Advances in Diffraction Studies of Light-Induced Transient Species in Molecular Crystals and Selected Complementary Techniques
Abstract
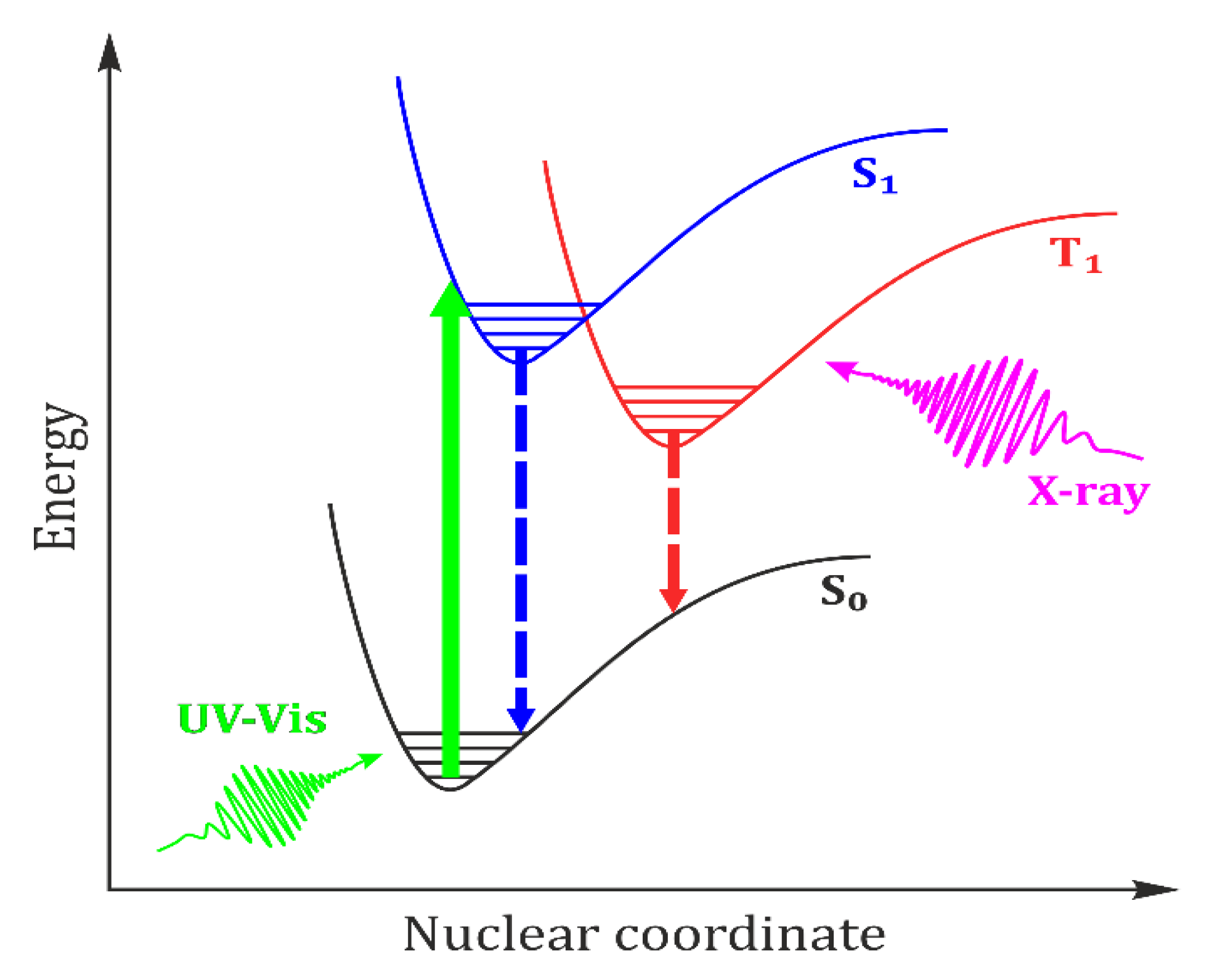
:1. Introduction
2. Time-Resolved Photocrystallography
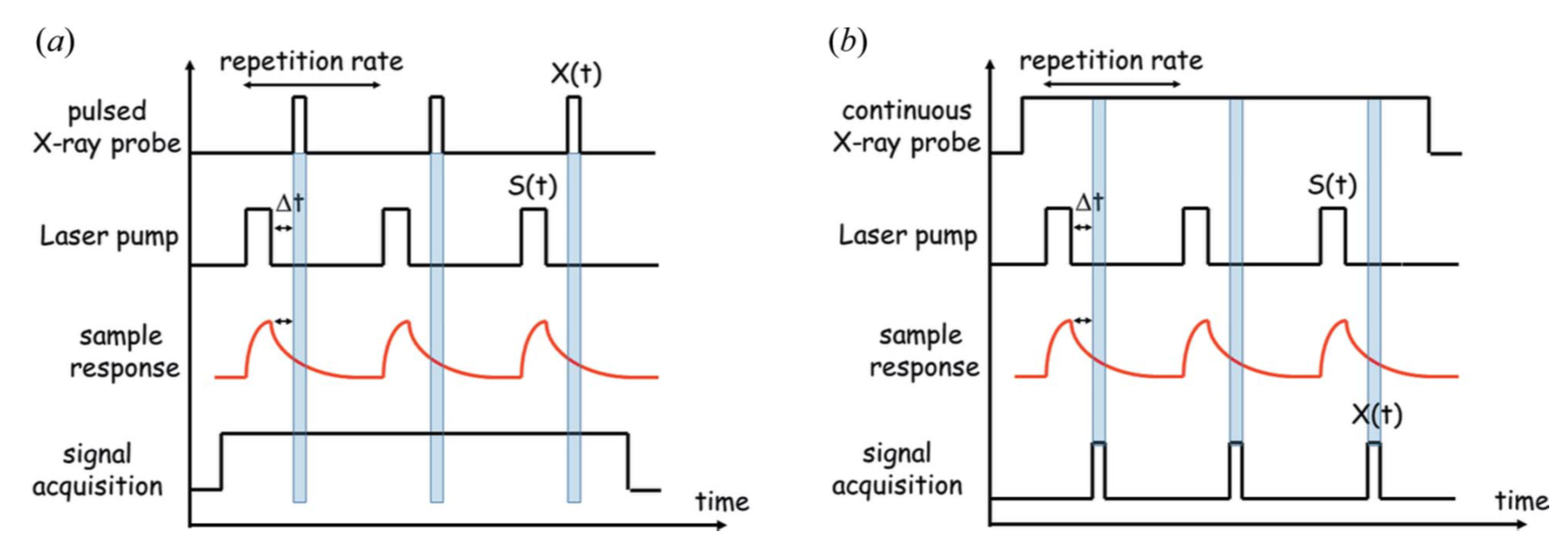
2.1. Experimental Approaches
2.2. Synchrotron Facilities for TR LAUE Studies
2.3. Processing of TR Laue Data
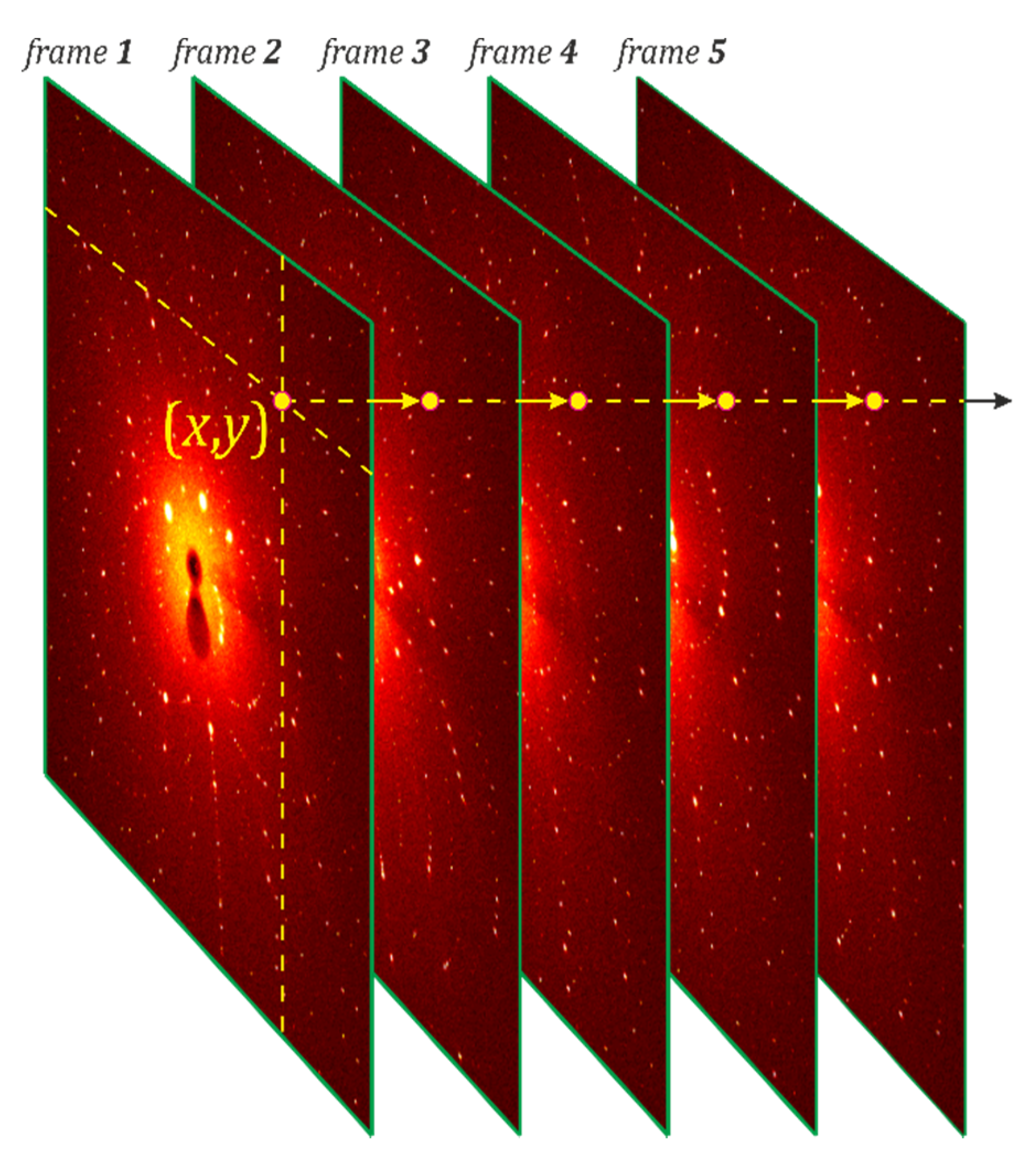
2.3.1. Signal Searching and Integration
2.3.2. Sample Orientation Determination
2.3.3. Structure Refinement
2.3.4. Software Comparison
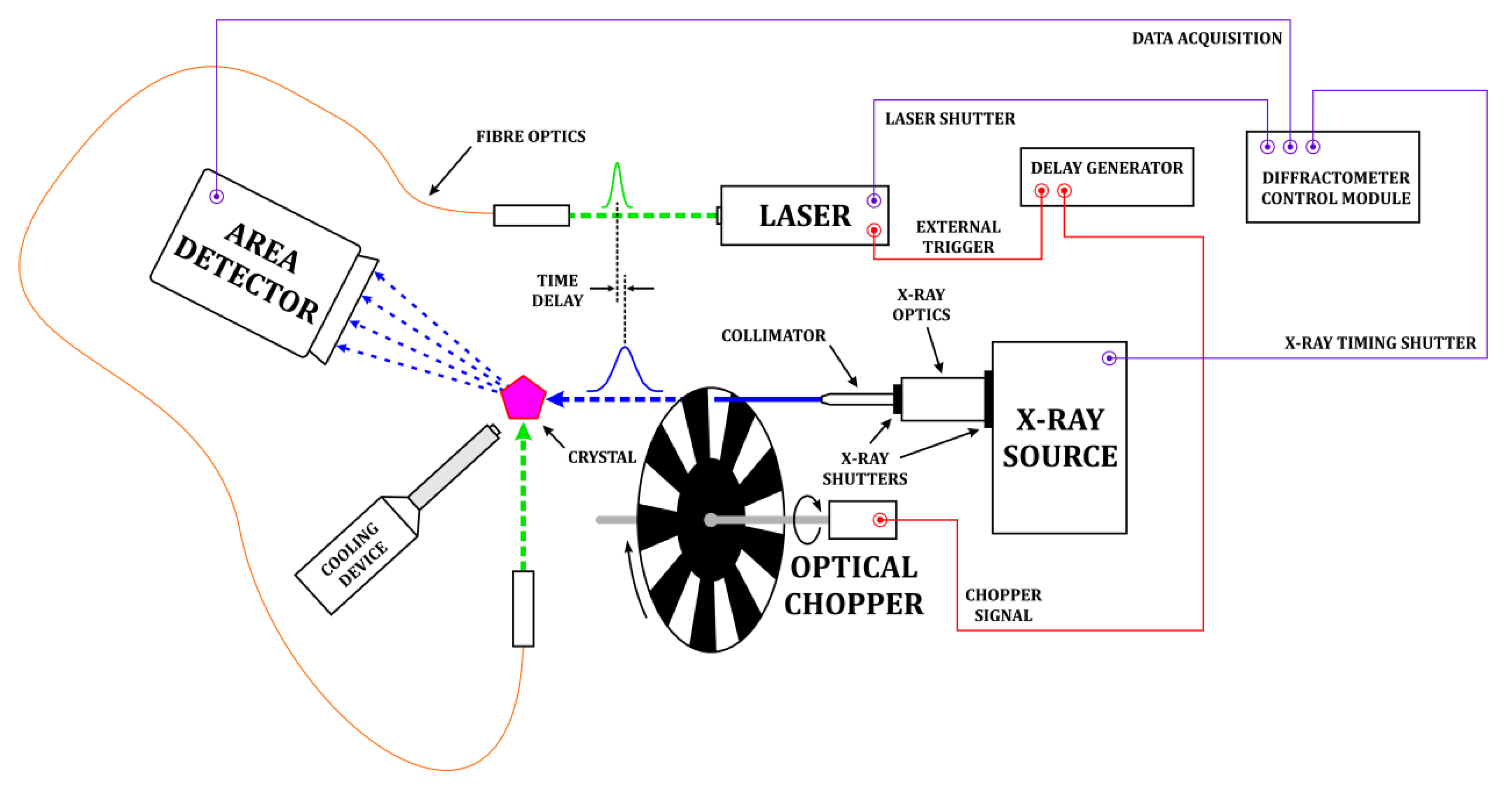
2.4. In-House TR Diffraction Studies
2.5. Case Studies
3. X-ray Free-Electron Lasers
3.1. Experimental Approaches
3.2. Facilities for TR XFEL Crystallography
3.2.1. LCLS
3.2.2. SACLA
3.2.3. European XFEL
3.3. Data Processing
3.4. Case Studies
4. Complementary Methods
4.1. Electron Diffraction
4.2. X-ray Absorption Spectroscopy
5. Summary and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wald, G. The Molecular Basis of Visual Excitation. Nature 1968, 219, 800–807. [Google Scholar] [CrossRef]
- Dowling, J.E.; Wald, G. The biological function of vitamin-A acid. Nutr. Rev. 1981, 39, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Sancar, A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 2003, 103, 2203–2238. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.S.; Manahan, C.C.; Crane, B.R. Photochemistry of flavoprotein light sensors. Nat. Chem. Biol. 2014, 10, 801–809. [Google Scholar] [CrossRef]
- Genick, U.K.; Gloria, E.O.B.; Ng, K.; Ren, Z.; Pradervand, C.; Burke, P.M.; Šrajer, V.; Teng, T.-Y.; Schildkamp, W.; McRee, D.E.; et al. Structure of a Protein Photocycle Intermediate by Millisecond Time-Resolved Crystallography. Science 1997, 275, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Šrajer, V.; Teng, T.-Y.; Ursby, T.; Pradervand, C.; Ren, Z.; Adachi, S.-I.; Schildkamp, W.; Bourgeois, D.; Wulff, M.; Moffat, K. Photolysis of the Carbon Monoxide Complex of Myoglobin: Nanosecond Time-Resolved Crystallography. Science 1996, 274, 1726–1729. [Google Scholar] [CrossRef]
- Coppens, P. The dramatic development of X-ray photocrystallography over the past six decades. Struct. Dyn. 2017, 4, 032102. [Google Scholar] [CrossRef] [Green Version]
- Coppens, P. What can time-resolved diffraction tell us about transient species?: Excited-state structure determination at atomic resolution. Chem. Commun. 2003, 12, 1317–1320. [Google Scholar] [CrossRef]
- Naumov, P. Chemical X-Ray Photodiffraction: Principles, Examples, and Perspectives. In Advanced X-Ray Crystallography; Rissanen, K., Ed.; Springer: Heidelberg/Berlin, Germany, 2012; pp. 111–131. [Google Scholar]
- Coppens, P.; Fournier, B. New methods in time-resolved Laue pump-probe crystallography at synchrotron sources. J. Synchrotron Rad. 2015, 22, 280–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, P. Molecular excited-state structure by time-resolved pump-probe X-ray diffraction. What is new and what are the prospects for further progress? J. Phys. Chem. Lett. 2011, 2, 616–621. [Google Scholar] [CrossRef]
- Coppens, P.; Benedict, J.B.; Messerschmidt, M.; Novozhilova, I.; Graber, T.; Chen, Y.-S.; Vorontsov, I.; Scheins, S.; Zheng, S.-L. Time-resolved synchrotron diffraction and theoretical studies of very short-lived photo-induced molecular species. Acta Cryst. Sect. A 2010, 66, 179–188. [Google Scholar] [CrossRef]
- Ren, Z.; Bourgeois, D.; Helliwell, J.R.; Moffat, K.; Šrajer, V.; Stoddard, B.L. Laue crystallography: Coming of age. J. Synchrotron Rad. 1999, 6, 891–917. [Google Scholar] [CrossRef]
- Hatcher, L.E.; Raithby, P.R. Dynamic single-crystal diffraction studies using synchrotron radiation. Coord. Chem. Rev. 2014, 277–278, 69–79. [Google Scholar] [CrossRef]
- Coppens, P.; Kamiński, R.; Schmøkel, M.S. On R factors for dynamic structure crystallography. Acta Cryst. Sect. A 2010, 66, 626–628. [Google Scholar] [CrossRef]
- Hajdu, J.; Machin, P.A.; Campbell, J.W.; Greenhough, T.J.; Clifton, I.J.; Zurek, S.; Gover, S.; Johnson, L.N.; Elder, M. Millisecond X-ray diffraction and the first electron density map from Laue photographs of a protein crystal. Nature 1987, 329, 178. [Google Scholar] [CrossRef]
- Šrajer, V.; Schmidt, M. Watching proteins function with time-resolved x-ray crystallography. J. Phys. D 2017, 50, 373001. [Google Scholar] [CrossRef]
- Jarzembska, K.N.; Hapka, M.; Kamiński, R.; Bury, W.; Kutniewska, S.E.; Szarejko, D.; Szczęśniak, M.M. On the nature of luminescence thermochromism of multinuclear copper(I) benzoate complexes in the crystalline state. Crystals 2019, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Coppens, P.; Zhang, L.; Thomas, R.; Chen, Y.; Jarzembska, K.; Kaminski, R.; Trzop, E.; Fournier, B. Can we deconvolute electron density changes from the dominant influence of the atomic rearrangement on molecular excitation in time-resolved diffraction studies? Phys. Scr. 2016, 91, 023003. [Google Scholar] [CrossRef]
- Jarzembska, K.N.; Kamiński, R.; Fournier, B.; Trzop, E.; Sokolow, J.D.; Henning, R.; Chen, Y.; Coppens, P. Shedding light on the photochemistry of coinage-metal phosphorescent materials: A time-resolved Laue diffraction study of an AgI-CuI tetranuclear complex. Inorg. Chem. 2014, 53, 10594–10601. [Google Scholar] [CrossRef] [Green Version]
- Makal, A.; Benedict, J.; Trzop, E.; Sokolow, J.; Fournier, B.; Chen, Y.; Kalinowski, J.A.; Graber, T.; Henning, R.; Coppens, P. Restricted photochemistry in the molecular solid state: Structural changes on photoexcitation of Cu(I) phenanthroline metal-to-ligand charge transfer (MLCT) complexes by time-resolved diffraction. J. Phys. Chem. A 2012, 116, 3359–3365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, E.; Moisan, N.; Baldé, C.; Bertoni, R.; Trzop, E.; Laulhé, C.; Lorenc, M.; Servol, M.; Cailleau, H.; Tissot, A.; et al. Ultrafast spin-state photoswitching in a crystal and slower consecutive processes investigated by femtosecond optical spectroscopy and picosecond X-ray diffraction. Phys. Chem. Chem. Phys. 2012, 14, 6192–6199. [Google Scholar] [CrossRef]
- Makal, A.; Trzop, E.; Sokolow, J.; Kalinowski, J.; Benedict, J.; Coppens, P. The development of Laue techniques for single-pulse diffraction of chemical complexes: Time-resolved Laue diffraction on a binuclear rhodium metal-organic complex. Acta Cryst. Sect. A 2011, 67, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Benedict, J.B.; Makal, A.; Sokolow, J.D.; Trzop, E.; Scheins, S.; Henning, R.; Graber, T.; Coppens, P. Time-resolved Laue diffraction of excited species at atomic resolution: 100 ps single-pulse diffraction of the excited state of the organometallic complex Rh2(µ-PNP)2(PNP)2•BPh4. Chem. Commun. 2011, 47, 1704–1706. [Google Scholar] [CrossRef] [Green Version]
- Brayshaw, S.K.; Knight, J.W.; Raithby, P.R.; Savarese, T.L.; Schiffers, S.; Teat, S.J.; Warren, J.E.; Warren, M.R. Photocrystallography—Design and methodology for the use of a light-emitting diode device. J. Appl. Cryst. 2010, 43, 337–340. [Google Scholar] [CrossRef] [Green Version]
- Fullagar, W.K.; Wu, G.; Kim, C.; Ribaud, L.; Sagerman, G.; Coppens, P. Instrumentation for photocrystallographic experiments of transient species. J. Synchrotron Rad. 2000, 7, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukaddem, K.T.; Cole, J.M.; Beyer, K.A.; Sylvester, S.O. Local Atomic Structure in Photoisomerized Ruthenium Sulfur Dioxide Complexes Revealed by Pair Distribution Function Analysis. J. Phys. Chem. C 2020, 124, 10094–10104. [Google Scholar] [CrossRef]
- Kovalevsky, A.Y.; Bagley, K.A.; Cole, J.M.; Coppens, P. Light-induced metastable linkage isomers of ruthenium sulfur dioxide complexes. Inorg. Chem. 2003, 42, 140–147. [Google Scholar] [CrossRef]
- Bowes, K.F.; Cole, J.M.; Husheer, S.L.G.; Raithby, P.R.; Savarese, T.L.; Sparkes, H.A.; Teat, S.J.; Warren, J.E. Photocrystallographic structure determination of a new geometric isomer of [Ru(NH3)4(H2O)(η1-OSO)][MeC6H4SO3]2. Chem. Commun. 2006, 2448–2450. [Google Scholar] [CrossRef]
- Sylvester, S.O.; Cole, J.M.; Waddell, P.G.; Nowell, H.; Wilson, C. SO2 phototriggered crystalline nanomechanical transduction of aromatic rotors in tosylates: Rationalization via photocrystallography of [Ru(NH3)4SO2X]tosylate2 (X = pyridine, 3-Cl-pyridine, 4-Cl-pyridine). J. Phys. Chem. C 2014, 118, 16003–16010. [Google Scholar] [CrossRef]
- Cole, J.M.; Velazquez-Garcia, J.d.J.; Gosztola, D.J.; Wang, S.G.; Chen, Y.-S. η2-SO2 Linkage photoisomer of an osmium coordination complex. Inorg. Chem. 2018, 57, 2673–2677. [Google Scholar] [CrossRef] [Green Version]
- Hatcher, L.E.; Skelton, J.M.; Warren, M.R.; Raithby, P.R. Photocrystallographic studies on transition metal nitrito metastable linkage isomers: Manipulating the metastable state. Acc. Chem. Res. 2019, 52, 1079–1088. [Google Scholar] [CrossRef]
- Hatcher, L.E.; Skelton, J.M.; Warren, M.R.; Stubbs, C.; Silvaa, E.L.d.; Raithby, P.R. Monitoring photo-induced population dynamics in metastable linkage isomer crystals: A crystallographic kinetic study of [Pd(Bu4dien)NO2]BPh4. Phys. Chem. Chem. Phys. 2018, 20, 5874–5886. [Google Scholar] [CrossRef] [Green Version]
- Hatcher, L.E.; Raithby, P.R. The impact of hydrogen bonding on 100% photo-switching in solid-state nitro-nitrito linkage isomers. Cryst. Eng. Comm. 2017, 19, 6297–6304. [Google Scholar] [CrossRef] [Green Version]
- Warren, M.R.; Easun, T.L.; Brayshaw, S.K.; Deeth, R.J.; George, M.W.; Johnson, A.L.; Schiffers, S.; Teat, S.J.; Warren, A.J.; Warren, J.E.; et al. Solid-state interconversions: Unique 100% reversible transformations between the ground and metastable states in single-crystals of a series of nickel(II) nitro complexes. Chem. Eur. J. 2014, 20, 5468–5477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatcher, L.E.; Christensen, J.; Hamilton, M.L.; Trincao, J.; Allan, D.R.; Warren, M.R.; Clarke, I.P.; Towrie, M.; Fuertes, S.; Wilson, C.C.; et al. Steady-state and pseudo-steady-state photocrystallographic studies on linkage isomers of [Ni(Et4dien)(η2-O,ON)(η1-NO2)]: Identification of a new linkage isomer. Chem. Eur. J. 2014, 20, 3128–3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, M.R.; Brayshaw, S.K.; Hatcher, L.E.; Johnson, A.L.; Schiffers, S.; Warren, A.J.; Teat, S.J.; Warren, J.E.; Woodall, C.H.; Raithby, P.R. Photoactivated linkage isomerism in single crystals of nickel, palladium and platinum di-nitro complexes—A photocrystallographic investigation. Dalton Trans. 2012, 41, 13173–13179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatcher, L.E.; Warren, M.R.; Allan, D.R.; Brayshaw, S.K.; Johnson, A.L.; Fuertes, S.; Schiffers, S.; Stevenson, A.J.; Teat, S.J.; Woodall, C.H.; et al. Metastable linkage isomerism in [Ni(Et4dien)(NO2)2]: A combined thermal and photocrystallographic structural investigation of a nitro/nitrito interconversion. Angew. Chem. Int. Ed. 2011, 50, 8371–8374. [Google Scholar]
- Warren, M.R.; Brayshaw, S.K.; Johnson, A.L.; Schiffers, S.; Raithby, P.R.; Easun, T.L.; George, M.W.; Warren, J.E.; Teat, S.J. Reversible 100% linkage isomerization in a single-crystal to single-crystal transformation: Photocrystallographic identification of the metastable [Ni(dppe)(η1-ONO)Cl] isomer. Angew. Chem. Int. Ed. 2009, 48, 5711–5714. [Google Scholar] [CrossRef]
- Rodenbough, P.P.; Karothu, D.P.; Gjorgjieva, T.; Commins, P.; Hara, H.; Naumov, P. Reversible Photolysis of Nitrosobenzene cis-Dimer Monitored In Situ by Single Crystal Photocrystallography. Cryst. Growth Des. 2018, 18, 1293–1296. [Google Scholar] [CrossRef]
- Ohashi, Y. Real-time in situ observation of chemical reactions. Acta Cryst. Sect. A 1998, 54, 842–849. [Google Scholar] [CrossRef]
- Nakamura, I.; Sumitani, R.; Mochida, T. Nitro–Nitrito Photoisomerization of Cationic Platinum(II) Complexes in the Solid State: Reactivity in Polymorphic Crystals and Glassy State. Cryst. Growth Des. 2021, 21, 1861–1868. [Google Scholar] [CrossRef]
- Nakamura, I.; Funasako, Y.; Mochida, T. Nitro–Nitrito Photoisomerization of Platinum(II) Complexes with Pt(NO2)42– and (FSO2)2N– Anions: Correlation between Isomerization Ratio and Reaction Cavity. Cryst. Growth Des. 2020, 20, 8047–8052. [Google Scholar] [CrossRef]
- Kutniewska, S.E.; Krówczyński, A.; Kamiński, R.; Jarzembska, K.N.; Pillet, S.; Wenger, E.; Schaniel, D. Photocrystallographic and spectroscopic studies of a model (N,N,O)-donor square-planar nickel(II) nitro complex: In search of high-conversion and stable photoswitchable materials. IUCrJ 2020, 7, 1188–1198. [Google Scholar] [CrossRef]
- Kutniewska, S.E.; Kamiński, R.; Buchowicz, W.; Jarzembska, K.N. Photo- and thermoswitchable half-sandwich nickel(II) complex: [Ni(η5-C5H5)(IMes)(η1-NO2)]. Inorg. Chem. 2019, 58, 16712–16721. [Google Scholar] [CrossRef]
- Kim, C.D.; Pillet, S.; Wu, G.; Fullagar, W.K.; Coppens, P. Excited-state structure by time-resolved X-ray diffraction. Acta Cryst. Sect. A 2002, 58, 133–137. [Google Scholar] [CrossRef]
- Vorontsov, I.I.; Kovalevsky, A.Y.; Chen, Y.-S.; Graber, T.; Gembicky, M.; Novozhilova, I.V.; Omary, M.A.; Coppens, P. Shedding light on the structure of a photoinduced transient excimer by time-resolved diffraction. Phys. Rev. Lett. 2005, 94, 193003. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, M.; Nozawa, S.; Sato, T.; Tomita, A.; Adachi, S.-I.; Koshihara, S.-y. Time-resolved X-ray crystal structure analysis for elucidating the hidden ‘over-neutralized’ phase of TTF-CA. RSC Adv. 2013, 3, 16313–16317. [Google Scholar] [CrossRef]
- Guérin, L.; Hébert, J.; Buron-Le Cointe, M.; Adachi, S.-I.; Koshihara, S.-Y.; Cailleau, H.; Collet, E. Capturing One-Dimensional Precursors of a Photoinduced Transformation in a Material. Phys. Rev. Lett. 2010, 105, 246101. [Google Scholar] [CrossRef]
- Schotte, F.; Cho, H.S.; Kaila, V.R.I.; Kamikubo, H.; Dashdorj, N.; Henry, E.R.; Graber, T.J.; Henning, R.; Wulff, M.; Hummer, G.; et al. Watching a signaling protein function in real time via 100-ps time-resolved Laue crystallography. Proc. Natl. Acad. Sci. USA 2012, 109, 19256–19261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotte, F.; Lim, M.; Jackson, T.A.; Smirnov, A.V.; Soman, J.; Olson, J.S.; Phillips, G.N., Jr.; Wulff, M.; Anfinrud, P.A. Watching a protein as it functions with 150-ps time-resolved x-ray crystallography. Science 2003, 300, 1944–1947. [Google Scholar] [CrossRef] [Green Version]
- Knapp, J.E.; Pahl, R.; Srajer, V.; Royer, W.E., Jr. Allosteric action in real time: Time-resolved crystallographic studies of a cooperative dimeric hemoglobin. Proc. Natl. Acad. Sci. USA 2006, 103, 7649–7654. [Google Scholar] [CrossRef] [Green Version]
- Graber, T.; Anderson, S.; Brewer, H.; Chen, Y.-S.; Cho, H.; Dashdorj, N.; Henning, R.W.; Kosheleva, I.; Macha, G.; Meron, M.; et al. BioCARS: A synchrotron resource for time-resolved X-ray science. J. Synchrotron Rad. 2011, 18, 658–670. [Google Scholar] [CrossRef] [Green Version]
- Wulff, M.; Plech, A.; Eybert, L.; Randler, R.; Schotte, F.; Anfinrud, P. Realisation of sub-nanosecond pump and probe experiments at the ESRF. Farad. Discuss. 2002, 122, 13–26. [Google Scholar] [CrossRef]
- Cammarata, M.; Ewald, F.; Eybert, L.; Reichenbach, W.; Wulff, M.; Anfinrud, P.; Schotte, F.; Kong, Q.; Lindenau, B.; Rabiger, J.; et al. Chopper system for single pulse experiments with synchrotron radiation. Rev. Sci. Instrum. 2009, 80, 015101–015111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nozawa, S.; Adachi, S.-I.; Takahashi, J.-I.; Tazaki, R.; Guérin, L.; Daimon, M.; Tomita, A.; Sato, T.; Chollet, M.; Collet, E.; et al. Developing 100 ps-resolved X-ray structural analysis capabilities on beamline NW14A at the Photon Factory Advanced Ring. J. Synchrotron Rad. 2007, 14, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.-C.; Lee, J.-J.; Chang, S.-H.; Lee, M.-H.; Liao, B.-Y. Micro-crystal x-ray diffraction beamline—Advanced and non-ambient crystallography. AIP Conf. Proc. 2019, 2054, 060029. [Google Scholar]
- Helliwell, J.R.; Habash, J.; Cruickshank, D.W.J.; Harding, M.M.; Greenhough, T.J.; Campbell, J.W.; Clifton, I.J.; Elder, M.; Machin, P.A.; Papiz, M.Z.; et al. The recording and analysis of synchrotron X-radiation Laue diffraction photographs. J. Appl. Cryst. 1989, 22, 483–497. [Google Scholar] [CrossRef]
- Campbell, J.W. LAUEGEN, an X-windows-based program for the processing of Laue diffraction data. J. Appl. Cryst. 1995, 28, 228–236. [Google Scholar] [CrossRef]
- Šrajer, V.; Crosson, S.; Schmidt, M.; Key, J.; Schotte, F.; Anderson, S.; Perman, B.; Ren, Z.; Teng, T.-Y.; Bourgeois, D.; et al. Extraction of accurate structure factor amplitudes from Laue data: Wavelength normalization with wiggler and undulator X-ray sources. J. Synchrotron Rad. 2000, 7, 236–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messerschmidt, M.; Tschentscher, T. LaueGUI—An open source Matlab tool for online inspection of time-resolved Laue diffraction patterns. Acta Cryst. Sect. A 2008, 64, C611. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, J.A.; Makal, A.; Coppens, P. The LaueUtil toolkit for Laue photocrystallography: I. Rapid orientation matrix determination for intermediate size unit-cell Laue data. J. Appl. Cryst. 2011, 44, 1182–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinowski, J.A.; Fournier, B.; Makal, A.; Coppens, P. The LaueUtil toolkit for Laue photocrystallography. II. Spot finding and integration. J. Synchrotron Rad. 2012, 19, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Bolotovsky, R.; White, M.E.; Darovsky, A.; Coppens, P. The ‘seed-skewness’ method for integration of peaks on imaging plates. J. Appl. Cryst. 1995, 28, 86–95. [Google Scholar] [CrossRef]
- Bolotovsky, R.; Coppens, P. The ‘seed-skewness’ method for integration of peaks on imaging plates II. Analysis of bias due to finite size of the peak mask and treatment of α1-α2 splitting. J. Appl. Cryst. 1997, 30, 244–253. [Google Scholar] [CrossRef]
- Peters, J. The ‘seed-skewness’ integration method generalized for three-dimensional Bragg peaks. J. Appl. Cryst. 2003, 36, 1475–1479. [Google Scholar] [CrossRef]
- Szarejko, D.; Kamiński, R.; Łaski, P.; Jarzembska, K.N. Seed-skewness algorithm for X-ray diffraction signal detection in the time-resolved synchrotron Laue photocrystallography. J. Synchrotron Rad. 2020, 27, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Pierre, S. Morphological Image Anaysis. Principles and Aplications; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Straasø, T.; Müter, D.; Sørensen, H.O.; Als-Nielsen, J. Objective algorithm to separate signal from noise in a Poisson-distributed pixel data set. J. Appl. Cryst. 2013, 46, 663–671. [Google Scholar] [CrossRef]
- Kamiński, R.; Szarejko, D.; Pedersen, M.N.; Hatcher, L.E.; Łaski, P.; Raithby, P.R.; Wulff, M.; Jarzembska, K.N. Instrument-model refinement in normalised reciprocal-vector space for X-ray Laue diffraction. J. Appl. Cryst. 2020, 53, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Gevorkov, Y.; Barty, A.; Brehm, W.; White, T.A.; Tolstikova, A.; Wiedorn, M.O.; Meents, A.; Grigat, R.-R.; Chapman, H.N.; Yefanov, O. PinkIndexer—A universal indexer for pink-beam X-ray and electron diffraction snapshots. Acta Cryst. Sect. A 2020, 76, 121–131. [Google Scholar] [CrossRef]
- Coppens, P.; Pitak, M.; Gembicky, M.; Messerschmidt, M.; Scheins, S.; Benedict, J.B.; Adachi, S.-I.; Sato, T.; Nozawa, S.; Ichiyanagi, K.; et al. The RATIO method for time-resolved Laue crystallography. J. Synchrotron Rad. 2009, 16, 226–230. [Google Scholar] [CrossRef]
- Blessing, R.H. DREAM—Data reduction and error analysis routines for accurate single-crystal diffraction intensity measurements. J. Appl. Cryst. 1986, 19, 412. [Google Scholar] [CrossRef]
- Blessing, R.H. Data reduction and error analysis for accurate single crystal diffraction intensities. Cryst. Rev. 1987, 1, 3–58. [Google Scholar] [CrossRef]
- Blessing, R.H.; Langs, D.A. Data averaging with normal down-weighting of outliers. J. Appl. Cryst. 1987, 20, 427–428. [Google Scholar] [CrossRef]
- Blessing, R.H. DREADD—Data reduction and error analysis for single-crystal diffractometer data. J. Appl. Cryst. 1989, 22, 396–397. [Google Scholar] [CrossRef]
- Blessing, R.H. Outlier treatment in data merging. J. Appl. Cryst. 1997, 30, 421–426. [Google Scholar] [CrossRef]
- Fournier, B.; Sokolow, J.; Coppens, P. Analysis of multicrystal pump-probe data sets. II. Scaling of ratio data sets. Acta Cryst. Sect. A 2016, 72, 250–260. [Google Scholar] [CrossRef]
- Coppens, P.; Fournier, B. On the scaling of multicrystal data sets collected at high-intensity X-ray and electron sources. Struct. Dyn. 2015, 2, 064101. [Google Scholar] [CrossRef]
- Fournier, B.; Coppens, P. Analysis of multicrystal pump-probe data sets. I. Expressions for the RATIO model. Acta Cryst. Sect. A 2014, 70, 514–517. [Google Scholar] [CrossRef] [Green Version]
- Vorontsov, I.; Pillet, S.; Kamiński, R.; Schmøkel, M.S.; Coppens, P. LASER—A program for response-ratio refinement of time-resolved diffraction data. J. Appl. Cryst. 2010, 43, 1129–1130. [Google Scholar] [CrossRef]
- Velazquez-Garcia, J.d.J.; Wong, J.; Basuroy, K.; Storozhuk, D.; Saouane, S.; Henning, R.; Techert, S. A Comparative Analysis on LaueUtil and PRECOGNITION Software Packages as Tools in Treating the Small Molecule Time-Resolved Laue Diffraction Measurements at High Flux X-ray facilities. arXiv 2020, arXiv:2010.05781v1. [Google Scholar]
- Coppens, P.; Makal, A.; Fournier, B.; Jarzembska, K.N.; Kamiński, R.; Basuroy, K.; Trzop, E. A priori checking of the light-response and data quality before extended data collection in pump-probe photocrystallography experiments. Acta Cryst. Sect. B 2017, 73, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Casaretto, N.; Schaniel, D.; Alle, P.; Wenger, E.; Parois, P.; Fournier, B.; Bendeif, E.-E.; Palin, C.; Pillet, S. In-house time-resolved photocrystallography on the millisecond timescale using a gated X-ray hybrid pixel area detector. Acta Cryst. Sect. B 2017, 73, 696–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzop, E.; Fournier, B.; Jarzembska, K.; Sokolow, J.; Kaminski, R.; Benedict, J.; Chen, Y.; Henning, R.; Coppens, P. Selective time-dependent changes of Cu(DPPE)(DMP)·PF6 on photoexcitation. Acta Cryst. Sect. A 2014, 70, C776. [Google Scholar] [CrossRef] [Green Version]
- Basuroy, K.; Chen, Y.; Sarkar, S.; Benedict, J.; Coppens, P. Exploring the structural changes on excitation of a luminescent organic bromine-substituted complex by in-house time-resolved pump-probe diffraction. Struct. Dyn. 2017, 4, 024501. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, R.; Benedict, J.; Trzop, E.; Jarzembska, K.; Fournier, B.; Coppens, P. First steps towards time-resolved ‘in-house’ X-ray diffraction experiments. Acta Cryst. Sect. A 2014, 70, C775. [Google Scholar] [CrossRef]
- Cox, J.M.; Walton, I.M.; Patel, D.G.D.; Xu, M.; Chen, Y.-S.; Benedict, J.B. The temperature dependent photoswitching of a classic diarylethene monitored by in situ X-ray diffraction. J. Phys. Chem. A 2015, 119, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, R.; Jarzembska, K.N.; Kutyła, S.E.; Kamiński, M. A portable light-delivery device for in situ photocrystallographic experiments at home laboratory. J. Appl. Cryst. 2016, 49, 1383–1387. [Google Scholar] [CrossRef]
- Kamiński, R.; Benedict, J.B.; Nottingham, G.; Coppens, P. An optical chopper for generation of short X-ray pulses to allow in-house time-resolved photocrystallography. J. Appl. Cryst. 2014, 47, 1765–1768. [Google Scholar] [CrossRef]
- Cruickshank, D.W.J.; Helliwell, J.R.; Moffat, K. Multiplicity distribution of reflections in Laue diffraction. Acta Cryst. Sect. A 1987, 43, 656–674. [Google Scholar] [CrossRef]
- Cruickshank, D.W.J.; Helliwell, J.R.; Moffat, K. Angular distribution of reflections in Laue diffraction. Acta Cryst. Sect. A 1991, 47, 352–373. [Google Scholar] [CrossRef]
- Ren, Z.; Moffat, K. Structure refinement against synchrotron Laue data: Strategies for data collection and reduction. J. Appl. Cryst. 1995, 28, 461–481. [Google Scholar] [CrossRef]
- Schmidt, M.; Rajagopal, S.; Ren, Z.; Moffat, K. Application of singular value decomposition to the analysis of time-resolved macromolecular X-ray data. Biophys. J. 2003, 84, 2112–2129. [Google Scholar] [CrossRef] [Green Version]
- Blakley, R.L.; Yin, Y.; Lloyd, C.; Mague, J.T.; McPherson, G.L. Photophysics of a flexible ligand bridged rhodium(I) dimer: An excited state conformational change in a crystalline solid. Chem. Phys. Lett. 1989, 157, 398–402. [Google Scholar] [CrossRef]
- Mague, J.T. Conformational diversity in the solid state structures of [Rh2(μ-CH3N(P(OCH3)2)2)2(CH3N(P(OCH3)2)2)2]X2 (X = O3SCF3, B(C6H5)4). Inorg. Chim. Acta 1995, 229, 17–25. [Google Scholar] [CrossRef]
- Schubert, H.; Wesemann, L. Silver dimer, tetramer, polymer, and network structures with the stannylene stanna-closo-dodecaborate. Organometallics 2010, 29, 4906–4913. [Google Scholar] [CrossRef]
- Jarzembska, K.N.; Kamiński, R.; Dziubek, K.F.; Citroni, M.; Paliwoda, D.; Durka, K.; Fanetti, S.; Bini, R. Impact of high pressure on metallophilic interactions and its consequences for spectroscopic properties of a model tetranuclear silver(I)-copper(I) complex in the solid state. Inorg. Chem. 2018, 57, 8509–8520. [Google Scholar] [CrossRef] [PubMed]
- Vorontsov, I.I.; Graber, T.; Kovalevsky, A.Y.; Novozhilova, I.V.; Gembicky, M.; Chen, Y.-S.; Coppens, P. Capturing and analyzing the excited-state structure of a Cu(I) phenanthroline complex by time-resolved diffraction and theoretical calculations. J. Am. Chem. Soc. 2009, 131, 6566–6573. [Google Scholar] [CrossRef] [PubMed]
- Caleman, C.; Martin, A.V. When Diffraction Stops and Destruction Begins. In X-ray Free Electron Lasers: A Revolution in Structural Biology; Boutet, S., Fromme, P., Hunter, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 185–207. [Google Scholar]
- Spence, J.C.H.; Weierstall, U.; Chapman, H.N. X-ray lasers for structural and dynamic biology. Rep. Prog. Phys. 2012, 75, 102601. [Google Scholar] [CrossRef] [Green Version]
- Howells, M.R.; Beetz, T.; Chapman, H.N.; Cui, C.; Holton, J.M.; Jacobsen, C.J.; Kirz, J.; Lima, E.; Marchesini, S.; Miao, H.; et al. An assessment of the resolution limitation due to radiation-damage in x-ray diffraction microscopy. J. Electron. Spectrosc. Relat. Phenom. 2009, 170, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, R.G.; Batyuk, A.; Sun, Z.; Aquila, A.; Hunter, M.S.; Lane, T.J.; Liang, M.; Yoon, C.H.; Alonso-Mori, R.; Armenta, R.; et al. The Macromolecular Femtosecond Crystallography Instrument at the Linac Coherent Light SourceThis article will form part of a virtual special issue on X-ray free-electron lasers. J. Synchrotron Rad. 2019, 26, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Martiel, I.; Muller-Werkmeister, H.M.; Cohen, A.E. Strategies for sample delivery for femtosecond crystallography. Acta Cryst. Sect. D 2019, 75, 160–177. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.-Z.; Zhang, B.; Yan, E.-K.; Sun, B.; Wang, Z.-J.; He, J.-H.; Yin, D.-C. A guide to sample delivery systems for serial crystallography. FEBS J. 2019, 286, 4402–4417. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M. Mix and Inject: Reaction Initiation by Diffusion for Time-Resolved Macromolecular Crystallography. Adv. Condens. Matter Phys. 2013, 2013, 167276. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, P.; Muller-Werkmeister, H.M.; Leimkohl, J.-P.; Schikora, H.; Ninkovic, J.; Krivokuca, S.; Andricek, L.; Epp, S.W.; Sherrell, D.; Owen, R.L.; et al. The HARE chip for efficient time-resolved serial synchrotron crystallography. J. Synchrotron Rad. 2020, 27, 360–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostedt, C.; Boutet, S.; Fritz, D.M.; Huang, Z.; Lee, H.J.; Lemke, H.T.; Robert, A.; Schlotter, W.F.; Turner, J.J.; Williams, G.J. Linac Coherent Light Source: The first five years. Rev. Mod. Phys. 2016, 88, 015007. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Brachmann, A.; Decker, F.J.; Dowell, D.; Emma, P.; Frisch, J.; Gilevich, S.; Hays, G.; Hering, P.; Huang, Z.; et al. Measurements and Simulations of Ultralow Emittance and Ultrashort Electron Beams in the Linac Coherent Light Source. Phys. Rev. Lett. 2009, 102, 254801. [Google Scholar] [CrossRef] [Green Version]
- Emma, P.; Bane, K.; Cornacchia, M.; Huang, Z.; Schlarb, H.; Stupakov, G.; Walz, D. Femtosecond and Subfemtosecond X-Ray Pulses from a Self-Amplified Spontaneous-Emission-Based Free-Electron Laser. Phys. Rev. Lett. 2004, 92, 074801. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, M.; Budday, D.; de Oliveira, S.H.P.; Madzelan, P.; Marchany-Rivera, D.; Seravalli, J.; Hayes, B.; Sierra, R.G.; Boutet, S.; Hunter, M.S.; et al. Mix-and-inject XFEL crystallography reveals gated conformational dynamics during enzyme catalysis. Proc. Natl. Acad. Sci. USA 2019, 116, 25634–25640. [Google Scholar] [CrossRef] [PubMed]
- Chollet, M.; Alonso-Mori, R.; Cammarata, M.; Damiani, D.; Defever, J.; Delor, J.T.; Feng, Y.; Glownia, J.M.; Langton, J.B.; Nelson, S.; et al. The X-ray Pump-Probe instrument at the Linac Coherent Light Source. J. Synchrotron Rad. 2015, 22, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Yabashi, M.; Tanaka, H.; Ishikawa, T. Overview of the SACLA facility. J. Synchrotron Rad. 2015, 22, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Shintake, T.; Tanaka, H.; Hara, T.; Tanaka, T.; Togawa, K.; Yabashi, M.; Otake, Y.; Asano, Y.; Bizen, T.; Fukui, T.; et al. A compact free-electron laser for generating coherent radiation in the extreme ultraviolet region. Nat. Photonics 2008, 2, 555–559. [Google Scholar] [CrossRef]
- Yabashi, M.; Tanaka, H.; Tono, K.; Ishikawa, T. Status of the SACLA Facility. Appl. Sci. 2017, 7, 604. [Google Scholar] [CrossRef] [Green Version]
- Palmer, G.; Kellert, M.; Wang, J.; Emons, M.; Wegner, U.; Kane, D.; Pallas, F.; Jezynski, T.; Venkatesan, S.; Rompotis, D.; et al. Pump-probe laser system at the FXE and SPB/SFX instruments of the European X-ray Free-Electron Laser FacilityThis article will form part of a virtual special issue on X-ray free-electron lasers. J. Synchrotron Rad. 2019, 26, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Geloni, G.; Saldin, E.; Samoylova, L.; Schneidmiller, E.; Sinn, H.; Tschentscher, T.; Yurkov, M. Coherence properties of the European XFEL. New J. Phys. 2010, 12, 035021. [Google Scholar] [CrossRef]
- Mancuso, A.P.; Aquila, A.; Batchelor, L.; Bean, R.J.; Bielecki, J.; Borchers, G.; Doerner, K.; Giewekemeyer, K.; Graceffa, R.; Kelsey, O.D.; et al. The Single Particles, Clusters and Biomolecules and Serial Femtosecond Crystallography instrument of the European XFEL: Initial installationThis article will form part of a virtual special issue on X-ray free-electron lasers. J. Synchrotron Rad. 2019, 26, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Barty, A.; Kirian, R.A.; Maia, F.R.N.C.; Hantke, M.; Yoon, C.H.; White, T.A.; Chapman, H. Cheetah: Software for high-throughput reduction and analysis of serial femtosecond X-ray diffraction data. J. Appl. Crystallogr. 2014, 47, 1118–1131. [Google Scholar] [CrossRef] [Green Version]
- White, T.A.; Kirian, R.A.; Martin, A.V.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Crystallogr. 2012, 45, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Duisenberg, A. Indexing in single-crystal diffractometry with an obstinate list of reflections. J. Appl. Cryst. 1992, 25, 92–96. [Google Scholar] [CrossRef]
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Cryst. Sect. D 2011, 67, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodhouse, J.; Kovacs, G.N.; Coquelle, N.; Uriarte, L.M.; Adam, V.; Barends, T.R.M.; Byrdin, M.; de la Mora, E.; Bruce Doak, R.; Feliks, M.; et al. Photoswitching mechanism of a fluorescent protein revealed by time-resolved crystallography and transient absorption spectroscopy. Nat. Commun. 2020, 11, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grotjohann, T.; Testa, I.; Reuss, M.; Brakemann, T.; Eggeling, C.; Hell, S.W.; Jakobs, S. rsEGFP2 enables fast RESOLFT nanoscopy of living cells. eLife 2012, 1, e00248. [Google Scholar] [CrossRef]
- Gustafsson, M.G.L. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA 2005, 102, 13081. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, M.; Eggeling, C.; Jakobs, S.; Hell, S.W. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 17565. [Google Scholar] [CrossRef] [Green Version]
- Adam, V.; Berardozzi, R.; Byrdin, M.; Bourgeois, D. Phototransformable fluorescent proteins: Future challenges. Curr. Opin. Chem. Biol. 2014, 20C, 92–102. [Google Scholar] [CrossRef]
- Nienhaus, K.; Ulrich Nienhaus, G. Fluorescent proteins for live-cell imaging with super-resolution. Chem. Soc. Rev. 2014, 43, 1088–1106. [Google Scholar] [CrossRef] [PubMed]
- Barends, T.; Foucar, L.; Ardevol, A.; Nass, K.; Aquila, A.; Botha, S.; Doak, R.; Falahati, K.; Hartmann, E.; Hilpert, M.; et al. Direct observation of ultrafast collective motions in CO myoglobin upon ligand dissociation. Science 2015, 350, 445–450. [Google Scholar] [CrossRef]
- Coquelle, N.; Sliwa, M.; Woodhouse, J.; Schirò, G.; Adam, V.; Aquila, A.; Barends, T.; Boutet, S.; Byrdin, M.; Carbajo, S.; et al. Chromophore twisting in the excited state of a photoswitchable fluorescent protein captured by time-resolved serial femtosecond crystallography. Nat. Chem. 2017, 10, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Nango, E.; Royant, A.; Kubo, M.; Nakane, T.; Wickstrand, C.; Kimura, T.; Tanaka, T.; Tono, K.; Song, C.; Tanaka, R.; et al. A three-dimensional movie of structural changes in bacteriorhodopsin. Science 2016, 354, 1552–1557. [Google Scholar] [CrossRef]
- Pande, K.; Hutchison, C.; Groenhof, G.; Aquila, A.; Robinson, J.; Tenboer, J.; Basu, S.; Boutet, S.; DePonte, D.; Liang, M.; et al. Femtosecond Structural Dynamics Drives the Trans/Cis Isomerization in Photoactive Yellow Protein. Science 2016, 352, 725–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, A.; Kubo, M.; Baba, S.; Yamashita, K.; Hirata, K.; Ueno, G.; Nomura, T.; Kimura, T.; Shinzawa-Itoh, K.; Baba, J.; et al. A nanosecond time-resolved XFEL analysis of structural changes associated with CO release from cytochrome C oxidase. Sci. Adv. 2017, 3, e1603042. [Google Scholar] [CrossRef] [Green Version]
- Suga, M.; Akita, F.; Sugahara, M.; Kubo, M.; Yoshiki, N.; Nakane, T.; Yamashita, K.; Umena, Y.; Nakabayashi, M.; Yamane, T.; et al. Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 2017, 543, 131–135. [Google Scholar] [CrossRef]
- Tenboer, J.; Basu, S.; Zatsepin, N.; Pande, K.; Milathianaki, D.; Frank, M.; Hunter, M.; Boutet, S.; Williams, G.; Koglin, J.; et al. Time-Resolved Serial Crystallography Captures High Resolution Intermediates of Photoactive Yellow Protein. Science 2014, 346, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Grünbein, M.L.; Bielecki, J.; Gorel, A.; Stricker, M.; Bean, R.; Cammarata, M.; Dörner, K.; Fröhlich, L.; Hartmann, E.; Hauf, S.; et al. Megahertz data collection from protein microcrystals at an X-ray free-electron laser. Nat. Commun. 2018, 9, 3487. [Google Scholar] [CrossRef] [PubMed]
- Wiedorn, M.; Oberthuer, D.; Bean, R.; Schubert, R.; Werner, N.; Abbey, B.; Aepfelbacher, M.; Adriano, L.; Allahgholi, A.; Al-Qudami, N.; et al. Megahertz serial crystallography. Nat. Commun. 2018, 9, 4025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.; Bean, R.; Sato, T.; Poudyal, I.; Bielecki, J.; Cruz Villarreal, J.; Yefanov, O.; Mariani, V.; White, T.A.; Kupitz, C.; et al. Time-resolved serial femtosecond crystallography at the European XFEL. Nat. Methods 2020, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Creelman, M.; Kumauchi, M.; Hoff, W.D.; Mathies, R.A. Chromophore Dynamics in the PYP Photocycle from Femtosecond Stimulated Raman Spectroscopy. J. Phys. Chem. B 2014, 118, 659–667. [Google Scholar] [CrossRef]
- Carlos, J.L.; Karl, R.R.; Bauer, S.H. Gas phase electron diffraction study of six fluoroethylenes. J. Chem. Soc. Faraday Trans. 1974, 70, 177–187. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.C.; Müller-Werkmeister, H.M.; Lu, C.; Zhang, D.; Field, R.L.; Sarracini, A.; Moriena, G.; Collet, E.; Miller, R.J.D. Structural Dynamics upon Photoexcitation in a Spin Crossover Crystal Probed with Femtosecond Electron Diffraction. Angew. Chem. Int. Ed. 2017, 56, 7130–7134. [Google Scholar] [CrossRef]
- Nannenga, B.L.; Gonen, T. The cryo-EM method microcrystal electron diffraction (MicroED). Nat. Methods 2019, 16, 369–379. [Google Scholar] [CrossRef]
- Mancini, G.F.; Latychevskaia, T.; Pennacchio, F.; Reguera, J.; Stellacci, F.; Carbone, F. Order/Disorder Dynamics in a Dodecanethiol-Capped Gold Nanoparticles Supracrystal by Small-Angle Ultrafast Electron Diffraction. Nano Lett. 2016, 16, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Nishina, Y.; Kato, T. Exploring Structures and Dynamics of Molecular Assemblies: Ultrafast Time-Resolved Electron Diffraction Measurements. Acc. Chem. Res. 2021, 54, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Jean-Ruel, H.; Gao, M.; Kochman, M.A.; Lu, C.; Liu, L.C.; Cooney, R.R.; Morrison, C.A.; Miller, R.J.D. Ring-closing reaction in diarylethene captured by femtosecond electron crystallography. J. Phys. Chem. B 2013, 117, 15894–15902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Jean-Ruel, H.; Cooney, R.R.; Stampe, J.; de Jong, M.; Harb, M.; Sciaini, G.; Moriena, G.; Dwayne Miller, R.J. Full characterization of RF compressed femtosecond electron pulses using ponderomotive scattering. Opt. Express 2012, 20, 12048–12058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean-Ruel, H.; Cooney, R.R.; Gao, M.; Lu, C.; Kochman, M.A.; Morrison, C.A.; Miller, R.J.D. Femtosecond dynamics of the ring closing process of diarylethene: A case study of electrocyclic reactions in photochromic single crystals. J. Phys. Chem. A 2011, 115, 13158–13168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofori-Okai, B.K.; Hoffmann, M.C.; Reid, A.H.; Edstrom, S.; Jobe, R.K.; Li, R.K.; Mannebach, E.M.; Park, S.J.; Polzin, W.; Shen, X.; et al. A terahertz pump mega-electron-volt ultrafast electron diffraction probe apparatus at the SLAC Accelerator Structure Test Area facility. J. Instrum. 2018, 13, P06014. [Google Scholar] [CrossRef]
- Zimmerman, H.E.; Zuraw, M.J. Photochemistry in a box. Photochemical reactions of molecules entrapped in crystal lattices: Mechanistic and exploratory organic photochemistry. J. Am. Chem. Soc. 1989, 111, 7974–7989. [Google Scholar] [CrossRef]
- Weathersby, S.P.; Brown, G.; Centurion, M.; Chase, T.F.; Coffee, R.; Corbett, J.; Eichner, J.P.; Frisch, J.C.; Fry, A.R.; Gühr, M.; et al. Mega-electron-volt ultrafast electron diffraction at SLAC National Accelerator Laboratory. Rev. Sci. Instrum. 2015, 86, 073702. [Google Scholar] [CrossRef] [PubMed]
- Kampfrath, T.; Tanaka, K.; Nelson, K.A. Resonant and nonresonant control over matter and light by intense terahertz transients. Nat. Photonics 2013, 7, 680–690. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Fleischer, S.; Brandt, N.C.; Perkins, B.G.; Liu, M.; Fan, K.; Sternbach, A.; Zhang, X.; Averitt, R.D.; Nelson, K.A. A review of non-linear terahertz spectroscopy with ultrashort tabletop-laser pulses. J. Mod. Opt. 2015, 62, 1447–1479. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirori, H.; Nagai, M. THz Nonlinear Spectroscopy of Solids. IEEE Trans. Terahertz Sci. Technol. 2011, 1, 301–312. [Google Scholar] [CrossRef]
- Peiponen, K.-E.; Zeitler, A.; Kuwata-Gonokami, M. Terahertz Spectroscopy and Imaging; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Wolf, T.J.A.; Sanchez, D.M.; Yang, J.; Parrish, R.M.; Nunes, J.P.F.; Centurion, M.; Coffee, R.; Cryan, J.P.; Gühr, M.; Hegazy, K.; et al. The photochemical ring-opening of 1,3-cyclohexadiene imaged by ultrafast electron diffraction. Nat. Chem. 2018, 11, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tromp, M.; Dent, A.J.; Headspith, J.; Easun, T.L.; Sun, X.-Z.; George, M.W.; Mathon, O.; Smolentsev, G.; Hamilton, M.L.; Evans, J. Energy Dispersive XAFS: Characterization of Electronically Excited States of Copper(I) Complexes. J. Phys. Chem. B 2013, 117, 7381–7387. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Moreno, I.; Díaz-Quintana, A.; Subías, G.; Mairs, T.; De la Rosa, M.A.; Díaz-Moreno, S. Detecting transient protein-protein interactions by X-ray absorption spectroscopy: The cytochrome c6-photosystem I complex. FEBS Lett. 2006, 580, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; DeBeer, S. The role of X-ray spectroscopy in understanding the geometric and electronic structure of nitrogenase. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1406–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, N.; Saida, T.; Uruga, T.; Nagamatsu, S.-I.; Sekizawa, O.; Nitta, K.; Yamamoto, T.; Ohkoshi, S.-I.; Iwasawa, Y.; Yokoyama, T.; et al. Operando Time-Resolved X-ray Absorption Fine Structure Study for Surface Events on a Pt3Co/C Cathode Catalyst in a Polymer Electrolyte Fuel Cell during Voltage-Operating Processes. ACS Catal. 2012, 2, 1319–1330. [Google Scholar] [CrossRef]
- Obara, Y.; Ito, H.; Ito, T.; Kurahashi, N.; Thürmer, S.; Tanaka, H.; Katayama, T.; Togashi, T.; Owada, S.; Yamamoto, Y.-I.; et al. Femtosecond time-resolved X-ray absorption spectroscopy of anatase TiO2 nanoparticles using XFEL. Struct. Dyn. 2017, 4, 044033. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Günther, B.; Achterhold, K.; Cui, Y.-T.; Gleich, B.; Dierolf, M.; Pfeiffer, F. Energy-Dispersive X-ray Absorption Spectroscopy with an Inverse Compton Source. Sci. Rep. 2020, 10, 8772. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Suarez-Alcantara, K.; Jennings, G.; Kurtz, C.A.; Lawson Daku, L.M.; Canton, S.E. Resolving the Ultrafast Changes of Chemically Inequivalent Metal–Ligand Bonds in Photoexcited Molecular Complexes with Transient X-ray Absorption Spectroscopy. ACS Omega 2019, 4, 6375–6381. [Google Scholar] [CrossRef] [Green Version]
- Gawelda, W.; Pham, V.-T.; Benfatto, M.; Zaushitsyn, Y.; Kaiser, M.; Grolimund, D.; Johnson, S.L.; Abela, R.; Hauser, A.; Bressler, C.; et al. Structural Determination of a Short-Lived Excited Iron(II) Complex by Picosecond X-Ray Absorption Spectroscopy. Phys. Rev. Lett. 2007, 98, 057401. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Holldack, K.; Kachel, T.; Mitzner, R.; Quast, T. Femtosecond Undulator Radiation from Sliced Electron Bunches. Phys. Rev. Lett. 2006, 97, 074801. [Google Scholar] [CrossRef]
- Beaud, P.; Johnson, S.L.; Streun, A.; Abela, R.; Abramsohn, D.; Grolimund, D.; Krasniqi, F.; Schmidt, T.; Schlott, V.; Ingold, G. Spatiotemporal Stability of a Femtosecond Hard--X-Ray Undulator Source Studied by Control of Coherent Optical Phonons. Phys. Rev. Lett. 2007, 99, 174801. [Google Scholar] [CrossRef]
- Zholents, A.A.; Zolotorev, M.S. Femtosecond X-Ray Pulses of Synchrotron Radiation. Phys. Rev. Lett. 1996, 76, 912–915. [Google Scholar] [CrossRef] [Green Version]
- Schoenlein, R.W.; Chattopadhyay, S.; Chong, H.H.W.; Glover, T.E.; Heimann, P.A.; Shank, C.V.; Zholents, A.A.; Zolotorev, M.S. Generation of Femtosecond Pulses of Synchrotron Radiation. Science 2000, 287, 2237–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, C.J.; Pham, V.-T.; Gawelda, W.; Veen, R.M.v.d.; Nahhas, A.E.; Johnson, S.L.; Beaud, P.; Ingold, G.; Lima, F.; Vithanage, D.A.; et al. Time-resolved x-ray absorption spectroscopy: Watching atoms dance. J. Phys. Conf. Ser. 2009, 190, 012052. [Google Scholar] [CrossRef]
- Schaniel, D.; Casaretto, N.; Bendeif, E.-E.; Woike, T.; Gallien, A.K.E.; Klüfers, P.; Kutniewska, S.E.; Kamiński, R.; Bouchez, G.; Boukheddaden, K.; et al. Evidence for a photoinduced isonitrosyl isomer in ruthenium dinitrosyl compounds. Cryst. Eng. Comm. 2019, 21, 5804–5810. [Google Scholar] [CrossRef] [Green Version]
- Schaniel, D.; Nicoul, M.; Woike, T. Ultrafast reversible ligand isomerisation in Na2[Fe(CN)5NO]·2H2O single crystals. Phys. Chem. Chem. Phys. 2010, 12, 9029–9033. [Google Scholar] [CrossRef]
- Liepuoniute, I.; Commins, P.; Karothu, D.P.; Schramm, S.; Hara, H.; Naumov, P. Reversible Multicolor Photochromism of Dihydroazulene Crystals. Chem. Eur. J. 2019, 25, 373–378. [Google Scholar] [CrossRef]
- Naumov, P.; Yu, P.; Sakurai, K. Electronic Tera-Order Stabilization of Photoinduced Metastable Species: Structure of the Photochromic Product of Spiropyran Determined with in situ Single Crystal X-ray Photodiffraction. J. Phys. Chem. A 2008, 112, 5810–5814. [Google Scholar] [CrossRef] [PubMed]



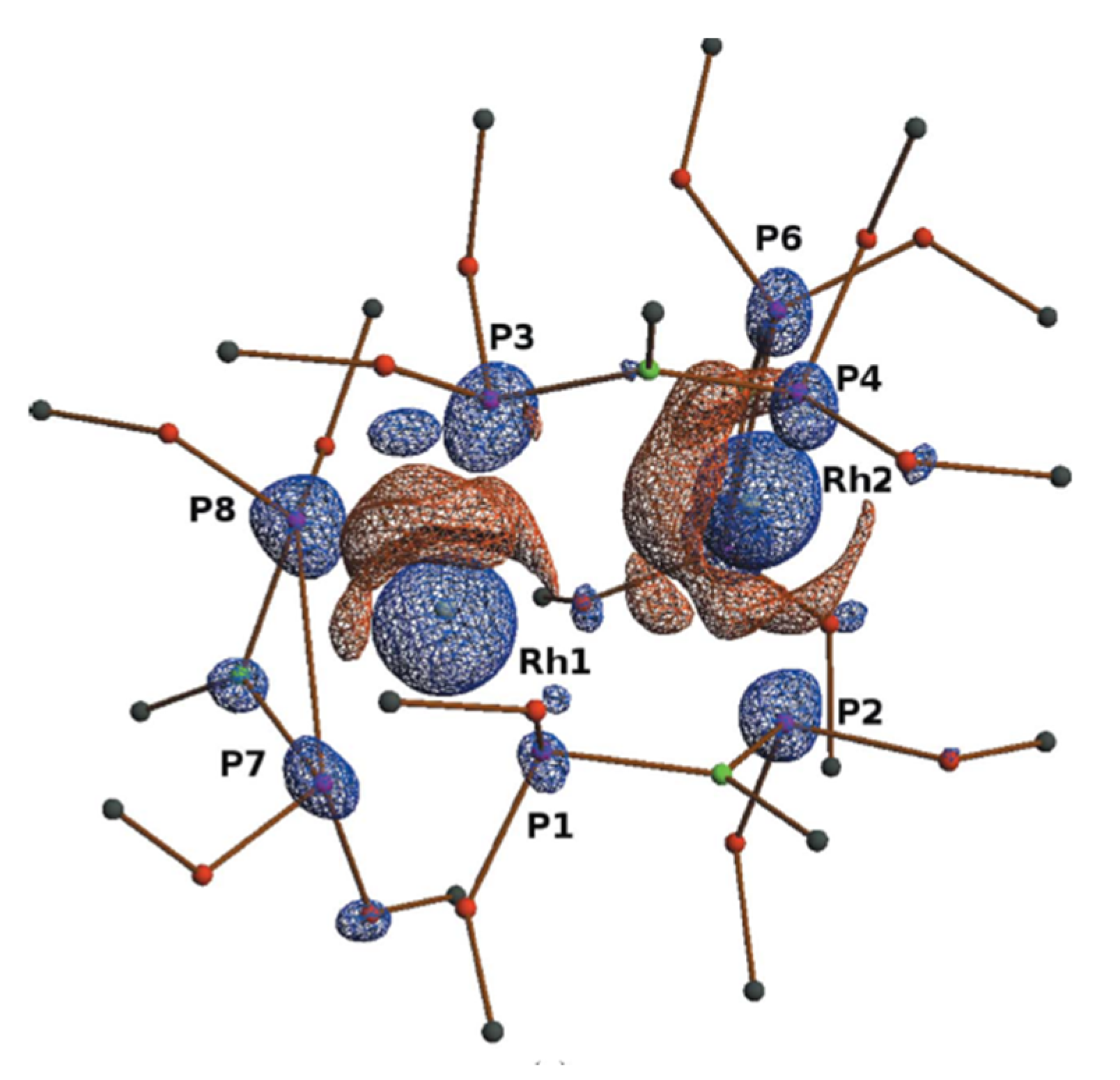
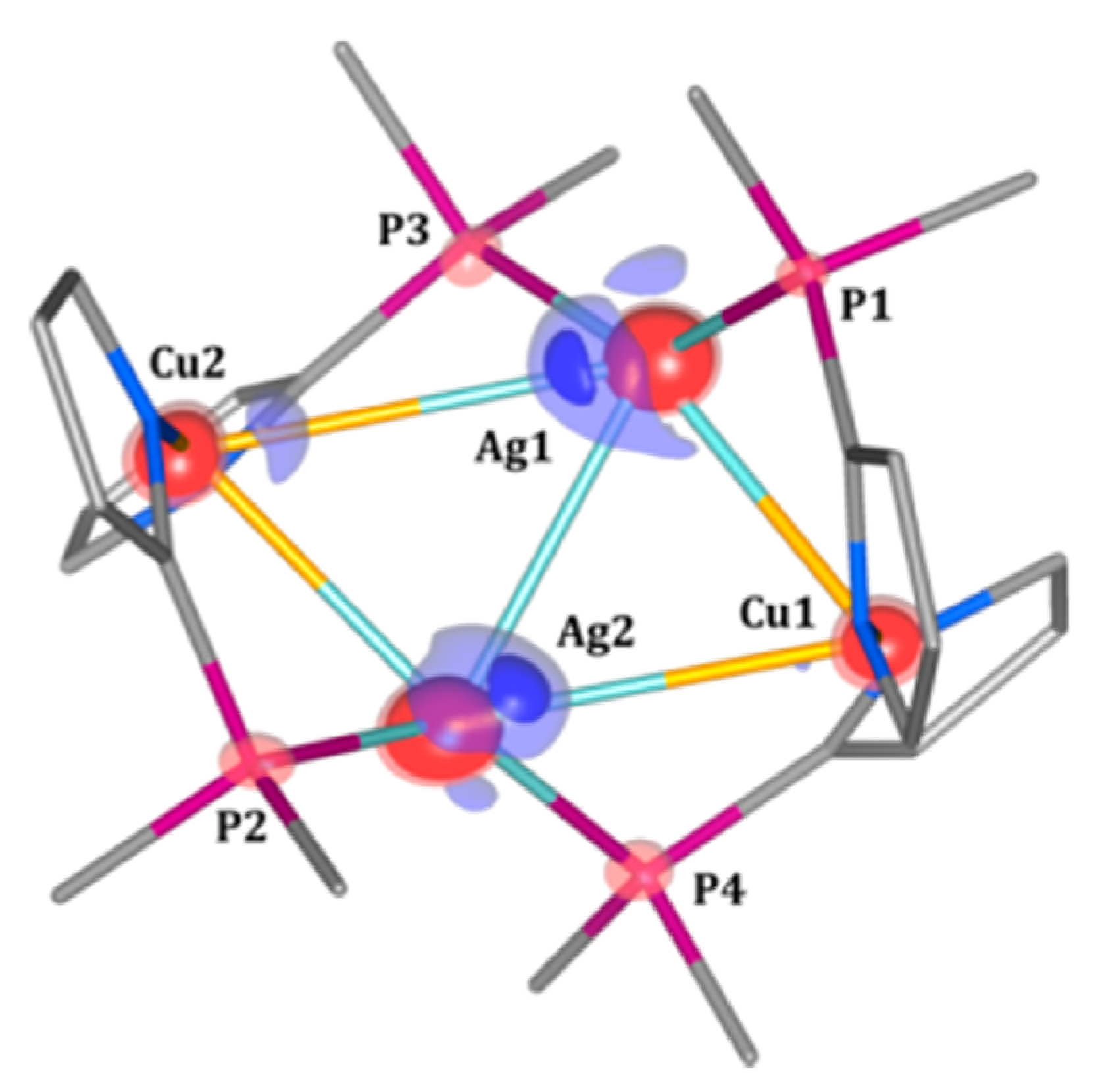
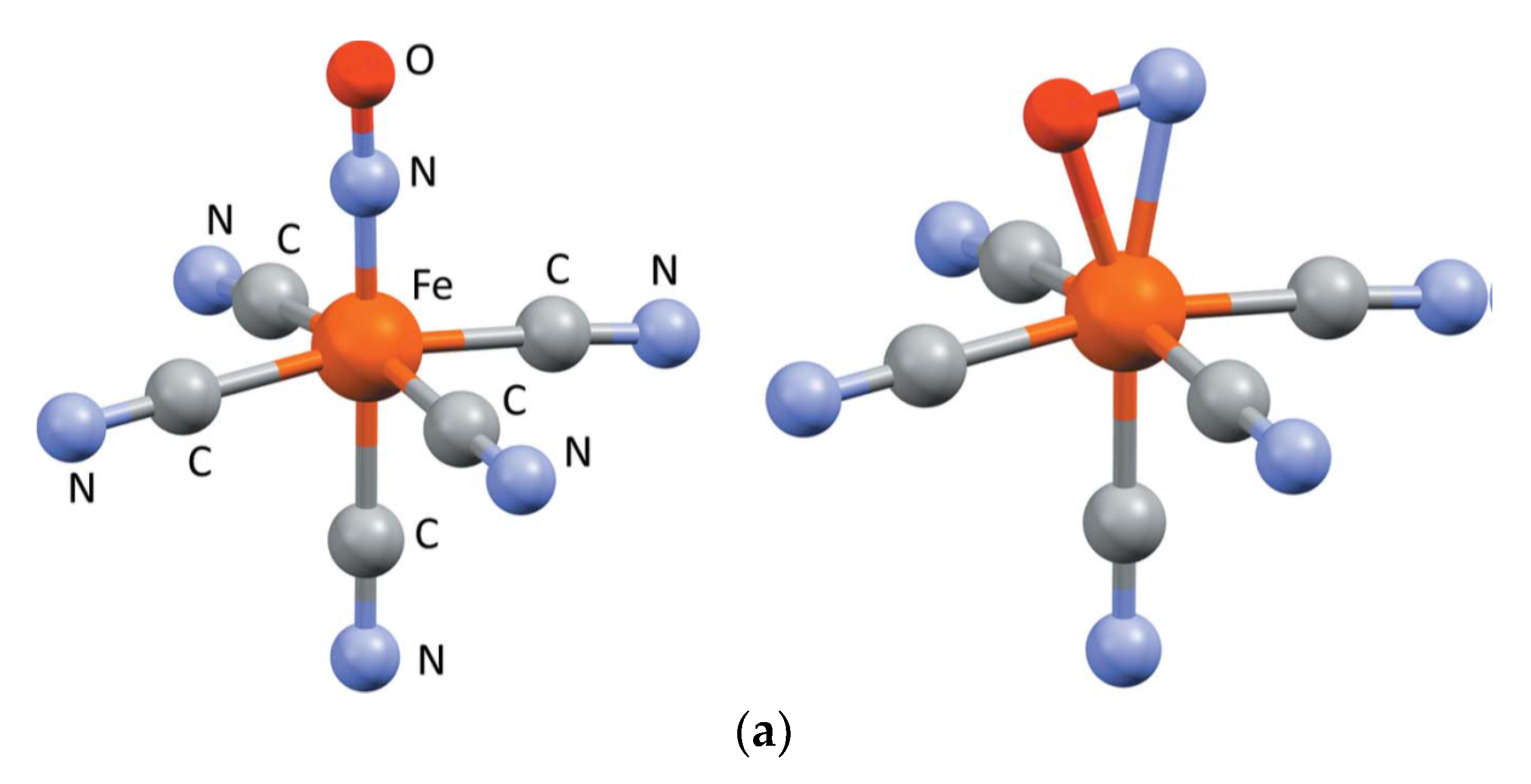
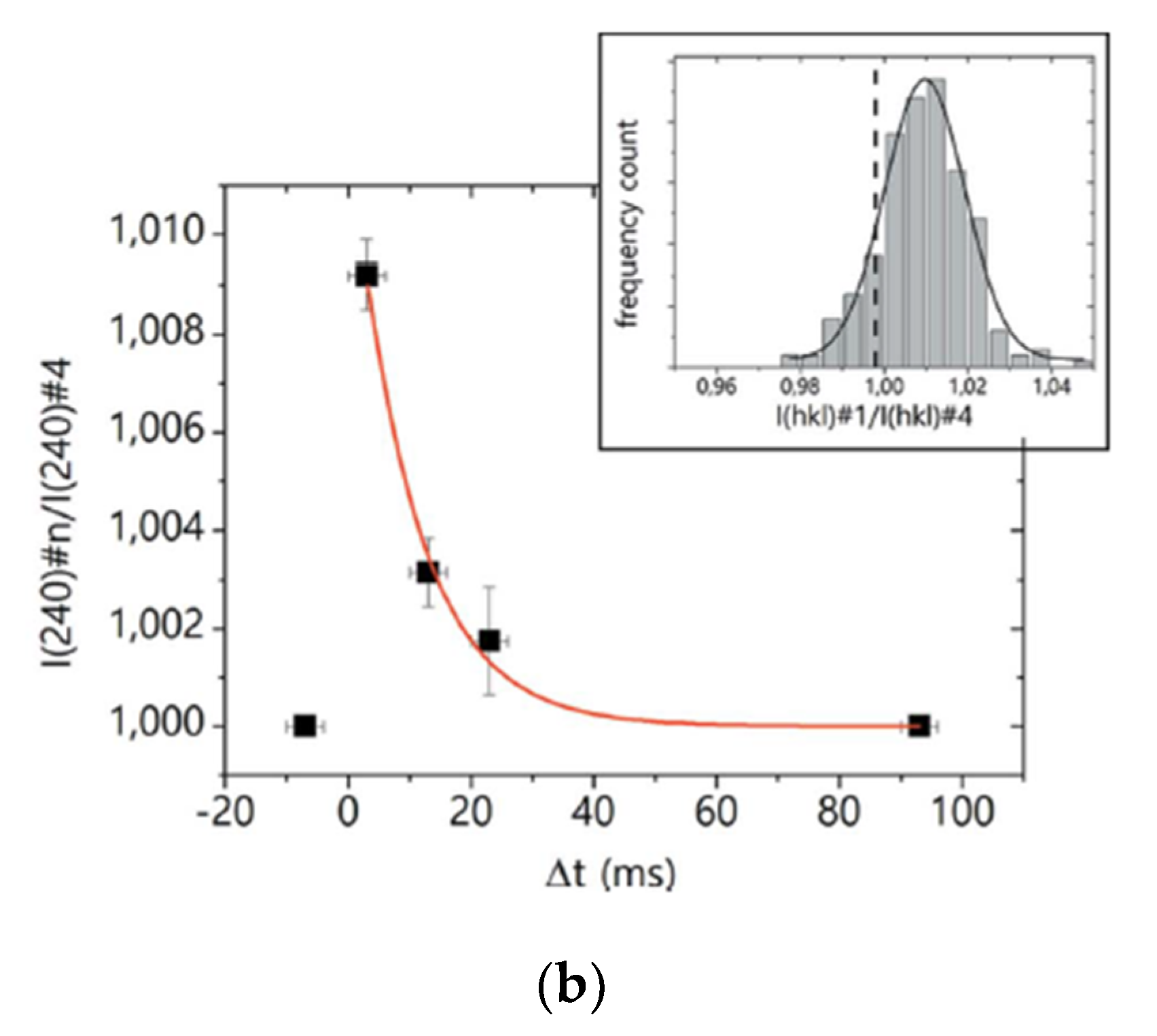

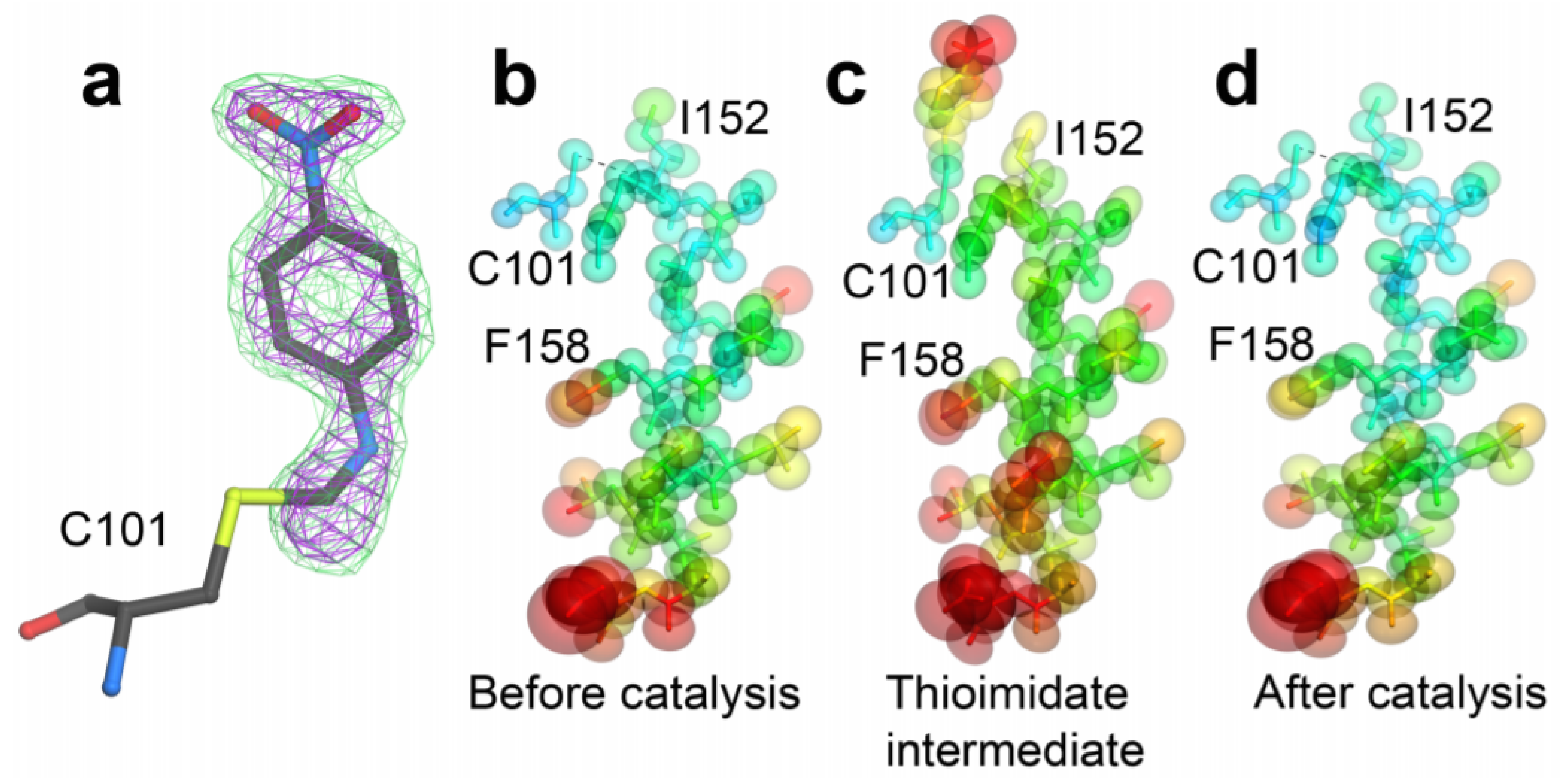
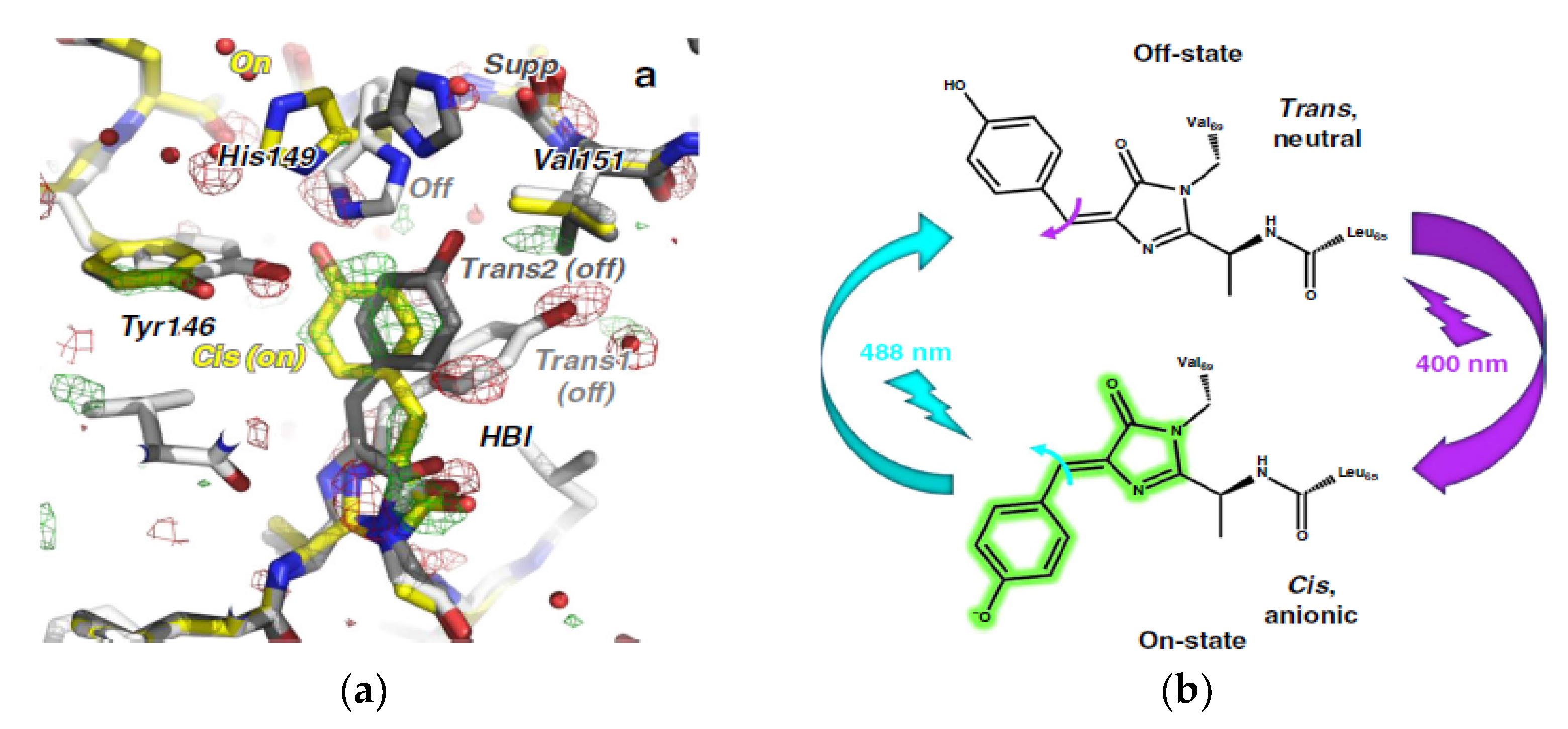
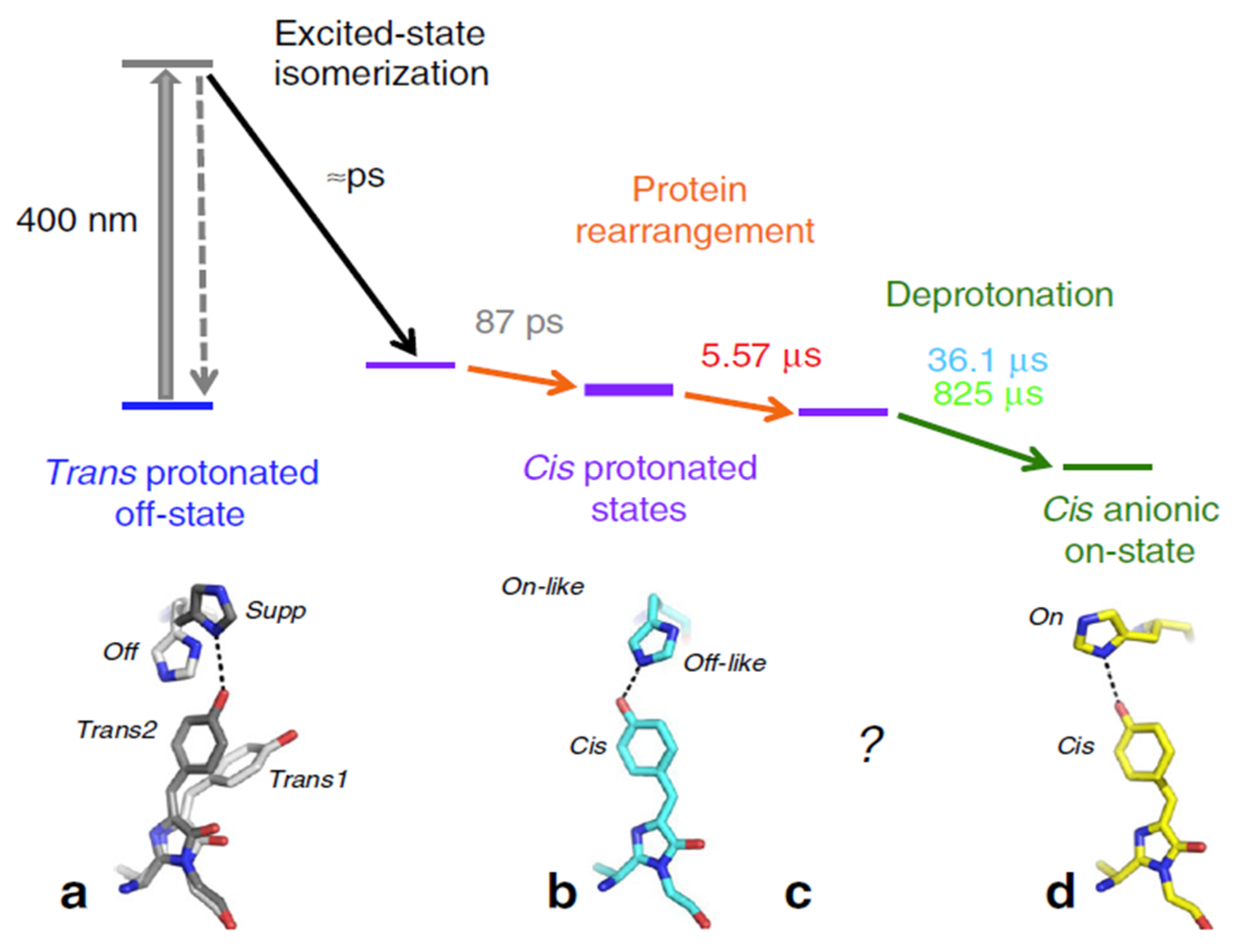
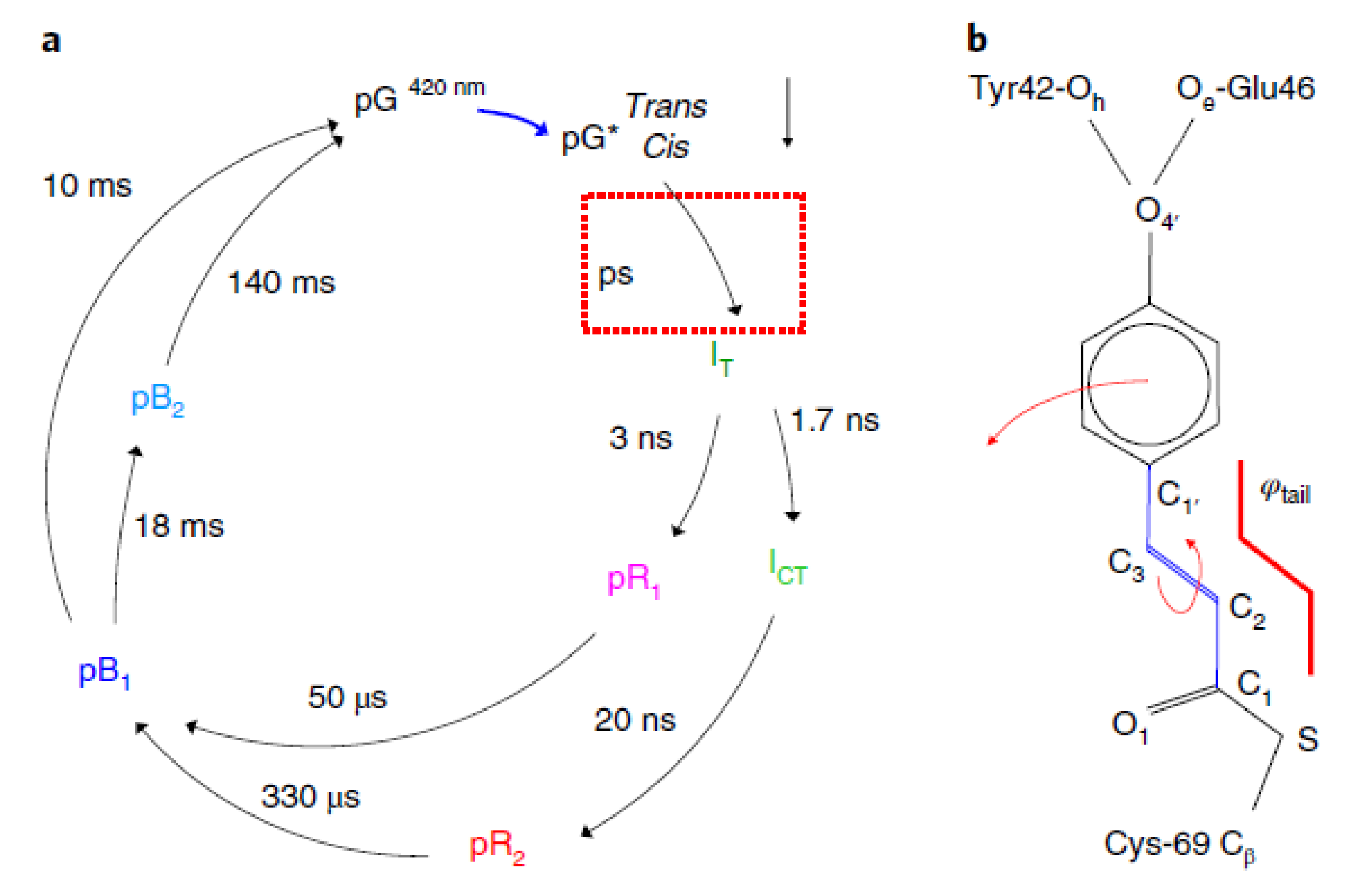
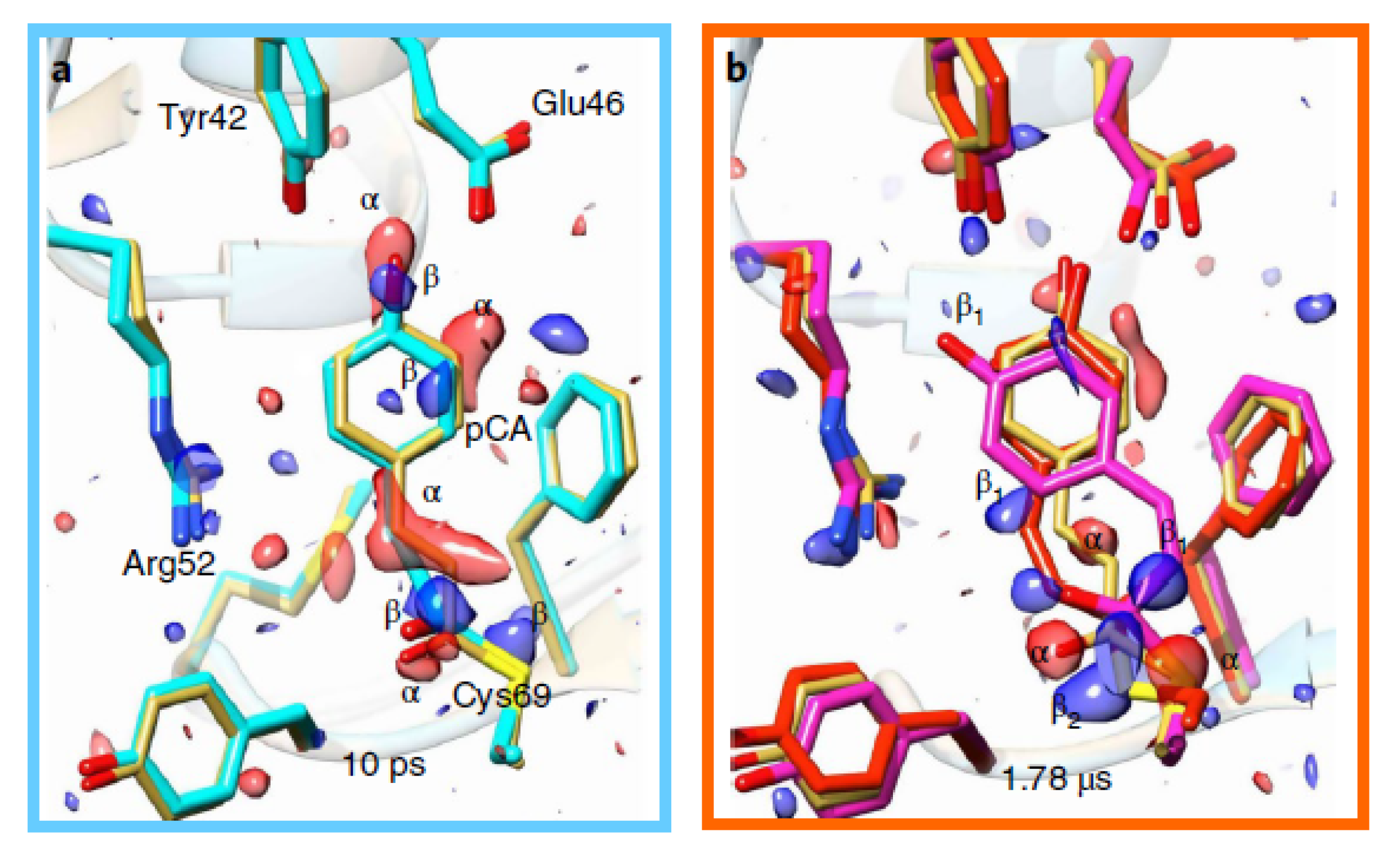
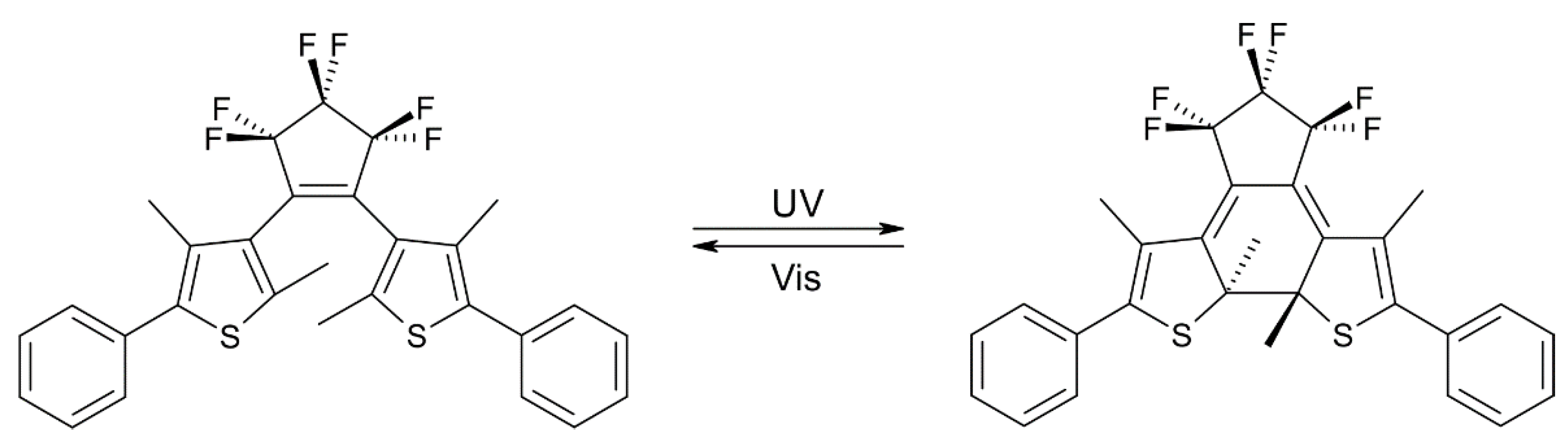

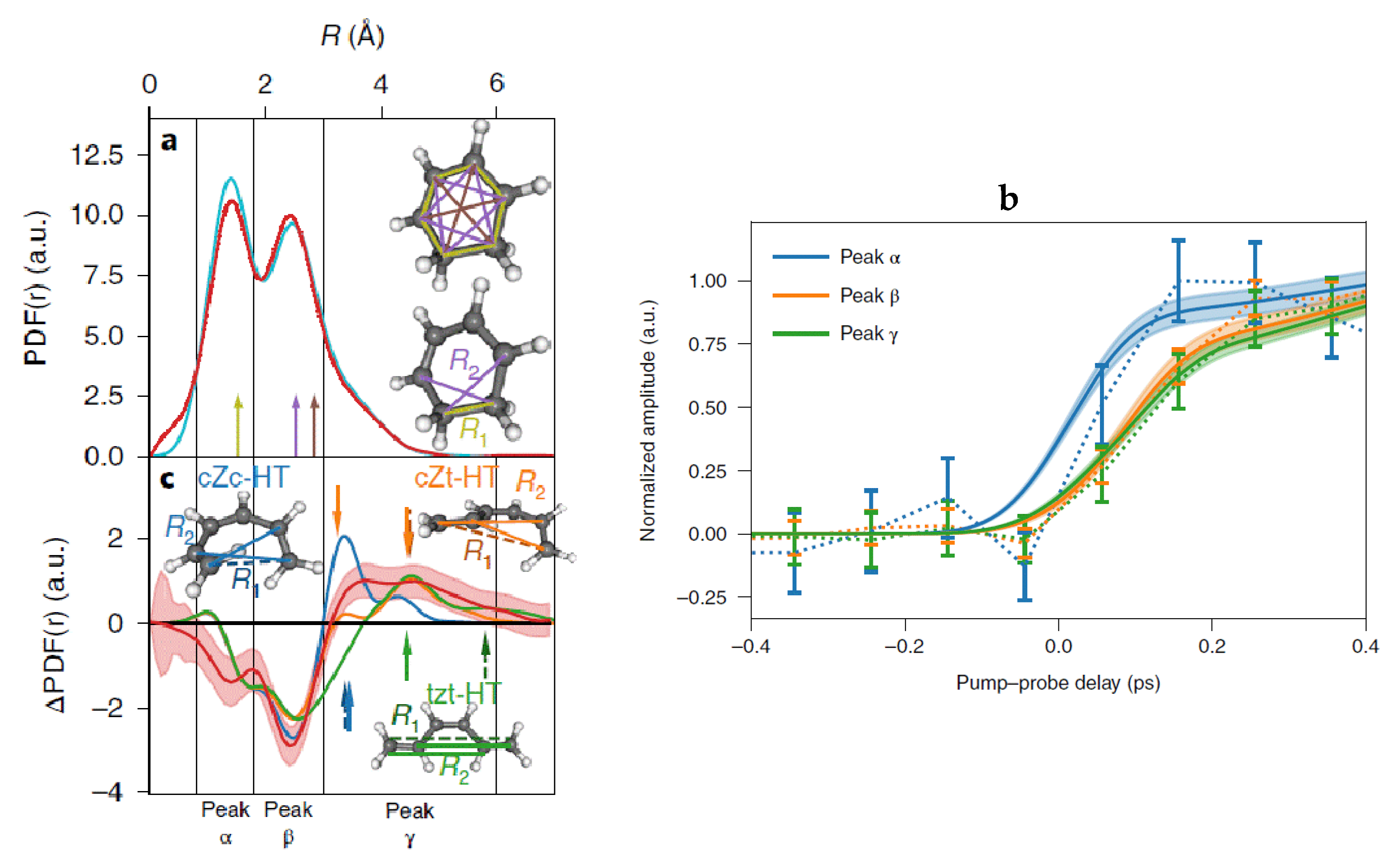



| Beamline Name | BioCARS 14-ID | ID09 | NW14A |
|---|---|---|---|
| Synchrotron source | APS, Chicago, USA | ESRF, Grenoble, France | PR-AR, Tsukuba, Japan |
| Storage ring energy | 7.0 GeV | 6.0 GeV | 6.5 GeV |
| Source type | Two undulators with 23 and 27 mm periods | Two undulators with 46 and 17 mm periods | Two undulators with 20 and 36 mm periods |
| Mirrors | Pair of Kirkpatrick–Beatz mirrors | Toroidal mirror | Cylindrical mirror |
| Monochromator | Cryogenically cooled Si (111) | ||
| Energy range | 6.8–19 keV | 8.5−28.0 keV | 5−20 keV |
| Photons per pulse | 1.1−4.2 × 1010 | 1.1 × 1010 | 1 × 109 |
| Focal spot size | 90 µm (h) × 20 µm (v) | 100 µm (h) × 60 µm (v) | 60 µm (h) × 60 µm (v) |
| X-ray pulse width | ~100 ps | ||
| Detectors a | Rayonix MX340-HS, b MarCCC 165 c | Rayonix MX170-HS | MarCCC 165, d Rigaku IP c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deresz, K.A.; Łaski, P.; Kamiński, R.; Jarzembska, K.N. Advances in Diffraction Studies of Light-Induced Transient Species in Molecular Crystals and Selected Complementary Techniques. Crystals 2021, 11, 1345. https://doi.org/10.3390/cryst11111345
Deresz KA, Łaski P, Kamiński R, Jarzembska KN. Advances in Diffraction Studies of Light-Induced Transient Species in Molecular Crystals and Selected Complementary Techniques. Crystals. 2021; 11(11):1345. https://doi.org/10.3390/cryst11111345
Chicago/Turabian StyleDeresz, Krystyna A., Piotr Łaski, Radosław Kamiński, and Katarzyna N. Jarzembska. 2021. "Advances in Diffraction Studies of Light-Induced Transient Species in Molecular Crystals and Selected Complementary Techniques" Crystals 11, no. 11: 1345. https://doi.org/10.3390/cryst11111345
APA StyleDeresz, K. A., Łaski, P., Kamiński, R., & Jarzembska, K. N. (2021). Advances in Diffraction Studies of Light-Induced Transient Species in Molecular Crystals and Selected Complementary Techniques. Crystals, 11(11), 1345. https://doi.org/10.3390/cryst11111345






