Influences of Powder Source Porosity on Mass Transport during AlN Crystal Growth Using Physical Vapor Transport Method
Abstract
:1. Introduction
2. Geometry Description and Numerical Modeling
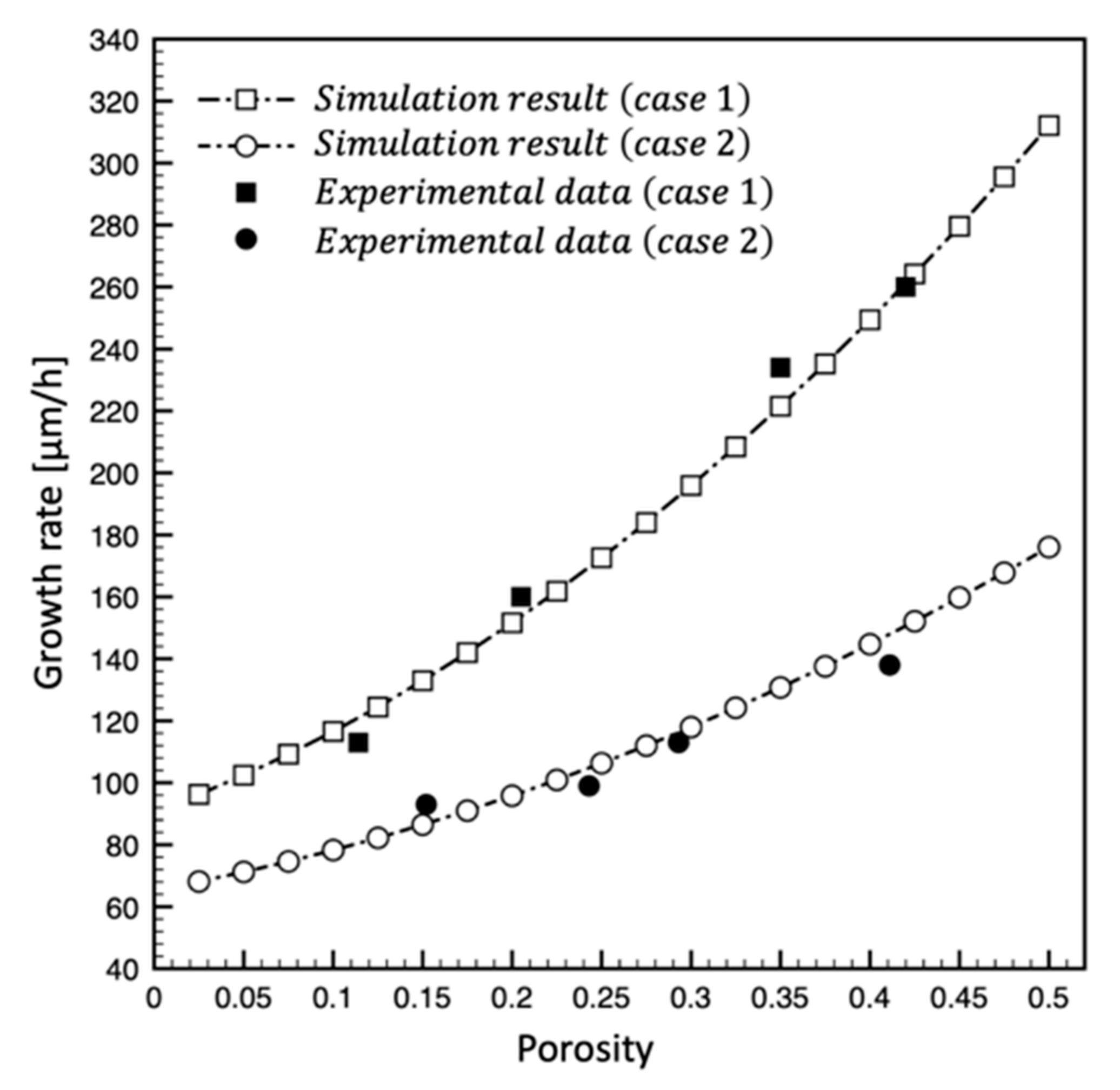
3. Validation of the Model
4. Simulation Results and Discussion
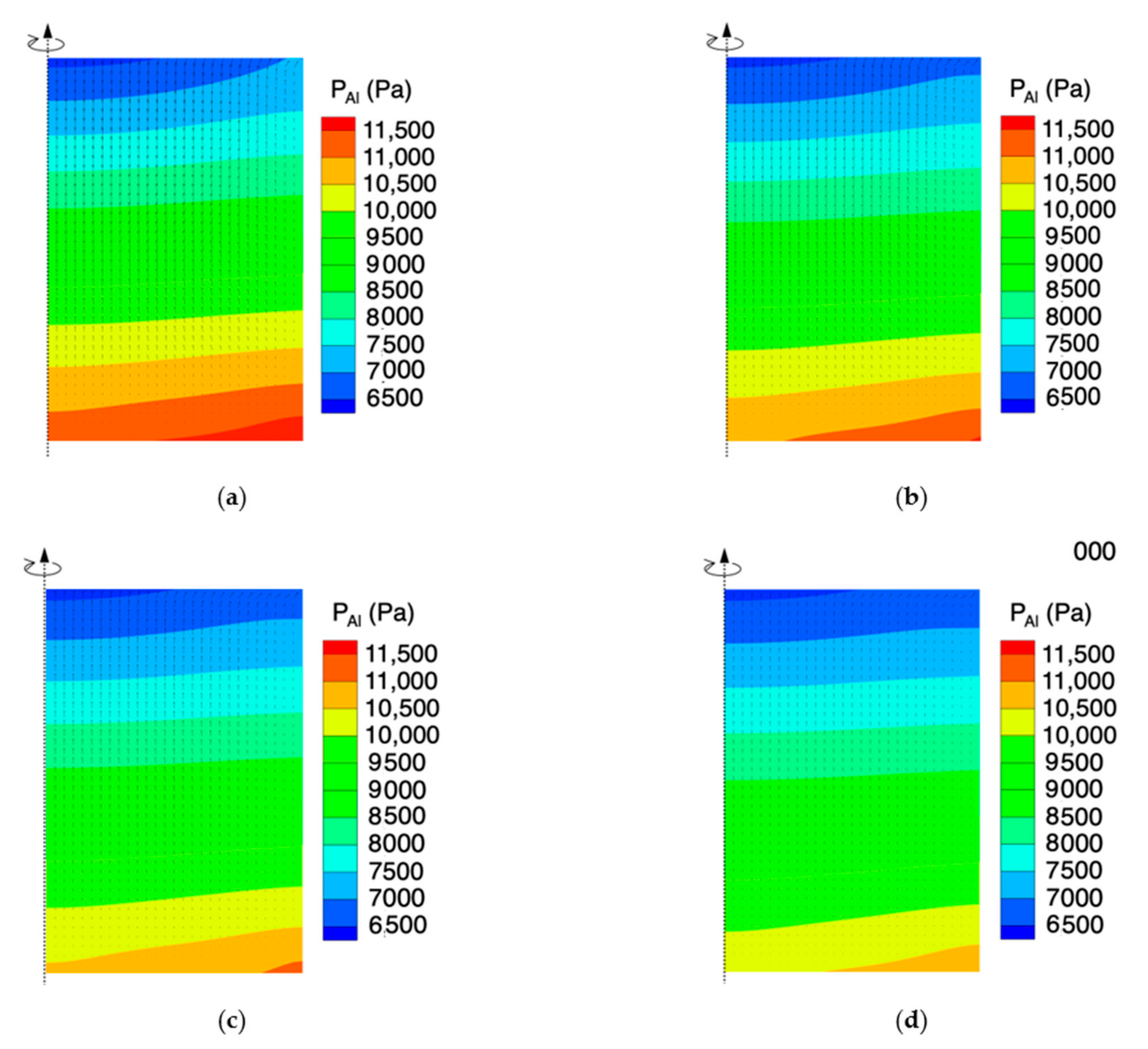
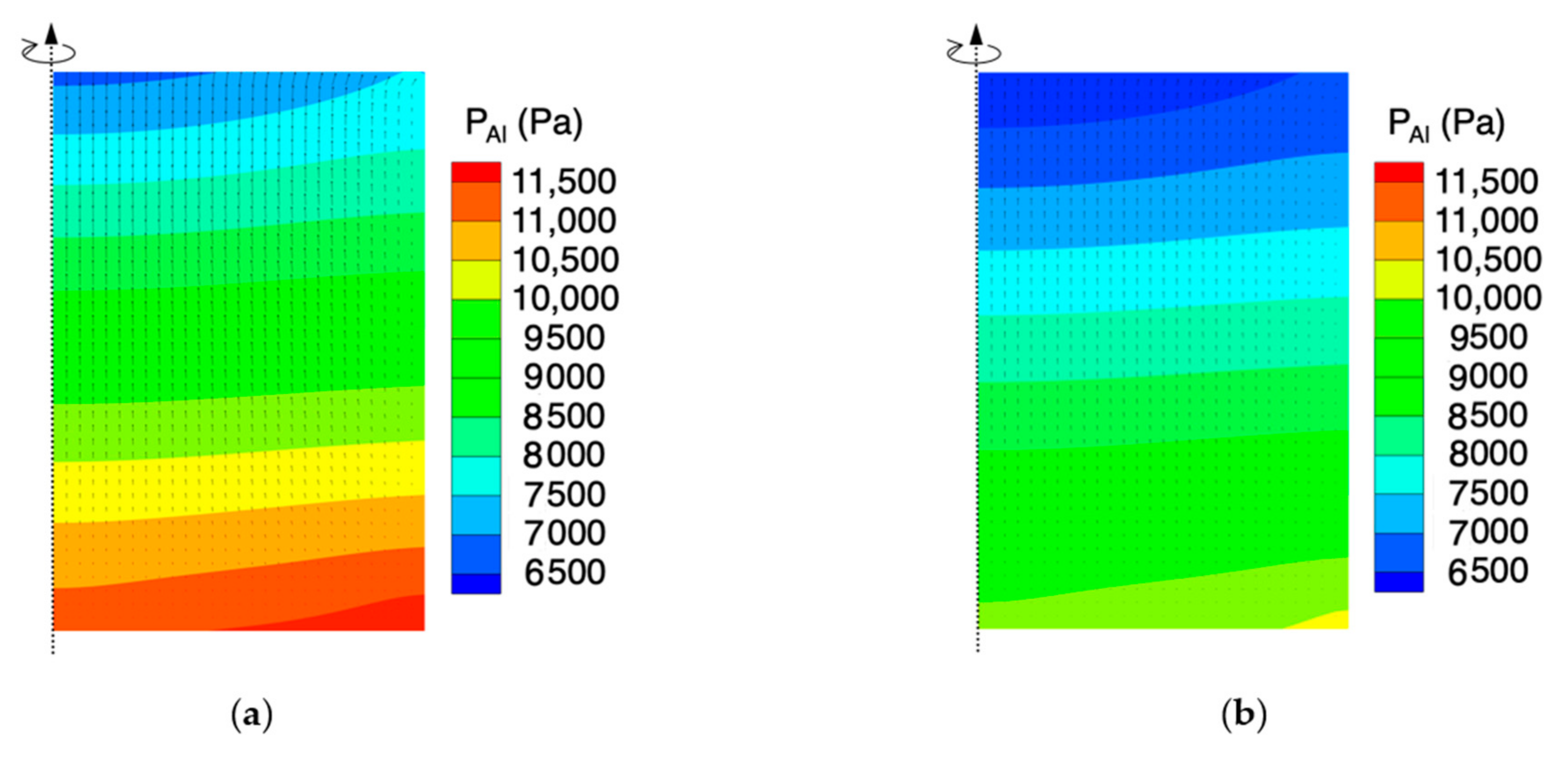
4.1. Effect of the Powder Porosity
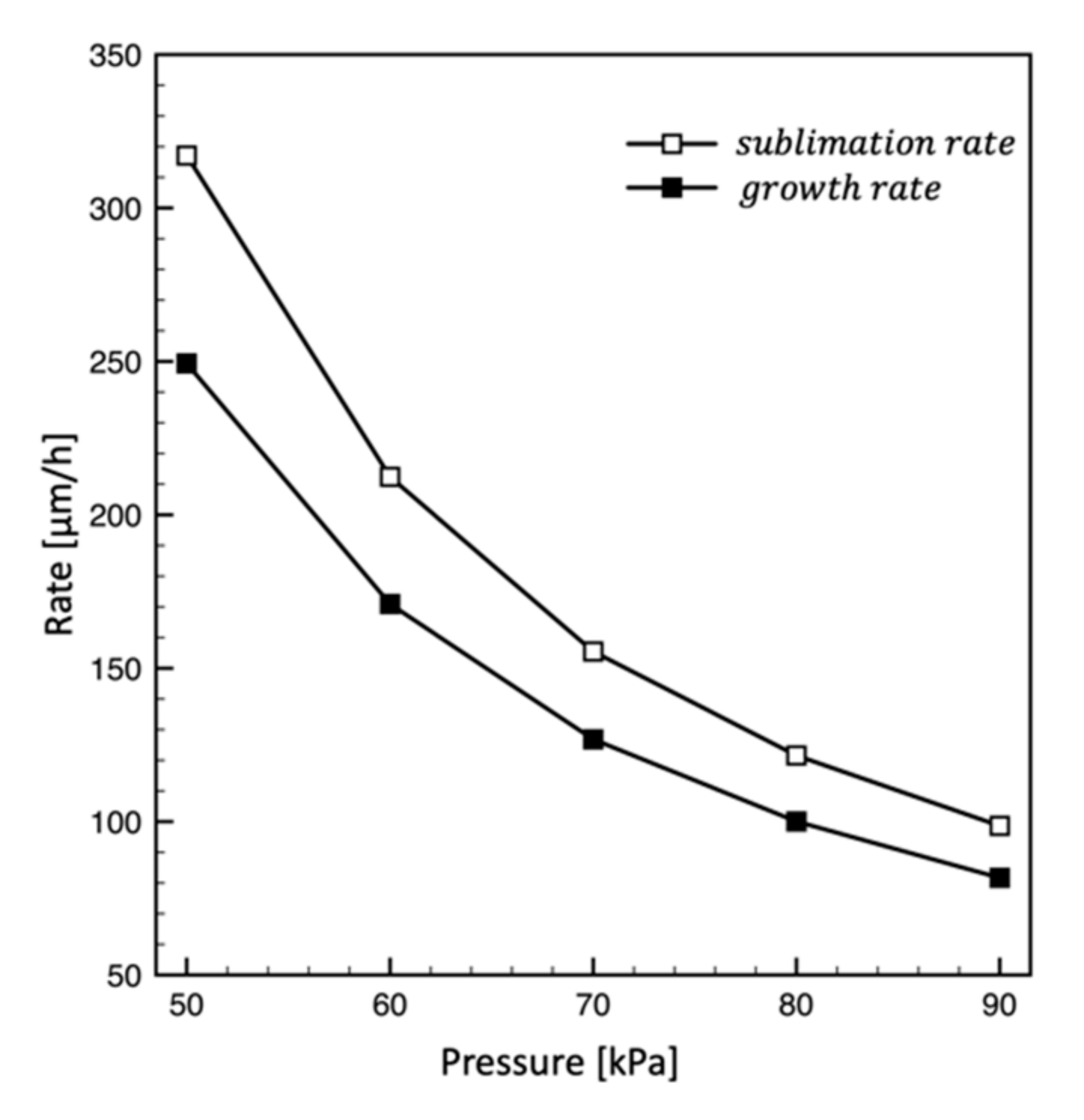
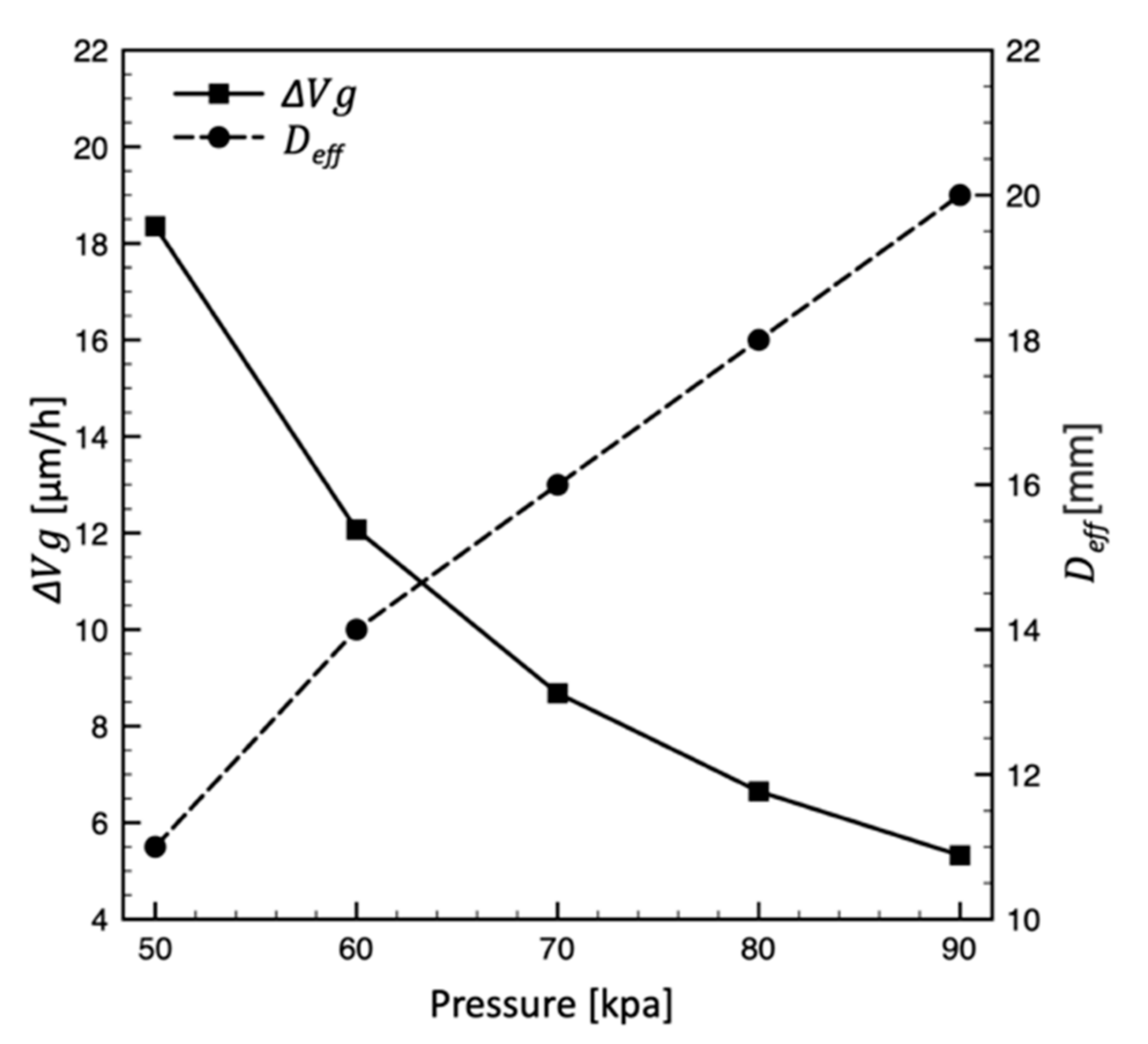
4.2. Effect of Pressure
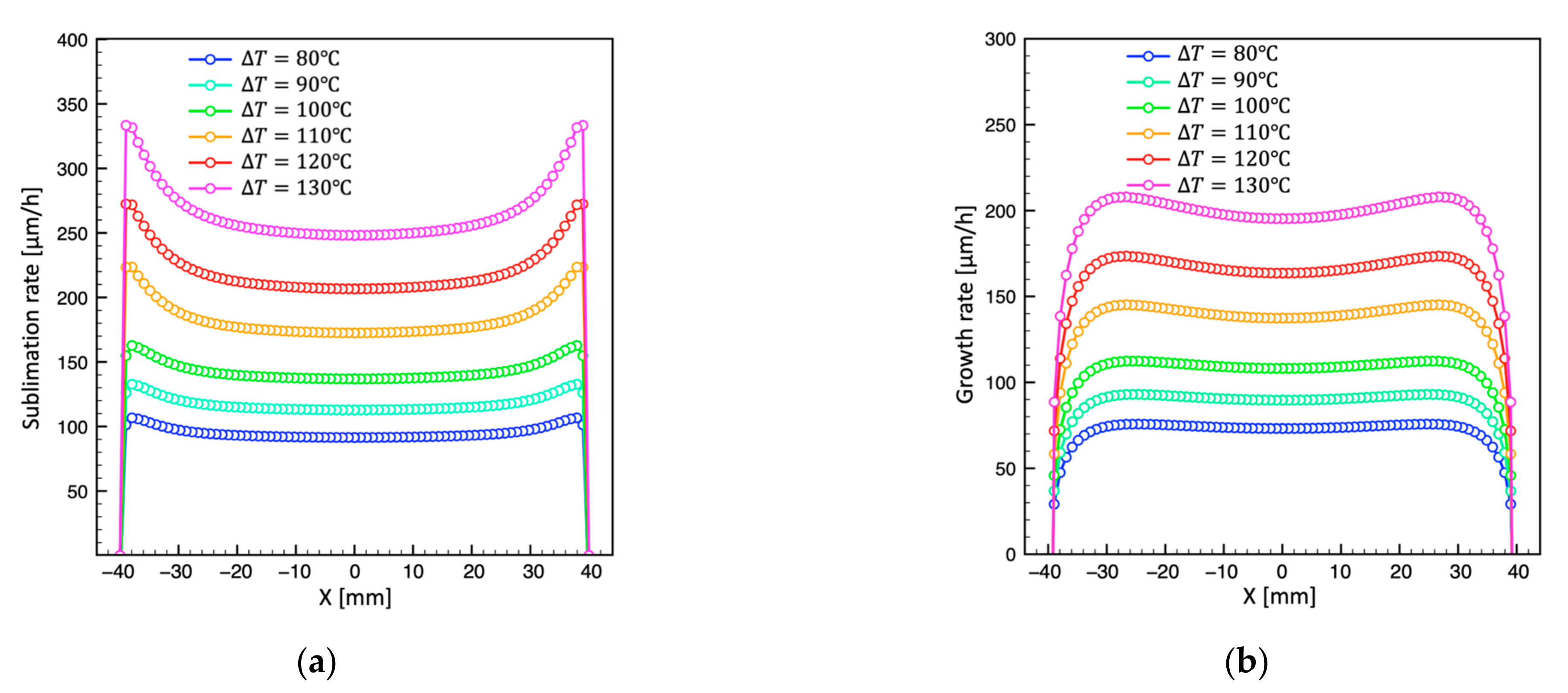
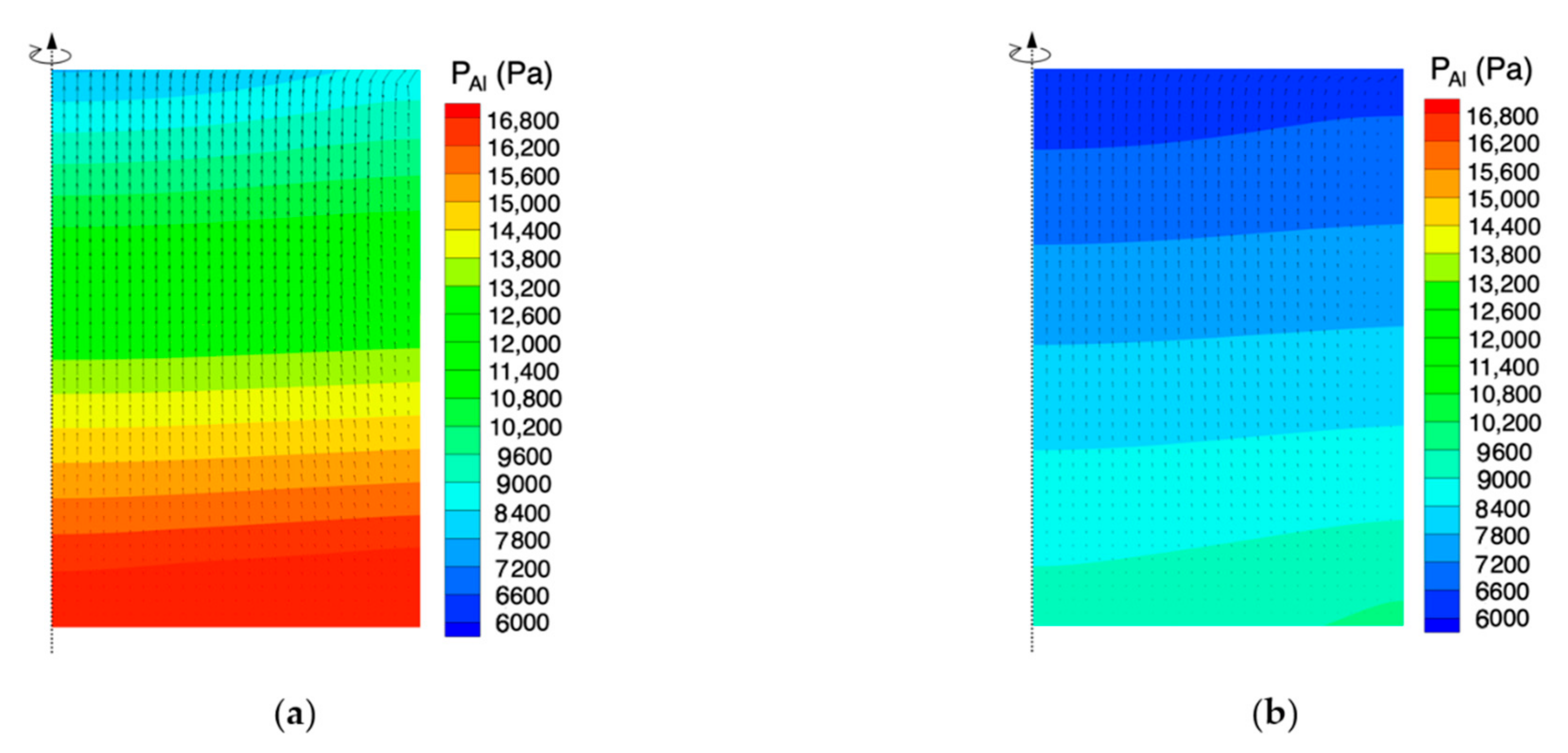
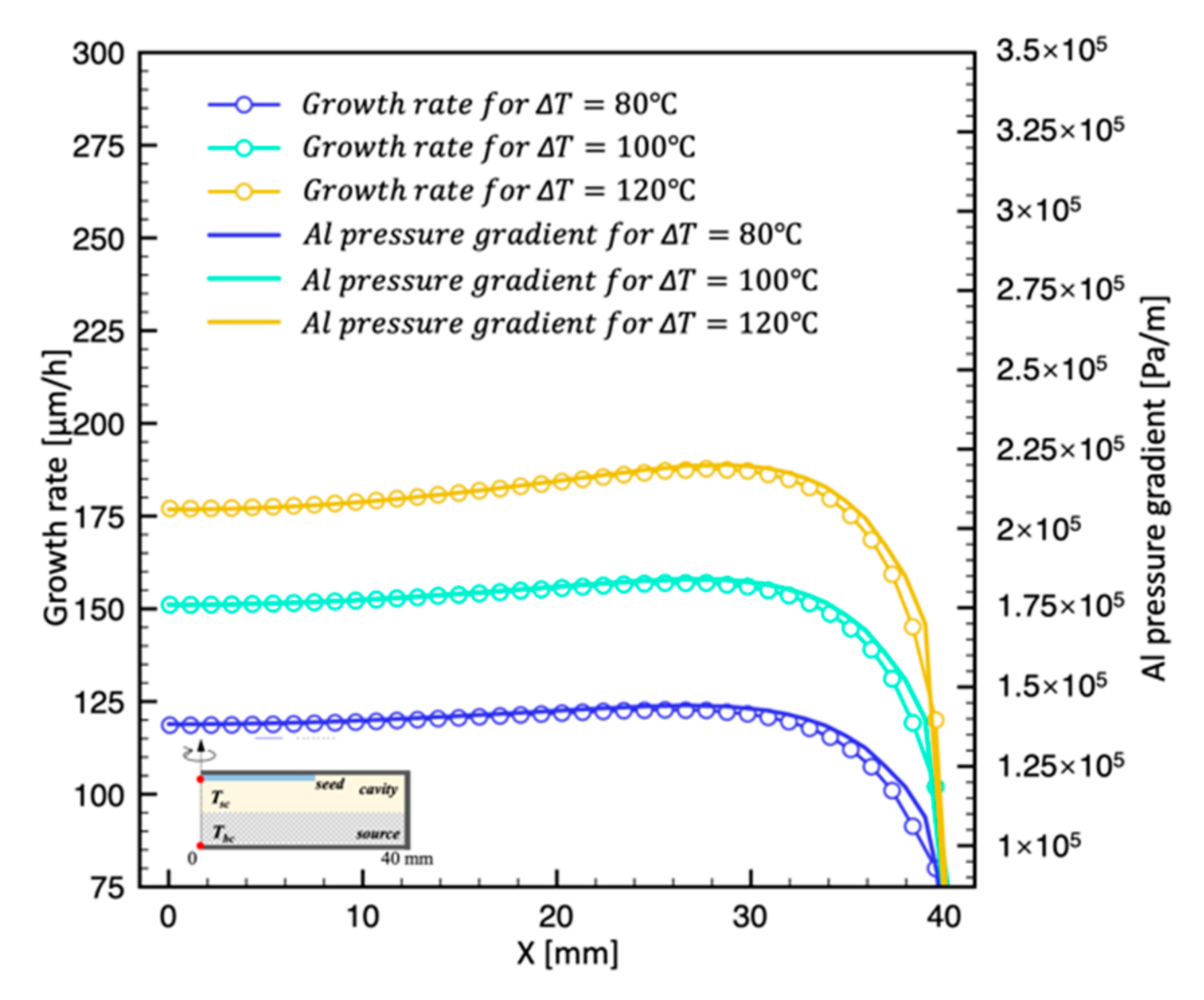
4.3. Effect of Temperature Difference
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambacher, O. Growth and applications of Group III-nitrides. J. Phys. D Appl. Phys. 1998, 31, 2653–2710. [Google Scholar] [CrossRef]
- Fu, D.; Gong, J.; Lei, D.; Huang, J.; Wang, Q.; Wu, L. Recent Progress and Future Challenges of AlN Single Crystal Growth by Physical Vapor Transport. J. Synth. Cryst. (Chin.) 2020, 49, 1141–1156. [Google Scholar]
- Hartmann, C.; Dittmar, A.; Wollweber, J.; Bickermann, M. Bulk AlN growth by physical vapour transport. Semicond. Sci. Technol. 2014, 29, 084002. [Google Scholar] [CrossRef]
- Noveski, V.; Schlesser, R.; Raghothamachar, B.; Dudley, M.; Mahajan, S.; Beaudoin, S.; Sitar, Z. Seeded growth of bulk AlN crystals and grain evolution in polycrystalline AlN boules. J. Cryst. Growth 2005, 279, 13–19. [Google Scholar] [CrossRef]
- Epelbaum, B.M.; Bickermann, M.; Nagata, S.; Heimann, P.; Filip, O.; Winnacker, A. Similarities and differences in sublimation growth of SiC and AlN. J. Cryst. Growth 2007, 305, 317–325. [Google Scholar] [CrossRef]
- Sumathi, R.R. Native seeding and silicon doping in bulk growth of AlN single crystals by PVT method. Phys. Status Solidi C 2014, 11, 545–548. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, D.; He, G.; Gong, J.; Huang, J.; Wu, J. Characterization of 60 mm AlN Single Crystal Wafers Grown by the Physical Vapor Transport Method. Phys. Status Solidi A 2019, 1900118. [Google Scholar] [CrossRef]
- Chemekova, T.Y.; Avdeev, O.V.; Barash, I.S.; Mokhov, E.N.; Nagalyuk, S.S.; Roenkov, A.D.; Segal, A.S.; Makarov, Y.N.; Ramm, M.G.; Davis, S.; et al. Sublimation growthof 2 inch diameter bulk AlN crystals. Phys. Status Solidi C 2008, 5, 1612–1614. [Google Scholar] [CrossRef]
- Schujman, S.B.; Schowalter, L.J.; Bondokov, R.T.; Morgan, K.E.; Liu, W.; Smart, J.A.; Bettles, T. Structural and surface characterization of large diameter, crystalline AlN substrates for device fabrication. J. Cryst. Growth 2008, 310, 887–890. [Google Scholar] [CrossRef]
- Liu, L.; Edgar, J.H. A Global Growth Rate Model for Aluminum Nitride Sublimation. J. Electrochem. Soc. 2002, 149, G12–G15. [Google Scholar] [CrossRef]
- Wu, B.; Ma, R.; Zhang, H.; Dudley, M.; Schlesser, R.; Sitar, R. Growth kinetics and thermal stress in AlN bulk crystal growth. J. Cryst. Growth 2003, 253, 326–339. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, H. Transport phenomena in an aluminum nitride induction heating sublimation growth system. Int. J. Heat Mass Transfer 2004, 47, 2989–3001. [Google Scholar] [CrossRef]
- Wu, B.; Ma, R.; Zhang, H.; Prasad, V. Modeling and simulation of AlN bulk sublimation growth systems. J. Cryst. Growth 2004, 266, 303–312. [Google Scholar] [CrossRef]
- Gao, B.; Nakano, S.; Kakimoto, K. The impact of pressure and temperature on growth rate and layer uniformity in the sublimation growth of AlN crystals. J. Cryst. Growth 2012, 338, 69–74. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Fu, D.; He, G.; Lei, D.; Wu, L. Influence of crucible shape on mass transport in AlN crystal growth by physical vapor transport process. J. Cryst. Growth 2019, 515, 21–25. [Google Scholar] [CrossRef]
- Fu, D.; Wang, Q.; Zhang, G.; Zhu, R.; Liu, H.; Li, Z.; Wu, L. Modelling and simulation of oxygen transport during AlN crystal growth by the PVT method. J. Cryst. Growth 2020, 551, 12592. [Google Scholar] [CrossRef]
- Wellmanna, P.J.; Hofmanna, D.; Kadinskib, L.; Selderb, M.; Straubingera, T.L.; Winnacker, A. Impact of source material on silicon carbide vapor transport growth process. J. Cryst. Growth 2001, 225, 312–316. [Google Scholar] [CrossRef]
- Wang, X.; Cai, D.; Zhang, H. Increase of SiC sublimation growth rate by optimizing of powder packaging. J. Cryst. Growth 2007, 305, 122–132. [Google Scholar] [CrossRef]
- Dupret, F.; Nicodème, P.; Ryckmans, Y.; Wouters, P.; Crochet, M.J. Global modelling of heat transfer in crystal growth furnaces. Int. J. Heat Mass Transfer 1990, 33, 1849–1871. [Google Scholar] [CrossRef]
- Khoei, A.R.; Salehi, S.A.; Hosseini, N. Modeling of reactive acid transport in fractured porous media with the Extended–FEM based on Darcy-Brinkman-Forchheimer framework. Comput. Geotech. 2020, 128, 103778. [Google Scholar] [CrossRef]
- Mueller, S.G.; Bondokov, R.T.; Morgan, K.E.; Slack, G.A.; Schujman, S.B.; Grandusky, J.; Smart, J.; Schowalter, L.J. The progress of AlN bulk growth and epitaxy for electronic applications. Phys. Status Solidi A 2009, 206, 1153–1159. [Google Scholar] [CrossRef]
- Liu, L.; Edgar, J.H. Transport effects in the sublimation growth ofaluminum nitride. J. Cryst. Growth 2000, 220, 243–253. [Google Scholar] [CrossRef]
- Karpov, S.Y.; Zimina, D.V.; Makarov, Y.N.; Mokhov, E.N.; Roenkov, A.D.; Ramm, M.G.; Vodakov, Y.A. Sublimation Growth of AlN in Vacuum and in a Gas Atmosphere. Phys. Status Solidi A 1999, 176, 435–438. [Google Scholar] [CrossRef]
- Wolfson, A.A.; Mokhov, E.N. Dependence of the Growth Rate of an AlN Layer on Nitrogen Pressure in a Reactor for Sublimation Growth of AlN Crystals. Semiconductors 2010, 44, 1430–1432. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, D.; Wang, Q.; Zhang, G.; Li, Z.; Huang, J.; Wang, J.; Wu, L. Influences of Powder Source Porosity on Mass Transport during AlN Crystal Growth Using Physical Vapor Transport Method. Crystals 2021, 11, 1436. https://doi.org/10.3390/cryst11111436
Fu D, Wang Q, Zhang G, Li Z, Huang J, Wang J, Wu L. Influences of Powder Source Porosity on Mass Transport during AlN Crystal Growth Using Physical Vapor Transport Method. Crystals. 2021; 11(11):1436. https://doi.org/10.3390/cryst11111436
Chicago/Turabian StyleFu, Danyang, Qikun Wang, Gang Zhang, Zhe Li, Jiali Huang, Jiang Wang, and Liang Wu. 2021. "Influences of Powder Source Porosity on Mass Transport during AlN Crystal Growth Using Physical Vapor Transport Method" Crystals 11, no. 11: 1436. https://doi.org/10.3390/cryst11111436





