, 2, 23–32. [Google Scholar] [CrossRef]
- Lee, K.; Adil, M.; Zaid, H.M.; Guan, B.H.; Soleimani, H.; Weis, M. Wettability, Interfacial Tension (IFT) and Viscosity Alteration of Nanofluids Under Electromagnetic (EM) Waves for Enhanced Oil Recovery (IFT) Applications. In Bioactive Natural Products for Pharmaceutical Applications; Springer Science and Business Media LLC: Berlin, Germany, 2018; pp. 305–311. [Google Scholar]
- Rezvani, H.; Riazi, M.; Tabaei, M.; Kazemzadeh, Y.; Sharifi, M. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@ Chitosan nanocomposites. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 544, 15–27. [Google Scholar] [CrossRef]
- Moslan, M.S.; Sulaiman, W.R.W.; Ismail, A.R.; Jaafar, M.Z. Applications of aluminium oxide and zirconium oxide nanoparticles in altering dolomite rock wettability using different dispersing medium. Chem. Eng. Trans. 2017, 56, 1339–1344. [Google Scholar]
- Ali, H.; Soleimani, H.; Yahya, N.; Khodapanah, L.; Sabet, M.; Demiral, B.M.; Hussain, T.; Adebayo, L.L. Enhanced oil recovery by using electromagnetic-assisted nanofluids: A review. J. Mol. Liq. 2020, 309, 113095. [Google Scholar] [CrossRef]
- Esmaeilnezhad, E.; Le Van, S.; Chon, B.H.; Choi, H.J.; Schaffie, M.; Gholizadeh, M.; Ranjbar, M. An experimental study on enhanced oil recovery utilizing nanoparticle ferrofluid through the application of a magnetic field. J. Ind. Eng. Chem. 2018, 58, 319–327. [Google Scholar] [CrossRef]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Stephen, K.D. Potential application of low-salinity polymeric-nanofluid in carbonate oil reservoirs: IFT reduction, wettability alteration, rheology and emulsification characteristics. J. Mol. Liq. 2019, 284, 735–747. [Google Scholar] [CrossRef]
- Adil, M.; Zaid, H.M.; Chuan, L.K. Electromagnetically-induced change in interfacial tension and contact angle of oil droplet using dielectric nanofluids. Fuel 2020, 259, 116274. [Google Scholar] [CrossRef]
- Bila, A.; Stensen, J.Å.; Torsæter, O. Experimental Investigation of Polymer-Coated Silica Nanoparticles for Enhanced Oil Recovery. Nanomaterials 2019, 9, 822. [Google Scholar] [CrossRef] [Green Version]
- Negin, C.; Ali, S.; Xie, Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum 2017, 3, 197–211. [Google Scholar] [CrossRef]
- ShamsiJazeyi, H.; Miller, C.A.; Wong, M.S.; Tour, J.M.; Verduzco, R. Polymer-coated nanoparticles for enhanced oil recovery. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Alvarez, N.J.; Anna, S.L.; Saigal, T.; Tilton, R.D.; Walker, L.M. Interfacial Dynamics and Rheology of Polymer-Grafted Nanoparticles at Air–Water and Xylene–Water Interfaces. Langmuir 2012, 28, 8052–8063. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Wang, S.; Li, Y.; Gao, M.; Liu, Y.; Sun, Y.; Zhao, M. The first study of surface modified silica nanoparticles in pressure-decreasing application. RSC Adv. 2015, 5, 61838–61845. [Google Scholar] [CrossRef]
- Choi, S.K.; Son, H.A.; Kim, H.T.; Kim, J.W. Nanofluid Enhanced Oil Recovery Using Hydrophobically Associative Zwitterionic Polymer-Coated Silica Nanoparticles. Energy Fuels 2017, 31, 7777–7782. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, M.; Dai, C.; Li, Y.; Lv, W. The Study of a Novel Modified Silica Nanofluid for Pressure-Decreasing Application in the Ultra-Low Permeable Formation. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition; Society of Petroleum Engineers (SPE), Jakarta, Indonesia, 17–19 October 2017. [Google Scholar]
- Wei, L.; Zhu, J.; Qi, J. Application of nano-nickel catalyst in the viscosity reduction of Liaohe extra-heavy oil by aqua-thermolysis. J. Fuel Chem. Technol. 2007, 35, 176–180. [Google Scholar]
- Adil, M.; Zaid, H.M.; Chuan, L.K.; Latiff, N.R.A. Influence of electromagnetic waves on viscosity and electrorheology of dielectric nanofluids-scale-based approach. J. Teknol. 2016, 78. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability Alteration in Carbonates using Zirconium Oxide Nanofluids: EOR Implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Moslan, M.S.; Sulaiman, W.R.W.; Ismail, A.R.; Jaafar, M.Z.; Ismail, I. Wettability Alteration of Dolomite Rock Using Nanofluids for Enhanced Oil Recovery. Mater. Sci. Forum 2016, 864, 194–198. [Google Scholar] [CrossRef]
- Shah, R.D. Application of Nanoparticle Saturated Injectant Gases for EOR of Heavy Oils. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V.; Ghazanfari, M.H. Enhanced Heavy Oil Recovery in Sandstone Cores Using TiO2 Nanofluids. Energy Fuels 2014, 28, 423–430. [Google Scholar] [CrossRef]
- Kasevich, R.; Price, S.; Faust, D.; Fontaine, M. Pilot testing of a radio frequency heating system for enhanced oil recovery from diatomaceous earth. Soc. Pet. Eng. 1994. [Google Scholar] [CrossRef]
- Bera, A.; Babadagli, T. Status of electromagnetic heating for enhanced heavy oil/bitumen recovery and future prospects: A review. Appl. Energy 2015, 151, 206–226. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Islam, M.R. A Critical Review of Electromagnetic Heating for Enhanced Oil Recovery. Pet. Sci. Technol. 2008, 26, 1619–1631. [Google Scholar] [CrossRef]
- Soleimani, H.; Latiff, N.R.A.; Yahya, N.; Zaid, H.M.; Sabet, M.; Guan, B.H.; Lee, K.C. Effect of Annealing Temperature on the Crystallization of Hematite-Alumina (Fe2O3-Al2O3) Nanocomposite and its Influence in EOR Application. J. Nano Res. 2014, 29, 105–113. [Google Scholar] [CrossRef]
- Yahya, N.; Kashif, M.; Nasir, N.; Akhtar, M.N.; Yusof, N.M. Cobalt Ferrite Nanoparticles: An Innovative Approach for Enhanced Oil Recovery Application. J. Nano Res. 2012, 17, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.; Huh, C.; Bryant, S.L. Focused Magnetic Heating Utilizing Superparamagnetic Nanoparticles for Improved Oil Production Applications. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Soleimani, H.; Yahya, N.; Latiff, N.R.A.; Zaid, H.M.; Demiral, B.; Amighian, J. Novel Enhanced Oil Recovery Method Using Co2+xFe2+1-xFe3+2O4 as Magnetic Nanoparticles Activated by Electromagnetic Waves. J. Nano Res. 2013, 26, 111–116. [Google Scholar] [CrossRef]
- Haroun, M.R.; Alhassan, S.M.; Ansari, A.A.; Al Kindy, N.A.M.; Sayed, N.A.; Kareem, B.A.A.; Sarma, H.K. Smart Nano-EOR Process for Abu Dhabi Carbonate Reservoirs. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Zaid, H.M.; Adil, M.; Lee, K.; Latiff, N.R. Influence of Frequency-Dependent Dielectric Loss on Electrorheology of Surface Modified ZnO Nanofluids. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Amsterdam, The Netherlands, 2018; Volume 350, p. 012014. [Google Scholar]
- Latiff, N.R.A.; Yahya, N.; Zaid, H.M.; Demiral, B. Novel enhanced oil recovery method using dielectric zinc oxide nanoparticles activated by electromagnetic waves. In Proceedings of the 2011 National Postgraduate Conference; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2011; pp. 1–7. [Google Scholar]
- Yahya, N.; Kashif, M.; Shafie, A.; Soleimani, H.; Zaid, H.M.; Latiff, N.R.A. Improved Oil Recovery by High Magnetic Flux Density Subjected to Iron Oxide Nanofluids. J. Nano Res. 2013, 26, 89–99. [Google Scholar] [CrossRef]
- Yusmaniar, Y.; Hutomo, D.; Handoko, E.J.M. Electromagnetic wave absorbing properties of husk silica-based SiO2/Fe3O4/UPR composite. Mater. Sci. Eng. Conf. Ser. 2018, 434, 012081. [Google Scholar] [CrossRef]
- Soleimani, H.; Latiff, N.R.A.; Yahya, N.; Zaid, H.M.; Sabet, M.; Lee, K.C.; Adil, M. Magnetization of Ferrofluid and its Influence on Improving Oil Recovery. Defect Diffusion Forum 2019, 390, 161–167. [Google Scholar] [CrossRef]
- Zaid, H.M.; Azahar, W.W.; Soleimani, H.; Latiff, N.A.; Shafie, A.; Lee, K.C.; Beh, H. Effect of Nickel: Zinc Ratio in Nickel-Zinc-Ferrite Nanoparticles as Surfactant on Recovery Efficiency in Enhanced Oil Recovery. J. Nano Res. 2014, 29, 115–120. [Google Scholar] [CrossRef]
- Tarek, M. Investigating Nano-Fluid Mixture Effects to Enhance Oil Recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015. [Google Scholar]
- Kazemzadeh, Y.; Sharifi, M.; Riazi, M.; Rezvani, H.; Tabaei, M. Potential effects of metal oxide/SiO2 nanocomposites in EOR processes at different pressures. Colloids Surfaces A Physicochem. Eng. Aspects. 2018, 559, 372–384. [Google Scholar] [CrossRef]
- Divandari, H.; Hemmati-Sarapardeh, A.; Schaffie, M.; Ranjbar, M. Integrating synthesized citric acid-coated magnetite nanoparticles with magnetic fields for enhanced oil recovery: Experimental study and mechanistic understanding. J. Pet. Sci. Eng. 2019, 174, 425–436. [Google Scholar] [CrossRef]
- Soleimani, H.; Latiff, N.R.A.; Yahya, N.; Sabet, M.; Khodapanah, L.; Kozlowski, G.; Chuan, L.K.; Guan, B.H. Synthesis and Characterization of Yttrium Iron Garnet (YIG) Nanoparticles Activated by Electromagnetic Wave in Enhanced Oil Recovery. J. Nano Res. 2016, 38, 40–46. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Dehdari, B.; Etemadan, Z.; Riazi, M.; Sharifi, M. Experimental investigation into Fe3O4/SiO2 nanoparticle performance and comparison with other nanofluids in enhanced oil recovery. Pet. Sci. 2019, 16, 578–590. [Google Scholar] [CrossRef] [Green Version]
- Vekas, L. Magnetic nanofluids properties and some applications. Rom. J. Phys. 2004, 49, 707–721. [Google Scholar]
- Baig, M.K.; Soleimani, H.; Yahya, N. Domain wall motion and Barkhausen effect in magnetic nanoparticles for EOR applications. In Proceedings of the 4th International Conference on Fundamental and Applied Sciences (Icfas2016); AIP Publishing: College Park, MD, USA, 2016; Volume 1787, p. 50015. [Google Scholar]
- Mukhametshina, A.; Martynova, E. Electromagnetic heating of heavy oil and bitumen: A review of experimental studies and field applications. J. Pet. Eng. 2013. [Google Scholar] [CrossRef]
- Adil, M.; Zaid, H.M.; Chuan, L.K.; Latiff, N.R.A. Effect of EM propagation medium on electrorheological characteristics of dielectric nanofluids. J. Dispers. Sci. Technol. 2016, 38, 570–576. [Google Scholar] [CrossRef]
- Primo, V.A.; Pérez-Rosa, D.; Garcia, B.; Cabanelas, J.C. Evaluation of the Stability of Dielectric Nanofluids for Use in Transformers under Real Operating Conditions. Nanomaterials 2019, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Yahya, N.; Qureshi, S. Interactions of ferro-nanoparticles (hematite and magnetite) with reservoir sandstone: Implications for surface adsorption and interfacial tension reduction. Pet. Sci. 2020, 17, 1037–1055. [Google Scholar] [CrossRef] [Green Version]
- Kothari, N.; Raina, B.; Chandak, K.B.; Iyer, V.; Mahajan, H.P. Application Of Ferrofluids For Enhanced Surfactant Flooding In IOR. In Proceedings of the SPE EUROPEC/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010. [Google Scholar]
- Chuan, L.K.; Guan, B.H.; Yusuf, Y.; Zainuddin, A.A.; Auni, N. Interfacial Tension and Contact Angle Alteration of Nanofluids by using Lanthanum Substituted Manganese-Zinc Ferrite Nanoparticles. DJNB 2019, 14, 1–5. [Google Scholar]
- Lau, Z.Y.; Lee, K.; Soleimani, H.; Beh, H.G. Experimental Study of Electromagnetic-Assisted Rare-Earth Doped Yttrium Iron Garnet (YIG) Nanofluids on Wettability and Interfacial Tension Alteration. Energies 2019, 12, 3806. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Sukri, M.N.M.; Guan, B.H.; Zaid, H.M.; Soleimani, H. Interfacial Tension and Viscosity Alteration of Samarium Doped Yttrium Iron Garnet (YIG) Nanofluid under the Presence of Electromagnetic Waves. Defect Diffus. Forum 2019, 390, 64–70. [Google Scholar] [CrossRef]
- Lee, K.; Shuhaili, F.M.; Zaid, H.M.; Guan, B.H. Neodymium (Nd) Doped Yttrium Iron Garnet (YIG) Nanofluid Activated By Electromagnetic Waves for Enhanced Oil Recovery (EOR). J. Phys. Conf. Ser. 2018, 1123, 012011. [Google Scholar] [CrossRef]
- Izadi, N.; Koochi, M.M.; Amrollahi, A.; Pourkhalil, M. Investigation of functionalized polyelectrolyte polymer-coated Fe3O4 nanoparticles stabilized in high salinity brine at high temperatures as an EOR agent. J. Pet. Sci. Eng. 2019, 178, 1079–1091. [Google Scholar] [CrossRef]
- Chang, H.; Chang, Y.-C. Fabrication of Al2O3 nanofluid by a plasma arc nanoparticles synthesis system. J. Mater. Process. Technol. 2008, 207, 193–199. [Google Scholar] [CrossRef]
- Tsuzuki, T.; McCormick, P.G. Mechanochemical synthesis of nanoparticles. J. Mater. Sci. 2004, 39, 5143–5146. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Tangsir, S.; Koponen, H.; Riikonen, J.; Karhunen, T.; Tapper, U.; Lehto, V.-P.; Moazed, H.; Naseri, A.A.; Hooshmand, A.; et al. Synthesis and characterization of Al2O3 nanoparticles by flame spray pyrolysis (FSP)—Role of Fe ions in the precursor. Powder Technol. 2016, 298, 42–49. [Google Scholar] [CrossRef]
- Ali, H.; Soleimani, H.; Yahya, N.; Lorimer, S.; Sabet, M.; Demiral, B.M.R.; Adebayo, L.L. Absorption of electromagnetic waves in sandstone saturated with brine and nanofluids for application in enhanced oil recovery. J. Taibah Univ. Sci. 2020, 14, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.; Huang, X.; Yang, S.; Lu, K.; Sheng, P. The giant electrorheological effect in suspensions of nanoparticles. Nat. Mater. 2003, 2, 727–730. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Gupta, A.K.; Basu, S. Deformation of an oil droplet on a solid substrate in simple shear flow. Chem. Eng. Sci. 2008, 63, 5496–5502. [Google Scholar] [CrossRef]
- Cui, M.; Emrick, T.; Russell, T.P. Stabilizing Liquid Drops in Nonequilibrium Shapes by the Interfacial Jamming of Nanoparticles. Science 2013, 342, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Zaid, H.M.; Adil, M.; Chuan, L.K.; Latiff, N.R.A. Stability and electrorheology of ZnO nanofluids in the presence of anionic surfactants. In Proceedings of the 4th International Conference on Fundamental and Applied Sciences (Icfas2016); AIP Publishing: College Park, MD, USA, 2016; Volume 1787, p. 050007. [Google Scholar]
- Zaid, H.M.; Yahya, N.; Latiff, N.R.A. The Effect of Nanoparticles Crystallite Size on the Recovery Efficiency in Dielectric Nanofluid Flooding. J. Nano Res. 2012, 21, 103–108. [Google Scholar] [CrossRef]


| Nanoparticles (NPs) | NPs Size | Interfacial Tension (IFT) (mN/m) | Porous Media | Fluids | Remark | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Clean | With NP | Clean | With NP | ||||||
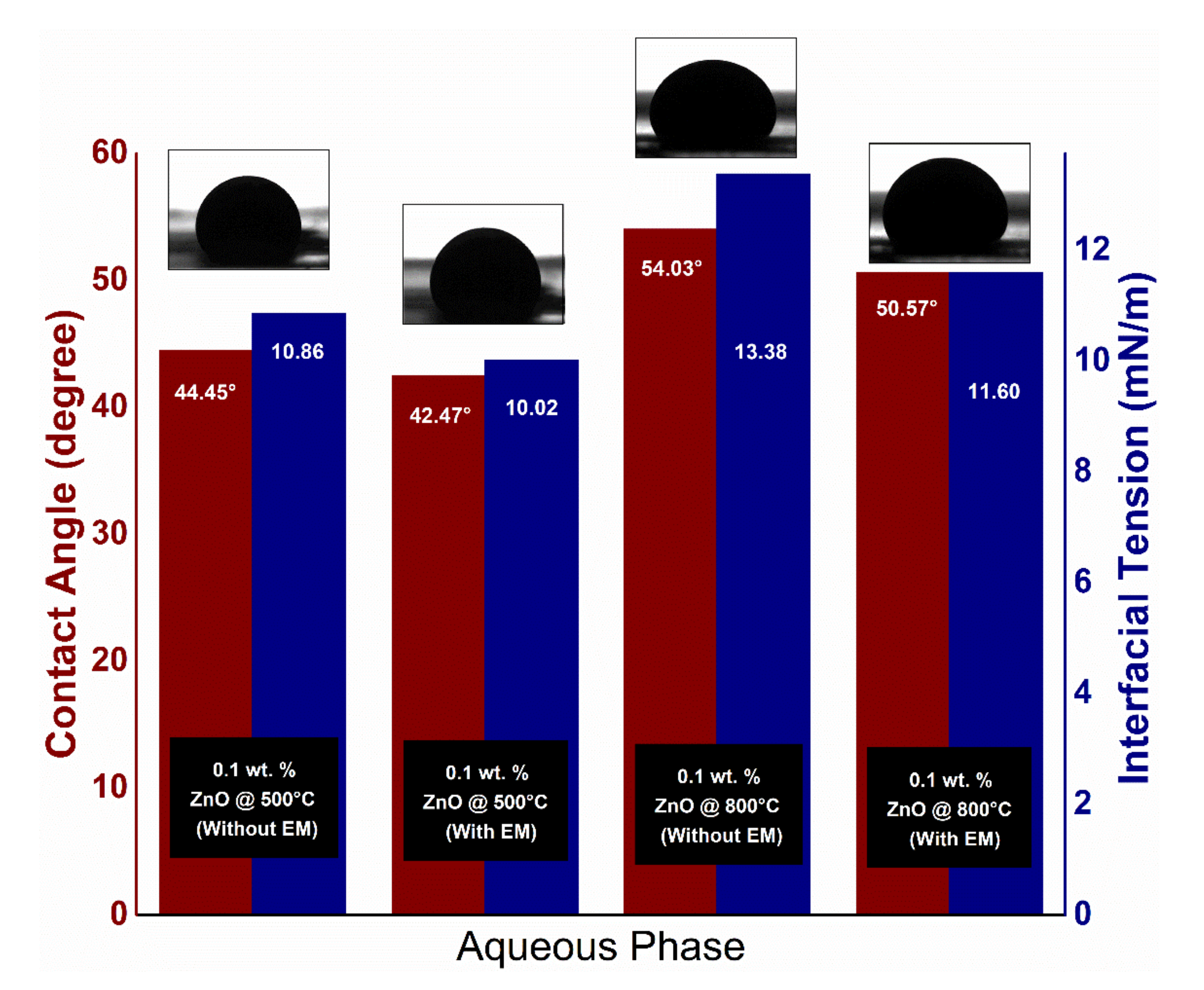
| ZnO | 117.1 | 13.38 | 11.60 | 54 | 50 | Sandstone | Brine | Electromagnetic wave has influenced | [49] |
| Fe2O3 | 20–35 | 38.50 | 2.75 | 132 | 103 | Core plugs | Propanol | IFT is the dominant factor | [2] |
| Al2O3 | 40 | 38.50 | 2.250 | 131 | 94 | Core plugs | Propanol | performance was satisfactory | [2] |
| SiO2 | 10–30 | 38.50 | 1.450 | 134 | 82 | Core plugs | Propanol | SiO2 was treated with silane | [2] |
| SiO2 | 40 | 19.20 | 17.50 | 131 | 38 | Quartz plate | Brine | Performance was satisfactory | [51] |
| Al2O3 | 17 | 19.20 | 12.80 | 131 | 28 | Quartz plate | Brine | IFT doesn’t reflect in Enhanced Oil Recovery (EOR) | [51] |
| TiO2 | 21 | 19.20 | - | 131 | 21 | Quartz plate | Brine | The nanofluids were mixed with Polyvinylpyrrolidone (PVP) polymer with significant results. | [51] |
| TiO2 | 10–30 | 21.10 | 17.50 | 57 | 46 | Limestone | Brine | Lower adsorption on the surface of limestone was observed. | [44] |
| Al2O3 | 40 | 26.5 | 18 | 71 | 61 | Limestone | Brine | The adsorption capacity was law | [44] |
| SiO2 | 20 | 26.5 | 17 | 26 | 18 | Limestone | Brine | High adsorption on limestone rock was observed, consequently, EOR was improved better. | [44] |
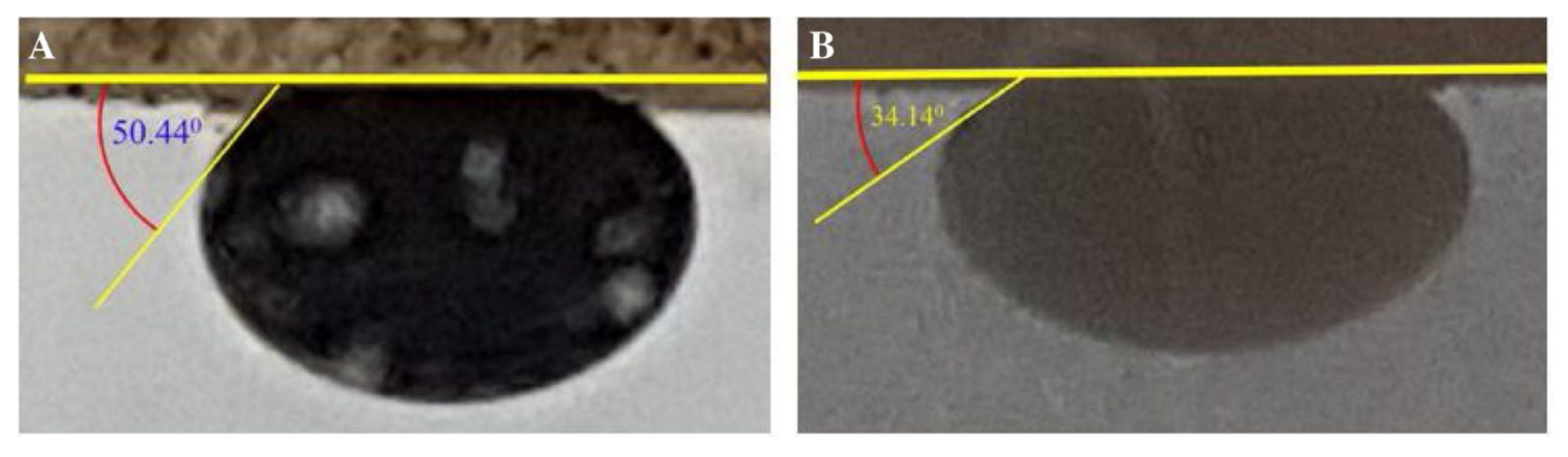
| Ferrite NPs | 200–500 | - | - | 50.44 | 34.14 | Sandstone | Brine | Fluids performed effectively | [58] |
| ZrO2 | 40 | 8.46 | 1.85 | 70 | 60 | Carbonate doomite | Cetyl Trimethyl Ammonium Bromide (CTAB) | Strong water wet was achieved | [56] |
| Al2O3 | 20 | 8.46 | 1.65 | 70 | 52 | Carbonate dolomite | CTAB | Strong water wet was achieved | [56] |
| ZrO2 | 40 | 9.88 | 2.78 | 92 | 84 | Carbonate | sodium dodecyl sulfate (SDS) | The NP performed satisfactory | [56] |
| Al2O3 | 20 | 9.88 | 2.75 | 92 | 75 | Carbonate | SDS | Al2O3 shows better performance than ZrO2 in all dispersion | [56] |
| ZnO/SiO2 | - | 19.68 | 9.45 | 137 | 34 | Carbonate rock | Seawater | Wettability was altered from strong oil-wet to strong water-wet | [59] |
| Fe2O4 | 50 | 30 | 17.3 | 145 | 90 | sandstone | Chitosan | Coating NPs is effective to EOR | [55] |
| NPs/Fluid Base | Oil Type | Rock Type | Recovery (%) | Reference |
|---|---|---|---|---|
| ZnO/brine | Heavy Crude oil | Glass parks | 14.8–26.2 | [83] |
| Co2+x Fe2+ 1-X Fe23+O4/brine | Heavy oil | Sand parks | 11.63–17.44 | [80] |
| Co0.4Fe0.6Fe2O4/brine | Miri Crude oil | Sand parks | 15.83 | [86] |
| Ni1-xZnxFe2O3/brine | Crude oil | Glass parks | 26.07 | [87] |
| ZnO/brine | Crude oil | Sand park | 9.00–10.40 | [49] |
| CoFe2O4/brine | Crude oil | Glass park | 8.70–31.58 | [78] |
| Al2O3/brine | Crude oil | Sand park | 13.3–24.1 | [18] |
| CuO, NiO/brine | Crude oil | Carbonate | 8.19 & 7.59 for CuO & NiO, respectively | [81] |
| SiO2, Al2O3, Fe2O3/brine | Mineral oil | Sandstone | 8.99--20.42 | [88] |
| Fe3O4/SiO2 & TiO2/SiO2/brine | Crude oil | Sand park | 24 & 23 for Fe3O4/SiO2 & TiO2/SiO2, respectively | [89] |
| Fe3O4//brine | Crude oil | Glass micromodel | 22 | [90] |
| Y3Fe5O12/brine | Heavy oil | Sand parks | 43.64 | [91] |
| Fe3O4/SiO2/brine | Crude oil | Glass micromodel | 13.2 | [92] |
| Fe2O3- Al2O3 | Heavy oil | Silica beads | 24.25–30.00 | [77] |
| Al2O3, Fe2O3 & SiO2 | Crude oil | Core plugs | 92.5, 88.6, and 95.3 for Al2O3, Fe2O3 & SiO2 | [2] |
| Nanoparticles | Scope of Study | EM Freq. (MHz) | Outcome | Remark | Ref. |
|---|---|---|---|---|---|
| ZnO | Determination of fluid viscosity, IFT, contact angle & Nano flooding test. | __ | Zinc oxide Nano flooding performed reasonably to EOR under the irradiation of EM waves at a reservoir temperature (95 °C). | Most of the experiments were conducted under ambient temp. It will be essential to have more studies under reservoir temp. | [49] |
| ZnO | Electrorheological effect of ZnO NPs activated by the electric field. | 167 & 18.8 | The viscosity increase was highly dependent on the increment of the applied electric field. | Varying the particle size of the material might influence changes in the fluid viscosity. | [82] |
| ZnO | Nano flooding was irradiated with EM waves | 0.001 | 26.2% of the oil was recovered during EM exposure to the fluids, whereas 14.8% was recovered in the absence of EM waves. | The EM wave facilitates ZnO nanofluids; however, other parameters that influenced the EOR mechanism have not been studied. | [83] |
| ZnO | Viscosity & IFT test | 18.8 | Viscosity and interfacial tension (IFT) were found to have increased with an increase in particle sizes | The crystal size of the material has performed effectively by annealing at different temp. | [46] |
| ZnO | Fluids stability and rheological effect using surfactants | __ | Increasing the surfactants led to the increment in the stability of the dispersed nanoparticles. | The presence of EM waves in the fluids has shown an increment to the viscosity. | [113] |
| ZnO | IFT, viscosity and wettability under EM waves | __ | The presence of EM waves has improved the IFT reduction, and increased fluid viscosity and wettability alteration. | The optimum frequency to be applied during EM wave irradiation is a matter of concern. | [54] |
| ZnO and Al2O3 | IFT and wettability under EM wave irradiation. | __ | IFT was reduced from 13.35 to 8.10 Mn/m for Al2O3 NPs, so the contact angle was equally reduced from 42.76° to 36.01°. | The nanofluids of Al2O3 performed better than those of ZnO nanofluids. | [60] |
| ZnO and Al2O3 | IFT | __ | Crystal sizes of the materials have influenced EOR. | It will be essential to investigate the effect of crystal sizes of the materials on viscosity and wettability. | [114] |
| ZnO and Al2O3 | Electrorheology | 167 | The particles of the dielectric NPs have revealed a very strong attraction at the high electric field. | Tap water, salt water, and air performed effectively as a dispersion media for conveying NPs to the reservoir when EM waves were applied. | [96] |
| ZnO and BiFeO3 | Adsorption capacity of the material. | 8500–12,500 | BiFeO3 nanofluids were observed to be superior in the adsorption capacity, dielectric, and magnetic properties. | BiFeO3 is a very good agent for EOR; hence, more analysis needs to be done by running core flooding analysis, IF, and wettability test. | [108] |
| ZnO and Al2O3 | Electrorheology and viscosity | 167 | The viscosity of the nanofluids for both ZnO and Al2O3 has improved when exposed to EM waves | The EM transmitter used in the analysis was observed to have propagated reasonably at an optimum voltage of 1.5 v. | [69] |
| ZnO and Al2O3 | Fluids Viscosity, wettability test, and nano flooding | 50 | When the EM wave was exposed to the dielectric nanofluids, more oil was recovered. | Nanofluids of ZnO have shown lower IFT compared to that of Al2O3 nanofluids | [45] |
| (CoFe2O4) | Nanofluids flooding analysis | __ | When the EM wave was exposed to the magnetic cobalt ferrite nanofluids, more oil was recovered. | High energy absorbance of the cobalt ferrite NPs leads to reduced oil viscosity, which in turn improves EOR. | [78] |
| Co2+xFe2+ 1-X Fe23+O4 | Core flooding test | 78 | The total recovery efficiency was observed to be 17.44% with EM and 11.63% without EM. | The mechanisms that influence the efficiency of the EOR have not been studied. | [80] |
| Co0.4Fe0.6Fe2O4 | Sand pack flooding | __ | 15.83% of the residual oil was recovered in the presence of a magnetic field, whereas 7.20% was recovered in the absence of a magnetic field. | The presence of the magnetic field leads to the generation of some resistance to the flowing fluids that consequently increased the viscosity of the ferrofluids. | [86] |
| Ni1-xZnxFe2O3 | Core flooding test | __ | 26% was observed to be the highest recovery for EOR at the value of x = 0.5 | More core flooding tests need to be done by varying injection fluids and reservoir rocks. | [87] |
| MnZnLaxFe2-XO4 | Wettability and IFT | __ | IFT reduction and wettability alteration was observed. | This material needs to be studied further on EOR by conducting a flooding test. | [100] |
| Y3Fe5O12 Y = Yttrium iron garnet | Core flooding | 13.6 | Y3Fe5O12 nanofluids flooding recovered 43.64 % when irradiated with EM waves. | The NPs annealed at a high temp. have shown the best recovery. | [91] |
| Y2.8R0.2 Fe5O12 (R = La, Nd, Sm) La = Lanthanum Nd = Neodymium Sm = Samarium | IFT, viscosity and wettability under EM waves | 100 | Wettability was improved in EM exposure to the nanofluids, contrary to IFT and viscosity | Employing material with high-temperature stability will be significant during the prospective analysis. | [101] |
| Y3Fe5O12 | IFT, and viscosity under EM waves | __ | IFT reduction and viscosity were improved more when EM waves were applied. | Y3Fe5O12 is a suitable NP that has performed reasonably for EOR. | [102] |
| Y3-xNdxFe5O12 Nd = Neodymium | IFT, and viscosity under EM waves | 18.8 | IFT reduction and viscosity were improved more when EM waves were applied. | It will be good to study further by varying the crystal structure of the material. | [103] |
| ZnO & Fe2O3 | Core flooding | Good recovery was achieved using a curve antenna with magnetic feeders during nanofluid suspensions. | Electric signal strength using curve antenna with magnetic feeders performed better than the case without magnetic feeders. | [84] | |
| Fe2O3–Al2O3 | Nano flooding test | 13.6 | The composite of hematite and magnetic NPs is essential when irradiated with EM waves for EOR. | More investigation is required of the energy activation fluids for the EOR application. | [77] |
| Fe3O4/SiO2 & TiO2/SiO2 | Sand pack flooding | __ | Fe3O4/SiO2 & TiO2/SiO2 nanofluids increased oil recovery by 24 and 23%, respectively. | Varying pressure can influence and affect the outcome of the oil recovery. | [89] |
| Fe3O4 | Glass micromodel flooding | __ | Coating the surface of the NPs with citric acid improved the IFT and wettability alteration. | If the coated magnetic NPs are stimulated by EM wave radiation, very good results are expected to be discovered. | [90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, Y.M.; Guan, B.H.; Zaid, H.M.; Hamza, M.F.; Adil, M.; Adam, A.A.; Hastuti, K. Application of Magnetic and Dielectric Nanofluids for Electromagnetic-Assistance Enhanced Oil Recovery: A Review. Crystals 2021, 11, 106. https://doi.org/10.3390/cryst11020106
Hassan YM, Guan BH, Zaid HM, Hamza MF, Adil M, Adam AA, Hastuti K. Application of Magnetic and Dielectric Nanofluids for Electromagnetic-Assistance Enhanced Oil Recovery: A Review. Crystals. 2021; 11(2):106. https://doi.org/10.3390/cryst11020106
Chicago/Turabian StyleHassan, Yarima Mudassir, Beh Hoe Guan, Hasnah Mohd Zaid, Mohammed Falalu Hamza, Muhammad Adil, Abdullahi Abbas Adam, and Kurnia Hastuti. 2021. "Application of Magnetic and Dielectric Nanofluids for Electromagnetic-Assistance Enhanced Oil Recovery: A Review" Crystals 11, no. 2: 106. https://doi.org/10.3390/cryst11020106






