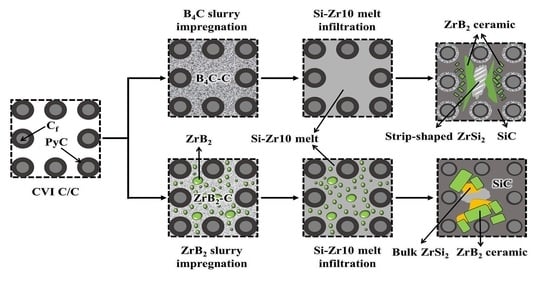
Fabrication of C/C–SiC–ZrB2 Ultra-High Temperature Composites through Liquid–Solid Chemical Reaction
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
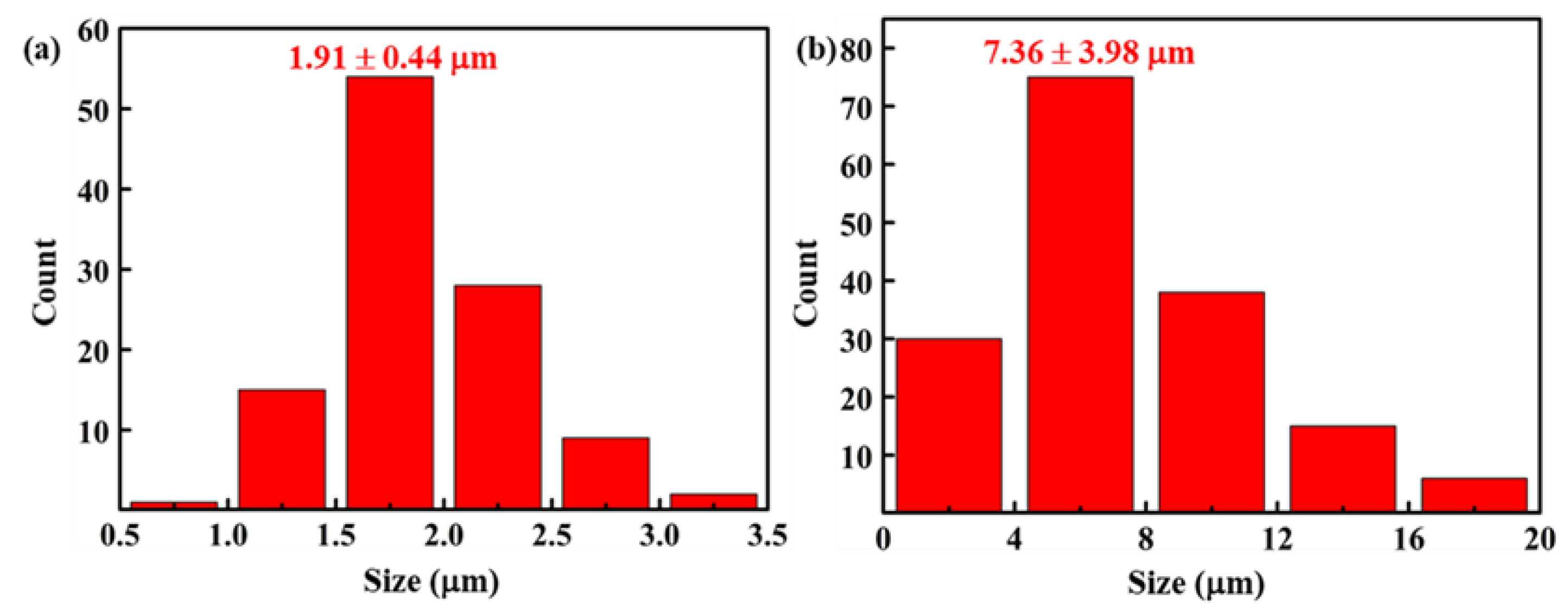
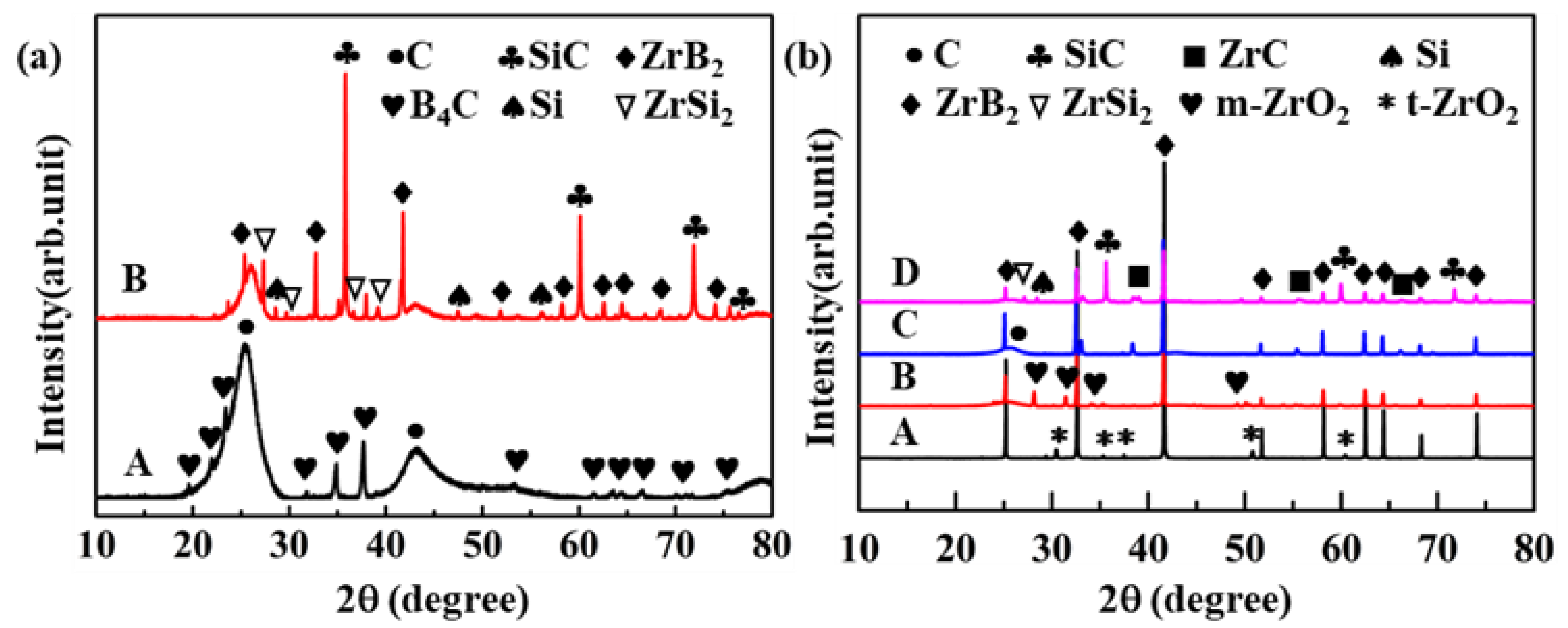
3.1. Microstructural Characterization of the C/C–SiC–ZrB2 Composites
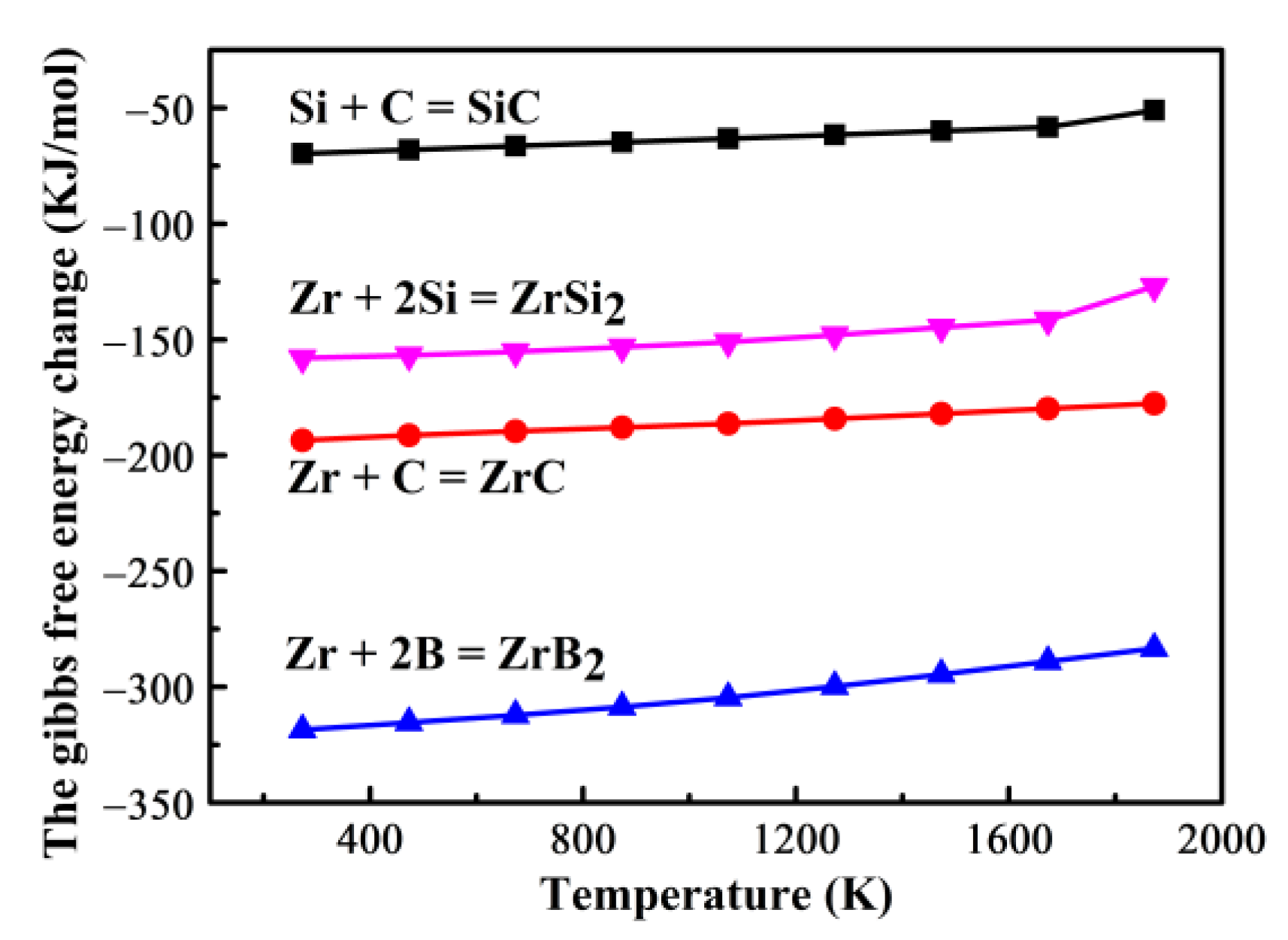
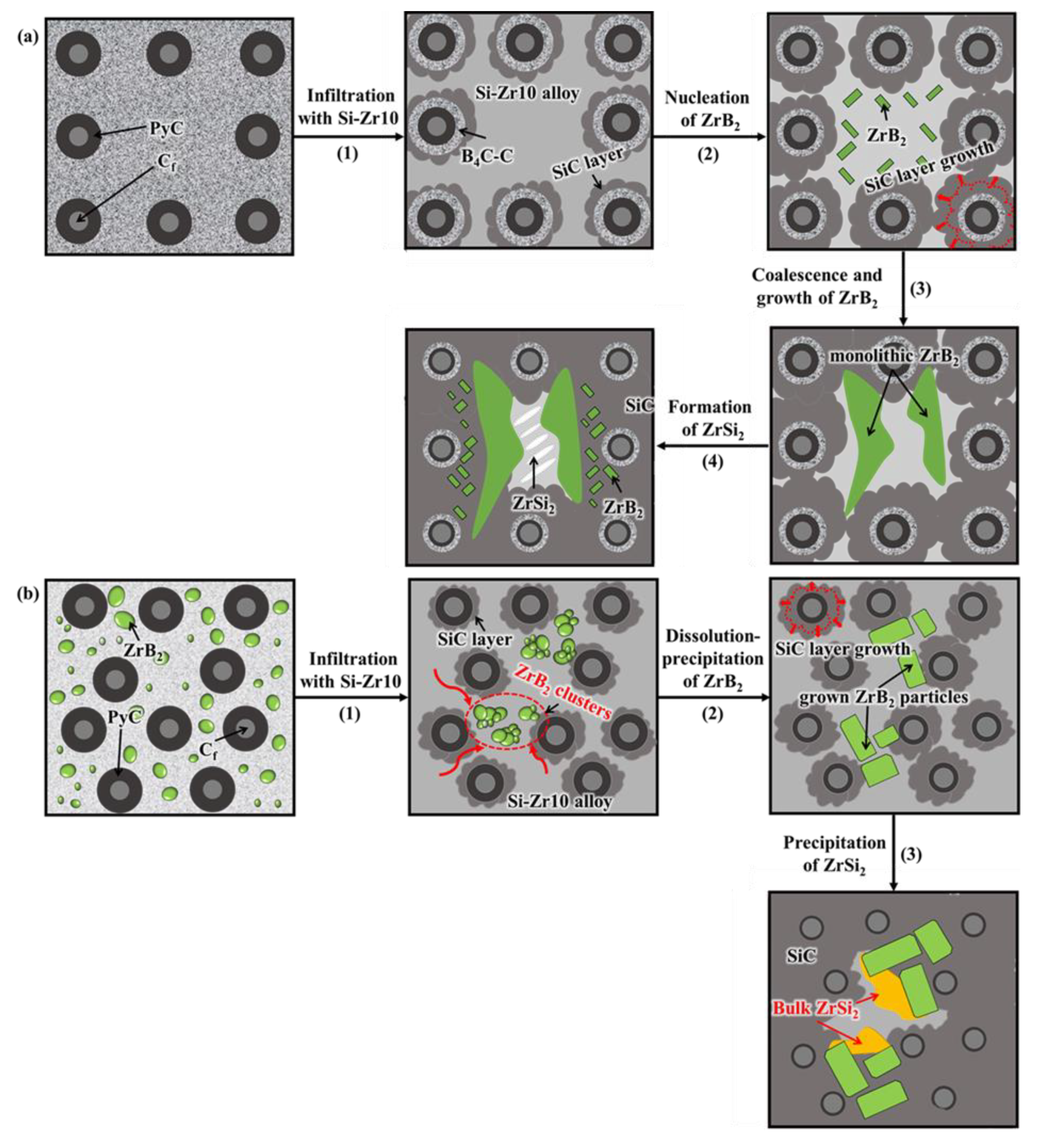
3.2. Microstructural Evolution and Formation Mechanism of the C/C–SiC–ZrB2 Composites
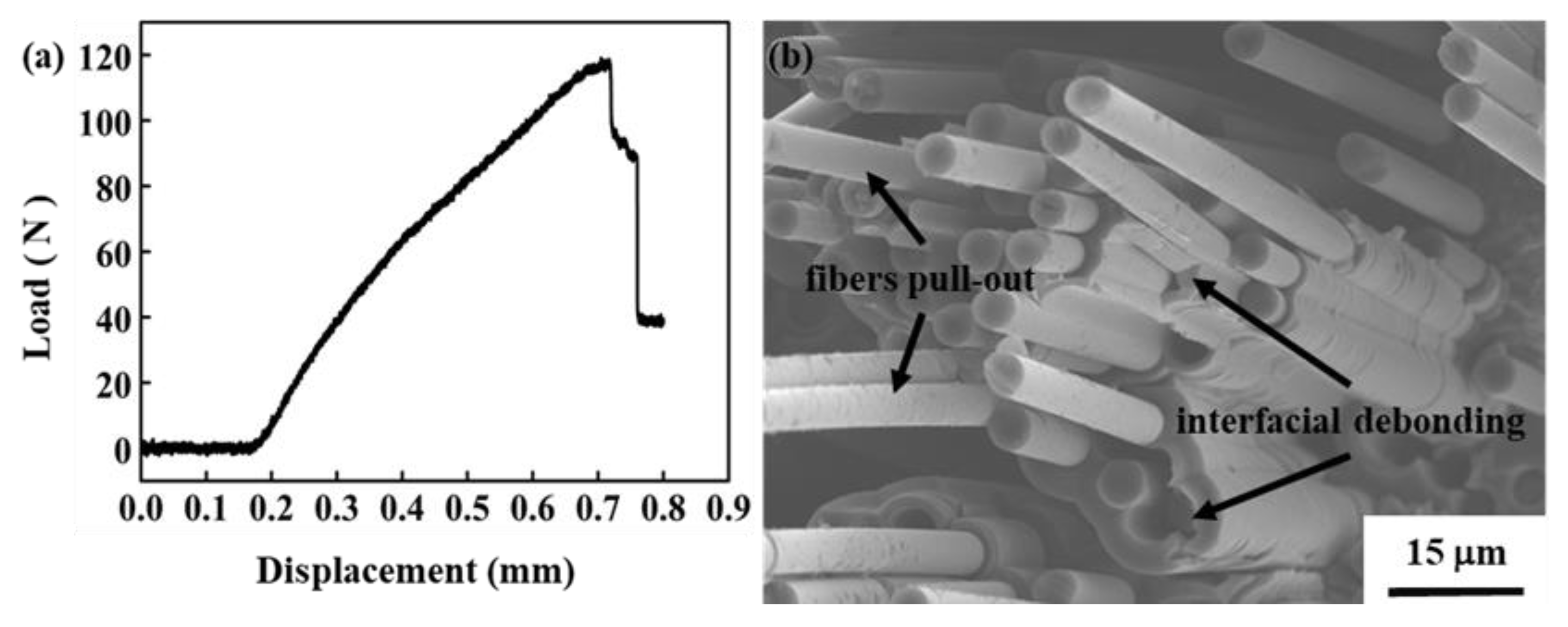
3.3. Mechanical Properties of C/C–SiC–ZrB2 Composite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fitzer, E. The future of carbon-carbon composites. Carbon 1987, 25, 163–190. [Google Scholar] [CrossRef]
- Windhorst, T.; Blount, G. Carbon-carbon composites: A summary of recent developments and applications. Mater. Des. 1997, 18, 11–15. [Google Scholar] [CrossRef]
- Li, T.; Yang, Z.; Qiao, S.; Zheng, X. High temperature oxidation resistance improvement of carbon-carbon composite. Carbon 1993, 31, 1361–1363. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.J.; Feng, W.; Shi, X.H.; Li, K.Z.; Guo, L.J. Ablation in different heat fluxes of C/C composites modified by ZrB2–ZrC and ZrB2–ZrC–SiC particles. Corros. Sci. 2013, 74, 159–167. [Google Scholar] [CrossRef]
- Jin, X.C.; Fan, X.L.; Lu, C.S.; Wang, T.J. Advances in oxidation and ablation resistance of high and ultra-high temperature ceramics modified or coated carbon/carbon composites. J. Eur. Ceram. Soc. 2018, 38, 1–28. [Google Scholar] [CrossRef]
- Arai, Y.; Inoue, R.; Goto, K.; Kogo, Y. Carbon fiber reinforced ultra-high temperature ceramic matrix composites: A review. Ceram. Int. 2019, 45, 14481–14489. [Google Scholar] [CrossRef]
- Li, Q.G.; Dong, S.M.; Wang, Z.; He, P.; Zhou, H.J.; Yang, J.S.; Wu, B.; Hu, J.B. Fabrication and properties of 3D Cf/SiC–ZrC composites, using ZrC precursor and polycarbosilane. J. Am. Ceram. Soc. 2012, 95, 1216–1219. [Google Scholar] [CrossRef]
- Zhou, H.J.; Ni, D.W.; He, P.; Yang, J.S.; Hu, J.B.; Dong, S.M. Ablation behavior of C/C-ZrC and C/SiC-ZrC composites fabricated by a joint process of slurry impregnation and chemical vapor infiltration. Ceram. Int. 2018, 44, 4777–4782. [Google Scholar] [CrossRef]
- Tang, S.F.; Deng, J.Y.; Wang, S.J.; Liu, W.C. Fabrication and characterization of an ultra-high-temperature carbon fiber-reinforced ZrB2–SiC matrix composite. J. Am. Ceram. Soc. 2007, 90, 3320–3322. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhu, X.J.; Zhang, L.T.; Cheng, L.F. C/C-SiC-ZrC composites fabricated by reactive melt infiltration with Si0.87Zr0.13 alloy. Ceram. Int. 2012, 38, 4337–4343. [Google Scholar] [CrossRef]
- Tang, S.F.; Hu, C.L. Design, preparation and properties of carbon fiber reinforced ultra-high temperature ceramic composites for aerospace applications: A review. J. Mater. Sci. Technol. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Xiang, X.; Chen, Z.K.; Xiao, P.; Huang, B.Y. Influence factors of C/C–SiC dual matrix composites prepared by reactive melt infiltration. Mater. Des. 2009, 30, 3738–3742. [Google Scholar] [CrossRef]
- Zhong, Q.; Zhang, X.Y.; Dong, S.M.; Yang, J.S.; Hu, J.B.; Gao, L.; He, P.; Zhou, H.J.; Wang, Z.; Ding, Y.S. Reactive melt infiltrated Cf/SiC composites with robust matrix derived from novel engineered pyrolytic carbon structure. Ceram. Int. 2017, 43, 5832–5836. [Google Scholar] [CrossRef]
- Guo, W.J.; Ye, Y.C.; Bai, S.X.; Zhu, L.A.; Li, S. Preparation and formation mechanism of C/C-SiC composites using polymer-Si slurry reactive melt infiltration. Ceram. Int. 2020, 46, 5586–5593. [Google Scholar] [CrossRef]
- Zou, L.; Wali, N.; Yang, J.M.; Bansal, N.P.; Dong, Y. Microstructural characterization of a Cf/ZrC composite manufactured by reactive melt infiltration. Int. J. Appl. Ceram. Technol. 2011, 8, 329–341. [Google Scholar] [CrossRef]
- Chen, S.A.; Zhang, C.R.; Zhang, Y.D.; Hu, H.F. Influence of pyrocarbon amount in C/C preform on the microstructure and properties of C/ZrC composites prepared via reactive melt infiltration. Mater. Des. 2014, 58, 570–576. [Google Scholar] [CrossRef]
- Ju, L.; Lenosky, T.J. Thermochemical and mechanical stabilities of the oxide scale of ZrB2+SiC and oxygen transport mechanisms. J. Am. Ceram. Soc. 2008, 91, 1475–1480. [Google Scholar] [CrossRef]
- Tong, Y.G.; Bai, S.X.; Zhang, H.; Chen, K. C/C–SiC composite prepared by Si–10Zr alloyed melt infiltration. Ceram. Int. 2012, 38, 3301–3307. [Google Scholar] [CrossRef]
- Tong, Y.G.; Bai, S.X.; Qin, Q.H.; Zhang, H.; Ye, Y.C. Effect of infiltration time on the microstructure and mechanical properties of C/C-SiC composite prepared by Si-Zr10 alloyed melt infiltration. Ceram. Int. 2015, 41, 4014–4020. [Google Scholar] [CrossRef]
- Tong, Y.G.; Cai, Z.H.; Bai, S.X.; Hua, M.Y.; Xie, W.; Ye, Y.C.; Li, Y. Microstructures and properties of Si-Zr alloy based CMCs reinforced by various porous C/C performs. Ceram. Int. 2018, 44, 16577–16582. [Google Scholar] [CrossRef]
- Chen, X.W.; Feng, Q.; Kan, Y.M.; Ni, D.W.; Zhou, H.J.; Gao, L.; Zhang, X.Y.; Ding, Y.S.; Dong, S.M. Effects of preform pore structure on infiltration kinetics and microstructure evolution of RMI-derived Cf/ZrC-ZrB2-SiC composite. J. Eur. Ceram. Soc. 2020, 40, 2683–2690. [Google Scholar] [CrossRef]
- Ni, D.W.; Wang, J.X.; Dong, S.M.; Chen, X.W.; Kan, Y.M.; Zhou, H.J.; Gao, L.; Zhang, X.Y. Fabrication and properties of Cf/ZrC-SiC-based composites by an improved reactive melt infiltration. J. Am. Ceram. Soc. 2018, 101, 3253–3258. [Google Scholar] [CrossRef]
- Chiang, Y.-M.; Messner, R.P.; Terwilliger, C.D.; Behrendt, D.R. Reaction-formed silicon carbide. Mater. Sci. Eng. A 1991, 144, 63–74. [Google Scholar] [CrossRef]
- Baxter, R.I.; Rawlings, R.D.; Iwashita, N.; Sawada, Y. Effect of chemical vapor infiltration on erosion and thermal properties of porous carbon/carbon composite thermal insulation. Carbon 2000, 38, 441–449. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory biborides of zirconium and hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Durand, F.; Duby, J.C. Carbon solubility in solid and liquid silicon—A review with reference to eutectic equilibrium. J. Phase Equilib. 1999, 20, 61–63. [Google Scholar] [CrossRef]
- Ikhmayies, S.J. Thermo-calc determination of phase diagram of Si-B binary system. JOM 2021, 73, 253–259. [Google Scholar] [CrossRef]
- Zou, L.H.; Wali, N.; Yang, J.-M.; Bansal, N.P. Microstructural development of a Cf/ZrC composite manufactured by reactive melt infiltration. J. Eur. Ceram. Soc. 2010, 30, 1527–1535. [Google Scholar] [CrossRef]
- Wang, D.K.; Dong, S.M.; Zhou, H.J.; Zhang, X.Y.; Ding, Y.S.; Zhu, G.X. Fabrication and microstructure of 3D Cf/ZrC-SiC composites: Through RMI method with ZrO2 powders as pore-making agent. Ceram. Int. 2016, 42, 6720–6727. [Google Scholar] [CrossRef]
- Sonber, J.K.; Suri, A.K. Synthesis and consolidation of zirconium diboride: Review. Adv. Appl. Ceram. 2011, 11, 321–334. [Google Scholar] [CrossRef]
- Monteverde, F.; Bellosi, A.; Guicciardi, S. Processing and properties of zirconium diboride-based composites. J. Eur. Ceram. Soc. 2002, 22, 279–288. [Google Scholar] [CrossRef]
- Bulanova, M.; Firstov, S.; Gornaya, I.; Miracle, D. The melting diagram of the Ti-corner of the Ti–Zr–Si system and mechanical properties of as-cast compositions. J. Alloys Compd. 2004, 384, 106–114. [Google Scholar] [CrossRef]
- Otani, S.; Ishizawa, Y. Preparation of ZrB2 single crystals by the floating zone method. J. Cryst. Growth 1996, 165, 319–322. [Google Scholar] [CrossRef]
- Ji, Z.; Sun, S.K.; Zhang, G.J.; Kan, Y.M.; Wang, P.L.; Ohji, T. Chemical reactions, anisotropic grain growth and sintering mechanisms of self-reinforced ZrB2–SiC doped with WC. J. Am. Ceram. Soc. 2011, 94, 1575–1583. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Cheng, L.; Yan, D. Microstructure and mechanical properties of three-dimensional carbon/silicon carbide composites fabricated by chemical vapor infiltration. Carbon 1998, 36, 1051–1056. [Google Scholar] [CrossRef]



| Samples | Density (g/cm3) | Open Porosity (%) | Phase Volume Fraction (%) | ||||
|---|---|---|---|---|---|---|---|
| Cf | ZrB2 | B4C | ZrSi2 | SiC | |||
| C/C-B4C | 1.27 | 32.07 | 17.60 | N/A | 6.74 | N/A | N/A |
| C/C-ZrB2 | 1.58 | 34.75 | 17.60 | 9.51 | N/A | N/A | N/A |
| B4C-composite | 1.98 | 18.04 | 17.60 | 1.47 | 5.87 | 2.45 | 23.10 |
| ZrB2-composite | 2.82 | 3.43 | 17.60 | 9.51 | N/A | 4.26 | 38.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Zhang, H.; Huang, C.; Zhang, W. Fabrication of C/C–SiC–ZrB2 Ultra-High Temperature Composites through Liquid–Solid Chemical Reaction. Crystals 2021, 11, 1352. https://doi.org/10.3390/cryst11111352
Sun Q, Zhang H, Huang C, Zhang W. Fabrication of C/C–SiC–ZrB2 Ultra-High Temperature Composites through Liquid–Solid Chemical Reaction. Crystals. 2021; 11(11):1352. https://doi.org/10.3390/cryst11111352
Chicago/Turabian StyleSun, Qian, Huifeng Zhang, Chuanbing Huang, and Weigang Zhang. 2021. "Fabrication of C/C–SiC–ZrB2 Ultra-High Temperature Composites through Liquid–Solid Chemical Reaction" Crystals 11, no. 11: 1352. https://doi.org/10.3390/cryst11111352
APA StyleSun, Q., Zhang, H., Huang, C., & Zhang, W. (2021). Fabrication of C/C–SiC–ZrB2 Ultra-High Temperature Composites through Liquid–Solid Chemical Reaction. Crystals, 11(11), 1352. https://doi.org/10.3390/cryst11111352