An Experimental and Theoretical Study on the Effect of Silver Nanoparticles Concentration on the Structural, Morphological, Optical, and Electronic Properties of TiO2 Nanocrystals
Abstract
1. Introduction
2. Experimental and Computational Methods
2.1. Experimental Details
2.1.1. Synthesis of AgNPs-Loaded TiO2
2.1.2. Characterizations
2.2. Computational Details
3. Results and Discussion
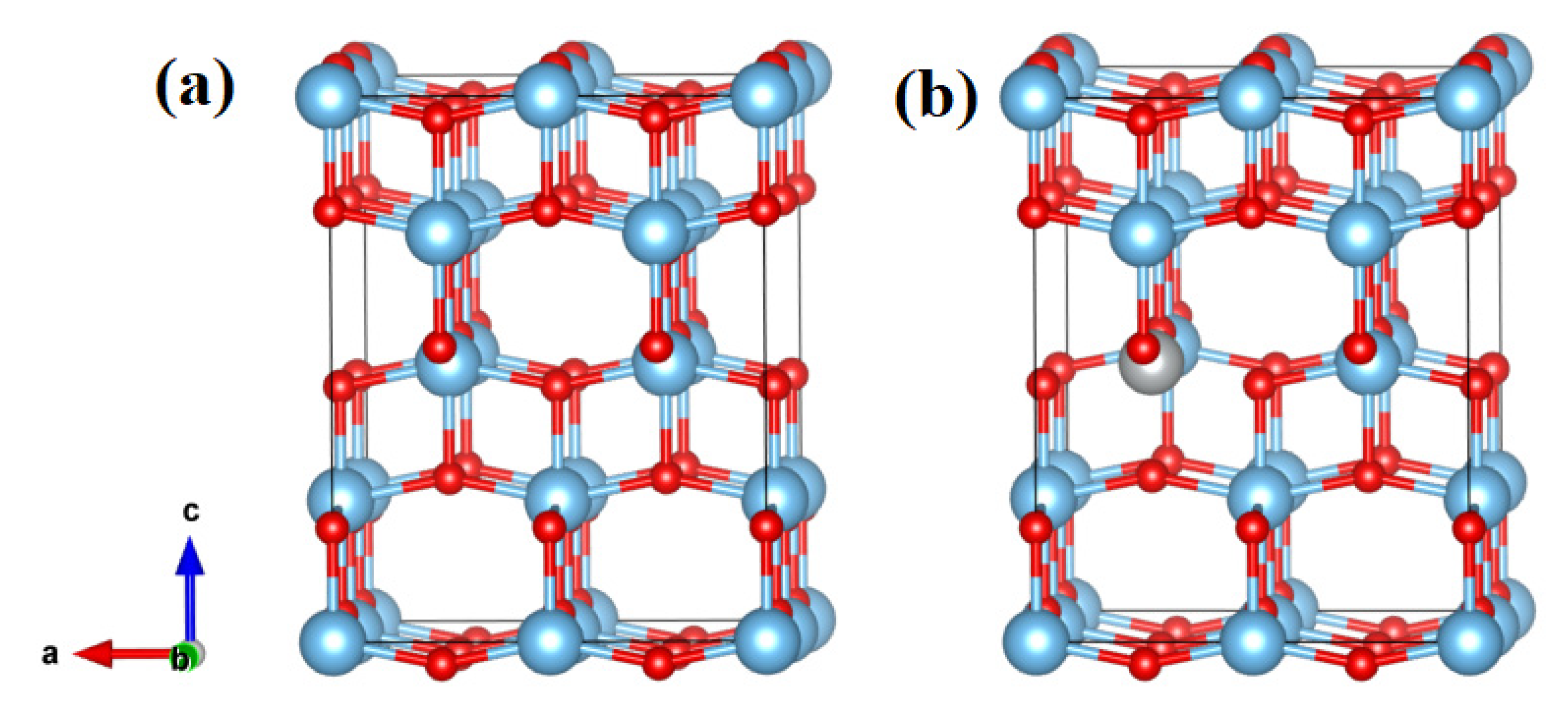
3.1. Structural Properties
3.1.1. X-ray Diffraction Analysis
3.1.2. FTIR Analysis
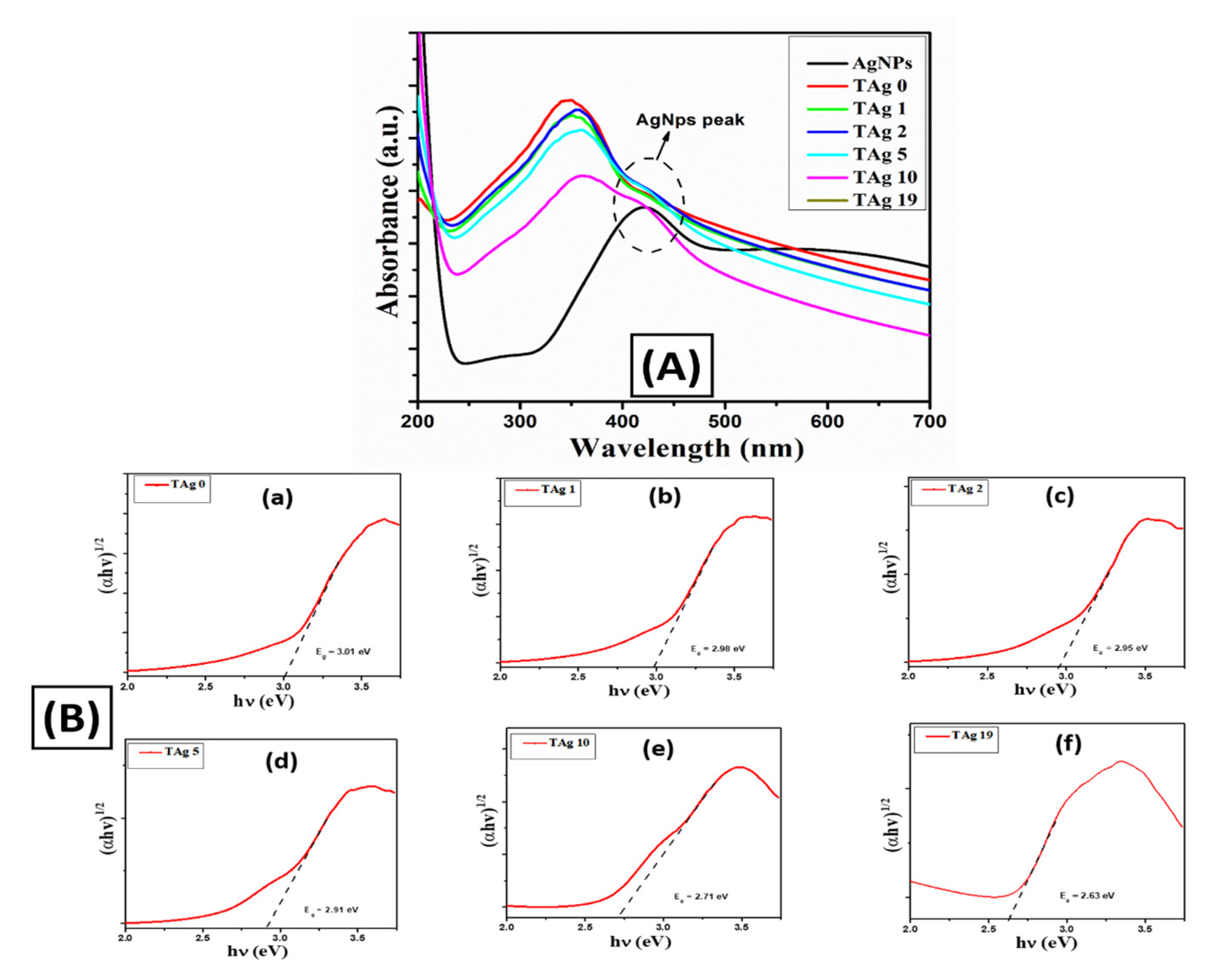
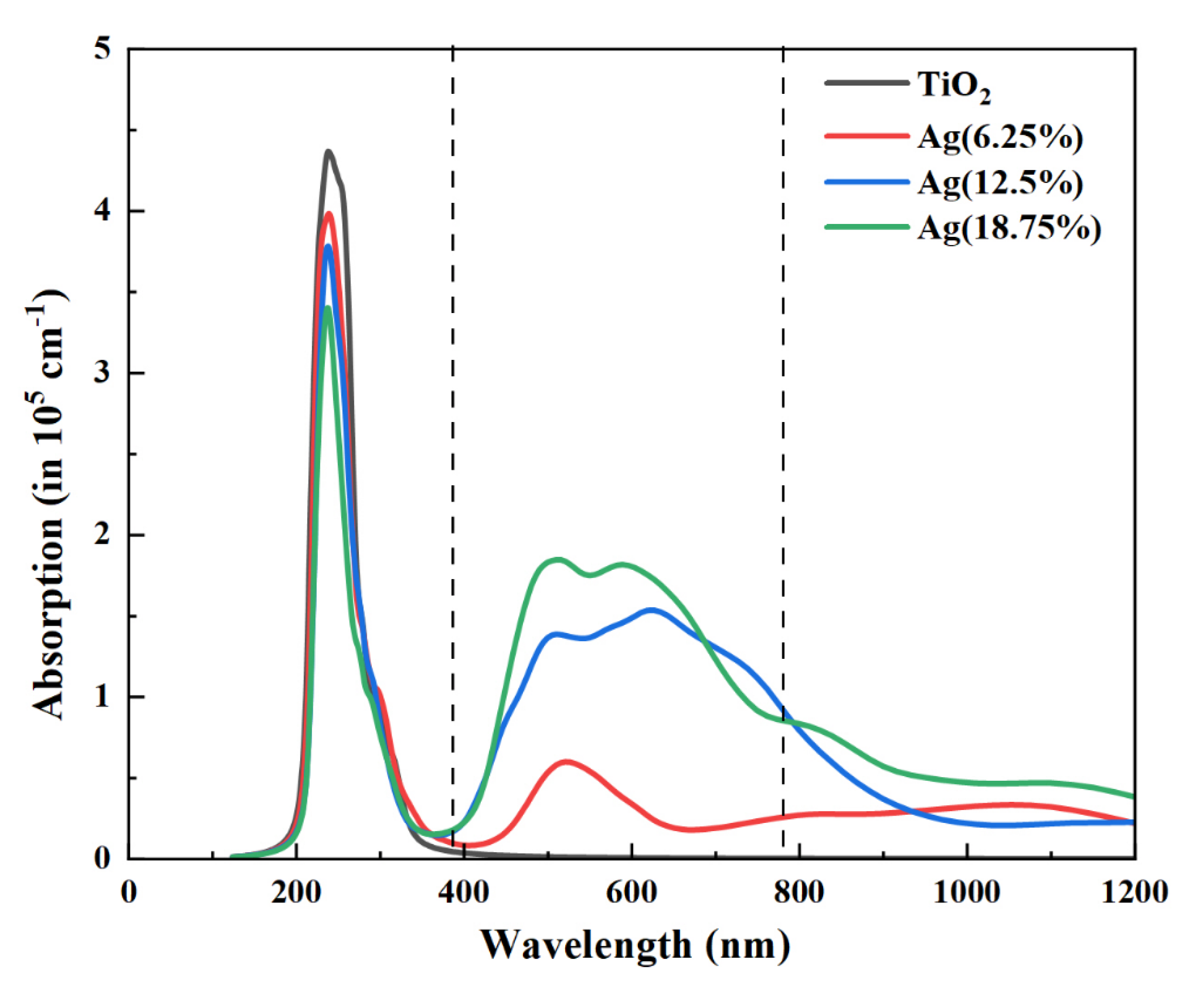
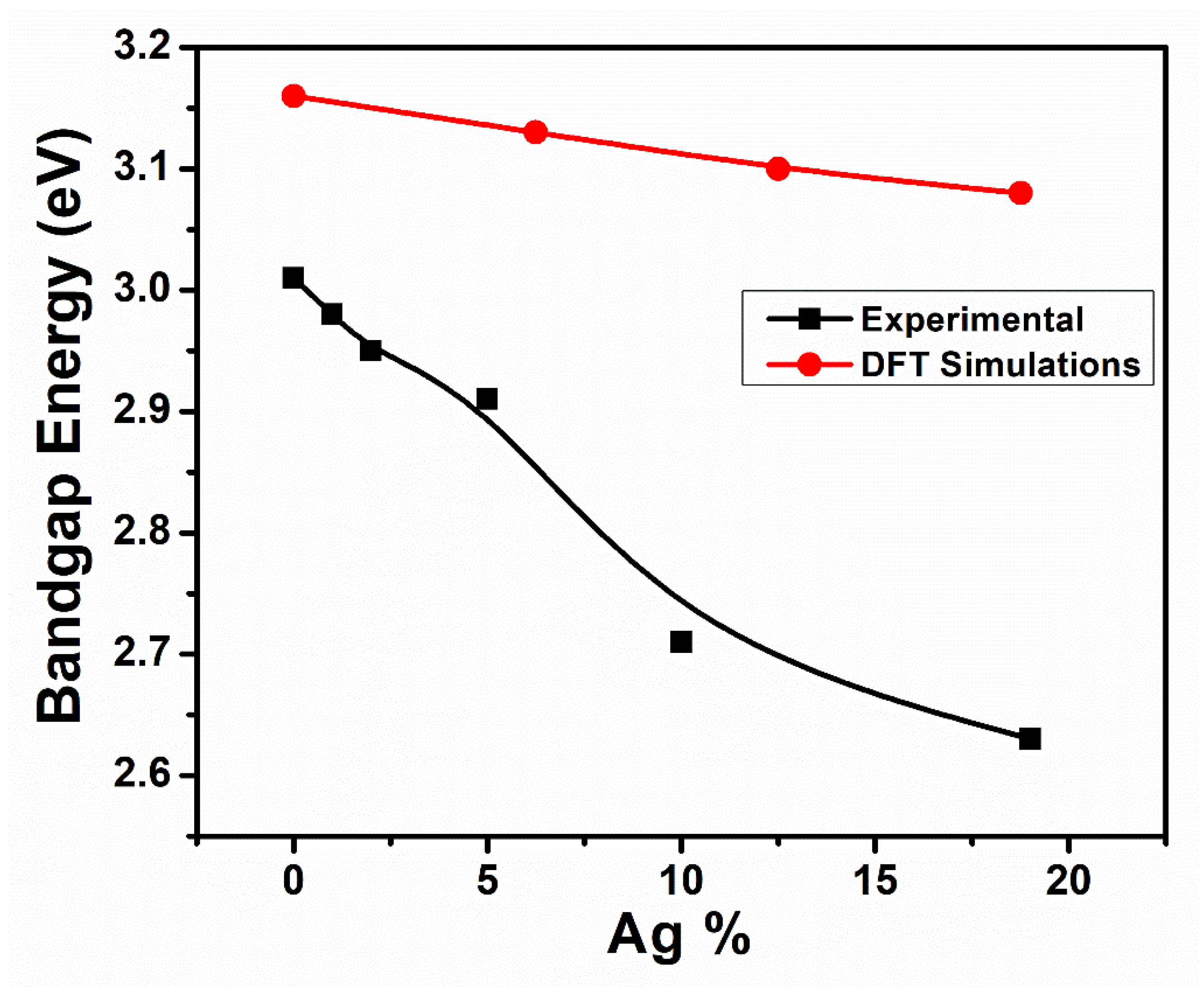
3.2. Optical Properties
3.3. Raman Analysis
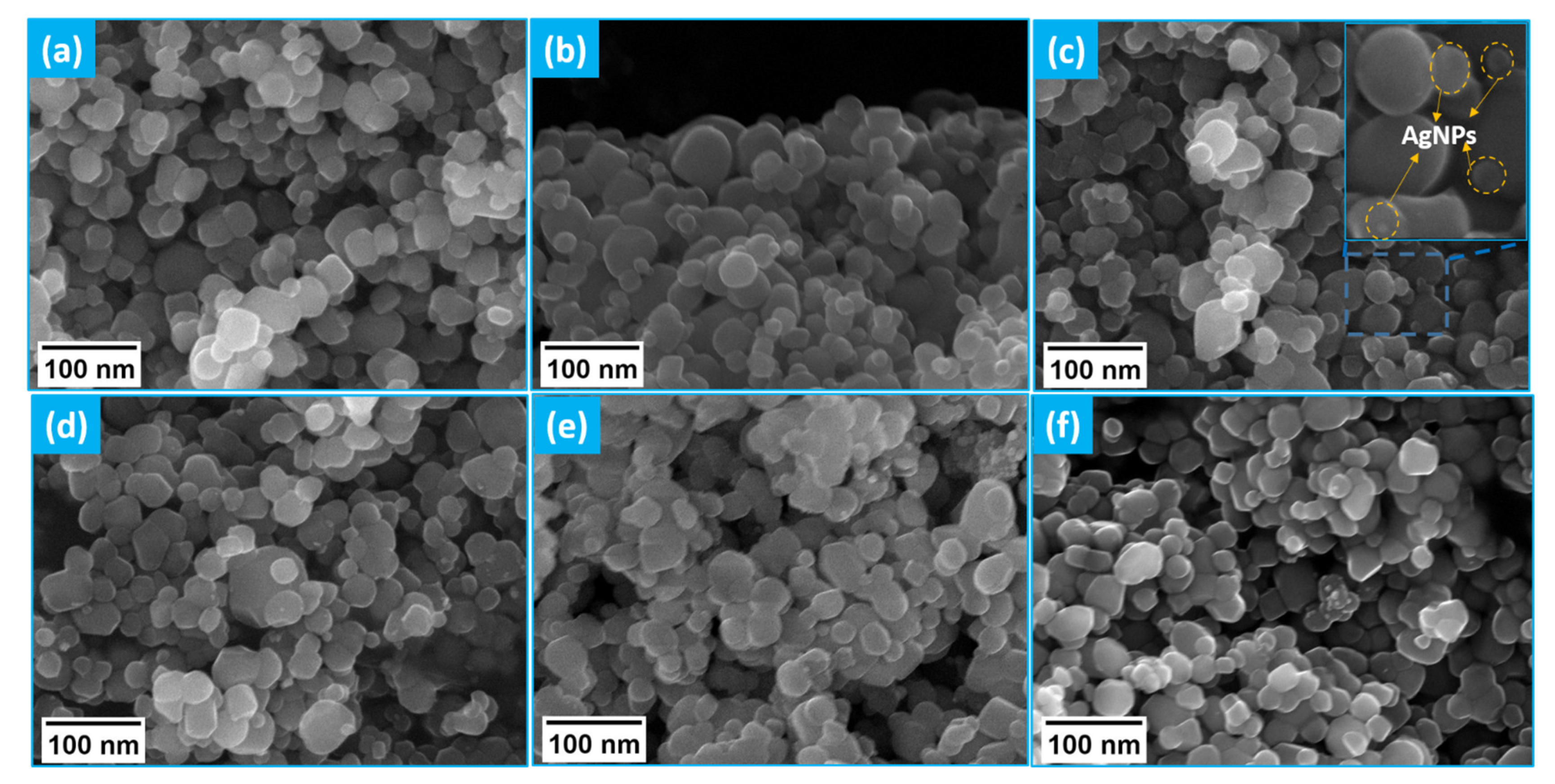
3.4. Morphological Studies
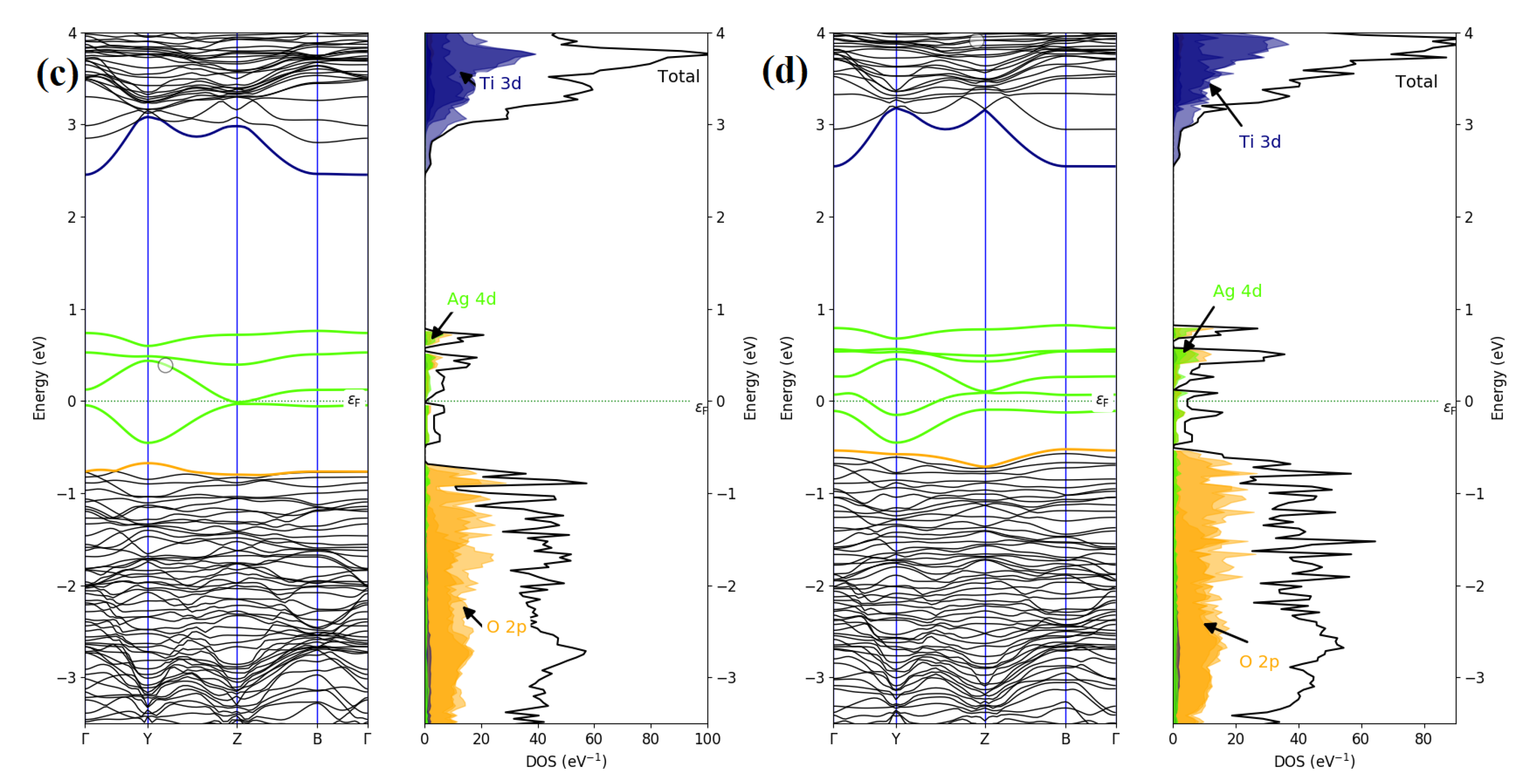
3.5. Computational Electronic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B., Jr. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, Z.; Zhu, C.; Hu, X.; Zhang, X.; Zhu, H.; Fan, J. Enhanced optical absorption and photocatalytic activity of anatase TiO2 through (Si,Ni) codoping. J. Appl. Phys. Lett. 2012, 101, 062106. [Google Scholar] [CrossRef][Green Version]
- Yin, W.J.; Tang, H.; Wei, S.H.; Al-Jassim, M.M.; Turner, J.; Yan, Y. Band structure engineering of semiconductors for enhanced photoelectrochemical water splitting: The case of TiO2. Phys. Rev. B Ondens. Matter Mater. Phys. 2010, 82, 045106. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Zhao, H.; Chen, J.; Cheng, J.; Yang, K.; Li, Y. Engineering Coexposed {001} and {101} Facets in Oxygen-Deficient TiO2 Nanocrystals for Enhanced CO2 Photoreduction under Visible Light. ACS Catal. 2016, 6, 1097–1108. [Google Scholar] [CrossRef]
- Wójcik, E.B.; Szwajgier, P.; Oleszczuk, A.; Mieczan, W. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health-a Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef]
- Hernandez-Alonso, M.D.; Fresno, F.; Suarez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Gai, Y.; Li, J.; Li, S.S.; Xia, J.B.; Wei, S.H. Design of Narrow-Gap TiO2: A Passivated Codoping Approach for Enhanced Photoelectrochemical Activity. Phys. Rev. Lett. 2009, 102, 036402. [Google Scholar] [CrossRef]
- Chang, J.; Jiang, Z.-Y.; Zhang, Z.Y.; Lin, Y.M.; Tian, P.-L.; Zhou, B.; Chen, L. Theoretical studies of photocatalytic behaviors of isoelectronic C/Si/Ge/Sn-doped TiO2: DFT+ U. Appl. Surf. Sci. 2019, 484, 1304–1309. [Google Scholar] [CrossRef]
- Wilke, K.; Breuer, H. The influence of transition metal doping on the physical and photocatalytic properties of titania. J. Photochem. Photobiol. A 1999, 121, 49–53. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Keulemans, M.; Filippousi, M.; Flahaut, D.; Tendeloo, G.V.; Lacombe, S.; Martens, J.A.; Lenaerts, S. Plasmonic gold–silver alloy on TiO2 photocatalysts with tunable visible light activity. Appl. Catal. B Environ. 2014, 156–157, 116–121. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Detlef, W.B. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, C.; Liu, J.; Tian, Z.; Wang, G.; Cai, W. Defect-Mediated Formation of Ag Cluster-Doped TiO2 Nanoparticles for Efficient Photodegradation of Pentachlorophenol. Langmuir 2012, 28, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, A.; Biju, K.; Rajesh, P.; Kumar, K.J.; Kiran, M.R. Surface modification of sol gel TiO2 surface with sputtered metallic silver for Sun light photocatalytic activity: Initial studies. Sol. Energy Mater. Sol. Cells 2012, 101, 241–248. [Google Scholar] [CrossRef]
- Li, Y.; Ma, M.; Chen, W.; Li, L.; Zen, M. Preparation of Ag-doped TiO2 nanoparticles by a miniemulsion method and their photoactivity in visible light illuminations. Mater. Chem. Phys. 2011, 129, 501–505. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrog. Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Liu, L.; Ouyang, S.; Ye, J. Gold-nanorod-photosensitized titanium dioxide with wide-range visible-light harvesting based on localized surface plasmon resonance. Angew. Chem. 2013, 125, 6821–6825. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.-H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef]
- De Souza, M.L.; dos Santos, D.P.; Corio, P. Localized surface plasmon resonance enhanced photocatalysis: An experimental and theoretical mechanistic investigation. RSC Adv. 2018, 8, 28753. [Google Scholar] [CrossRef]
- Quiñones-Jurado, Z.V.; Waldo-Mendoza, M.; Aguilera-Bandin, H.M.; Villabona-Leal, E.G.; Cervantes-Gonzalez, E.; Pérez, E. Silver Nanoparticles Supported on TiO2 and Their Antibacterial Properties: Effect of Surface Confinement and Nonexistence of Plasmon Resonance. Mater. Sci. Appl. 2014, 5, 895–903. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Sang, Q.; Du, J.; Yang, M.; Li, X.; Shen, Y.; Han, X.; Jiang, X.; Zhao, B. A Ag synchronously deposited and doped TiO2 hybrid as an ultrasensitive SERS substrate: A multifunctional platform for SERS detection and photocatalytic degradation. Phys. Chem. Chem. Phys. 2018, 20, 15149. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Lai, W.; Yang, X.; Meng, J.; Su, L.; Gu, C.; Jiang, T.; Pun, E.Y.B.; Shao, L. Irreversible accumulated SERS behavior of the molecule-linked silver and silver-doped titanium dioxide hybrid system. Nat. Commun. 2020, 11, 1785. [Google Scholar] [CrossRef]
- Al Suliman, N.; Awada, C.; Alshoaibi, A.; Shaalan, N.M. Simple Preparation of Ceramic-Like Materials Based on 1D-Agx (x= 0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance. Crystals 2020, 10, 1024. [Google Scholar] [CrossRef]
- Wang, F.; Di Valentin, C.; Pacchioni, G. Doping of WO3 for Photocatalytic Water Splitting: Hints from Density Functional Theory. J. Phys. Chem. C 2012, 116, 8901–8909. [Google Scholar] [CrossRef]
- Islam, M.M.; Calatayud, M.; Pacchioni, G. Hydrogen Adsorption and Diffusion on the Anatase TiO2(101) Surface: A First-Principles Investigation. J. Phys. Chem. C 2011, 115, 6809–6814. [Google Scholar] [CrossRef]
- Kubacka, A.; Fern´andez-Garcıa, M.; Colon, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Henrich, V.E. Metal-oxide surfaces. Prog. Surf. Sci. 1995, 50, 77–90. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Shwetharani, R.; Sakar, M.; Fernando, C.A.N.; Binas, V. Recent advances and strategies to tailor the energy levels, active sites and electron mobility in titania and its doped/composite analogues for hydrogen evolution in sunlight. Sci. Technol. 2019, 9, 12–46. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, Z.; Zhu, C.; Hu, X.; Zhang, X.; Zhu, H.; Fand, J.; Linbe, S.H. C/B codoping effect on band gap narrowing and optical performance of TiO2 photocatalyst: A spin-polarized DFT study. J. Mater. Chem. A 2013, 1, 4516. [Google Scholar] [CrossRef]
- Niu, M.; Cheng, D.; Cao, D. Understanding Photoelectrochemical Properties of B–N Codoped Anatase TiO2 for Solar Energy Conversion. J. Phys. Chem. C 2013, 117, 15911–15917. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, H. A Theoretical Perspective on Charge Separation and Transfer in Metal Oxide Photocatalysts for Water Splitting. ChemCatChem 2019, 11, 3688–3715. [Google Scholar] [CrossRef]
- Vorontsov, V.A.; Valdés, H.; Smirniotisc, P.G.; Paza, Y. Computational models of (001) faceted anatase TiO2 nanoparticles. J. Chem. Technol. Biotechnol. 2020, 95, 2750–2760. [Google Scholar]
- Tamura, T.; Ishibashi, S.; Terakura, K.; Weng, H. First-principles study of the rectifying properties of Pt/TiO2 interface. Phys. Rev. B 2009, 80, 195302. [Google Scholar] [CrossRef]
- Shahzad, N.; Hussain, A.; Mustafa, N.; Ali, N.; Kanoun, M.B.; Goumri-Said, S. First principles study of the adsorption and dissociation mechanisms of H2S on a TiO2 anatase (001) surface. RSC Adv. 2016, 6, 7941–7949. [Google Scholar] [CrossRef]
- Okazaki, K.; Morikawa, Y.; Tanaka, S.; Tanaka, K.; Kohyama, M. Electronic structures of Au on TiO2 (100) by first-principles calculations. Phys. Rev. B 2004, 69, 235404. [Google Scholar] [CrossRef]
- Marri, I.; Ossicini, S. Oxygen vacancy effects on the Schottky barrier height at the Au/TiO2 (110) interface: A first principle study. Solid State Commun. 2008, 147, 205–207. [Google Scholar] [CrossRef]
- Boonchun, A.; Umezawa, N.; Ohno, T.; Ouyanga, S.; Jinhua, Y. Role of photoexcited electrons in hydrogen evolution from platinum co-catalysts loaded on anatase TiO2: A first-principles study. Mater. Chem. A 2013, 1, 6664. [Google Scholar] [CrossRef]
- Hammer, A.; Norskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Smidstrup, S.; Markussen, T.; Vancraeyveld, P.; Wellendorff, J.; Schneider, J.; Gunst, T.; Verstichel, B.; Stradi, D.; Khomyakov, P.A.; Vej-Hansen, U.G.; et al. QuantumATK: An integrated platform of electronic and atomic-scale modelling tools. J. Phys. Condens. Matter 2020, 32, 015901. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Van Setten, M.; Giantomassi, M.; Bousquet, E.; Verstraete, M.; Hamann, D.; Gonze, X.; Rignanese, G.-M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Marques, M.; Teles, L.K. Approximation to density functional theory for the calculation of band gaps of semiconductors. Phys. Rev. B 2008, 78, 125116. [Google Scholar] [CrossRef]
- MKanoun, M.-B.; Goumri-Said, S.; Schwingenschlögl, U.; Manchon, A. Magnetism in Sc-doped ZnO with zinc vacancies: A hybrid density functional and GGA+U approaches. Chem. Phys. Lett. 2012, 532, 96–99. [Google Scholar] [CrossRef]
- Tian, J. Large Oriented Arrays and Continuous Films of TiO2-Based Nanotubes. Am. Chem. Soc. 2003, 125, 12384. [Google Scholar] [CrossRef] [PubMed]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Rajakumar, A.; Abdul Rauman, S.; Mohana Roopan, V.G.; Khanna, G.; Elango, C.; Kamaraj, A.A.; Zahir, K.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Palencia, M.; Córdoba, A.; Bernabé, L.R. Concentration–polarization effect of poly(sodium styrenesulfonate) on size distribution of colloidal silver nanoparticlesduring diafiltration experiments. Colloid. Polym. Sci. 2014, 292, 619–626. [Google Scholar] [CrossRef]
- Begam, J.N. Biosynthesis and characterization of silver nanoparticles (AgNPs) using marine bacteria against certain human pathogens. Int. J. Adv. Sci. Res. 2016, 2, 152–156. [Google Scholar] [CrossRef]
- Wang, S.; Han, Z.; Di, T.; Li, R.; Liu, S.; Cheng, Z. Preparation of pod-shaped TiO2 and Ag@TiO2 nano burst tubes and their photocatalytic activity. R. Soc. Open Sci. 2019, 6, 191019. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Manonmani, J.; Senthilselvan, J. Effect on interfacial charge transfer resistance by hybrid co-sensitization in DSSC applications. J. Mater. Sci. Mater. Electron. 2014, 25, 5296–5301. [Google Scholar] [CrossRef]
- Chowdhury, J.; Ghosh, M. Concentration-dependent surface-enhanced Raman scattering of 2-benzoylpyridine adsorbed on colloidal silver particles. J. Colloid Interface Sci. 2004, 277, 121–127. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungusT. asperellum. Nanotechnology 2008, 19, 075103. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Khalid, M.; Abu-Salah, L.; Dutta, J. Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Ionova, G.V.; Mikhalko, V.K.; Ionova, I.S.; Gerasimova, G.A. Plasmon properties of silver spherical nanoparticles and films. Prot. Met. Phys. Chem. Surfaces 2013, 49, 169–172. [Google Scholar] [CrossRef]
- Burdett, J.K.; Hughbanks, T.; Miller, G.J.; Richardson, J.W.; Smith, J.V. Structural-electronic relationships in inorganic solids: Powder neutron diffraction studies of the rutile and anatase polymorphs of titanium dioxide at 15 and 295 K. J. Am. Chem. Soc. 1987, 109, 3639. [Google Scholar] [CrossRef]
- Ikram, M.; Wakeel, M.; Hassan, J.; Haider, A.; Naz, S.; Ul-Hamid, A.; Haider, J.; Ali, S.; Goumri-Said, S.; Kanoun, M.B. Impact of Bi Doping into Boron Nitride Nanosheets on Electronic and Optical Properties Using Theoretical Calculations and Experiments. Nanoscale Res. Lett. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Xu, J.; Chen, N.; Cao, W.; Ullah, A.; Usman, Z.; Khan, D.F. Effect of Ag doping concentration on the electronic and optical properties of anatase TiO2: A DFT-based theoretical study. Res. Chem. Intermed. 2013, 39, 1633–1644. [Google Scholar] [CrossRef]
- Guo, M.; Du, J. First-principles study of electronic structures and opticalproperties of Cu, Ag, and Au-dopedanataseTiO2. Physica B 2012, 407, 1003–1007. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Alshoaibi, A.; Kanoun, M.B.; Haq, B.U.; AlFaify, S.; Goumri-Said, S. Insights into the Impact of Yttrium Doping at the Ba and Ti Sites of BaTiO3 on the Electronic Structures and Optical Properties: A First-Principles Study. ACS Omega 2020, 5, 15502–15509. [Google Scholar] [CrossRef]




| Title | a (Å) | c (Å) | V (Å) | Ti–O | O–O | O–Ag |
|---|---|---|---|---|---|---|
| Pristine | 3.782 | 9.496 | 543.34 | 1.960 | 2.47 | - |
| Ag(6.25%) | 3.800 | 9.528 | 550.40 | 1.950 | 2.50 | 2.030 |
| Ag(12.5%) | 3.818 | 9.572 | 557.08 | 1.951 | 2.48 | 2.064 |
| Ag(18.75%) | 3.845 | 9.586 | 561.11 | 1.950 | 2.478 | 2.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, F.; Kanoun, M.B.; Awada, C.; Jonin, C.; Brevet, P.-F. An Experimental and Theoretical Study on the Effect of Silver Nanoparticles Concentration on the Structural, Morphological, Optical, and Electronic Properties of TiO2 Nanocrystals. Crystals 2021, 11, 1488. https://doi.org/10.3390/cryst11121488
Ahmed F, Kanoun MB, Awada C, Jonin C, Brevet P-F. An Experimental and Theoretical Study on the Effect of Silver Nanoparticles Concentration on the Structural, Morphological, Optical, and Electronic Properties of TiO2 Nanocrystals. Crystals. 2021; 11(12):1488. https://doi.org/10.3390/cryst11121488
Chicago/Turabian StyleAhmed, Faheem, Mohammed Benali Kanoun, Chawki Awada, Christian Jonin, and Pierre-Francois Brevet. 2021. "An Experimental and Theoretical Study on the Effect of Silver Nanoparticles Concentration on the Structural, Morphological, Optical, and Electronic Properties of TiO2 Nanocrystals" Crystals 11, no. 12: 1488. https://doi.org/10.3390/cryst11121488
APA StyleAhmed, F., Kanoun, M. B., Awada, C., Jonin, C., & Brevet, P.-F. (2021). An Experimental and Theoretical Study on the Effect of Silver Nanoparticles Concentration on the Structural, Morphological, Optical, and Electronic Properties of TiO2 Nanocrystals. Crystals, 11(12), 1488. https://doi.org/10.3390/cryst11121488








