Modification of FA0.85MA0.15Pb(I0.85Br0.15)3 Films by NH2-POSS
Abstract
:1. Introduction
2. Experimental
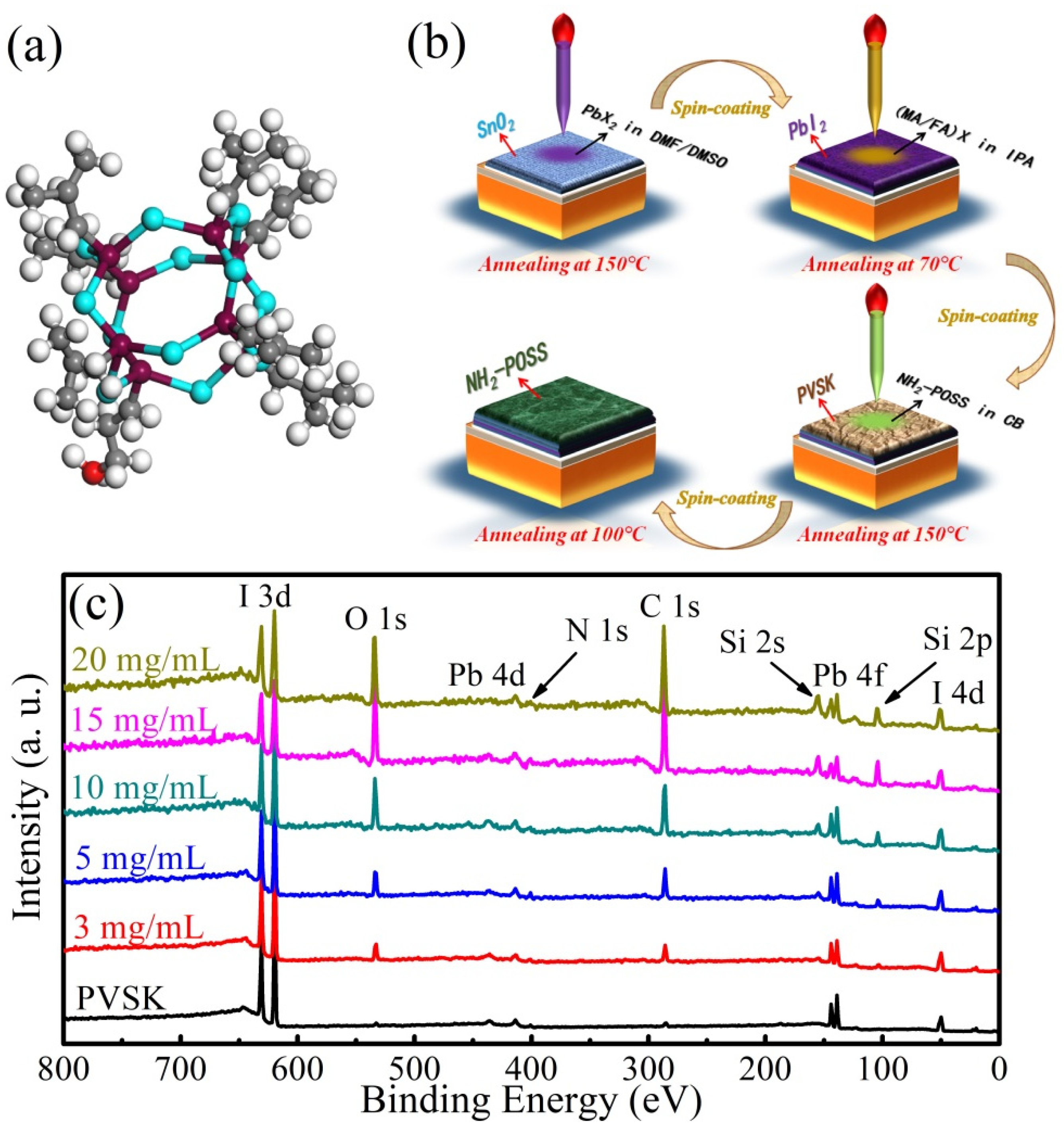
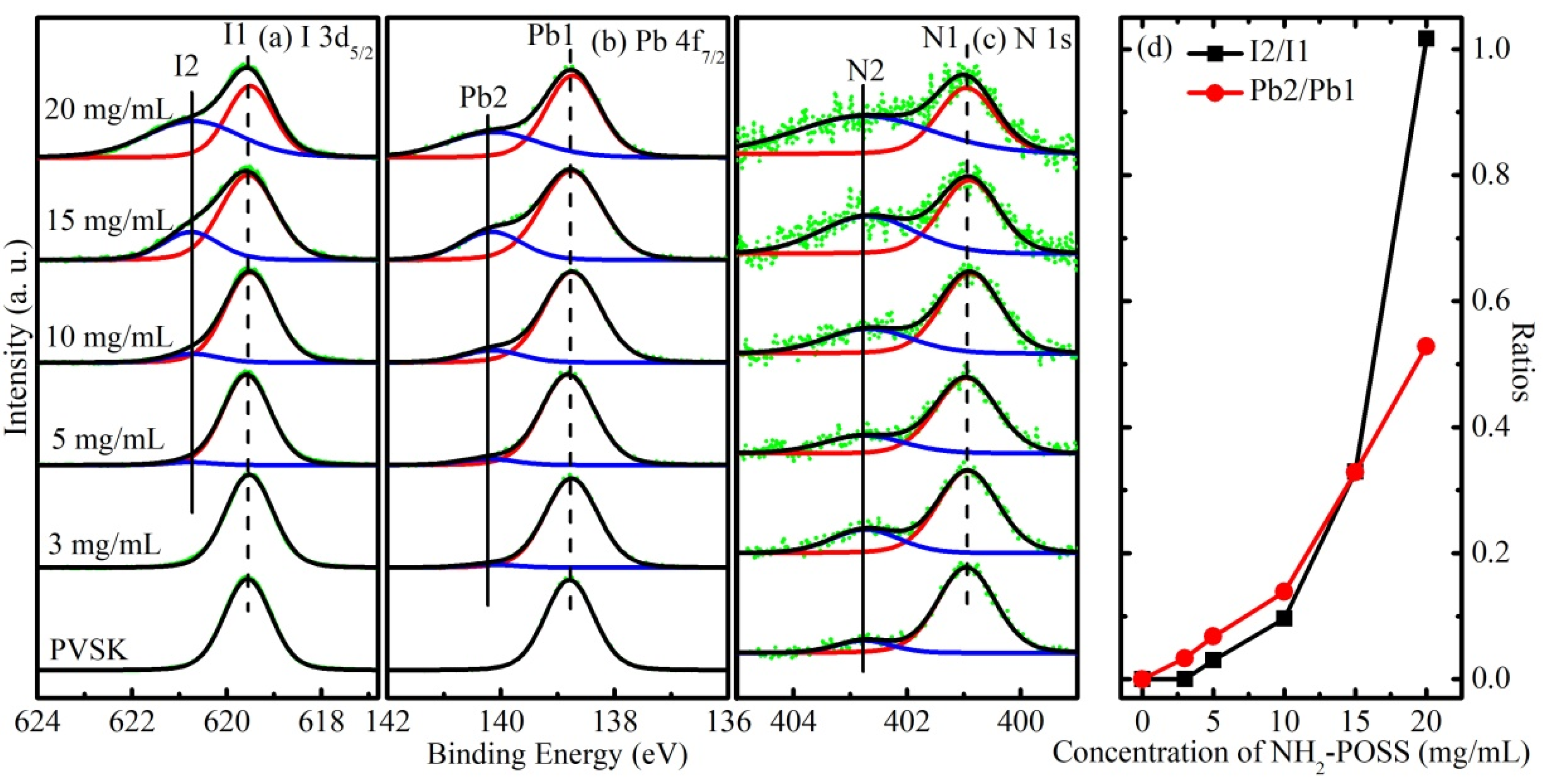
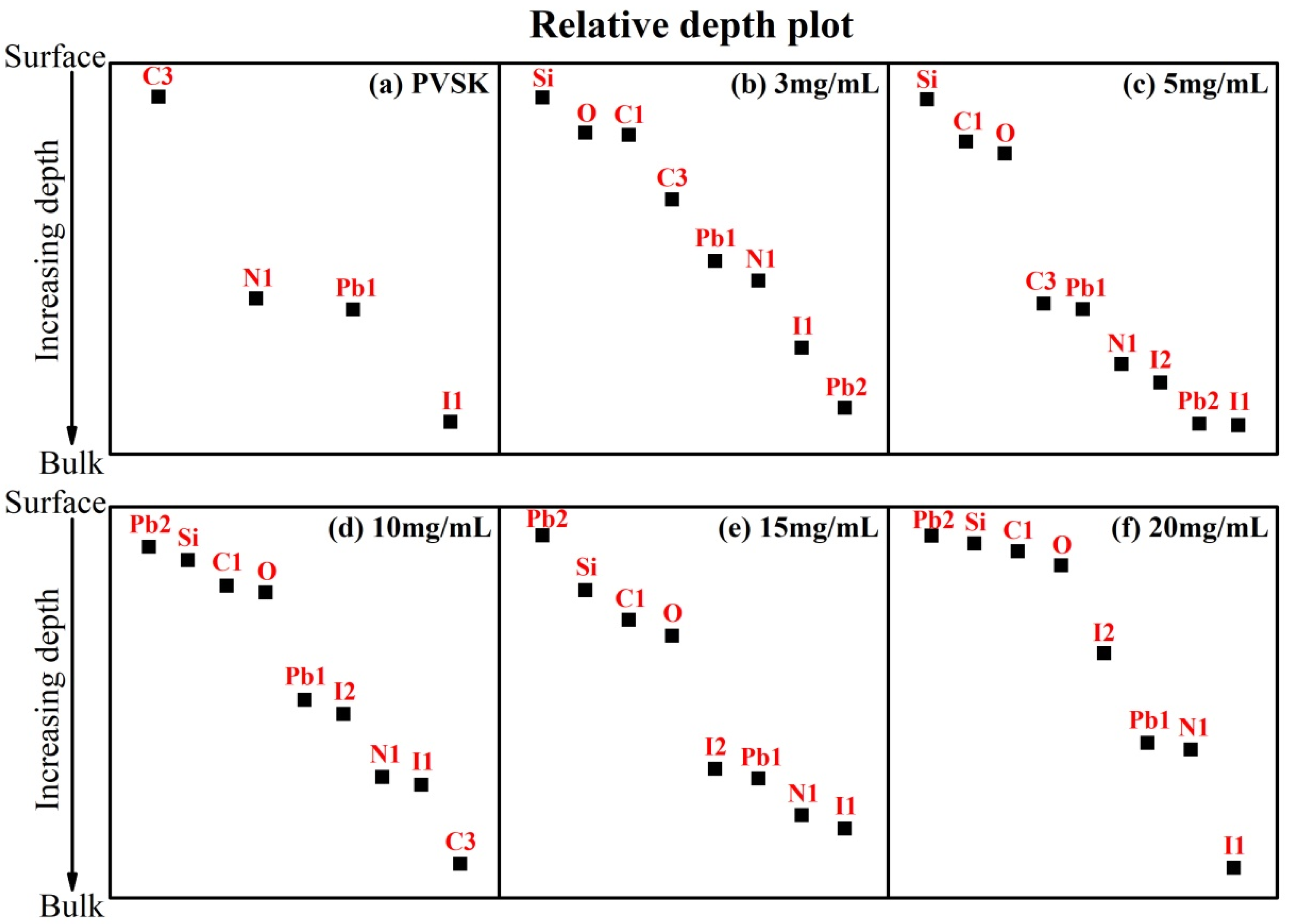
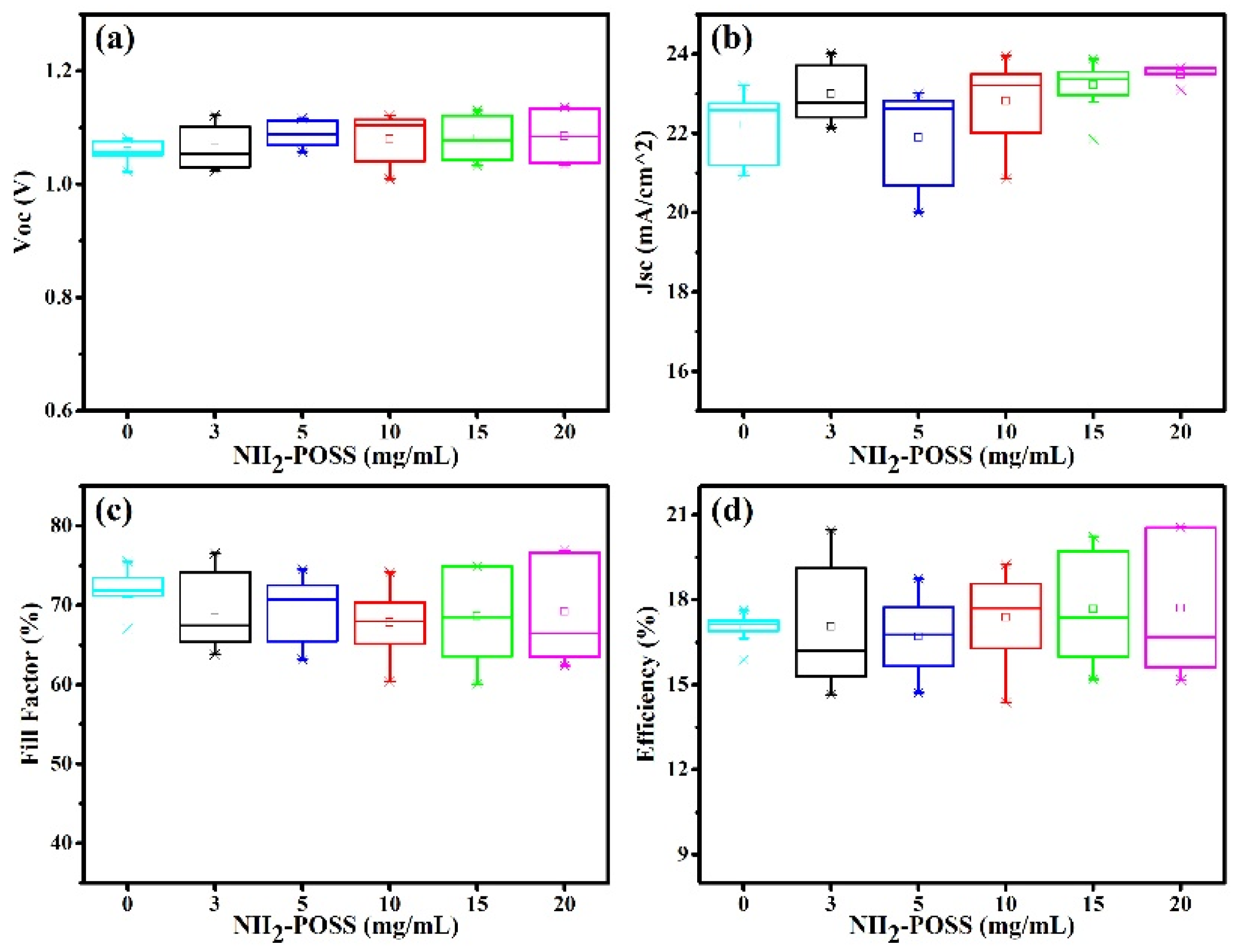
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.L. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 17042. [Google Scholar] [CrossRef]
- Xing, G.C.; Mathews, N.; Sun, S.Y.; Lim, S.S.; Lam, Y.M.; Gratzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General working principles of CH3NH3PbX3 perovskite solar cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.W.; Liu, L.; Zhang, D.L.; De Marco, N.; Lee, J.W.; Lin, O.; Chen, Q.; Yang, Y. The emergence of the mixed perovskites and their applications as solar cells. Adv. Energy Mater. 2017, 7, 1700491. [Google Scholar] [CrossRef]
- NREL. Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-rev211117.pdf (accessed on 17 November 2021).
- Conings, B.; Baeten, L.; De Dobbelaere, C.; D’Haen, J.; Manca, J.; Boyen, H.G. Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach. Adv. Mater. 2014, 26, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Geissbuhler, J.; Werner, J.; de Nicolas, S.M.; Barraud, L.; Hessler-Wyser, A.; Despeisse, M.; Nicolay, S.; Tomasi, A.; Niesen, B.; De Wolf, S.; et al. 22.5% efficient silicon heterojunction solar cell with molybdenum oxide hole collector. Appl. Phys. Lett. 2015, 107, 081601. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.P.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.R.; You, J.B.; Liu, Y.S.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Sanehira, E.M.; de Villers, B.J.T.; Schulz, P.; Reese, M.O.; Ferrere, S.; Zhu, K.; Lin, L.Y.; Berry, J.J.; Luther, J.M. Influence of electrode interfaces on the stability of perovskite solar cells: Reduced degradation using MoOx/Al for hole collection. ACS Energy Lett. 2016, 1, 38–45. [Google Scholar] [CrossRef]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.B.; Huang, J.S. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef]
- Steirer, K.X.; Schulz, P.; Teeter, G.; Stevanovic, V.; Yang, M.; Zhu, K.; Berry, J.J. Defect tolerance in methylammonium lead triiodide perovskite. ACS Energy Lett. 2016, 1, 360–366. [Google Scholar] [CrossRef]
- Li, N.X.; Tao, S.X.; Chen, Y.H.; Niu, X.X.; Onwudinanti, C.K.; Hu, C.; Qiu, Z.W.; Xu, Z.Q.; Zheng, G.H.J.; Wang, L.G.; et al. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat. Energy 2019, 4, 408–415. [Google Scholar] [CrossRef]
- Zhu, X.J.; Yang, D.; Yang, R.X.; Yang, B.; Yang, Z.; Ren, X.D.; Zhang, J.; Niu, J.Z.; Feng, J.S.; Liu, S.Z. Superior stability for perovskite solar cells with 20% efficiency using vacuum co-evaporation. Nanoscale 2017, 9, 12316–12323. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Marco, N.; Zhou, H.P.; Chen, Q.; Sun, P.Y.; Liu, Z.H.; Meng, L.; Yao, E.P.; Liu, Y.S.; Schiffer, A.; Yang, Y. Guanidinium: A route to enhanced carrier lifetime and open-circuit voltage in hybrid perovskite solar cells. Nano Lett. 2016, 16, 1009–1016. [Google Scholar] [CrossRef]
- Noel, N.K.; Abate, A.; Stranks, S.D.; Parrott, E.S.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Enhanced photoluminescence and solar cell performance via Lewis base passivation of organic-inorganic lead halide perovskites. ACS Nano 2014, 8, 9815–9821. [Google Scholar] [CrossRef] [PubMed]
- Son, D.Y.; Kim, S.G.; Seo, J.Y.; Lee, S.H.; Shin, H.; Lee, D.; Park, N.G. Universal approach toward hysteresis-free perovskite solar cell via defect engineering. J. Am. Chem. Soc. 2018, 140, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Guo, R.Q.; Li, C.; Lu, C.F.; Yang, G.C.; Wang, F.Y.; Nie, J.Q.; Ma, C.; Gao, M. POSS hybrid hydrogels: A brief review of synthesis, properties and applications. Eur. Polym. J. 2021, 143, 110180. [Google Scholar] [CrossRef]
- Peng, B.; Liu, R.; Han, G.; Wang, W.; Zhang, W.Q. The synthesis of thermoresponsive POSS-based eight-arm star poly (N-isopropylacrylamide): Comparison between Z-RAFT and R-RAFT strategies. Polym. Chem. 2021, 12, 2063–2074. [Google Scholar] [CrossRef]
- Zhao, B.J.; Mei, H.G.; Liu, N.; Zheng, S.X. Organic–inorganic polycyclooctadienes with double-decker silsesquioxanes in the main chains: Synthesis, self-healing, and shape memory properties regulated with quadruple hydrogen bonds. Macromolecules 2020, 53, 7119–7131. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, L.C.; Ni, H.L.; Pittman, C.U. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: A review. J. Inorg. Organomet. Polym. Mater. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Huang, H.; Lin, H.; Kershaw, S.V.; Susha, A.S.; Choy, W.C.H.; Rogach, A.L. Polyhedral oligomeric silsesquioxane enhances the brightness of perovskite nanocrystal-based green light-emitting devices. J. Phys. Chem. Lett. 2016, 7, 4398–4404. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Du, Q.; Yin, G.Z.; Liu, P.F.; Li, L.; Xie, H.P.; Zhu, C.; Li, Y.J.; Zhou, H.P.; Zhang, W.B.; et al. Extremely low trap-state energy level perovskite solar cells passivated using NH2-POSS with improved efficiency and stability. J. Mater. Chem. A 2018, 6, 6806–6814. [Google Scholar] [CrossRef]
- Xie, H.P.; Niu, D.M.; Lyu, L.; Zhang, H.; Zhang, Y.H.; Liu, P.; Wang, P.; Wu, D.; Gao, Y.L. Evolution of the electronic structure of C60/La0.67Sr0.33MnO3 interface. Appl. Phys. Lett. 2016, 108, 011603. [Google Scholar] [CrossRef]
- Xie, H.P.; Huang, H.; Cao, N.T.; Zhou, C.H.; Niu, D.M.; Gao, Y.L. Effects of annealing on structure and composition of LSMO thin films. Phys. B Condens. Matter 2015, 477, 14–19. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.L.; Lyu, L.; Xie, H.P.; Zhang, H.; Niu, D.M.; Huang, H.; Bi, C.; Xiao, Z.G.; Huang, J.S.; et al. Interfacial electronic structure at the CH3NH3PbI3/MoOx interface. Appl. Phys. Lett. 2015, 106, 506–902. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, Y.G.; Huang, H.; Lyu, L.; Zhang, H.; Cao, N.T.; Xie, H.P.; Gao, X.Y.; Niu, D.M.; Gao, Y.L. Thickness-dependent air-exposure-induced phase transition of CuPc ultrathin films to well-ordered one-dimensional nanocrystals on layered substrates. J. Phys. Chem. C 2015, 119, 4217–4223. [Google Scholar] [CrossRef]
- Gao, D.G.; Wang, P.P.; Shi, J.B.; Li, F.; Li, W.B.; Lyu, B.; Ma, J.Z. A green chemistry approach to leather tanning process: Cage-like octa (aminosilsesquioxane) combined with Tetrakis (hydroxymethyl) phosphonium sulfate. J. Clean. Prod. 2019, 229, 1102–1111. [Google Scholar] [CrossRef]
- Xie, H.P.; Liu, X.L.; Lyu, L.; Niu, D.M.; Wang, Q.; Huang, J.S.; Gao, Y.L. Effects of precursor ratios and annealing on electronic structure and surface composition of CH3NH3PbI3 perovskite films. J. Phys. Chem. 2015, 120, 215–220. [Google Scholar] [CrossRef]
- Feng, W.; Geng, W.; Zhou, Y.; Fang, H.H.; Tong, C.J.; Loi, M.A.; Liu, L.M.; Zhao, N. Phenylalkylamine passivation of organolead halide perovskites enabling high-efficiency and air-stable photovoltaic cells. Adv. Mater. 2016, 28, 9986–9992. [Google Scholar]
- Liu, B.T.; Lin, H.R.; Lee, R.H.; Gorji, N.E.; Chou, J.C. Fabrication and characterization of an efficient inverted perovskite solar cells with POSS passivating hole transport layer. Nanomaterials 2021, 11, 974. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zai, H.C.; Xie, H.P.; Liu, B.X.; Wang, S.T.; Zhao, Y.; Niu, D.M.; Huang, H.; Chen, Q.; Gao, Y.L. Effects of CsPbBr3 nanocrystals concentration on electronic structure and surface composition of perovskite films. Org. Electron. 2019, 73, 327–331. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, N.; Xie, H.; Liu, J.; Yuan, P.; Wei, J.; Zhao, Y.; Yang, B.; Zhang, J.; Wang, S.; et al. Modification of FA0.85MA0.15Pb(I0.85Br0.15)3 Films by NH2-POSS. Crystals 2021, 11, 1544. https://doi.org/10.3390/cryst11121544
Zhang Y, Liu N, Xie H, Liu J, Yuan P, Wei J, Zhao Y, Yang B, Zhang J, Wang S, et al. Modification of FA0.85MA0.15Pb(I0.85Br0.15)3 Films by NH2-POSS. Crystals. 2021; 11(12):1544. https://doi.org/10.3390/cryst11121544
Chicago/Turabian StyleZhang, Yangyang, Na Liu, Haipeng Xie, Jia Liu, Pan Yuan, Junhua Wei, Yuan Zhao, Baopeng Yang, Jianhua Zhang, Shitan Wang, and et al. 2021. "Modification of FA0.85MA0.15Pb(I0.85Br0.15)3 Films by NH2-POSS" Crystals 11, no. 12: 1544. https://doi.org/10.3390/cryst11121544
APA StyleZhang, Y., Liu, N., Xie, H., Liu, J., Yuan, P., Wei, J., Zhao, Y., Yang, B., Zhang, J., Wang, S., Huang, H., Niu, D., Chen, Q., & Gao, Y. (2021). Modification of FA0.85MA0.15Pb(I0.85Br0.15)3 Films by NH2-POSS. Crystals, 11(12), 1544. https://doi.org/10.3390/cryst11121544






