Charge–Transfer Fluorescence and Room-Temperature Phosphorescence from a Bisamide-Based Derivative
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
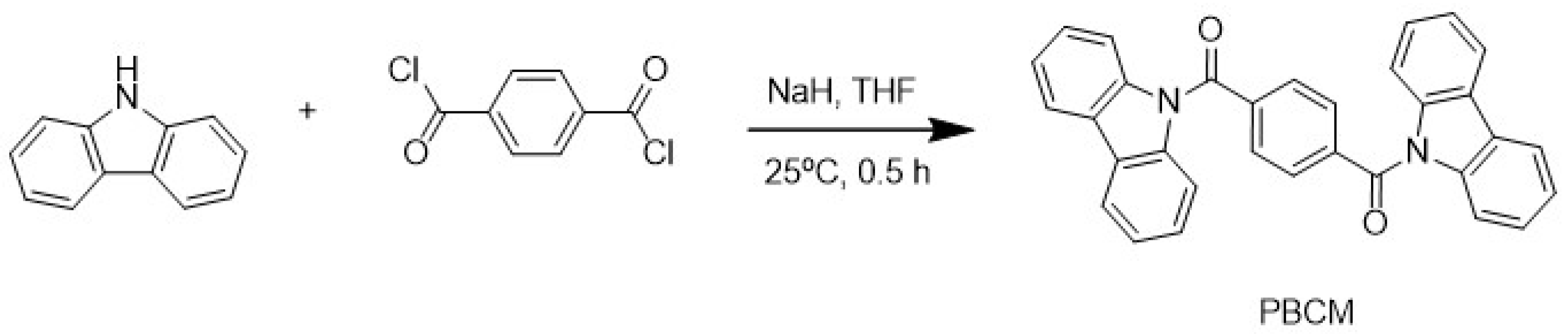
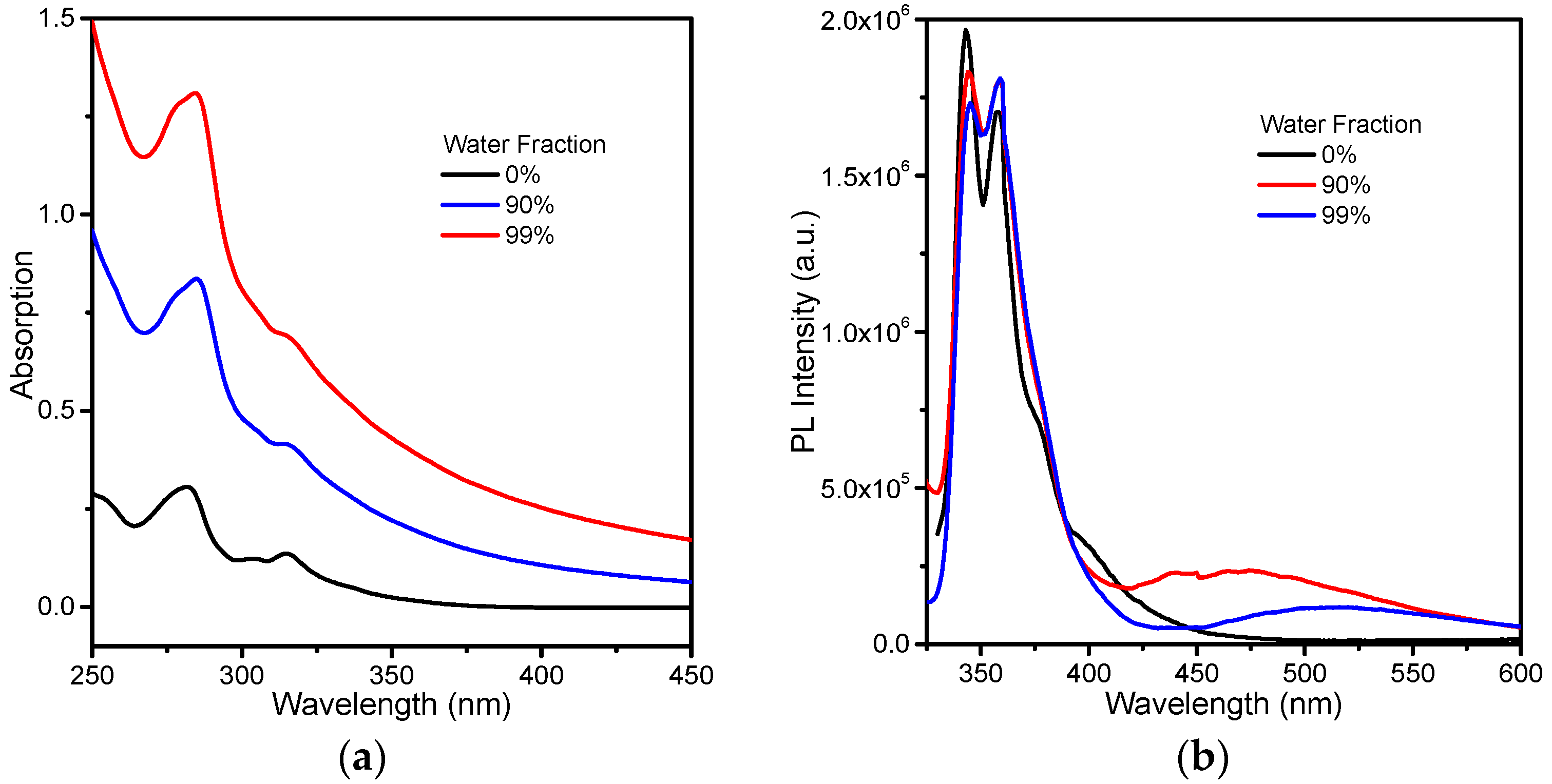
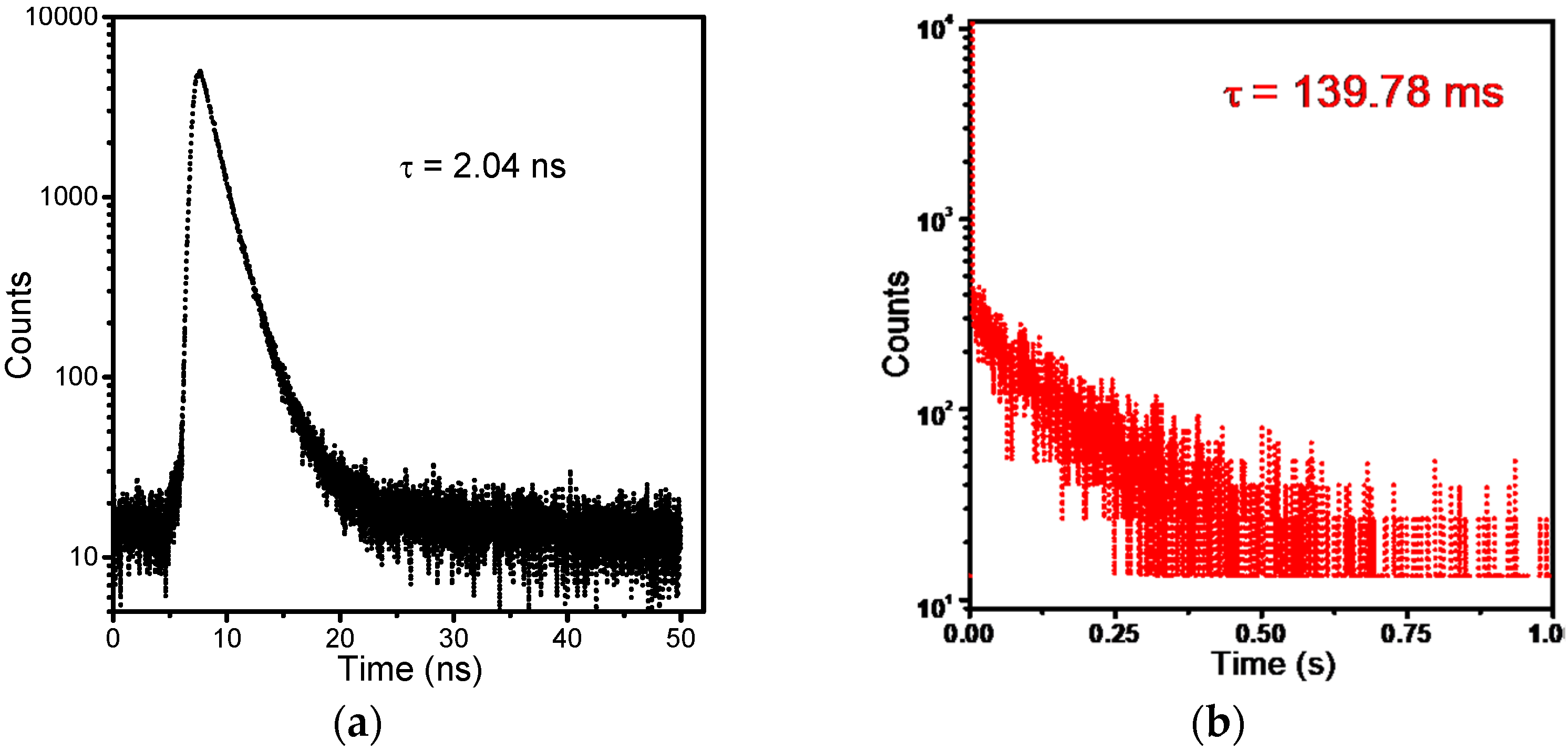
3.1. Charge-Transfer Fluorescence of PBCM
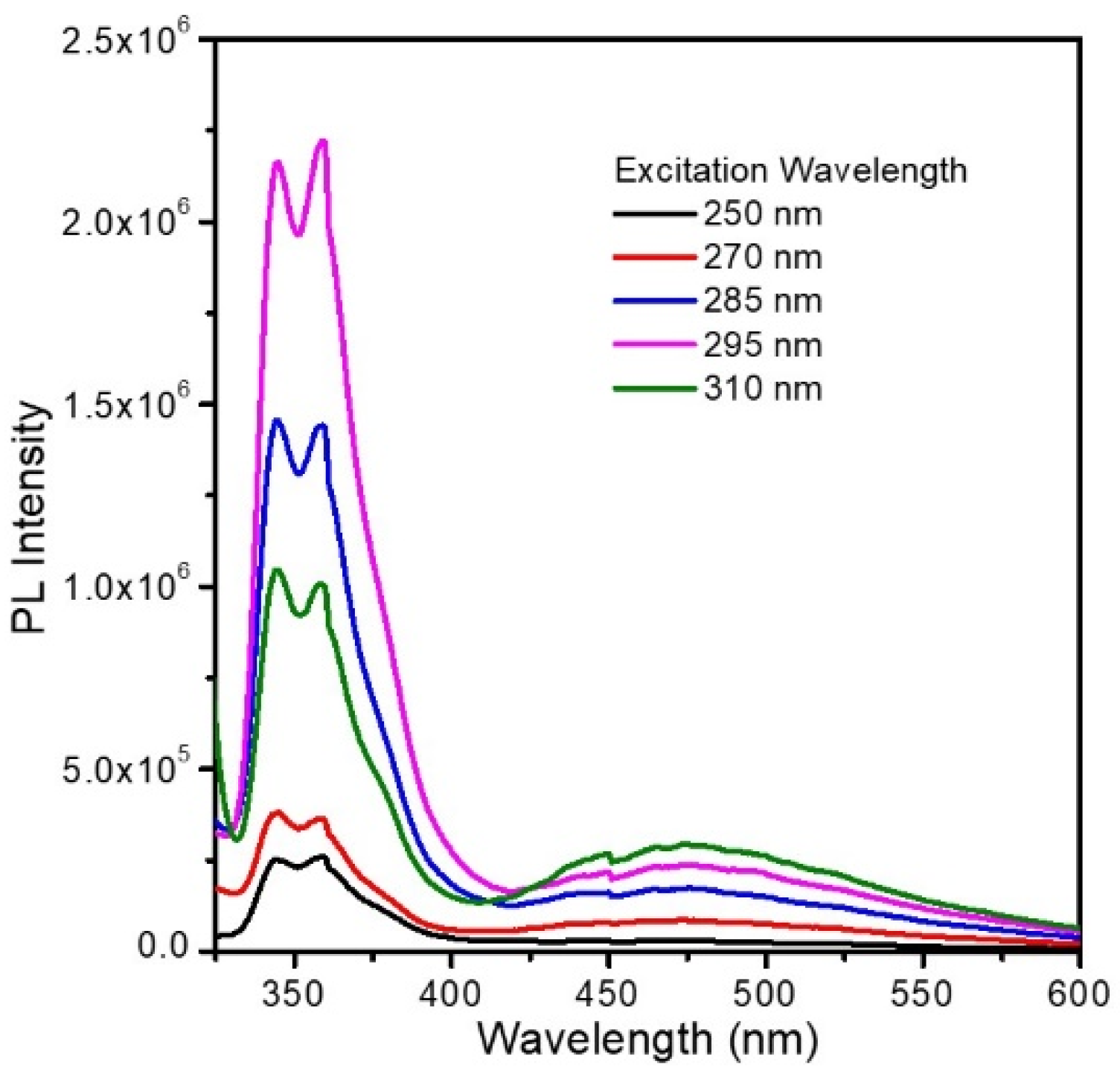
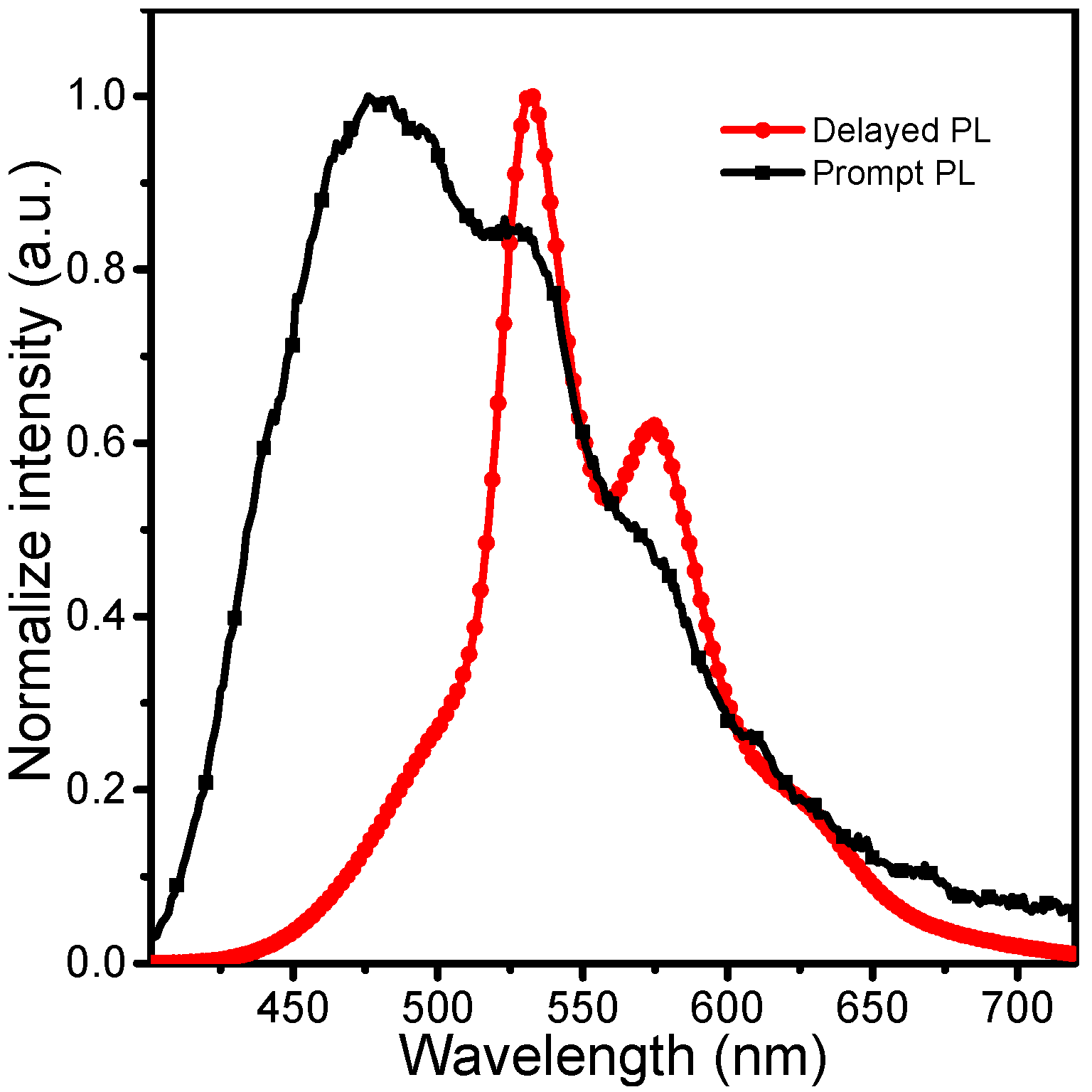
3.2. Bimodal Luminescence from PBCM Crystals
3.3. Single-Crystal X-ray Structure of PBCM
3.4. Thermal Properties of PBCM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S. Recent Advances in Materials with Room-Temperature Phosphorescence: Photophysics for Triplet Exciton Stabilization. Adv. Opt. Mater. 2017, 5, 1700116. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, D.; Heeger, A.J. Visible light emission from semiconducting polymer diodes. Appl. Phys. Lett. 1991, 58, 1982–1984. [Google Scholar] [CrossRef] [Green Version]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Tong, B.; Mei, Q.; Wang, S.; Fang, Y.; Meng, Y.; Wang, B. Nearly 100% internal phosphorescence efficiency in a polymer light-emitting diode using a new iridium complex phosphor. J. Mater. Chem. 2008, 18, 1636–1639. [Google Scholar] [CrossRef]
- Hoshino, S.; Suzuki, H. Electroluminescence from triplet excited states of benzophenone. Appl. Phys. Lett. 1996, 69, 224–226. [Google Scholar] [CrossRef]
- Jankus, V.; Chiang, C.-J.; Dias, F.; Monkman, A.P. Deep Blue Exciplex Organic Light-Emitting Diodes with Enhanced Efficiency; P-type or E-type Triplet Conversion to Singlet Excitons? Adv. Mater. 2012, 25, 1455–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, D.; Sigmund, E.; Meyer, A.; Rock, L.; Klemm, P.; Lautenschlager, S.; Schmid, A.; Yost, S.R.; Voorhis, T.V.; Bange, S.; et al. Metal-free OLED triplet emitters by side-stepping Kasha’s Rule. Angew. Chem. Int. Ed. 2013, 52, 13449–13452. [Google Scholar] [CrossRef] [PubMed]
- Marriott, G.; Clegg, R.M.; Arndt-Jovin, D.J.; Jovin, T.M. Time resolved imaging microscopy. Phosphorescence and delayed fluorescence imaging. Biophys. J. 1991, 60, 1374–1387. [Google Scholar] [CrossRef]
- Lee, D.R.; Han, S.H.; Lee, J.Y. Metal-free and purely organic phosphorescent light-emitting diodes using phosphorescence harvesting hosts and organic phosphorescent emitters. J. Mater. Chem. C 2019, 7, 11500–11506. [Google Scholar] [CrossRef]
- Li, B.; Gong, Y.; Wang, L.; Lin, H.; Li, Q.; Guo, F.; Li, Z.; Peng, Q.; Shuai, Z.; Zhao, L.; et al. Highly efficient organic room-temperature phosphorescent luminophores through tuning triplet states and spin-orbit coupling with incorporation of a secondary group. J. Phys. Chem. Lett. 2019, 10, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Ouyang, M.; Li, C.; Zhang, Y.; Cao, F.; Pan, G.; Lv, C.; Zhang, X.; Sun, J. Organic Luminophores Exhibiting Bimodal Emissions of Fluorescence and Room-Temperature Phosphorescence for Versatile Applications. Chem. Sel. 2020, 5, 12770–12776. [Google Scholar] [CrossRef]
- Zhao, W.; He, Z.; Lam, J.W.Y.; Peng, Q.; Ma, H.; Shuai, Z.; Bai, G.; Hao, J.; Tang, B.Z. Rational Molecular Design for Achieving Persistent and Efficient Pure Organic Room-Temperature Phosphorescence. Chemistry 2016, 1, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.Z.; Shen, X.Y.; Zhao, H.; Lam, J.W.Y.; Tang, L.; Lu, P.; Wang, C.; Liu, Y.; Wang, Z.; Zheng, Q.; et al. Crystallization-Induced Phosphorescence of Pure Organic Luminogens at Room Temperature. J. Phys. Chem. C 2010, 114, 6090–6099. [Google Scholar] [CrossRef]
- Scypinski, S.; Love, L.J.C. Room-temperature phosphorescence of polynuclear aromatic hydrocarbons in cyclodextrins. Anal. Chem. 1984, 56, 322–327. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Li, L.; Wang, S.; Tang, X.; Wang, H.; Li, M.; Zheng, C.; Chen, R. Activating intersystem crossing and aggregation coupling by CN-substitution for efficient organic ultralong room temperature phosphorescence. J. Phys. Chem. C 2020, 124, 10129–10134. [Google Scholar] [CrossRef]
- Kuila, S.; Garain, S.; Bandi, S.; George, S.J. All-organic, temporally pure white afterglow in amorphous films using complementary blue and greenish-yellow ultralong room temperature phosphors. Adv. Funct. Mater. 2020, 30, 2003693. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, X.; Liu, J.; Shan, G.-G.; Zhang, H.; Qi, J.; Zhao, W.; Sung, H.H.-Y.; Williams, I.D.; Lam, J.W.Y.; et al. New Wine in old bottles: Prolonging room-temperature phosphorescence of crown ethers by supramolecular interactions. Angew. Chem. Int. Ed. 2020, 59, 9293–9298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Xu, W.-W.; Xu, W.-S.; Niu, J.; Sun, X.-H.; Liu, Y. A synergistic enhancement strategy for realizing ultralong and efficient room-temperature phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 18748–18754. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Dai, W.; Tian, Y.; Yang, J.; Li, P.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Revealing insight into long-lived room-temperature phosphorescence of host-guest systems. J. Phys. Chem. Lett. 2019, 10, 6019–6025. [Google Scholar] [CrossRef]
- Park, S.K.; Varghese, S.; Kim, J.H.; Yoon, S.-J.; Kwon, O.K.; An, B.-K.; Gierschner, J.; Park, S.Y. Tailor-Made Highly Luminescent and Ambipolar Transporting Organic Mixed Stacked Charge-Transfer Crystals: An Isometric Donor−Acceptor Approach. J. Am. Chem. Soc. 2013, 135, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Du, J.; Nie, X.; Wu, Z.; Su, H.; Huang, W.; Wang, T.; Chen, B.; Jiang, J.; Zhang, X.; et al. Modulation of red organic room-temperature phosphorescence in heavy atom-free phosphors. Dye. Pigment. 2021, 193, 109505. [Google Scholar] [CrossRef]
- Ouyang, M.; Zhuo, C.; Cao, F.; Pan, G.; Lv, C.; Yang, S.; Li, C.; Zhang, C.; Sun, J.; Zhang, Y. Organogelator based on long alkyl chain attached excimer precursor: Two channels of TICT, highly efficient and switchable luminescence. Dye. Pigment. 2020, 180, 108433. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhuo, C.; Sun, J.; Ouyang, M. Charge–Transfer Fluorescence and Room-Temperature Phosphorescence from a Bisamide-Based Derivative. Crystals 2021, 11, 1370. https://doi.org/10.3390/cryst11111370
Li C, Zhuo C, Sun J, Ouyang M. Charge–Transfer Fluorescence and Room-Temperature Phosphorescence from a Bisamide-Based Derivative. Crystals. 2021; 11(11):1370. https://doi.org/10.3390/cryst11111370
Chicago/Turabian StyleLi, Chengjian, Chaozheng Zhuo, Jingwei Sun, and Mi Ouyang. 2021. "Charge–Transfer Fluorescence and Room-Temperature Phosphorescence from a Bisamide-Based Derivative" Crystals 11, no. 11: 1370. https://doi.org/10.3390/cryst11111370
APA StyleLi, C., Zhuo, C., Sun, J., & Ouyang, M. (2021). Charge–Transfer Fluorescence and Room-Temperature Phosphorescence from a Bisamide-Based Derivative. Crystals, 11(11), 1370. https://doi.org/10.3390/cryst11111370







