Comparison of Mechanical and Microstructural Properties of as-Cast Al-Cu-Mg-Ag Alloys: Room Temperature vs. High Temperature
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
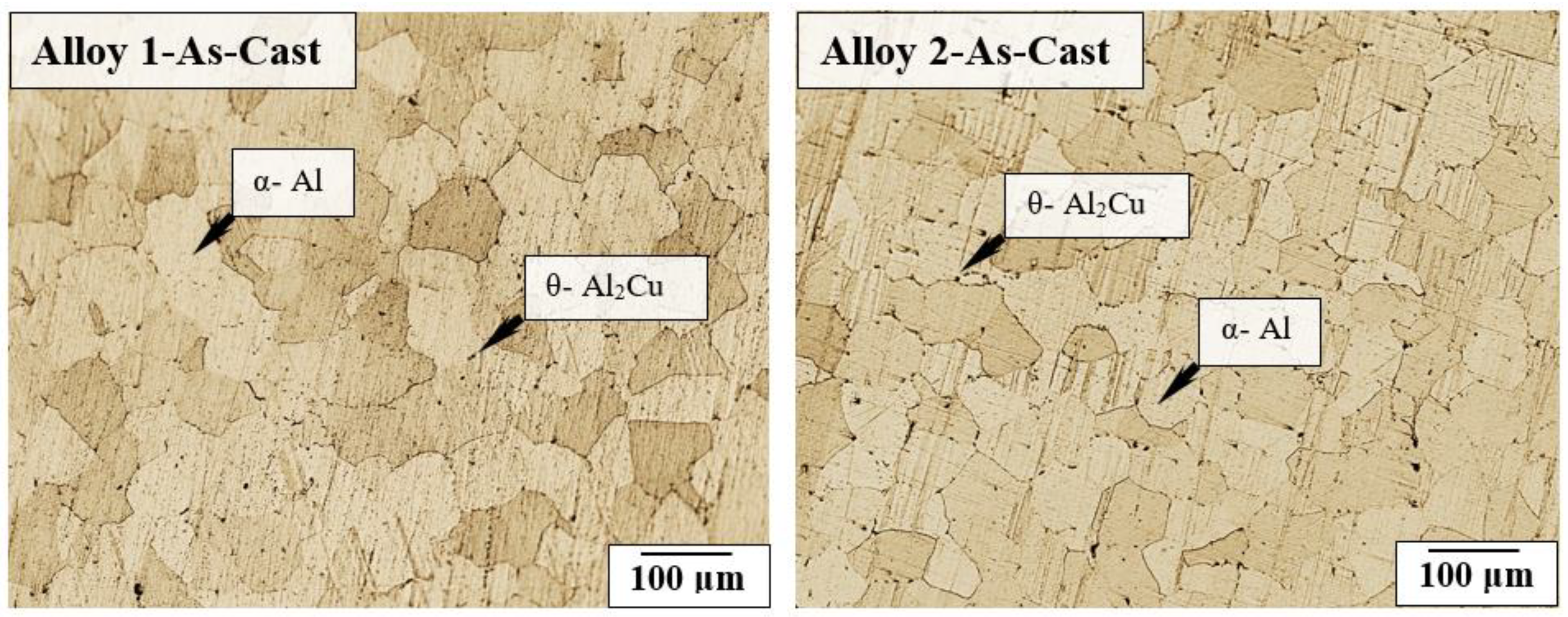
3.1. Effect of Cu/Mg Ratio on the As-Cast Microstructure
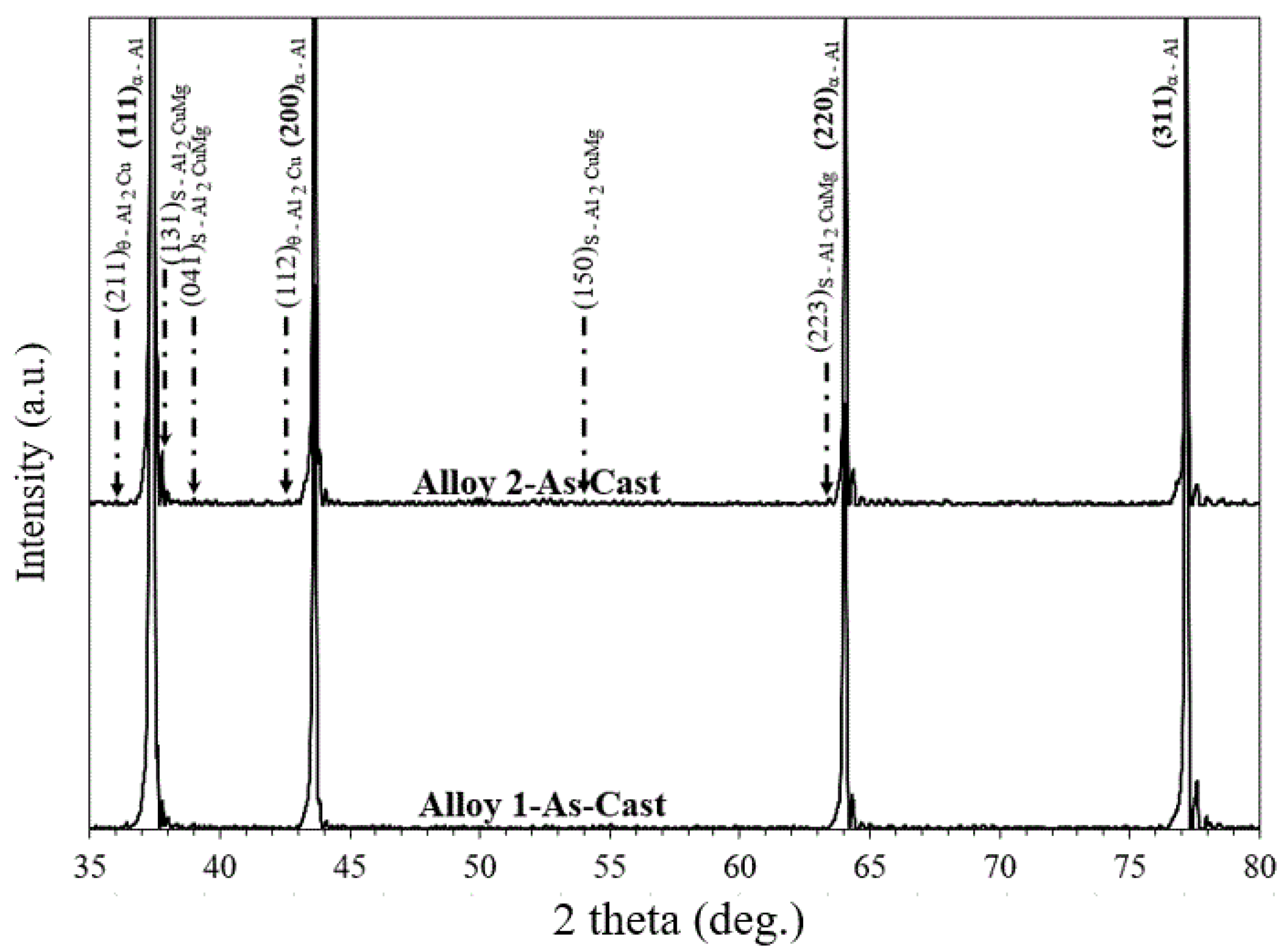
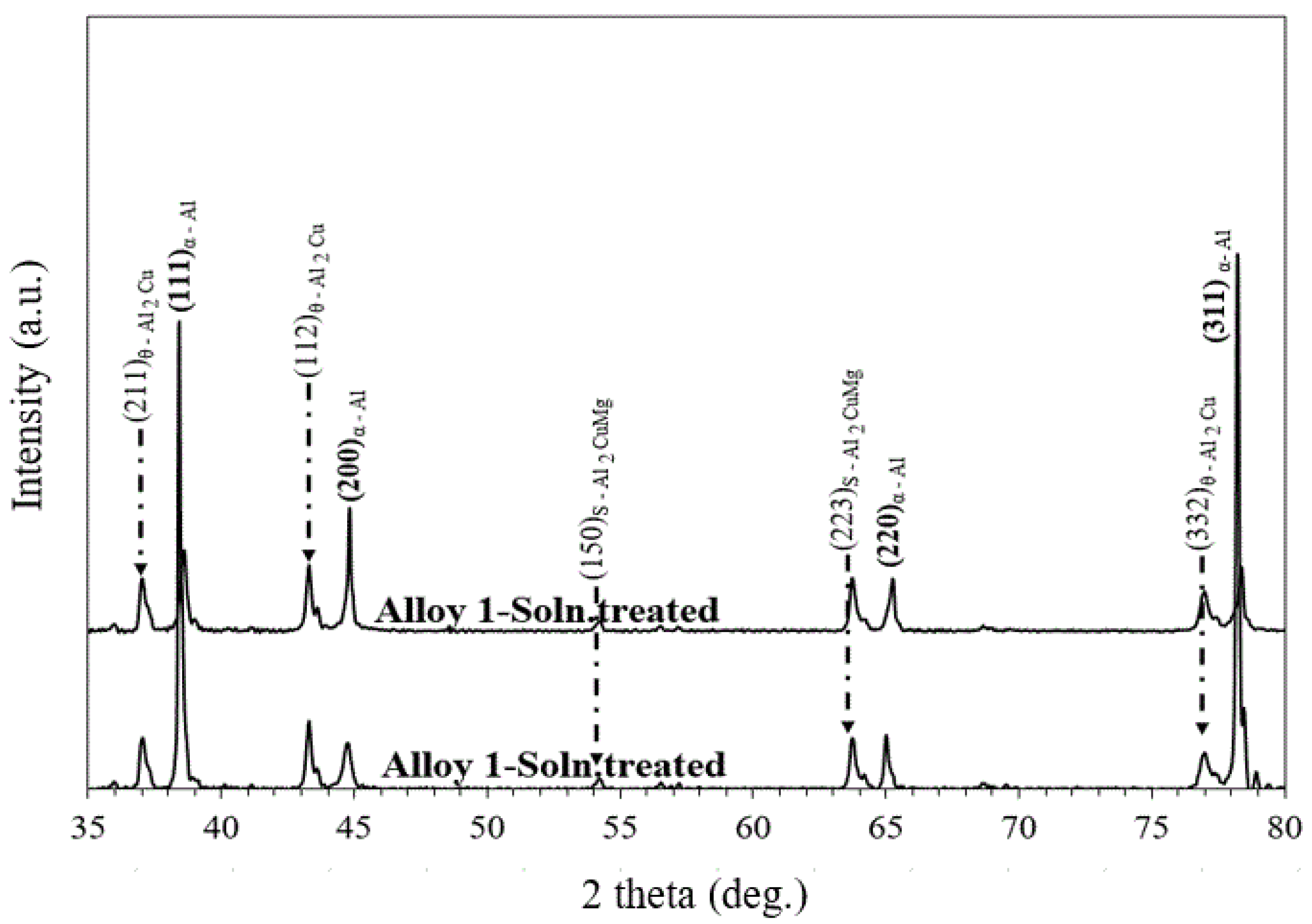
3.2. Effect of Cu/Mg Ratio on Phase Constitution of As-Cast Solution-Treated Alloys
3.3. Effect of Cu/Mg Ratio on the Age-Hardening Behavior
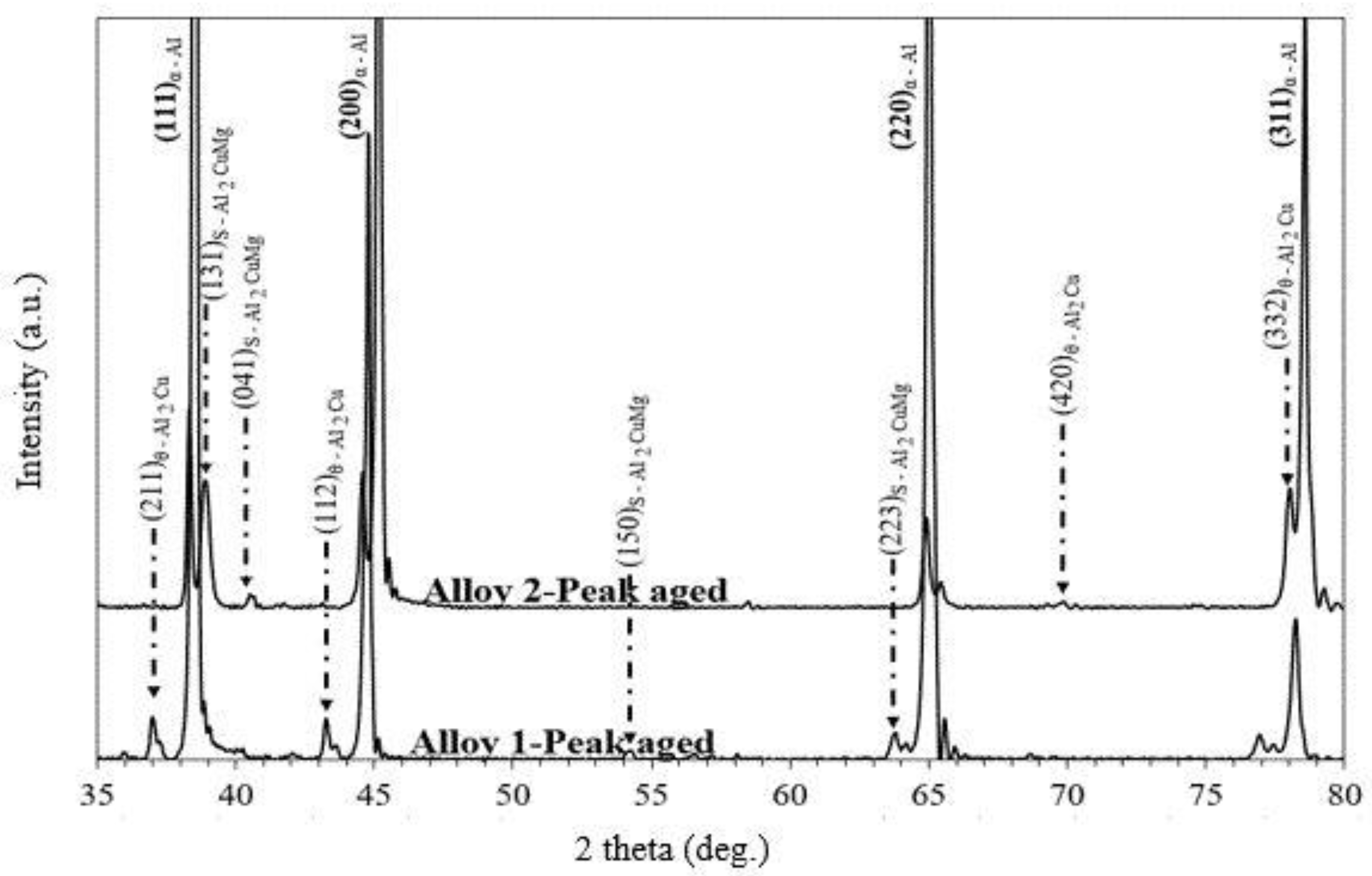
3.4. Effect of Cu/Mg Ratio on Phase Constitution of Peak-Aged Alloys
3.5. Effect of Cu/Mg Ratio on the Microstructure Evolution of Peak Aged Alloys
3.6. Effect of Cu/Mg Ratio on the Room-Temperature Mechanical Properties of Peak-Aged Alloys
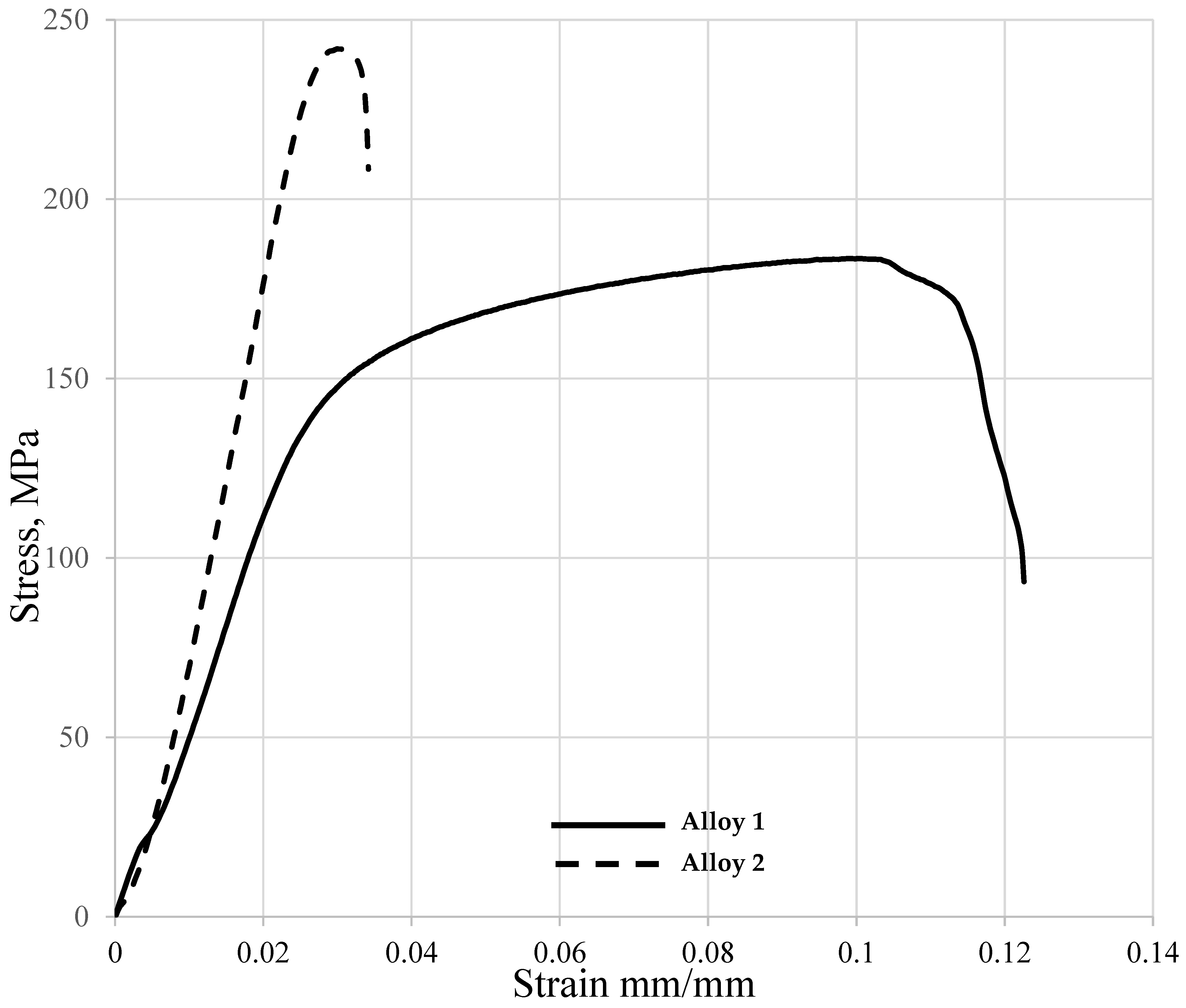
3.7. Effect of Cu/Mg Ratio on the High-Temperature Mechanical Properties of Peak-Aged Alloys
4. Conclusions
- From XRD profiles, it was found that the Al2Cu phase was more denominated in the higher Cu/Mg ratio alloy (Alloy 1) when compared to the lower Cu/Mg ratio alloy (Alloy 2).
- The peak intensity of Alloy 2 after ageing treatment showed stronger intensity and larger grain size, which is attributed to lower pinning pressure of the second phase particles.
- The mechanical properties were evidently sensitive to the testing environment and possible microstructure developments, which was manifested in high tensile strength of Alloy 2 at 180 °C.
- Increasing the Cu/Mg ratio was advantageous to the mechanical properties at room temperature for the peak-aged specimens but decreased the mechanical strength properties at 180 °C. The high Cu/Mg ratio alloy had an ultimate tensile strength of 224 MPa at room temperature, which decreased to 183 MPa at 180 °C.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cole, C.S.; Sherman, A.M. Lightweight materials for automotive applications. Mater. Charact. 1995, 35, 3–9. [Google Scholar] [CrossRef]
- Miller, W.S.; Zhuang, L.; Bottema, J.; Wittebrood, A.; de Smet, P.; Haszler, A.; Vieregge, A.J.M.S. Recent development in aluminum alloys for the automotive industry. Mater. Sci. Eng. A 2000, 280, 37–49. [Google Scholar] [CrossRef]
- Goehler, D.D. Procedings of Innovations and Advancements in Aluminum Casting Technology—AFS Special Conference, City of Industry, CA, 1988; American Foundarymen’s Society: Des Plaines, IL, USA, 1998; pp. 103–106. [Google Scholar]
- Palazzo, F. The future of aluminum in the automotive industry. Aluminio 1977, 46, 323–334. [Google Scholar]
- Frid lyander, I.N. Aluminum alloys in aircraft in the periods of 1970–2000 and 2001–2015. Met. Sci. Heat Treat. 2001, 43, 6–10. [Google Scholar] [CrossRef]
- Spada, A.T. Search of lightweight components: Automotive cast aluminum conversion. Eng. Cast. Solut. 2002, 4, 28–31. [Google Scholar]
- Hosford, W.F.; Fleischer, R.L.; Backofen, W.A. Tensile deformation of aluminium single crystals. Acta Met. 1960, 8, 187–199. [Google Scholar] [CrossRef]
- Dumoulin, S.; Tabourot, L. Experimental data on aluminium single crystals behaviour. Proc. IMechE Part C J. Mech. Eng. Sci. 2005, 219, 1159. [Google Scholar]
- Moszczyńska, D.; Adamczyk-Cieślak, B.; Osiak, B.; Lipiec, R.; Kulczyk, M.; Mizera, J. Microstructure and texture development in a polycrystal and different aluminium single crystals subjected to hydrostatic extrusion. Bull. Mater. Sci. 2019, 42, 110. [Google Scholar] [CrossRef] [Green Version]
- Filippov, P.; Koch, U. Nanoindentation of aluminum single crystals: Experimental study on influencing factors. Materials 2019, 12, 3688. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, J.G.; Rooy, E.L. Aluminum Alloy Castings: Properties, Processes, and Applications; ASM International, Materials Park: Novelty, OH, USA, 2004. [Google Scholar]
- Yang, C.; Zhu, K.; Liu, Y.; Cai, Y.; Liu, W.; Zhang, K.; Huang, J. A comparative study of fatigue energy dissipation of additive manufactured and cast AlSi10Mg alloy. Metals 2021, 11, 1274. [Google Scholar] [CrossRef]
- So, H.; Won, S.; Park, J.; Ju Oh, S.; Kang, L.; Kim, K. Mechanical properties and microstructural evolution in Al–Cu–Mg–Ag alloy with a CuxMgx/10 content. Mater. Sci. Eng. A 2021, 824, 141573. [Google Scholar] [CrossRef]
- Huda, Z.; Taib, N.; Zaharinie, T. Characterization of 2024-T3: An aerospace aluminum alloy. Mater. Chem. Phys. 2009, 113, 515–517. [Google Scholar] [CrossRef]
- Wang, Q.G. Microstructural effects on the tensile and fracture behavior of aluminum casting alloys A356/357. Metall. Mater. Trans. A 2003, 34, 2887–2899. [Google Scholar] [CrossRef]
- Gazizov, M.; Kaibyshev, R. Precipitation structure and strengthening mechanisms in an Al-Cu-Mg-Ag alloy. Mater. Sci. Eng. A 2017, 702, 29–40. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gaunt, P.; Chaturvedi, M.C. The crystallography and morphology of the S’-phase precipitate in an Al(CuMg) alloy. Philos. Mag. A 1987, 55, 375–387. [Google Scholar] [CrossRef]
- Alshammari, T.T.; Alharbi, H.F.; Soliman, M.S.; Ijaz, M.F. Effects of Mg content on the microstructural and mechanical properties of Al-4Cu-xMg-0.3Ag alloys. Crystals 2020, 10, 895. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Muhammad, J.A.; Soliman, M.S. Alloying elements effects on electrical conductivity and mechanical properties of newly fabricated Al based alloys produced by conventional casting process. Materials 2021, 14, 3971. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiao, W.L.; Ge, S.J.; Zhao, W.T.; Hanada, S.; Ma, C.L. Effects of Cu content and Cu/Mg ratio on the microstructure and mechanical properties of Al-Si-Cu-Mg alloys. J. Alloys Compd. 2015, 649, 291–296. [Google Scholar] [CrossRef]
- Bakavos, D.; Prangnell, P.B.; Bes, B.; Eberl, F. The effect of silver on microstructuralevolution in two 2xxx series Al-alloys with a high Cu:Mg ratio during ageing to a T8 temper. Mater. Sci. Eng. 2008, A491, 214–223. [Google Scholar] [CrossRef]
- Gable, B.M.; Shiflet, G.J.; Starke, E.A. Alloy development for the enhanced stabilityof Ω precipitates in Al-Cu-Mg-Ag alloys. Metall. Mater. Trans. 2006, 37, 1091–1105. [Google Scholar] [CrossRef]
- Ringer, S.P.; Yeung, W.; Muddle, B.C.; Polmear, I.J. Precipitate stability in Al-Cu-MgAg alloys aged at high temperatures. Acta Metall. Mater. 1994, 42, 1715–1725. [Google Scholar] [CrossRef]
- Reich, L.; Murayama, M.; Hono, K. Evolution of Ω phase in an Al-Cu-Mg-Ag alloy-Athree-dimensional atom probe study. Acta Mater. 1998, 46, 6053–6062. [Google Scholar] [CrossRef]
- Hutchinson, C.R.; Fan, X.; Penyciik, S.J.; Shiflet, G.J. On the origin of the high coarsening resistance of Ω plates in Al-Cu-Mg-Ag alloys. Acta Mater. 2001, 49, 2827. [Google Scholar] [CrossRef]
- Taylor, J.A.; Parker, B.A.; Polmear, I.J. Precipitation in AI-Cu-Mg-Ag casting alloy. Met. Sci. 1978, 12, 478–482. [Google Scholar] [CrossRef]
- Teixeira, J.D.; Cram, D.G.; Bourgeois, L.; Bastow, T.J.; Hill, A.J.; Hutchinson, C.R. On the strengthening response of aluminum alloys containing shear-resistant plate shaped precipitates. Acta Mater. 2008, 56, 6109–6122. [Google Scholar] [CrossRef]
- Molina, R.; Amalberto, P.; Rosso, M. Mechanical characterization of aluminium alloys for high temperature applications. Part1: Al-Si-Cu alloys. Metall. Sci. Technol. 2011, 29, 5–15. [Google Scholar]
- Xiao, D.H.; Wang, J.N.; Ding, D.Y.; Yang, H.L. Effect of rare earth Ce addition on the microstructure and mechanical properties of an Al–Cu–Mg–Ag alloy. J. Alloy. Compd. 2003, 352, 84–88. [Google Scholar] [CrossRef]
- Shyam, A.; Roy, S.; Shin, D.; Poplawsky, J.D.; Allard, L.F.; Yamamoto, Y.; Morris, J.R.; Mazumder, B.; Idrobo, J.C.; Rodriguez, A.; et al. Elevated temperature microstructural stability in cast AlCuMnZr alloys through solute segregation. Mater. Sci. Eng. 2019, A765, 138279. [Google Scholar] [CrossRef]
- Gao, Y.H.; Yang, C.; Zhang, J.Y.; Cao, L.F.; Liu, G.; Sun, J.; Ma, E. Stabilizing nano precipitates in Al-Cu alloys for creep resistance at 300 degrees C. Mater. Res. Lett. 2019, 7, 18–25. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.; Huang, T.; Xia, P.; Li, F. Enhanced fracture toughness in an annealed Al-Cu-Mg alloy by increasing Goss/Brass texture ratio. Mater. Charact. 2016, 119, 47–54. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.; Cao, L.; Luo, J.; Zhang, J.; Zhuang, L. Effect of heating rate on mechanical property, microstructure and texture evolution of Al-Mg-Si-Cu alloy during solution treatment. Mater. Sci. Eng. A 2015, 621, 8–17. [Google Scholar] [CrossRef]
- Buken, H.; Kozeschnik, E. Modeling static recrystallization in Al-Mg Alloys. Metall. Mater. Trans. A 2021, 52, 544–552. [Google Scholar] [CrossRef]
- Mei, Z.; Liu, Z.; Bai, S.; Wang, J.; Cao, J. Effects of Yatrium addittions on microstructure and mechanical properties of cast Al-Cu-Mg alloys. J. Alloys Compd. 2021, 870, 159435. [Google Scholar] [CrossRef]
- Xie, H.; Zhao, J.; Cao, J.; Luo, L.; Guo, S.; Ou, L.; Liu, Z.; Bai, S. Effects of minor Er addittions on the microstructure and mechanical properties of cast Al-Cu-Mg-Ag alloys. Materials 2021, 14, 4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Bai, S.; Cao, J.; Zhao, J.; Luo, L.; Li, J. Microstructure evolution and mechanical properties of the electron-beam welded joints of cast Al-Cu-Mg-Ag alloy. Mater. Sci. Eng. 2021, A801, 140363. [Google Scholar] [CrossRef]
- Tiryakioglu, M.; Campbell, J. Ductility, structural quality, and fracture toughnessof Al-Cu-Mg-Ag (A201) alloy castings. Mater. Sci. Technol. 2009, 25, 784–789. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Liu, K.; Pan, L.; Breton, F.; Chen, X.G. Enhanced mechanical properties of high-temperature-resistant Al-Cu cast alloy by microalloying with Mg. J. Alloy. Compd. 2020, 827, 154305. [Google Scholar] [CrossRef]
- Zamani, M.; Toschi, S.; Morri, A.; Ceschini, L.; Seifeddine, S. Optimisation of heat treatment of Al-Cu-(Mg-Ag) cast alloys. J. Therm. Anal. Calorim. 2020, 139, 3427–3440. [Google Scholar] [CrossRef] [Green Version]
- Feng Li, J.; Ziqiao, Z.; Na, J.; Chengyu, T. Localized corrosion mechanism of 2×××-series Al alloy containing S(Al2CuMg) and θ (Al2Cu) precipitates in 4.0% NaCl solution at pH 6.1. Mater. Chem. Phys. 2005, 91, 325–329. [Google Scholar]
- Liu, Q.; Zhu, R.; Li, J.; Chen, Y.; Zhang, X.; Zhang, L.; Zheng, Z. Microstructure evolution of Mg, Ag and Zn micro-alloyed Al-Cu-Li alloy during homogenization. Trans. Nonferrous Met. Soc. China 2016, 26, 607–619. [Google Scholar] [CrossRef]
- García-Hernández, J.L.; Garay-Reyes, C.G.; Gómez-Barraza, I.K.; Ruiz-Esparza-Rodríguez, M.A.; Gutiérrez-Castañeda, E.J.; Estrada-Guel, I.; Maldonado-Orozco, M.C.; Martínez-Sánchez, R. Influence of plastic deformation and Cu/Mg ratio on the strengthening mechanisms and precipitation behavior of AA2024 aluminum alloys. J. Mater. Res. Technol. 2019, 8, 5471. [Google Scholar] [CrossRef]
- Deschamps, A.; Bastow, T.J.; de Geuser, F.; Hill, A.J.; Hutchinson, C.R. In situ evaluation of the microstructure evolution during rapid hardening of an Al-2.5Cu-1.5Mg (wt.%) alloy. Acta Mater. 2011, 59, 2918–2927. [Google Scholar] [CrossRef]
- Basu, I.; Pradeep, K.G.; Mieβen, C.; Barrales-Mora, L.A.; Al-Samman, T. The role of atomic scale segregation in designing highly ductile magnesium alloys. Acta Mater. 2016, 116, 77–94. [Google Scholar] [CrossRef]
- Gladman, T. On the theory of the effect of precipitate particles on grain growth in metals. Proc. R. Soc. Lond. A 1966, 294, 298–309. [Google Scholar]
- Humphreys, F.J. A unified theory of recovery recrystallization and grain growth, based on the stability and growth of cellular microstructures-II. The effect of second-phase particles. Acta Mater. 1977, 45, 5031–5039. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena; Pergamon: Oxford, UK; Elsevier: Amsterdan, The Netherlands, 2004. [Google Scholar]
- Koch, C.C.; Ovid’ko, I.A.; Seal, S.; Veprek, S. Structural Nanocrystalline Materials: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Nes, E.; Ryum, N.; Hunderi, O. On the zener drag. Acta Metall. 1985, 33, 11–22. [Google Scholar] [CrossRef]
- Takata, N.; Yoshida, F.; Ikeda, K.; Nikashima, H.; Abe, H. Abnormal grain growth of off-cube grains in high purity aluminum foils with cube texture. Mater. Trans. 2005, 46, 2975–2980. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Naguami, H.; Han, Y.; Zhang, G.; Zhai, T. The deformation behavior and microstructure evolution of a Mn- and Cr-containing Al-Mg-Si-Cu alloy during hot compression and subsequent heat treatment. Metall. Mater. Trans. A 2017, 48, 1355–1365. [Google Scholar] [CrossRef]
- Dennis, J.; Bate, P.S.; Humphreys, F.J. Abnormal grain growth in Al-3.5Cu. Acta Mater. 2009, 57, 4539–4547. [Google Scholar] [CrossRef]
- Sztwiertnia, K.; Bieda, M.; Korneva, A. Continuous and discontinuous recrystallization of 6013 aluminum alloy. Mater. Sci. Forum 2013, 753, 221–224. [Google Scholar] [CrossRef]
- Uttarasak, K.; Chongchitnan, W.; Matsuda, K.; Chairuangsri, T.; Kajornchaiyakul, J.; Banjongprasert, C. Evolution of fe-containing intermetallic phases and abnormal grain growth in 6063 aluminum alloy during homogenization. Results Phys. 2019, 15, 102535. [Google Scholar] [CrossRef]
- King, S.J.; Kim, Y.; Kim, M.; Zuo, J. Determination of interfacial atomic structure, misfits and energetics of Ω phase in Al–Cu–Mg–Ag alloy. Acta Mater. 2014, 81, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Ryen, Ø.; Holmedal, B.; Nijs, O.; Nes, E.; Sjölander, E.; Ekström, H.E. Strengthening mechanisms in solid solution aluminum alloys. Metall. Mater. Trans. A 2006, 37, 1999. [Google Scholar] [CrossRef]




| Alloys | Cu/Mg | Chemical Composition (wt.%) | |||
|---|---|---|---|---|---|
| Cu | Mg | Ag | Al | ||
| Alloy 1 | 12.6 | 2.89 | 0.23 | 0.65 | Balance |
| Alloy 2 | 6.3 | 2.96 | 0.47 | 0.65 | Balance |
| Mechanical Properties/Environment | Alloy 1 | Alloy 2 |
|---|---|---|
| UTS—[180 °C] | 183 | 242 |
| UTS—[RT] | 224 | 190 |
| Elongation to Fracture (ef %) [RT] | 4.5 | 3.9 |
| Elongation to Fracture (ef %) [180 °C] | 12.2 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ijaz, M.F.; Soliman, M.S.; Alasmari, A.S.; Abbas, A.T.; Hashmi, F.H. Comparison of Mechanical and Microstructural Properties of as-Cast Al-Cu-Mg-Ag Alloys: Room Temperature vs. High Temperature. Crystals 2021, 11, 1330. https://doi.org/10.3390/cryst11111330
Ijaz MF, Soliman MS, Alasmari AS, Abbas AT, Hashmi FH. Comparison of Mechanical and Microstructural Properties of as-Cast Al-Cu-Mg-Ag Alloys: Room Temperature vs. High Temperature. Crystals. 2021; 11(11):1330. https://doi.org/10.3390/cryst11111330
Chicago/Turabian StyleIjaz, Muhammad Farzik, Mahmoud S. Soliman, Ahmed S. Alasmari, Adel T. Abbas, and Faraz Hussain Hashmi. 2021. "Comparison of Mechanical and Microstructural Properties of as-Cast Al-Cu-Mg-Ag Alloys: Room Temperature vs. High Temperature" Crystals 11, no. 11: 1330. https://doi.org/10.3390/cryst11111330
APA StyleIjaz, M. F., Soliman, M. S., Alasmari, A. S., Abbas, A. T., & Hashmi, F. H. (2021). Comparison of Mechanical and Microstructural Properties of as-Cast Al-Cu-Mg-Ag Alloys: Room Temperature vs. High Temperature. Crystals, 11(11), 1330. https://doi.org/10.3390/cryst11111330









