Influence of Rubber Powder Modification Methods on the Mechanical and Durability Properties of Rubberized Magnesium Oxychloride Cement
Abstract
:1. Introduction
2. Raw Materials and Test Method
2.1. Raw Materials
2.2. Sample Preparation
2.2.1. Modification of Rubber Powders
2.2.2. Preparation of Cement Specimens
2.3. Experiment Methods
2.3.1. Hydrophilic Performance Test
2.3.2. Mechanical Properties Tests
2.3.3. Freeze-Thaw Durability Test
2.3.4. Microstructural Properties
3. Experiment Results and Discussions
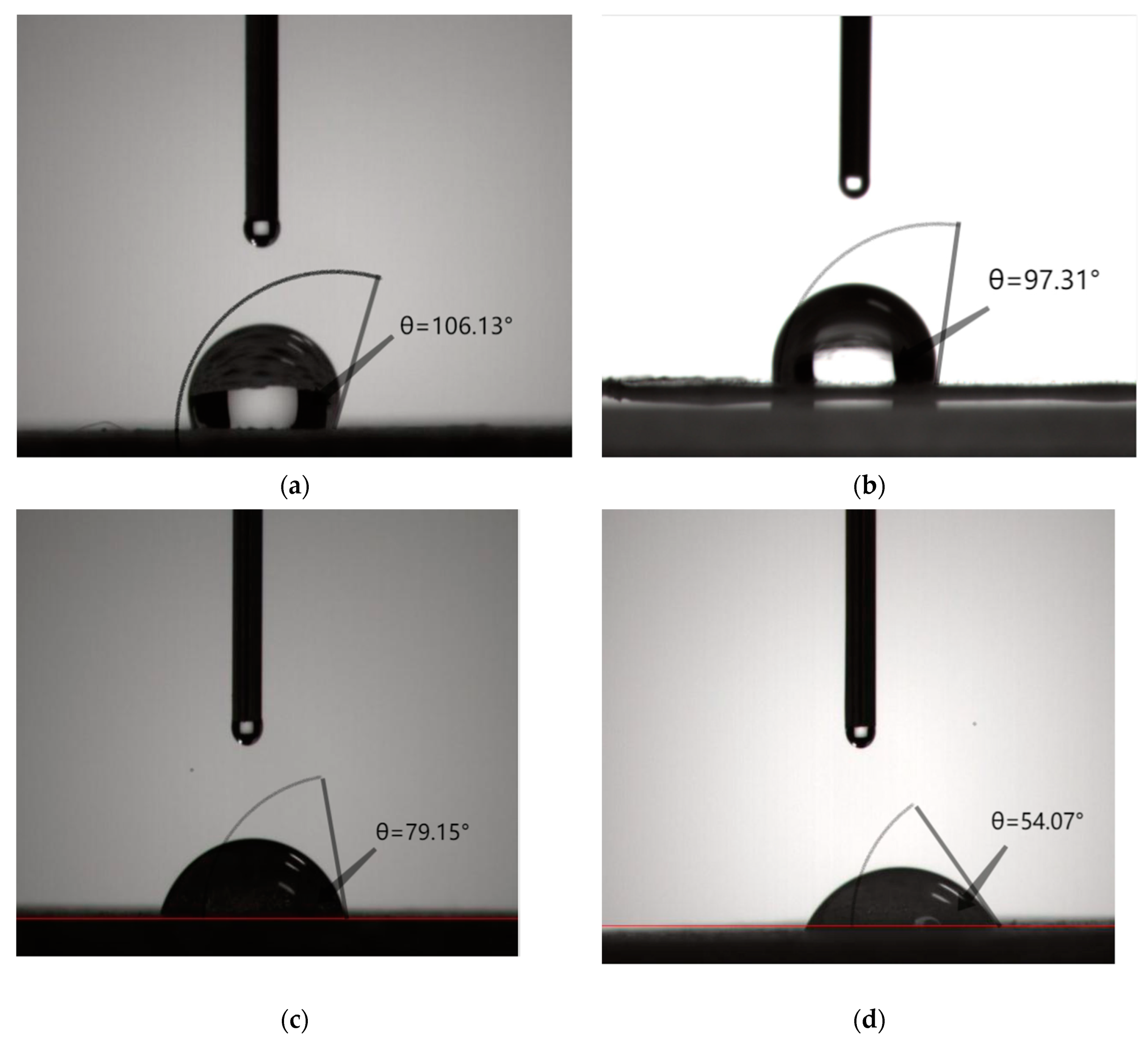
3.1. Measurement of Contact Angle
3.2. Mechanical Properties
3.2.1. Flexural Strength
3.2.2. Compressive Strength
3.3. Frost Resistance
3.3.1. Apparent Analysis
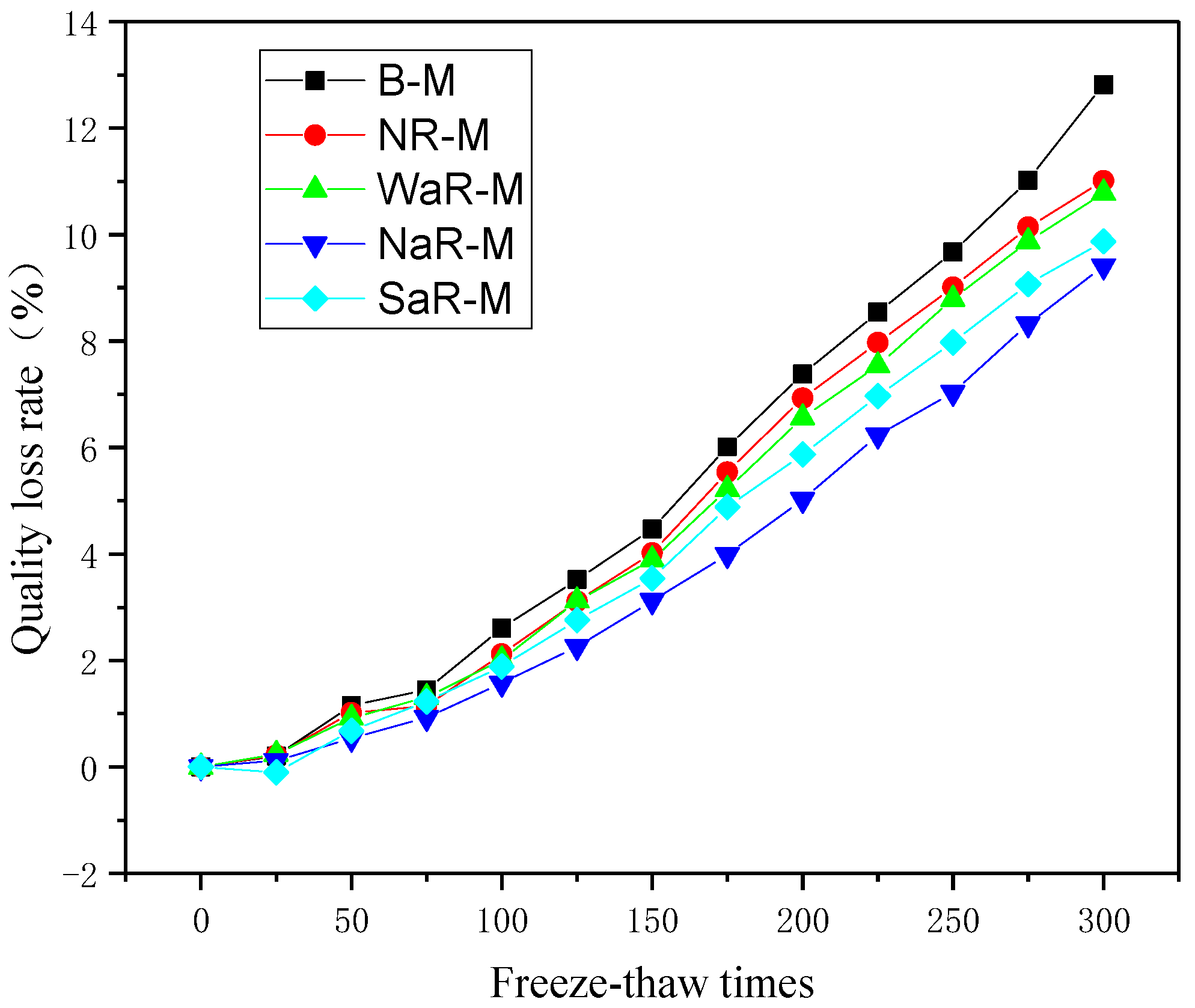
3.3.2. Mass Loss Rate
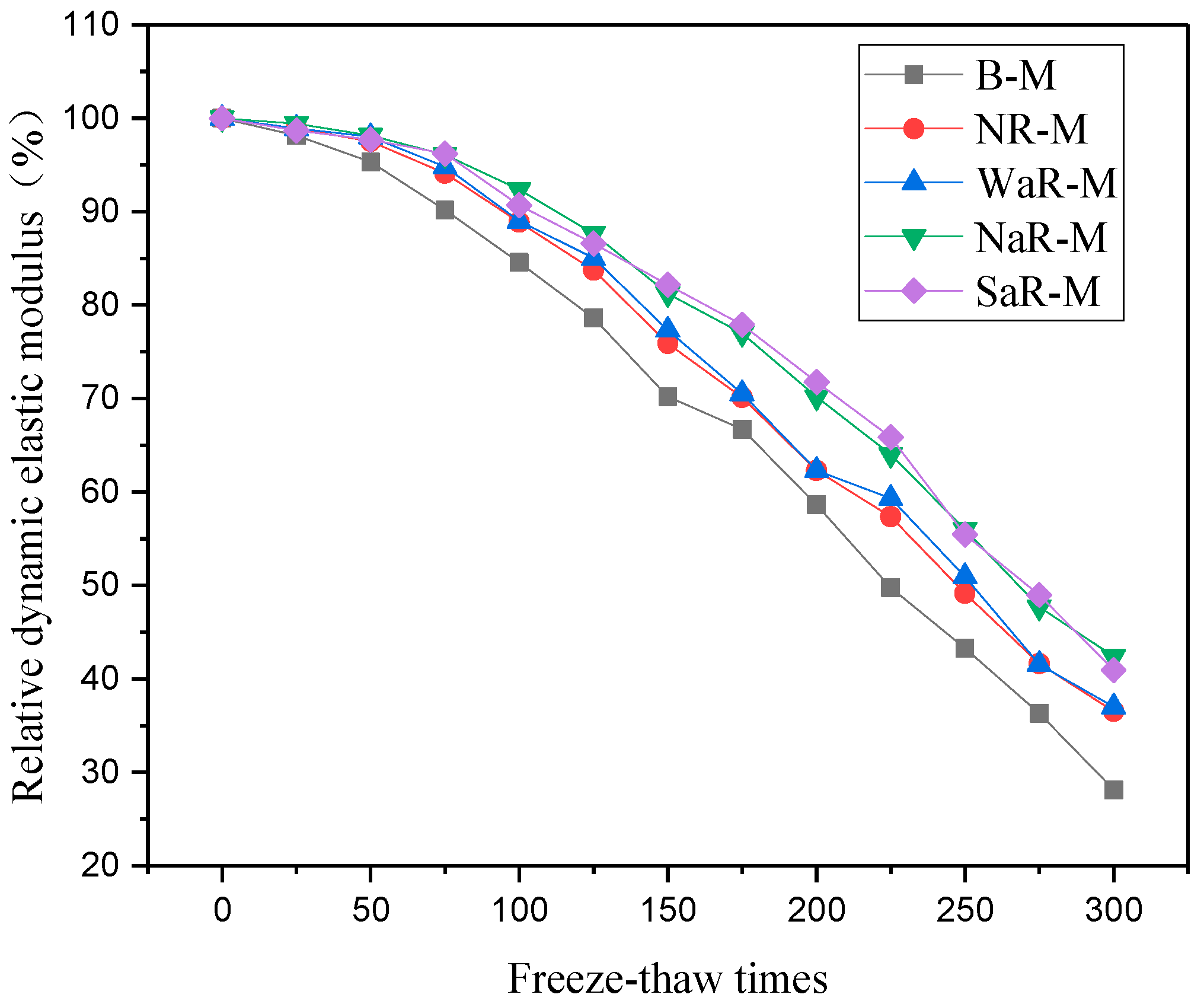
3.3.3. Relative Dynamic Modulus of Elasticity
4. Microstructure Analysis
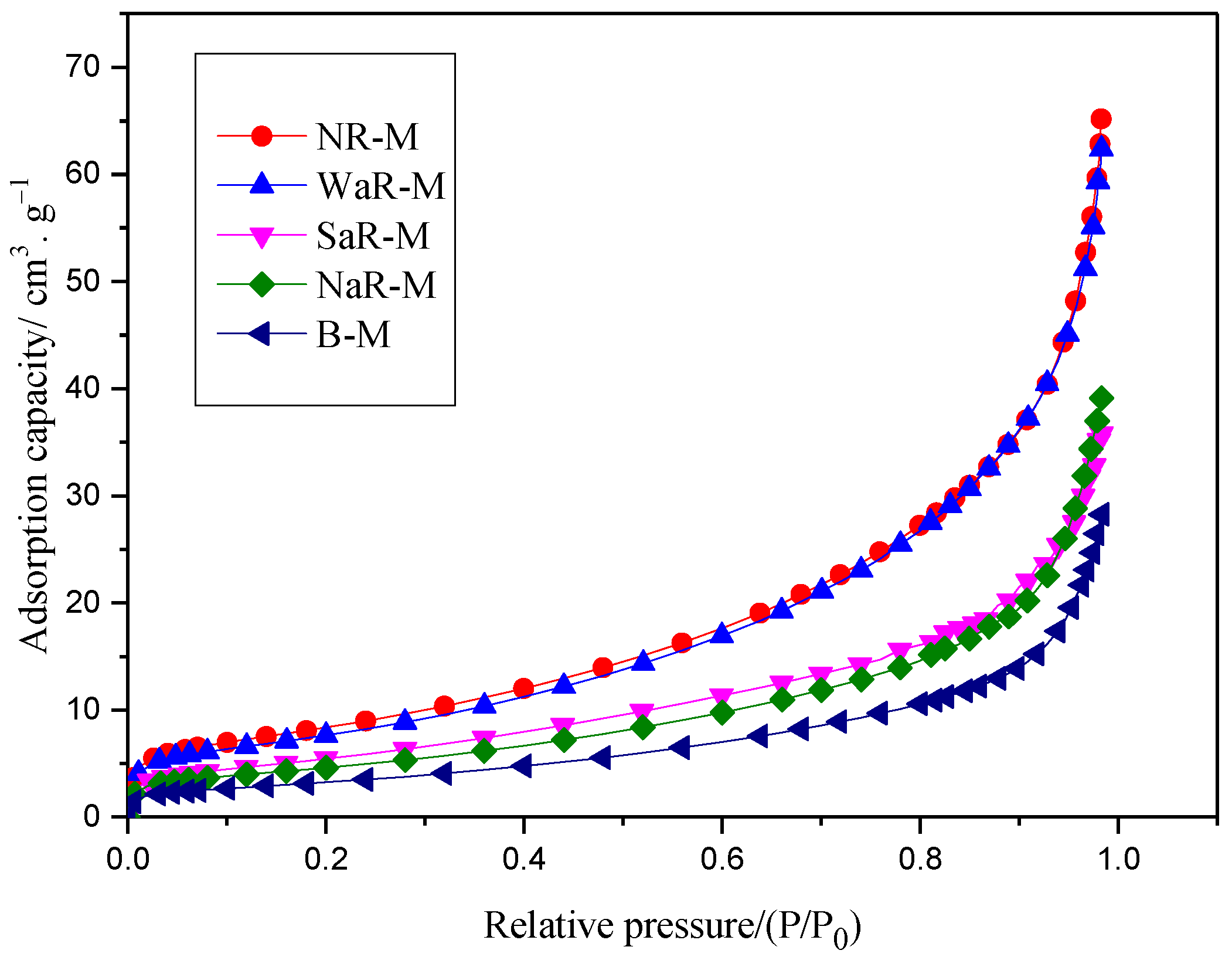
4.1. Pore Structure
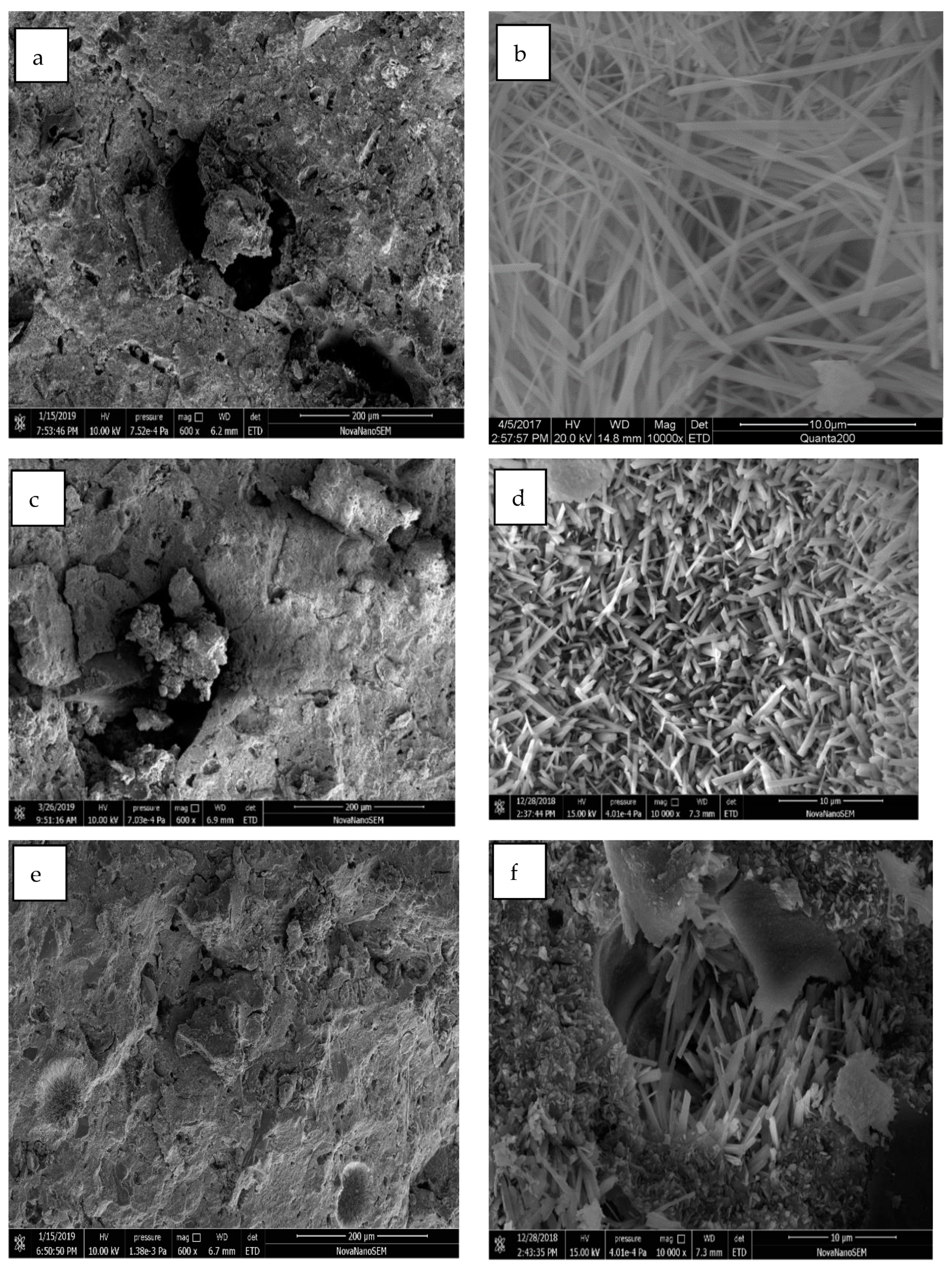
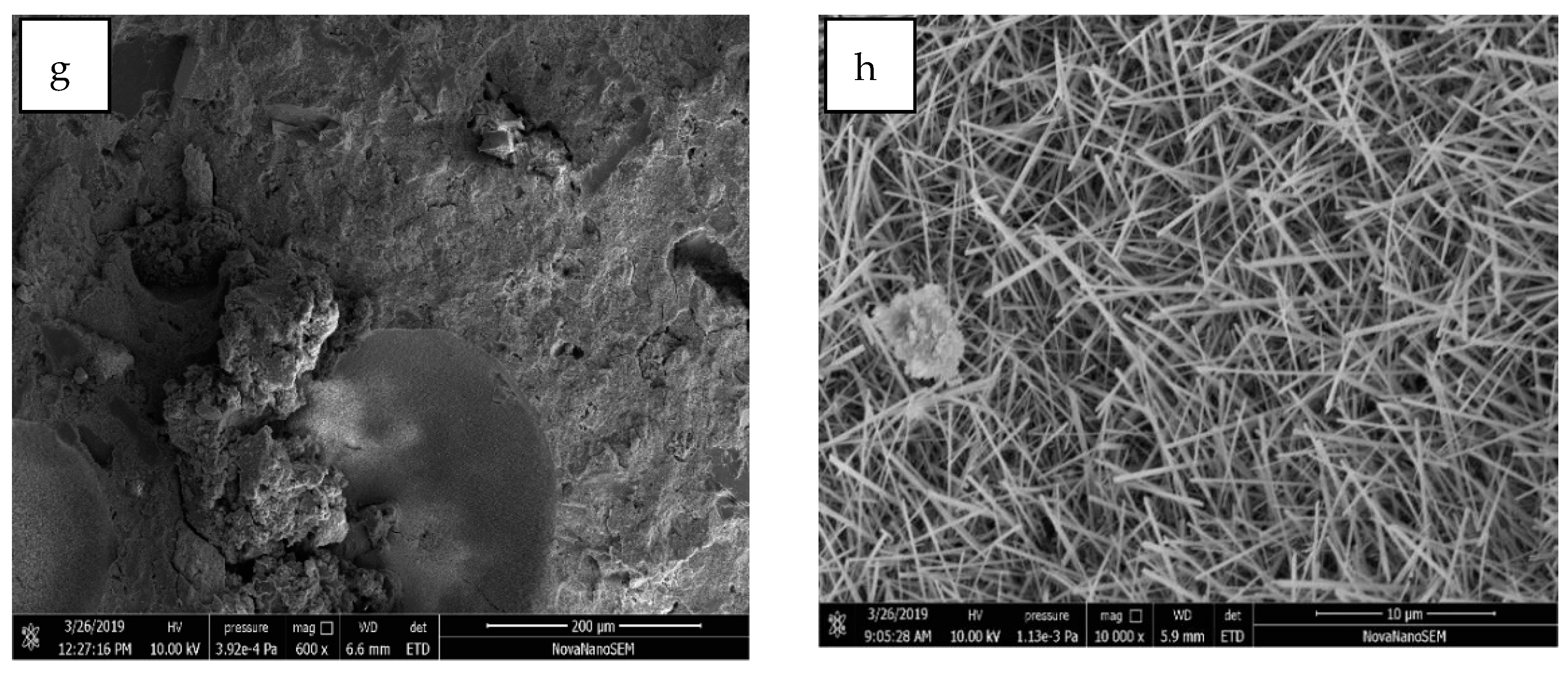
4.2. SEM Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahab, S.N.; Mo, K.H.; Yap, S.P.; Yang, T.; Ling, T.-C. Lightweight foamed concrete as a promising avenue for incorporating waste materials: A review. Resour. Conserv. Recycl. 2021, 164, 103–105. [Google Scholar]
- Abed, S.M.; Hadi, R.A.; Khaliefa, H.J. Improving the mechanical properties of lightweight foamed concrete using various types of fibres. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 12–29. [Google Scholar] [CrossRef]
- Zheng, W.; Xiao, X.; Wen, J.; Chang, C.; An, S.; Dong, J. Water-to-Cement Ratio of Magnesium Oxychloride Cement Foam Concrete with Caustic Dolomite Powder. Sustainability 2021, 13, 2429. [Google Scholar] [CrossRef]
- Aiken, T.A.; Russell, M.; McPolin, D.; Bagnall, L. Magnesium oxychloride boards: Understanding a novel building material. Mater. Struct. 2020, 53, 491–500. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Yao, N.; Zhang, A. Recent progress of magnesium oxychloride cement: Manufacture, curing, structure and performance. Constr. Build. Mater. 2020, 255, 119381. [Google Scholar] [CrossRef]
- Yu, K.; Guo, Y.; Zhang, Y.X.; Soe, K. Magnesium oxychloride cement-based strain-hardening cementitious composite: Mechanical property and water resistance. Constr. Build. Mater. 2020, 261, 119970. [Google Scholar] [CrossRef]
- Li, G.; Yu, Y.; Li, J.; Wang, Y.; Liu, H. Experimental study on urban refuse/magnesium oxychloride cement compound floor tile. Cem. Concr. Res. 2003, 33, 1663–1668. [Google Scholar] [CrossRef]
- Misra, A.K.; Mathur, R. Magnesium oxychloride cement concrete. Bull. Mater. Sci. 2007, 30, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.J.; Chau, C.K. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, D. Research on the water resistance of magnesium oxychloride cement and its improvment. J. Chin. Ceram. Soc. 1995, 23, 673–679. [Google Scholar]
- Wei, L.; Wang, Y.; Yu, J.; Xiao, J.; Xu, S. Feasibility study of strain hardening magnesium oxychloride cement-based composites. Constr. Build. Mater. 2018, 165, 750–760. [Google Scholar] [CrossRef]
- Sambucci, M.; Marini, D.; Sibai, A.; Valente, M. Preliminary Mechanical Analysis of Rubber-Cement Composites Suitable for Additive Process Construction. J. Compos. Sci. 2020, 4, 120. [Google Scholar] [CrossRef]
- Segre, N.; Joekes, I. Use of tire rubber particles as addition to cement paste. Cem. Concr. Res. 2000, 30, 1421–1425. [Google Scholar] [CrossRef]
- Longvinenko, A.A. Use of rubber crumbs in cement concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 327, 032034. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.C.; Gao, L.L.; Mostafa, M. Mechanical Performance Test of Rubber-Powder Modified Concrete. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 38. [Google Scholar]
- Ganjian, E.; Khorami, M.; Maghsoudi, A.A. Scrap-tyre-rubber replacement for aggregate and filler in concrete. Constr. Build. Mater. 2009, 23, 1828–1836. [Google Scholar] [CrossRef]
- Eldin, N.N.; Senouci, A.B. Rubber-Tire Particles as Concrete Aggregate. J. Mater. Civ. Eng. 1993, 5, 478–496. [Google Scholar] [CrossRef]
- Topcu, I.B. The properties of rubberized concretes. Cem. Concr. Res. 1995, 25, 304–310. [Google Scholar] [CrossRef]
- He, L.; Cai, H.; Huang, Y.; Ma, Y.; Van Den Bergh, W.; Gaspar, L.; Valentin, J.; Vasiliev, Y.E.; Kowalski, K.J.; Zhang, J. Research on the properties of rubber concrete containing surface-modified rubber powders. J. Build. Eng. 2021, 35, 101991. [Google Scholar] [CrossRef]
- Xue, H.; Cao, Y.; Liu, Q.; Zhang, H.; Zhang, M. Stability Evaluation and Mechanism of Asphalts Modified with Various Rubber Powder Contents. Front. Mater. 2021, 7, 479. [Google Scholar] [CrossRef]
- Huang, Z.; Sui, L.; Wang, F.; Du, S.; Zhou, Y.; Ye, J. Dynamic compressive behavior of a novel ultra-lightweight cement composite incorporated with rubber powder. Compos. Struct. 2020, 244, 112300. [Google Scholar] [CrossRef]
- Tantala., M.W.; Lepore, J.A.; Zandi, I. Quasi-elastic behavior of rubber included concrete. In Proceedings of the 12th International Conference on Solid Waste Technology and Management, Philadelphia, PA, USA, 17–20 November 1996. [Google Scholar]
- Goulias, D.G.; Ali, A.H. Non-Destructive Evaluation of Rubber Modified Concrete. In Infrastructure Condition Assessment: Art, Science, and Practice; American Society of Civil Engineers: New York, NY, USA, 2013. [Google Scholar]
- Topçu, I.B.; Avcular, N. Analysis of rubberized concrete as a composite material. Cem. Concr. Res. 1997, 27, 1135–1139. [Google Scholar] [CrossRef]
- Pavlikova, M.; Zaleska, M.; Pivak, A.; Jankovsky, O. Moc-diatomite composites filled with multi-walled carbon nanotubes. Materials 2021, 14, 4576. [Google Scholar] [CrossRef] [PubMed]
- Pavlik, A.Z.; Pavlíkova, M.; Zaleska, M. Properties of concrete with plastic polypropylene aggregates. In Use of Recycled Plastics in Eco-Efficient Concrete; Woodhead Publishing: Shaxton, UK, 2019; pp. 189–213. [Google Scholar]
- Singh, A.; Kumar, R.; Goel, P. Factors influencing strength of magnesium oxychloride cement. Constr. Build. Mater. 2021, 303, 124571. [Google Scholar] [CrossRef]
- Huang, J.; Ge, S.; Wang, H.; Chen, R. Study on the improvement of water resistance and water absorption of magnesium oxychloride cement using long-chain organosilane-nonionic surfactants. Constr. Build. Mater. 2021, 306, 124872. [Google Scholar] [CrossRef]
- Venkatesan, G.; Anjali, R.; Kapgate, B.; Rajkumar, K. A feasibility study on rubberized concrete mortar cubes used in construction industry. Mater. Today Proc. 2021, 45, 677–688. [Google Scholar] [CrossRef]





| Material | Sieve Residue % | Carbon Black Content % | Rubber Hydrocarbon Content % | Ash Content % | Tensile Strength MPa | Elongation at Break % | Bulk Density g/cm3 | Density g/cm3 |
|---|---|---|---|---|---|---|---|---|
| 80-mesh | 8 | 30 | 56 | 6.6 | 17.6 | 55.8 | 0.37 | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, D.; Wang, S.; Gao, Y.; Wang, L.; Li, Y.; Wang, J. Influence of Rubber Powder Modification Methods on the Mechanical and Durability Properties of Rubberized Magnesium Oxychloride Cement. Crystals 2021, 11, 1323. https://doi.org/10.3390/cryst11111323
Zhong D, Wang S, Gao Y, Wang L, Li Y, Wang J. Influence of Rubber Powder Modification Methods on the Mechanical and Durability Properties of Rubberized Magnesium Oxychloride Cement. Crystals. 2021; 11(11):1323. https://doi.org/10.3390/cryst11111323
Chicago/Turabian StyleZhong, Dongqing, Shuguang Wang, Yu Gao, Luming Wang, Yanbo Li, and Jiaqing Wang. 2021. "Influence of Rubber Powder Modification Methods on the Mechanical and Durability Properties of Rubberized Magnesium Oxychloride Cement" Crystals 11, no. 11: 1323. https://doi.org/10.3390/cryst11111323
APA StyleZhong, D., Wang, S., Gao, Y., Wang, L., Li, Y., & Wang, J. (2021). Influence of Rubber Powder Modification Methods on the Mechanical and Durability Properties of Rubberized Magnesium Oxychloride Cement. Crystals, 11(11), 1323. https://doi.org/10.3390/cryst11111323






