Crystal Structure, Thermodynamic Properties and DFT Studies of 5,6-dimethyl-1H-benzo[d]imidazol-3-ium 3-((2,4-dioxo-1,5-dioxaspiro[5.5]undecan-3-ylidene)methyl) -2,4-dioxo-1,5-dioxaspiro[5.5]undecane Hydrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical Measurements
2.2. Preparation of DBH
2.3. X-ray Diffraction Analysis of DBH
2.4. Computational Methods
3. Results and Discussion
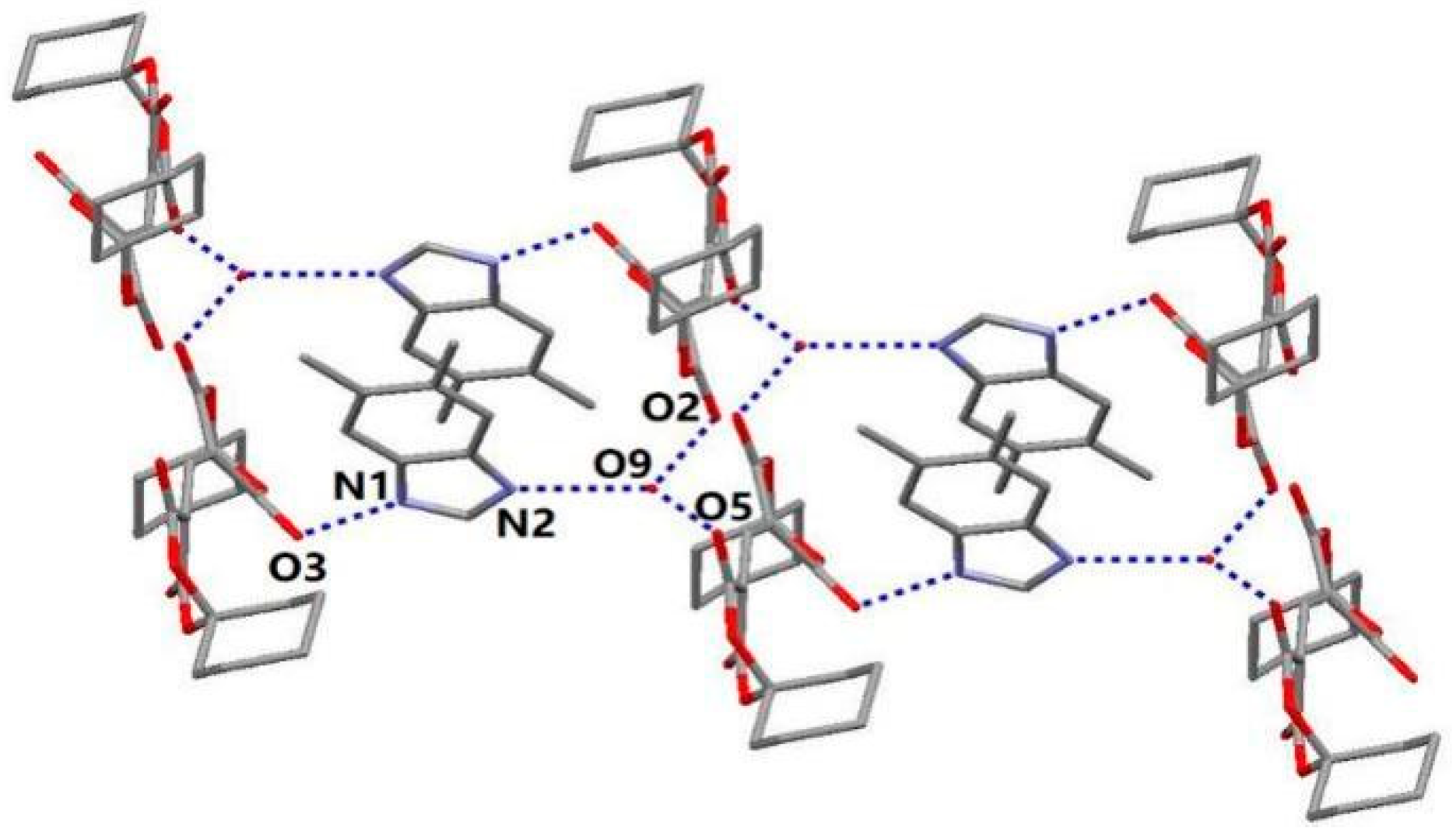
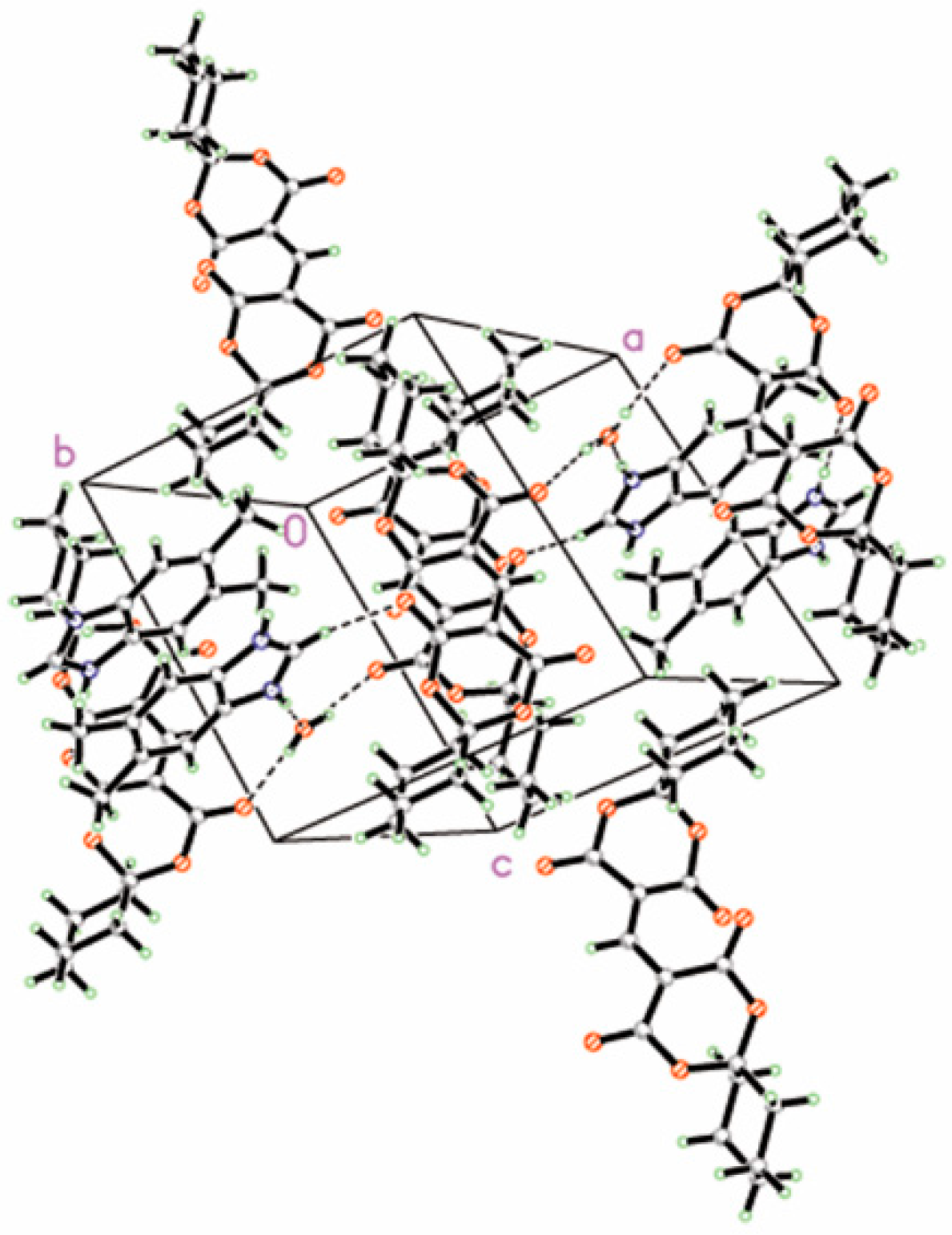
3.1. Crystal Structure of DBH
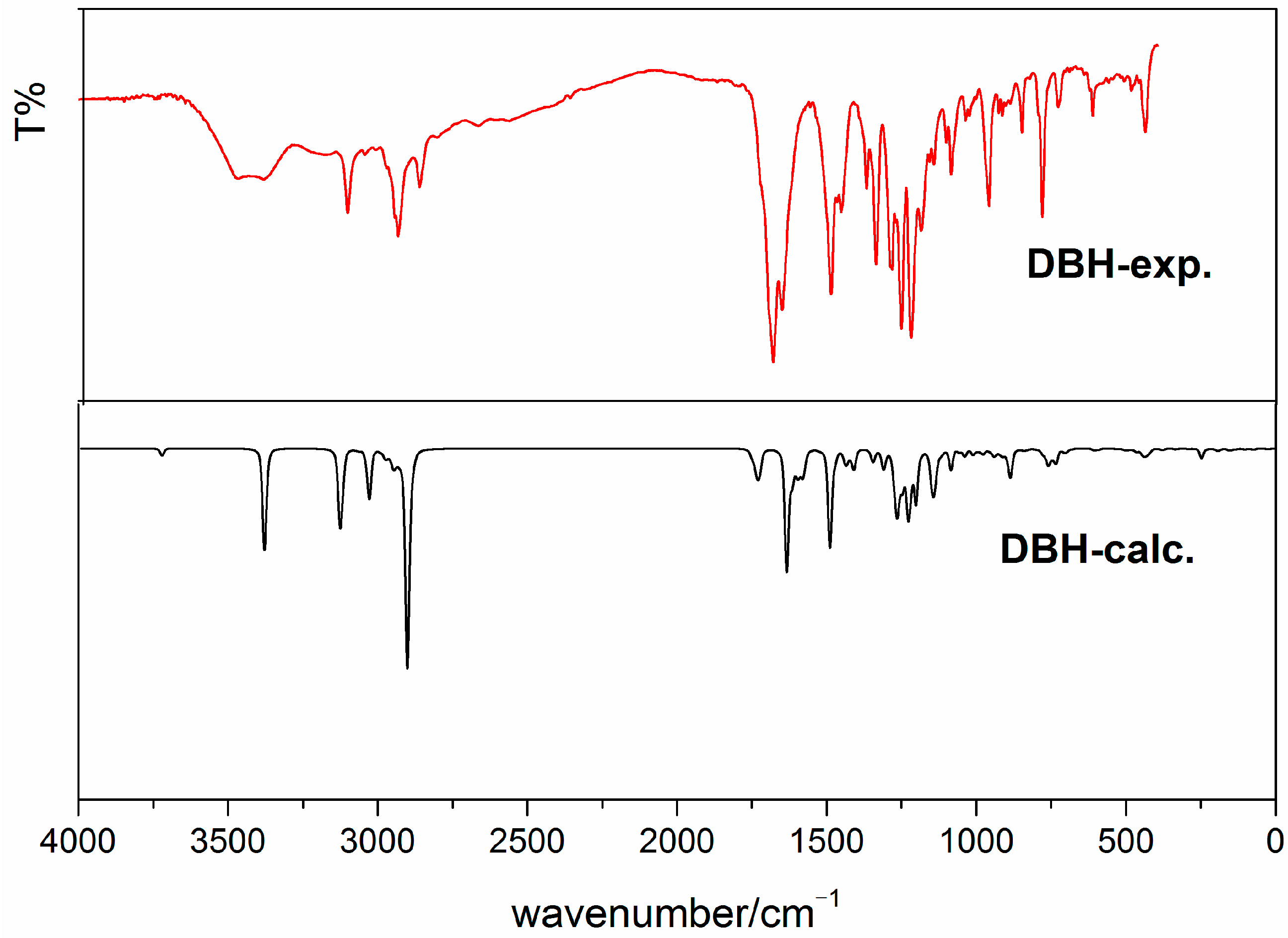
3.2. Vibrational Analysis
3.3. Thermodynamic Properties
3.4. Electronic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haoran, W.; Akhtar, W.; Nainwal, L.M.; Kaushik, S.K.; Akhter, M.; Shaquiquzzaman, M.; Alam, M.M. Synthesis and biological evaluation of benzimidazole pendant cyanopyrimidine derivatives as anticancer agents. J. Heterocycl. Chem. 2020, 57, 3350–3360. [Google Scholar] [CrossRef]
- Natha, J.; Paul, R.; Ghosh, S.K.; Paul, J.; Singha, B.; Debnath, N. Drug repurposing and relabeling for cancer therapy: Emerging benzimidazole antihelminthics with potent anticancer effects. Life Sci. 2020, 258, 118189. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.F.; Lal, C. Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur. J. Med. Chem. 2009, 44, 4028–4033. [Google Scholar] [CrossRef] [PubMed]
- Bektas, H.; Sökmen, B.B.; Aydın, S.; Menteşe, E.; Bektaş, A.; Dilekçi, G. Design, synthesis, and characterization of some new benzimidazole derivatives and biological evaluation. J. Heterocycl. Chem. 2020, 57, 2234–2242. [Google Scholar] [CrossRef]
- Khalifa, M.E. Synthesis and evaluation of new 2-mercaptomethyl benzimidazole scaffolds as potential antibacterial, antioxidant and cytotoxic agents. ChemistrySelect 2020, 5, 10562–10566. [Google Scholar] [CrossRef]
- Flores-Carrillo, P.; Velázquez-López, J.M.; Aguayo-Ortiza, R.; Hernández-Campos, A.; Trejo-Soto, J.; Yépez-Mulia, L.; Castillo, R. Synthesis, antiprotozoal activity, and chemoinformatic analysis of 2-(methylthio)-1H-benzimidazole-5-carboxamide derivatives: Identification of new selective. Eur. J. Med. Chem. 2017, 137, 211–220. [Google Scholar] [CrossRef]
- Beltran-Hortelano, I.; Alcolea, V.; Font, M.R.; Pérez-Silanes, S. The role of imidazole and benzimidazole heterocycles in Chagas disease: A review. Eur. J. Med. Chem. 2020, 206, 112692. [Google Scholar] [CrossRef] [PubMed]
- Akkoça, S.; Tüzün, B.; İlhan, I.Ö.; Akkurt, M. Efficient synthesis and molecular docking studies of new pyrimidine-chromeno hybrid derivatives as potential antiproliferative agents. J. Mol. Struct. 2020, 1219, 128582. [Google Scholar]
- Caymaz, B.; Yıldız, U.; Akkoç, S.; Gerçek, Z.; Şengül, A. Synthesis, characterization, and antiproliferative activity studies of novel benzimidazole-Imidazopyridine hybrids as DNA groove binders. ChemistrySelect 2020, 5, 8465–8474. [Google Scholar] [CrossRef]
- Agliano, F.; Karlinsey, K.S.; Ragazzi, M.; Ménoret, A.; Vella, A.T. A benzimidazole inhibitor attenuates sterile inflammation induced in a model of systemic autoinflammation in female mice. Sci. Rep. 2020, 10, 12100. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.M.; Rajvanshi, S.; Johar, M.; Bharti, N.; Azam, A.; Singh, A.K. Anti-inflammatory, analgesic and antiamoebic activity evaluation of pyrimido[1,6-a]benzimidazole derivatives synthesized by the reaction of ketoisothiocyanates with mono and diamines. Eur. J. Med. Chem. 2002, 37, 835–843. [Google Scholar] [CrossRef]
- Zeynep, A.A. Antimicrobial Activities of 1-H-Benzimidazole-based Molecules. Curr. Top. Med. Chem. 2016, 16, 2953–2962. [Google Scholar]
- Vasil’ev, P.M.; Kalitin, K.Y.; Spasov, A.A.; Grechko, O.Y.; Poroikov, V.V.; Filimonov, V.V.; Anisimova, V.A. Prediction and study of anticonvulsant properties of benzimidazole derivatives. Pharm. Chem. J. 2017, 50, 775–780. [Google Scholar] [CrossRef]
- Alpan, A.S.; Parlar, S.; Carlino, L.; Tarikogullari, A.H.; Alptüzün, V.; Günes, H.S. Synthesis, biological activity and molecular modeling studies on 1H-benzimidazole derivatives as acetylcholinesterase inhibitors. Bioorg. Med. Chem. 2013, 21, 4928–4937. [Google Scholar] [CrossRef]
- Chaouiki, A.; Chafiq, M.; Rbaa, M.; Salghi, R.; Lakhrissi, B.; Ali, I.H.; Bashir, S.; Chung, I.M.J. Comprehensive assessment of corrosion inhibition mechanisms of novel benzimidazole compounds for mild steel in HCl: An experimental and theoretical investigation. J. Mol. Liq. 2020, 320, 114383. [Google Scholar] [CrossRef]
- Allahresani, A.; Naghdi, E.; Nasseri, M.A. Catalytic activity of Co (II) Salen@ KCC-1 on the synthesis of 2, 4, 5-triphenyl-1H-imidazoles and benzimidazoles. Inorg. Chem. Commun. 2020, 119, 108137. [Google Scholar] [CrossRef]
- Maji, A.; Pal, S.; Lohar, S.; Mukhopadhyay, S.K.; Chattopadhyay, P. A new turn-on benzimidazole-based greenish-yellow fluorescent sensor for Zn 2+ ions at biological pH applicable in cell imaging. New J. Chem. 2017, 41, 7583–7590. [Google Scholar] [CrossRef]
- John, M.E.; Karnik, A.V. Chiral benzimidazole derived bis-phenyl triazoles as chiroptical sensors for iodide and chiral amines. J. Heterocycl. Chem. 2020, 57, 2844–2853. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Krishna, P.M. Asymmetric synthesis of nature-inspired bioactive spiro compounds through organocatalytic diels–alder reactions. Asian J. Org. Chem. 2016, 5, 729–734. [Google Scholar] [CrossRef]
- Niu, Q.; Xi, J.; Li, L.; Li, L.; Pan, C.; Lan, M.; Ronget, L. Isatins 3-C annulation vs ring-opening: Two different pathways for synthesis of spiro compounds via multicomponent reactions. Tetrahedron Lett. 2019, 60, 151181. [Google Scholar] [CrossRef]
- Guo, C.; Li, B.; Liu, H.; Zhang, X.; Zhang, X.; Fan, X. Synthesis of fused or spiro polyheterocyclic compounds via the dehydrogenative annulation reactions of 2-arylindazoles with maleimides. Org. Lett. 2019, 21, 7189–7193. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, X. Crystal structures of spiro derivatives including 6,10-dioxaspiro[4.5] decane-7,9-dione group and their spectral studies. J. Chem. Crystallogr. 2019, 49, 139–145. [Google Scholar] [CrossRef]
- Zeng, W.; Jiang, J.; Jiang, G.; Li, Y. Synthesis, characterization, and fluorescence properties of two new heterocyclic compounds containing 1,5-dioxaspiro group. Crystals 2018, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Jiang, J. Synthesis and crystal structure of a new hydrated benzimidazolium salt containing spiro structure. Crystals 2017, 7, 303. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zeng, W. The crystal structure of 8-((4-chlorophenylamino) methylene)-6,10-dioxaspiro[4.5]decane-7,9-dione, C15H14ClNO4. Kristallogr. NCS 2020, 235, 925–927. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian, Inc. Wallingford CT. 2013. Available online: https://gaussian.com/citation/ (accessed on 13 October 2021).
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Khemalapure, S.S.; Hiremath, S.M.; Hiremath, C.S.; Kattia, V.S.; Basanagouda, M. Structural, spectroscopic and computational investigations on (4,6-dimethyl-benzofuran-3-yl)-acetic acid hydrazide. J. Mol. Struct. 2020, 1220, 128748. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, X.; Song, J.; Sun, X.; Zeng, W.L. Synthesis, characterization, crystal structure and DFT studies on 3-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-1-m-tolylpropan-2-yl-nicotinate. Spectrochim. Acta Part A 2011, 79, 219–225. [Google Scholar] [CrossRef] [PubMed]

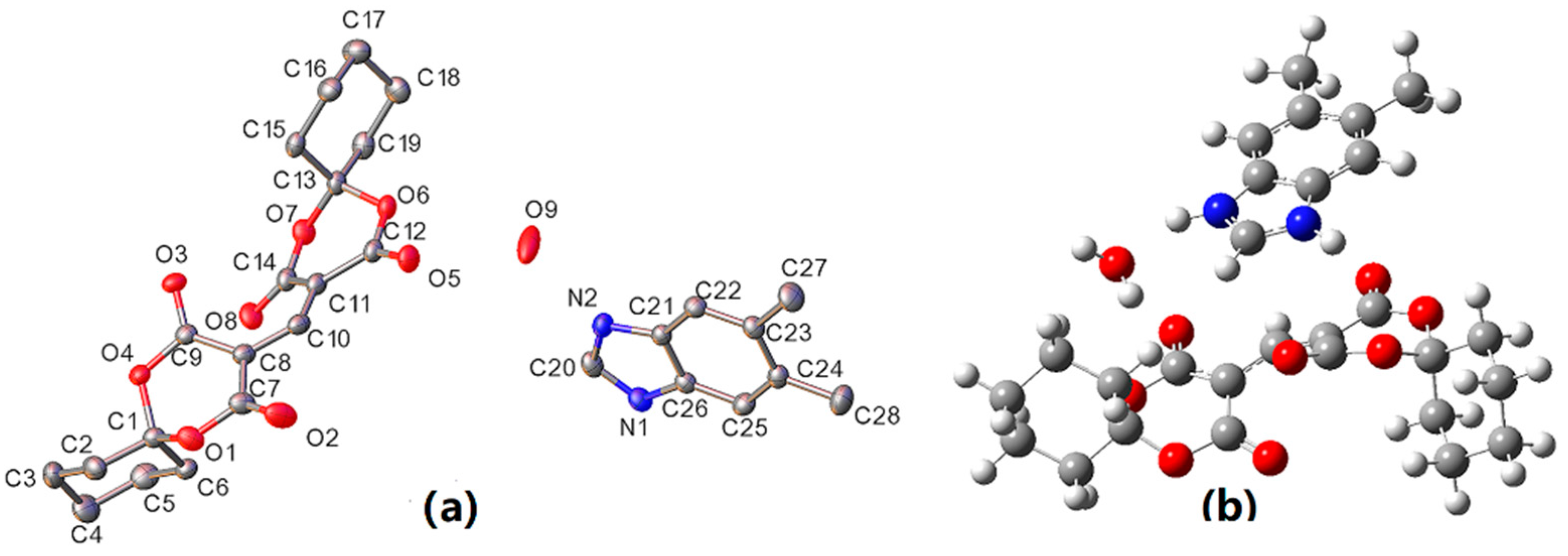
| Formula | C28H34N2O9 |
|---|---|
| CCDC | 1948628 |
| Mr | 380.35 |
| Color/shape | yellow/block |
| Temperature | 293(2) K |
| Crystal system, space group | Triclinic, P-1 |
| Unit cell dimensions | a = 10.5431(3) Å α = 73.9160(10)° b = 11.3397(4) Å β = 70.7600(10)° c = 12.4926(4) Å γ =89.8030(10)° |
| V | 1348.57(8) Å3 |
| Z, Density | 2, 1.336 Mg/m3 |
| μ | 0.100 mm−1 |
| F(000) | 576 |
| θ | 3.117 to 27.484° |
| Limiting indices | −13 ≤ h ≤ 12, −14 ≤ k ≤ 14, −16 ≤ l ≤ 16 |
| No. of reflections collected/unique | 13,425/6145 (Rint = 0.0188) |
| No. of parameters | 357 |
| GOF | 1.132 |
| R1 (I > 2σ (I)) | 0.0450 |
| wR2 (I > 2σ (I)) | 0.1099 |
| R1 (all data) | 0.0595 |
| wR2 (all data) | 0.1284 |
| Largest diff. peak and hole | 0.372 and −0.224 e. Å−3 |
| Bond Lengths | Exp. (Å) | Calc. (Å) | Bond | Exp. (Å) | Calc. (Å) |
|---|---|---|---|---|---|
| C8–C10 | 1.389(2) | 1.395 | O1–C1 | 1.436(16) | 1.419 |
| C10–C11 | 1.382(2) | 1.388 | O1–C7 | 1.365(18) | 1.385 |
| O8–C14 | 1.213(16) | 1.201 | C1–O4 | 1.441(15) | 1.449 |
| O5–C12 | 1.212(16) | 1.231 | O2–C7 | 1.218(17) | 1.201 |
| O6–C12 | 1.361(17) | 1.351 | O3–C9 | 1.221(16) | 1.231 |
| O6–C13 | 1.438 (15) | 1.456 | O4–C9 | 1.356(16) | 1.352 |
| O7–C14 | 1.363(18) | 1.382 | N1–C20 | 1.318(2) | 1.329 |
| N2–C20 | 1.322(19) | 1.333 | N2–C21 | 1.389(17) | 1.391 |
| Bond angle | Exp (°) | Calc. (°) | Bond angle | Exp (°) | Calc. (°) |
| C11–C10–C8 | 130.62(13) | 132.30 | C10–C8–C7 | 118.45(13) | 117.36 |
| C10–C11–C12 | 117.58(12) | 117.45 | C10–C8–C9 | 123.96(12) | 123.18 |
| C10–C11–C14 | 123.67(12) | 122.91 | C7–C8–C9 | 117.00(13) | 118.61 |
| C12–C11–C14 | 118.17(13) | 118.41 | N1–C20–N2 | 110.32(12) | 110.43 |
| Torsion angle | Exp (°) | Calc. (°) | Torsion angle | Exp (°) | Calc. (°) |
| C7–C8–C10–C11 | 167.64(14) | 168.64 | O1–C7–C8–C9 | 19.52(18) | 18.90 |
| C8–C10–C11–C12 | 168.36(13) | 166.71 | O2–C7–C8–C9 | −158.30(15) | −160.69 |
| C8–C10–C11–C14 | −20.6(2) | −24.00 | C9–C8–C10–C11 | −21.4(2) | −24.28 |
| D–H···A | Symmetry | D–H (Å) | H···A (Å) | D···A (Å) | ∠D–H···A (°) |
|---|---|---|---|---|---|
| N1–H1···O3 | x, y − 1, z | 0.86 | 1.94 | 2.777 (15) | 165.0 |
| N2−H2···O9 | intra | 0.86 | 1.79 | 2.650(18) | 172.3 |
| O9–H9A···O2 | −x + 2, −y + 1, z + 1 | 0.86 | 1.97 | 2.792(18) | 161.6 |
| O9–H9B···O5 | intra | 0.86 | 1.90 | 2.728(16) | 165.5 |
| T (K) | C0p,m (J·mol−1·K−1) | S0m (J·mol−1·K−1) | H0m (kJ·mol−1) |
|---|---|---|---|
| 100.0 | 245.91 | 520.42 | 14.32 |
| 200.0 | 423.94 | 745.99 | 47.88 |
| 298.1 | 601.87 | 948.26 | 98.18 |
| 300.0 | 605.24 | 951.99 | 99.29 |
| 400.0 | 780.04 | 1150.47 | 168.70 |
| 500.0 | 930.87 | 1341.24 | 254.48 |
| 600.0 | 1054.72 | 1522.29 | 353.97 |
| 700.0 | 1155.81 | 1692.73 | 464.66 |
| 800.0 | 1239.14 | 1852.68 | 584.54 |
| 900.0 | 1308.68 | 2002.76 | 712.03 |
| 1000.0 | 1367.29 | 2143.76 | 845.91 |
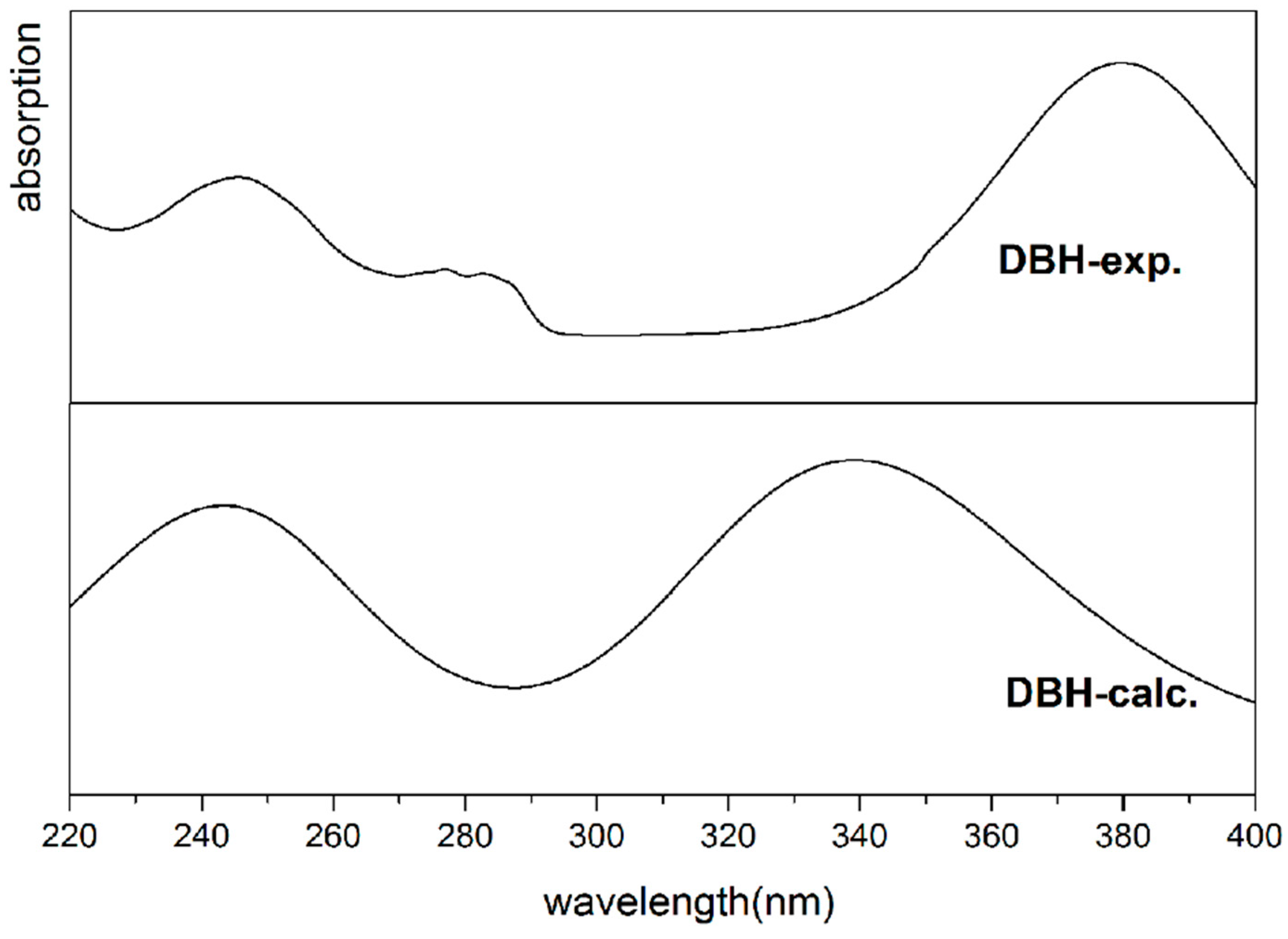
| Exp. | Calc. (TD-DFT) | ||
|---|---|---|---|
| Wavelength (nm) | Wavelength (nm) | Oscillator Strength(f) | Electronic Transition Modes |
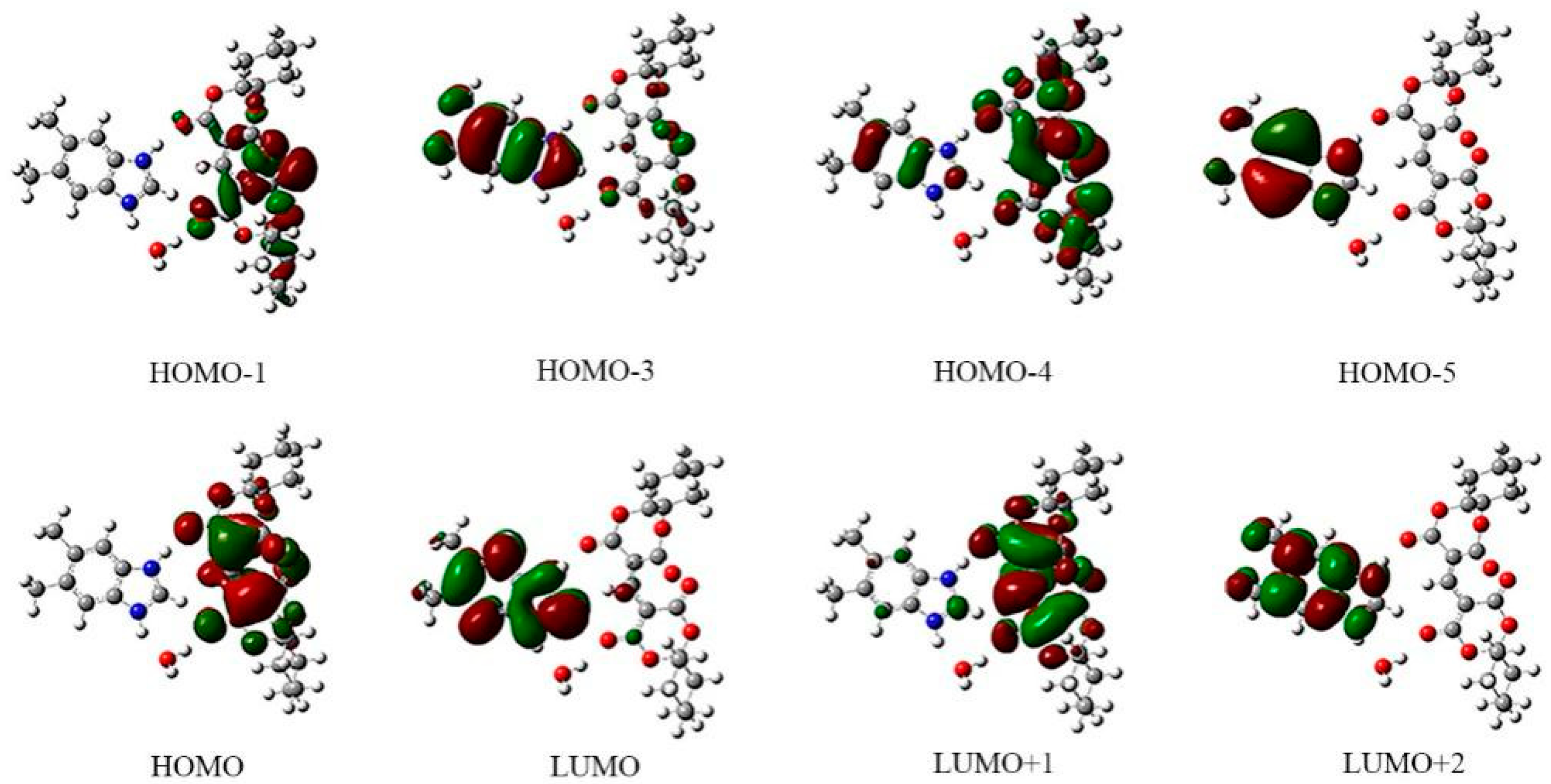
| 245 | 252 | 0.2079 | 139HOMO − 5→147LUMO + 2 |
| 140HOMO − 4→145LUMO | |||
| 141HOMO − 3→145LUMO | |||
| 378 | 339 | 0.4089 | 144HOMO→146LUMO + 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, W.; Wang, X.; Zhang, Y. Crystal Structure, Thermodynamic Properties and DFT Studies of 5,6-dimethyl-1H-benzo[d]imidazol-3-ium 3-((2,4-dioxo-1,5-dioxaspiro[5.5]undecan-3-ylidene)methyl) -2,4-dioxo-1,5-dioxaspiro[5.5]undecane Hydrate. Crystals 2021, 11, 1393. https://doi.org/10.3390/cryst11111393
Zeng W, Wang X, Zhang Y. Crystal Structure, Thermodynamic Properties and DFT Studies of 5,6-dimethyl-1H-benzo[d]imidazol-3-ium 3-((2,4-dioxo-1,5-dioxaspiro[5.5]undecan-3-ylidene)methyl) -2,4-dioxo-1,5-dioxaspiro[5.5]undecane Hydrate. Crystals. 2021; 11(11):1393. https://doi.org/10.3390/cryst11111393
Chicago/Turabian StyleZeng, Wulan, Xia Wang, and Yunju Zhang. 2021. "Crystal Structure, Thermodynamic Properties and DFT Studies of 5,6-dimethyl-1H-benzo[d]imidazol-3-ium 3-((2,4-dioxo-1,5-dioxaspiro[5.5]undecan-3-ylidene)methyl) -2,4-dioxo-1,5-dioxaspiro[5.5]undecane Hydrate" Crystals 11, no. 11: 1393. https://doi.org/10.3390/cryst11111393
APA StyleZeng, W., Wang, X., & Zhang, Y. (2021). Crystal Structure, Thermodynamic Properties and DFT Studies of 5,6-dimethyl-1H-benzo[d]imidazol-3-ium 3-((2,4-dioxo-1,5-dioxaspiro[5.5]undecan-3-ylidene)methyl) -2,4-dioxo-1,5-dioxaspiro[5.5]undecane Hydrate. Crystals, 11(11), 1393. https://doi.org/10.3390/cryst11111393






