Interfacial Microstructure Characteristics and Mechanical Properties of a Press Bonded Ti–5Al–2Sn–2Zr–4Mo–4Cr Alloy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
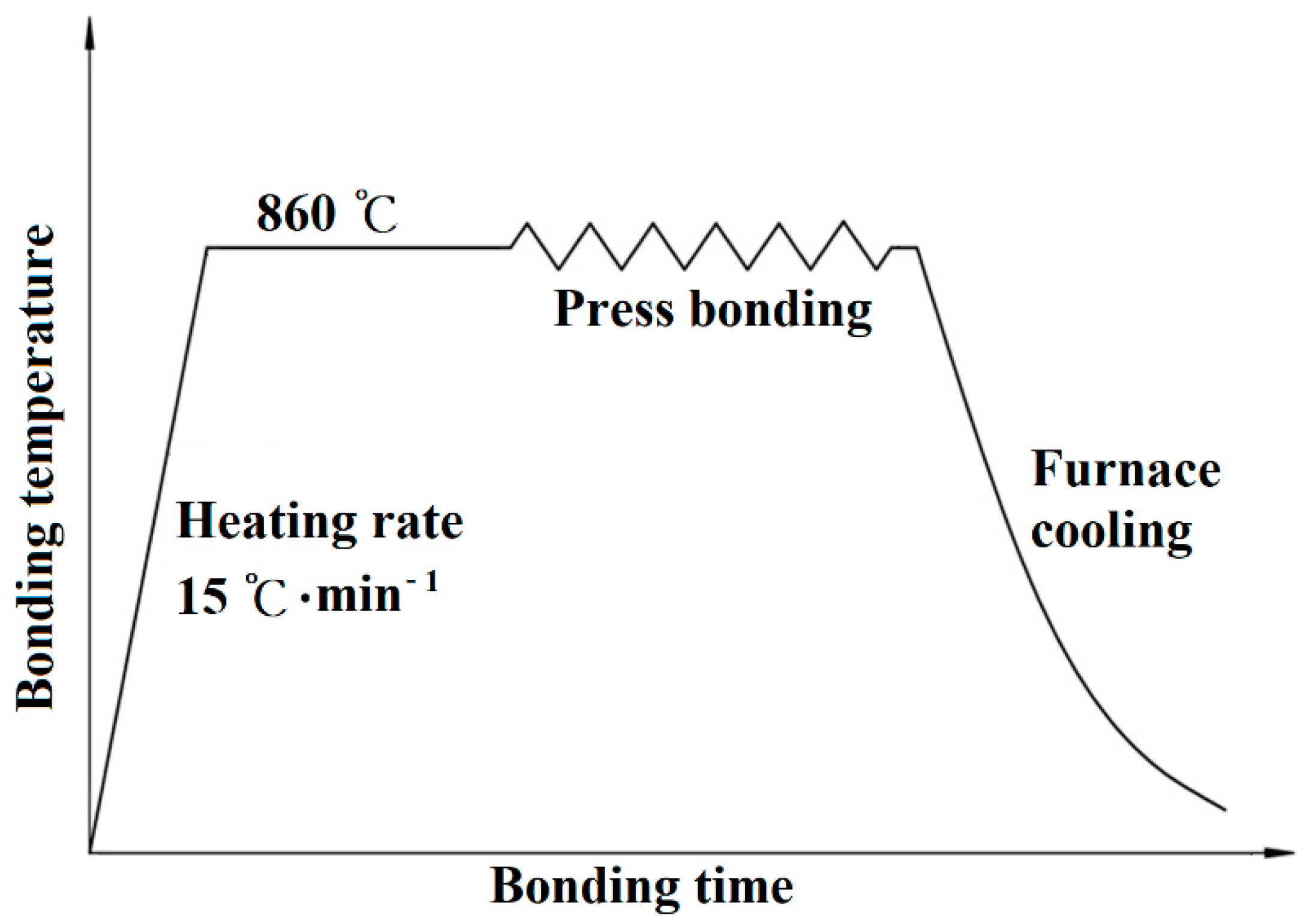
2.2. Press Bonding Procedure
2.3. Microstructure Examination
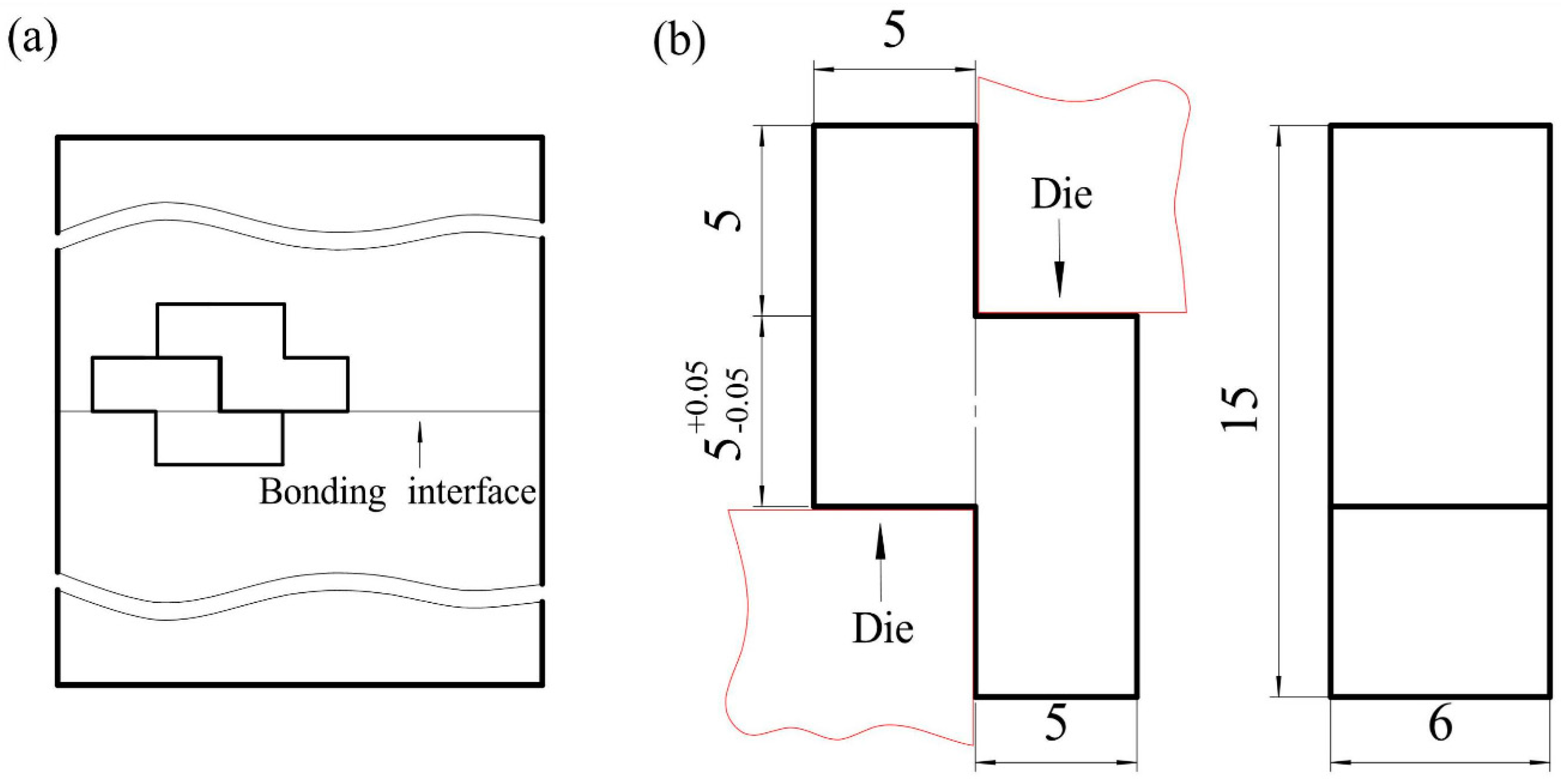
2.4. Lap Shear Tests
3. Results and Discussion
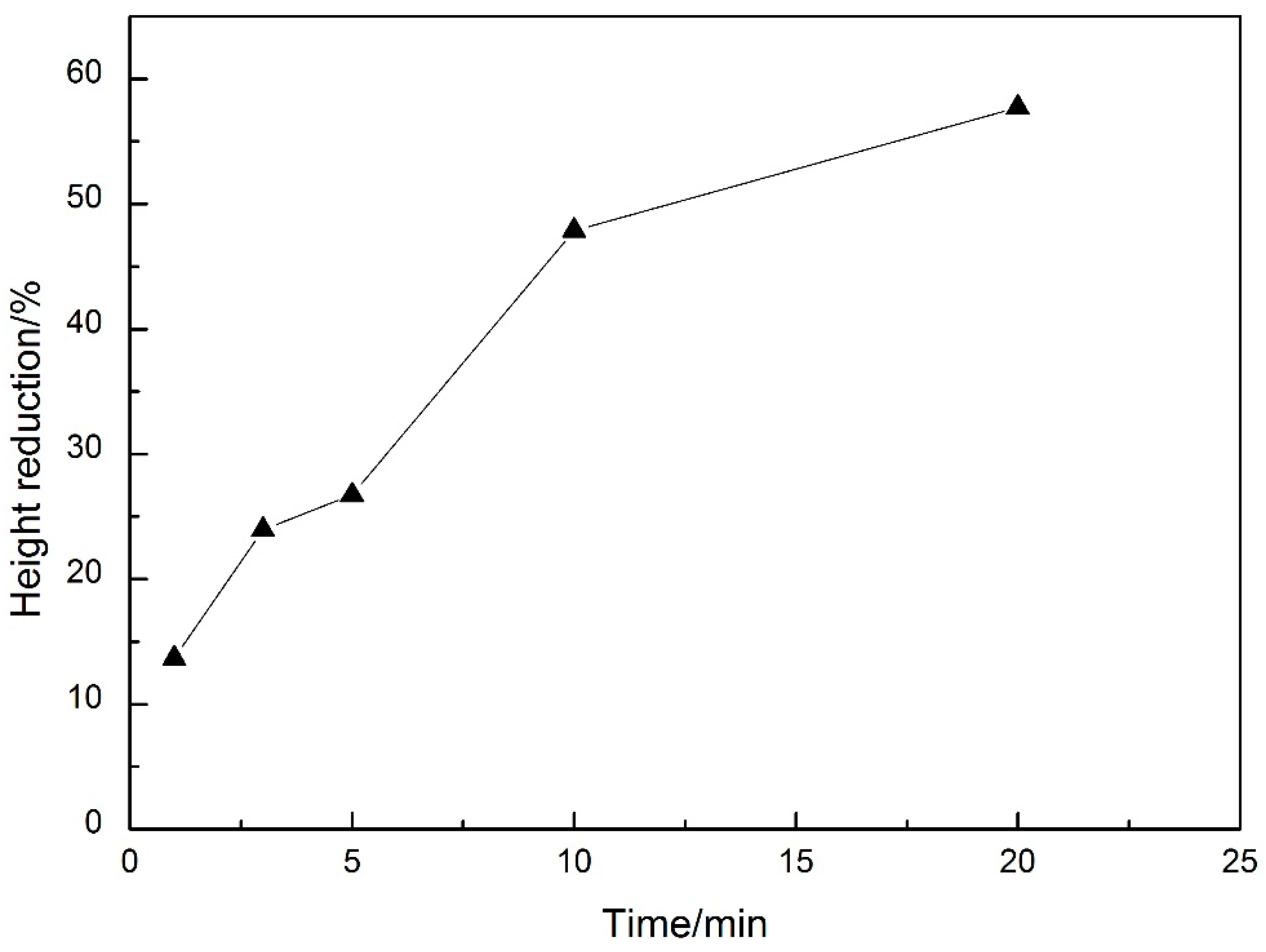
3.1. Deformation Ratio
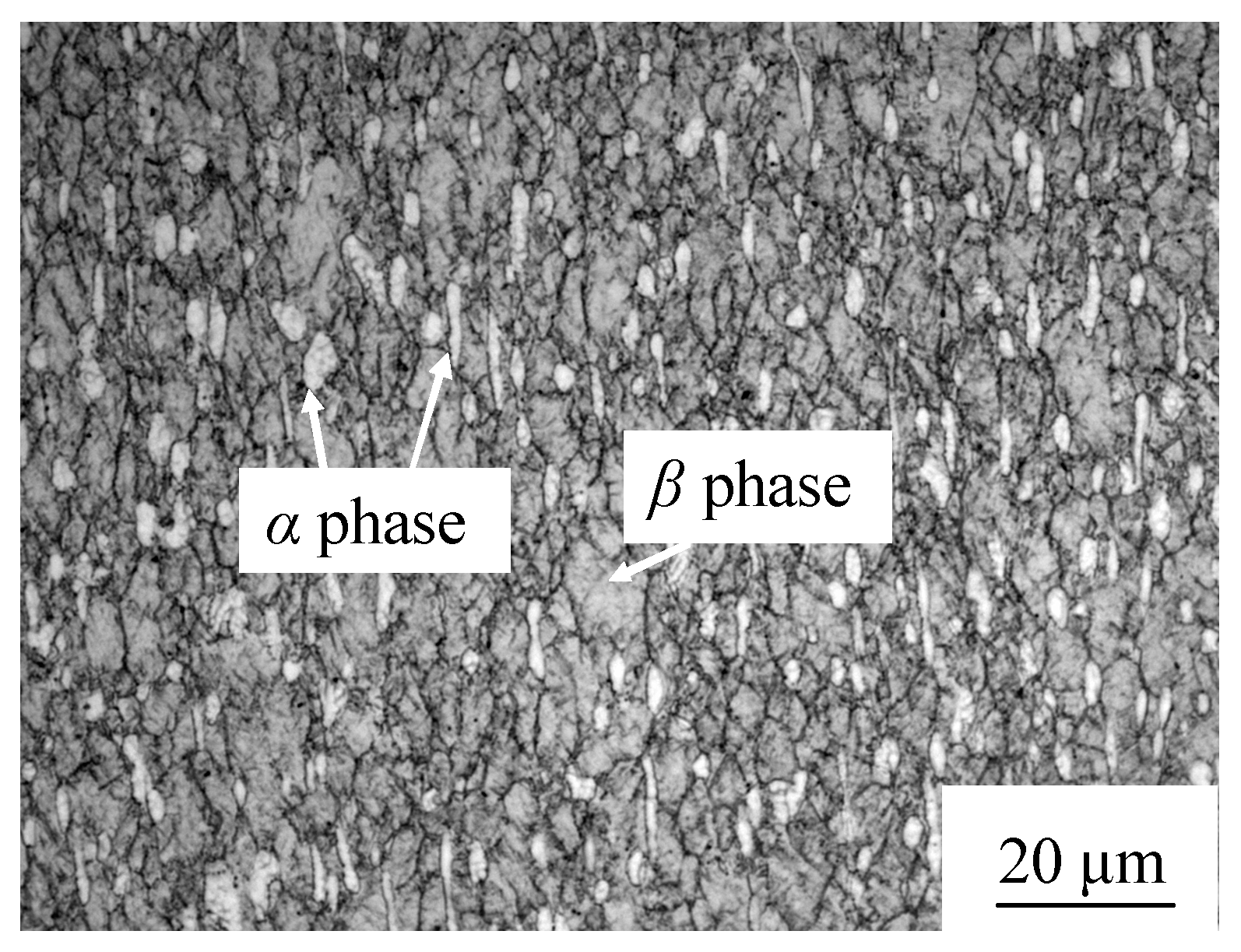
3.2. Microstructure Morphology of the Bond
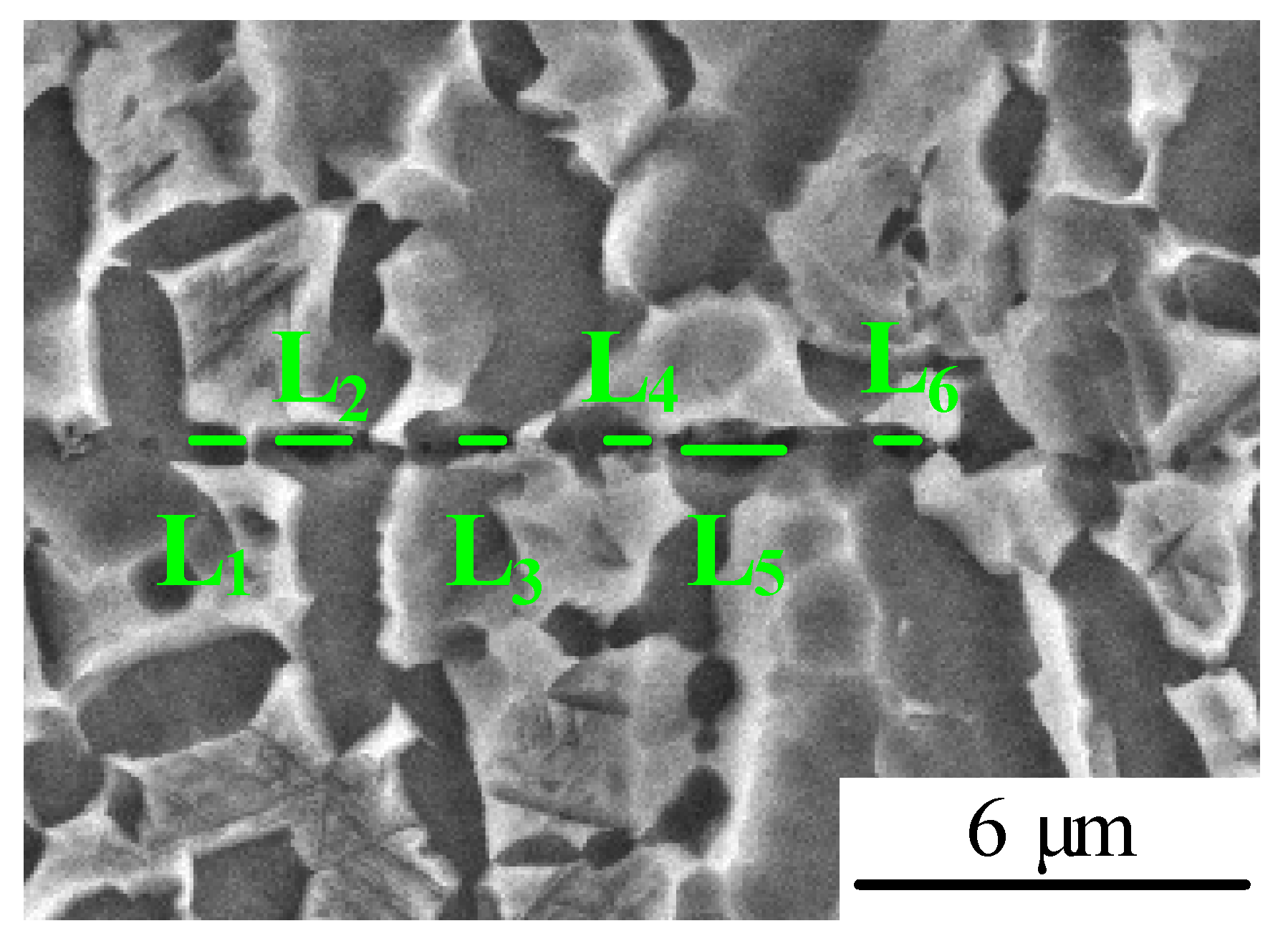
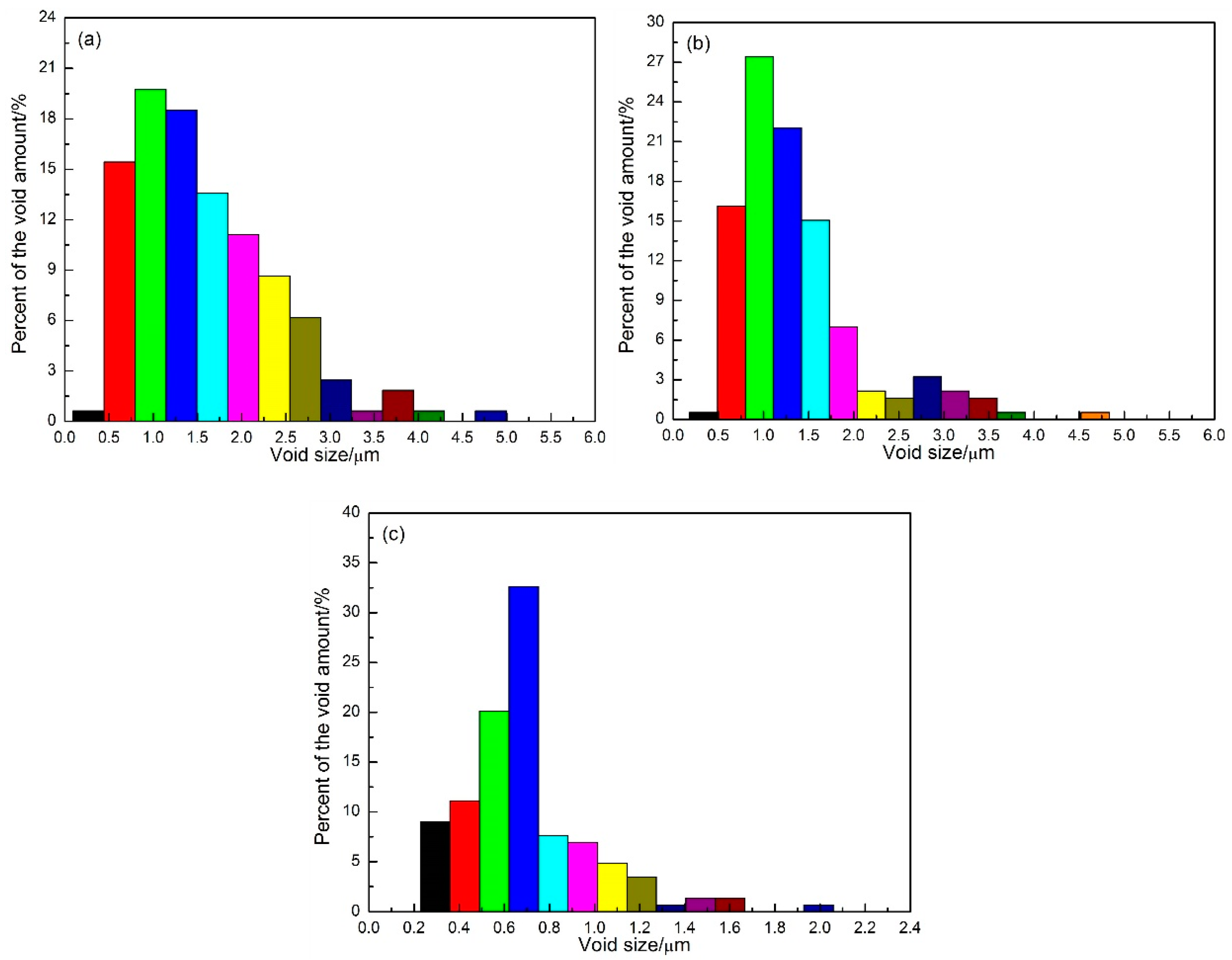
3.3. Interfacial Void Size and Distribution
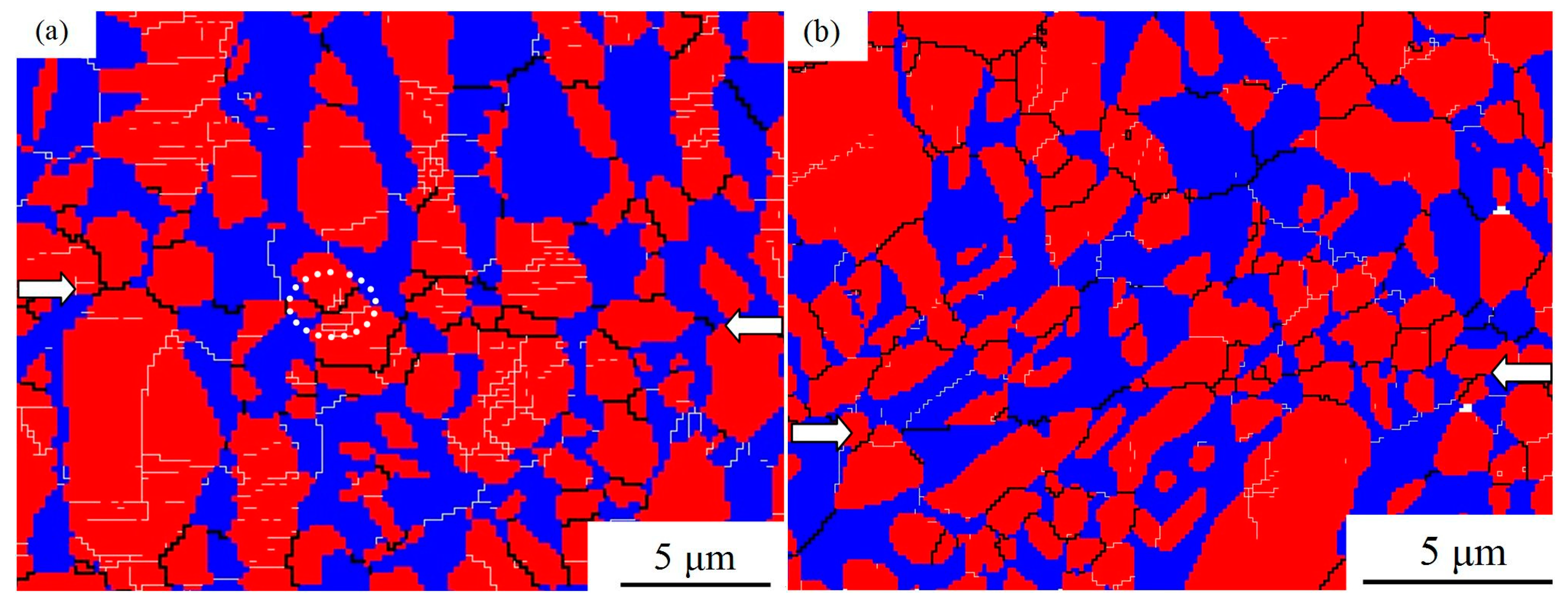
3.4. Volume Fraction and Grain Size of the Primary α Phase
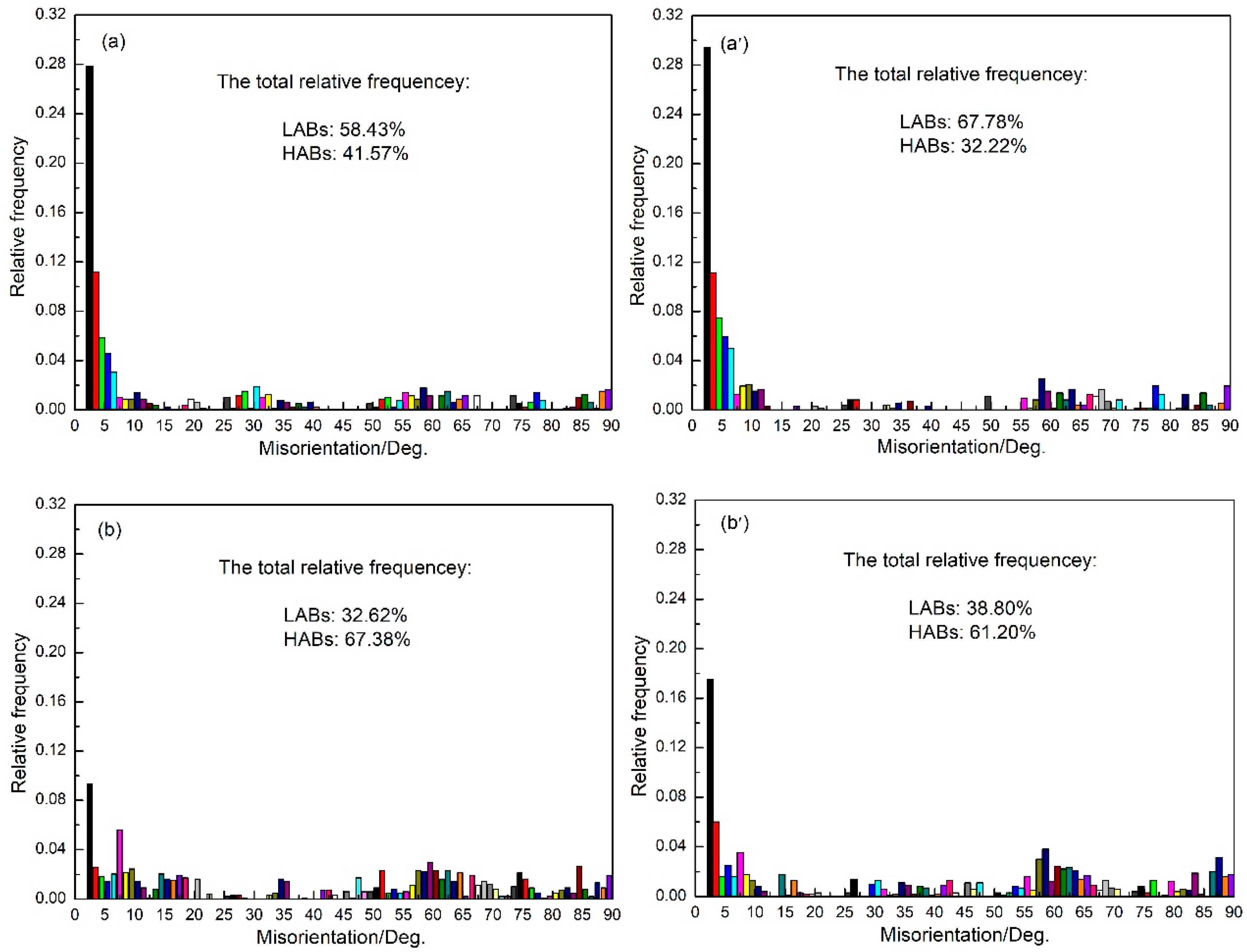
3.5. Grain Boundary Misorientation
3.6. Shear Strength of the Bond
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Li, M.Q.; Han, T.; Liu, H.B. The deformation behavior of isothermally compressed, Ti-17 titanium alloy in α + β field. Mater. Sci. Eng. A 2012, 546, 40–45. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Wei, Y.; Feng, G.; Deng, D. Microstructure and mechanical properties of vacuum diffusion bonded, Ti2AlNb/Ti/TC4 joint. Crystals 2021, 11, 770. [Google Scholar] [CrossRef]
- Lee, H.S.; Yoon, J.H.; Yi, Y.M. Oxidation behavior of titanium alloy under diffusion bonding. Thermochim. Acta 2007, 455, 105–108. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.Q.; Li, H. The behavior and mechanism of void self-shrinkage in diffusion bonded 1Cr11Ni2W2MoV steel joint: Effect of temperature and void morphology. J. Manuf. Process. 2018, 35, 71–78. [Google Scholar] [CrossRef]
- Li, H.; Yang, C.; Sun, L.X.; Li, M.Q. Hot press bonding of γ-TiAl and, TC 17 at a low bonding temperature by imposing plastic deformation and post heating. Mater. Lett. 2017, 187, 4–6. [Google Scholar] [CrossRef]
- Tuppen, S.J.; Bache, M.R.; Voice, W.E. A fatigue assessment of dissimilar titanium alloy diffusion bonds. Int. J. Fatigue 2005, 27, 651–658. [Google Scholar] [CrossRef]
- Wang, X.F.; Ma, M.; Liu, X.B.; Lin, J.G. Interface characteristics in diffusion bonding of γ-TiAl alloy to, Ti-6Al-4V. J. Mater. Sci. 2007, 42, 4004–4008. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.M.; Carreño, F.; Ruano, O.A.; Sarkeeva, A.A.; Kruglov, A.A.; Lutfullin, R.Y. Influence of interfacial defects on the impact toughness of solid state diffusion bonded, Ti-6Al-4V alloy based multilayer composites. Mater. Sci. Eng. A 2013, 563, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, C.; Liu, H.B.; Li, M.Q. Bonding interface characteristic and shear strength of diffusion bonded, Ti-17 titanium alloy, Trans. Nonferrous. Met. Soc. China 2015, 25, 80–87. [Google Scholar]
- Li, H.; Liu, H.B.; Yu, W.X.; Li, M.Q. Fabrication of high strength bond of, Ti-5Al-2Sn-2Zr-4Mo-4Cr alloy using press bonding under a high bonding pressure. Mater. Lett. 2013, 108, 212–214. [Google Scholar] [CrossRef]
- Chen, J.G.; Liu, C.X.; Wei, C.; Liu, Y.C.; Li, H.J. Study on microstructure and mechanical properties of direct diffusion bonded low-carbon, RAFM steels. J. Manuf. Process. 2019, 43, 192–199. [Google Scholar] [CrossRef]
- Wu, H.; Lee, S. Effect of bonding variables on bonding mechanisms in press bonding superplastic 8090 aluminium alloy. Mater. Sci. Technol. 2001, 17, 906–911. [Google Scholar] [CrossRef]
- Somekawa, H.; Watanabe, H.; Mukai, T.; Higashi, K. Low temperature diffusion bonding in a superplastic, AZ31 magnesium alloy. Scr. Mater. 2003, 48, 1249–1254. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhao, Y.Q.; Hou, H.L.; Wang, Y.Q. Effect of hydrogen content on superplastic forming/diffusion bonding of, TC21 alloys. J. Alloys Compd. 2010, 503, 151–154. [Google Scholar] [CrossRef]
- Derby, B.; Wallach, E.R. Theoretical model for diffusion bonding. Met. Sci. 1982, 16, 49–56. [Google Scholar] [CrossRef]
- Somekawa, H.; Tanaka, T.; Sasaki, H.; Kita, K.; Inoue, A.; Higashi, K. Diffusion bonding in ultra fine-grained, Al-Fe alloy indicating high-strain-rate superplasticity. Acta Mater. 2004, 52, 1051–1059. [Google Scholar] [CrossRef]
- Ma, R.F.; Li, M.Q.; Li, H.; Yu, W.X. Modeling of void closure in diffusion bonding process based on dynamic conditions. Sci. China Technol. Sci. 2012, 55, 2420–2431. [Google Scholar] [CrossRef]
- Hu, W.; Ponge, D.; Gottstein, G. Origin of grain boundary motion during diffusion bonding by hot pressing. Mater. Sci. Eng. A 1995, 190, 223–229. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Li, M.Q. Role of surface finish on interface grain boundary migration in vacuum diffusion bonding. Vacuum 2017, 137, 49–55. [Google Scholar] [CrossRef]
- Calvo, F.A.; De Salazar, J.M.G.; Urena, A.; Perosanz, F. Diffusion bonding of, Ti-6Al-4V alloy at low temperature: Metallurgical aspects. J. Mater. Sci. 1992, 27, 391–398. [Google Scholar] [CrossRef]
- Xun, Y.W.; Tan, M.J. Applications of superplastic forming and diffusion bonding to hollow engine blades. J. Mater. Process. Technol. 2000, 99, 80–85. [Google Scholar] [CrossRef]
- Han, W.B.; Zhang, K.F.; Wang, G.F. Superplastic forming and diffusion bonding for honeycomb structure of, Ti-6Al-4V alloy. J. Mater. Process. Technol. 2007, 183, 450–454. [Google Scholar] [CrossRef]
- Luo, J.; Li, M.; Yu, W.; Li, H. Effect of the strain on processing maps of titanium alloys in isothermal compression. Mater. Sci. Eng. A 2009, 504, 90–98. [Google Scholar] [CrossRef]
- Chen, H.Y.; Cao, J.; Song, X.G.; Feng, J.C. Contributions of atomic diffusion and plastic deformation to the plasma surface activation assisted diffusion bonding of zirconium-based bulk metallic glass. Appl. Phys. Lett. 2012, 100, 211602. [Google Scholar] [CrossRef]
- Jardim, P.M.; Acchar, W.; Losch, W. Grain boundary reactive diffusion during, Ni2Si formation in thin films and its dependence on the grain boundary angle. Appl. Surf. Sci. 1999, 137, 163–169. [Google Scholar] [CrossRef]
- Das, S.K.; Brodusch, N.; Gauvin, R.; Jung, I.H. Grain boundary diffusion of, Al in, Mg. Scr. Mater. 2014, 80, 41–44. [Google Scholar] [CrossRef]

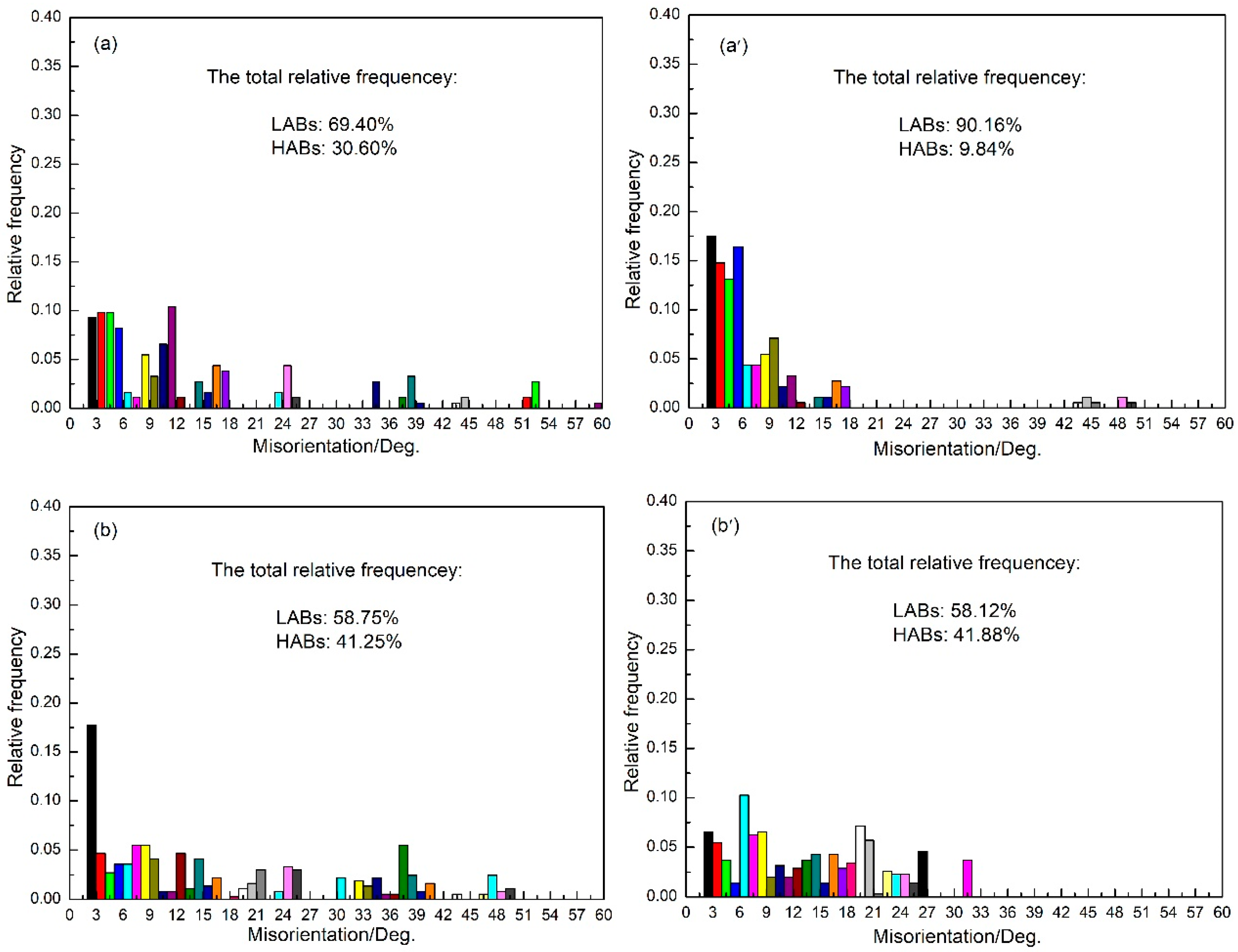
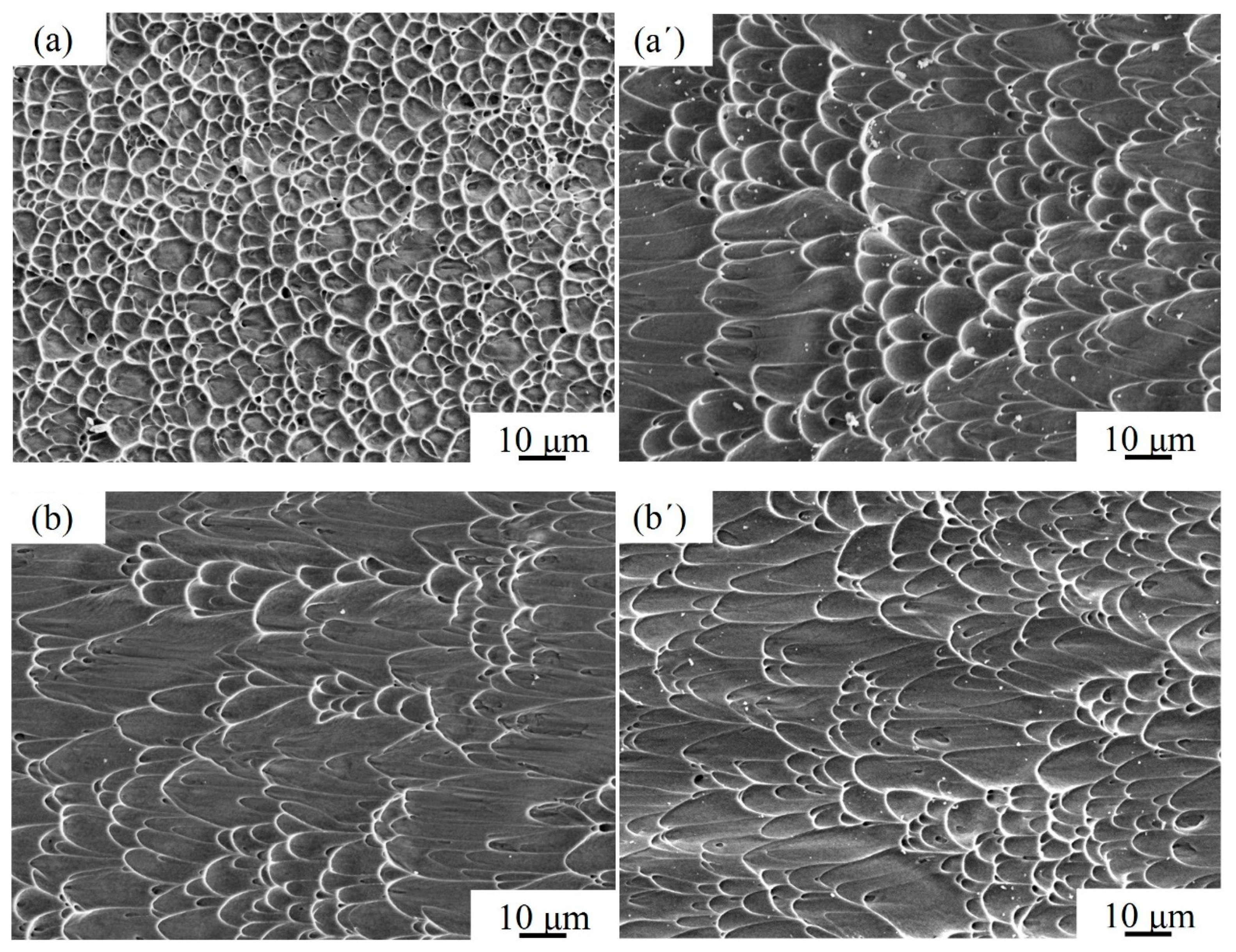
| Time/min | 1 | 3 | 5 | 10 | 20 |
|---|---|---|---|---|---|
| Average void size/μm | 1.2 | 1.0 | 0.6 | 0 | 0 |
| Bonding ratio/% | 89.4 | 91.7 | 94.9 | 100.0 | 100.0 |
| Time/min | 1 | 3 | 5 | 10 | 20 |
|---|---|---|---|---|---|
| Volume fraction/% | 25.45 | 23.99 | 26.75 | 31.43 | 29.51 |
| Grain size/μm | 3.98 ± 0.90 | 3.58 ± 0.78 | 4.23 ± 0.90 | 3.97 ± 1.05 | 4.06 ± 0.91 |
| Time/min | α Grain Boundary | β Grain Boundary | ||
|---|---|---|---|---|
| BZ | VBZ | BZ | VBZ | |
| 1 | 25.5° | 23.4° | 14.1° | 7.8° |
| 10 | 40.7° | 38.5° | 16.9° | 13.6° |
| Time/min | 1 | 3 | 5 | 10 | 20 | |
|---|---|---|---|---|---|---|
| Shear strength/MPa | Bond | 847.7 | 868.1 | 892.6 | 950.6 | 949.6 |
| Base alloy | 920.7 | 916.9 | 908.1 | 972.5 | 957.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, M. Interfacial Microstructure Characteristics and Mechanical Properties of a Press Bonded Ti–5Al–2Sn–2Zr–4Mo–4Cr Alloy. Crystals 2021, 11, 1395. https://doi.org/10.3390/cryst11111395
Li H, Li M. Interfacial Microstructure Characteristics and Mechanical Properties of a Press Bonded Ti–5Al–2Sn–2Zr–4Mo–4Cr Alloy. Crystals. 2021; 11(11):1395. https://doi.org/10.3390/cryst11111395
Chicago/Turabian StyleLi, Hong, and Miaoquan Li. 2021. "Interfacial Microstructure Characteristics and Mechanical Properties of a Press Bonded Ti–5Al–2Sn–2Zr–4Mo–4Cr Alloy" Crystals 11, no. 11: 1395. https://doi.org/10.3390/cryst11111395




