The Different Inclusions’ Characteristics between Natural and Heat-Treated Tanzanite: Evidence from Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Conventional Gemological Features
3.2. Raman Spectra Comparison of Inclusions between Natural and Heat-treated Tanzanite
3.2.1. Calcite Inclusions
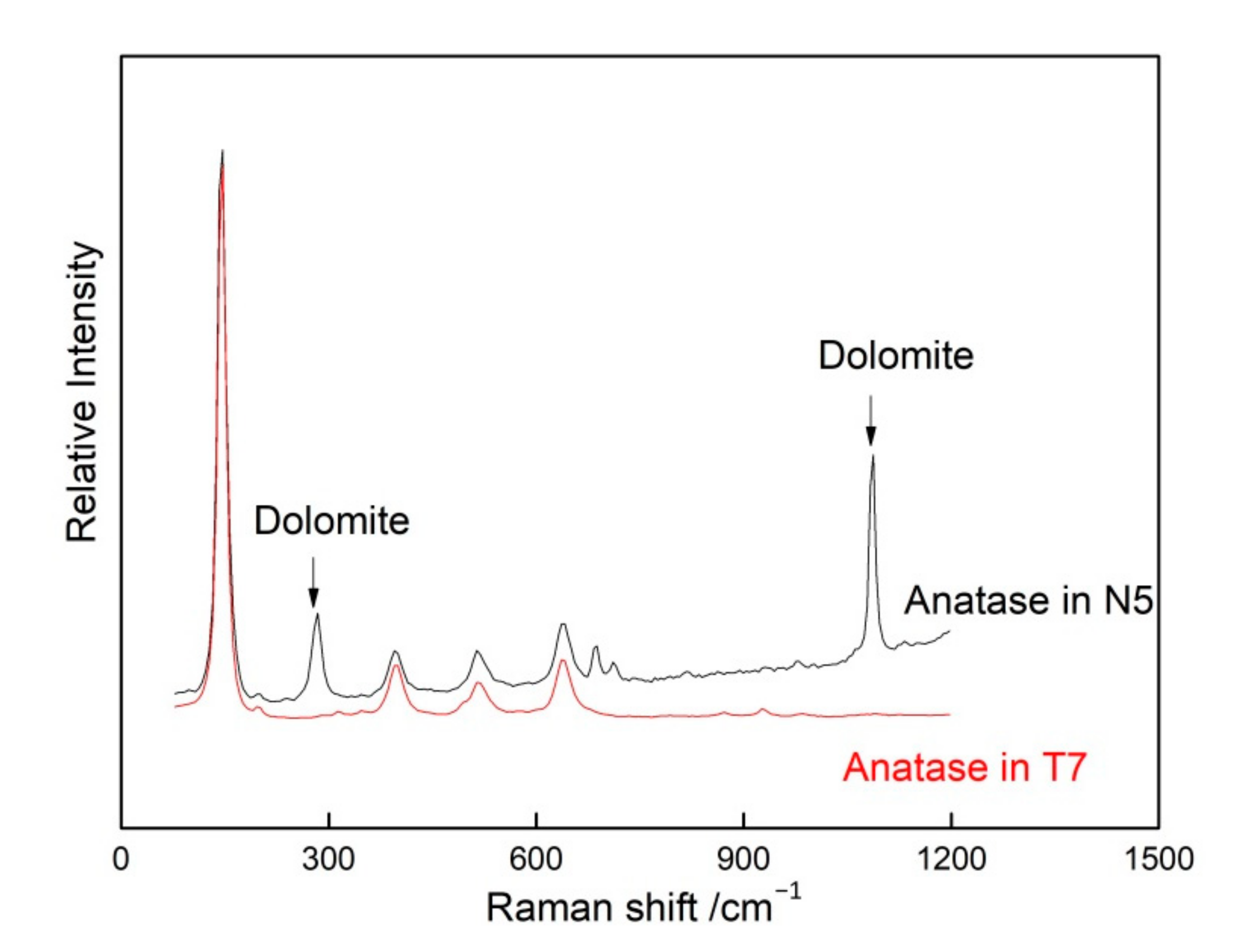
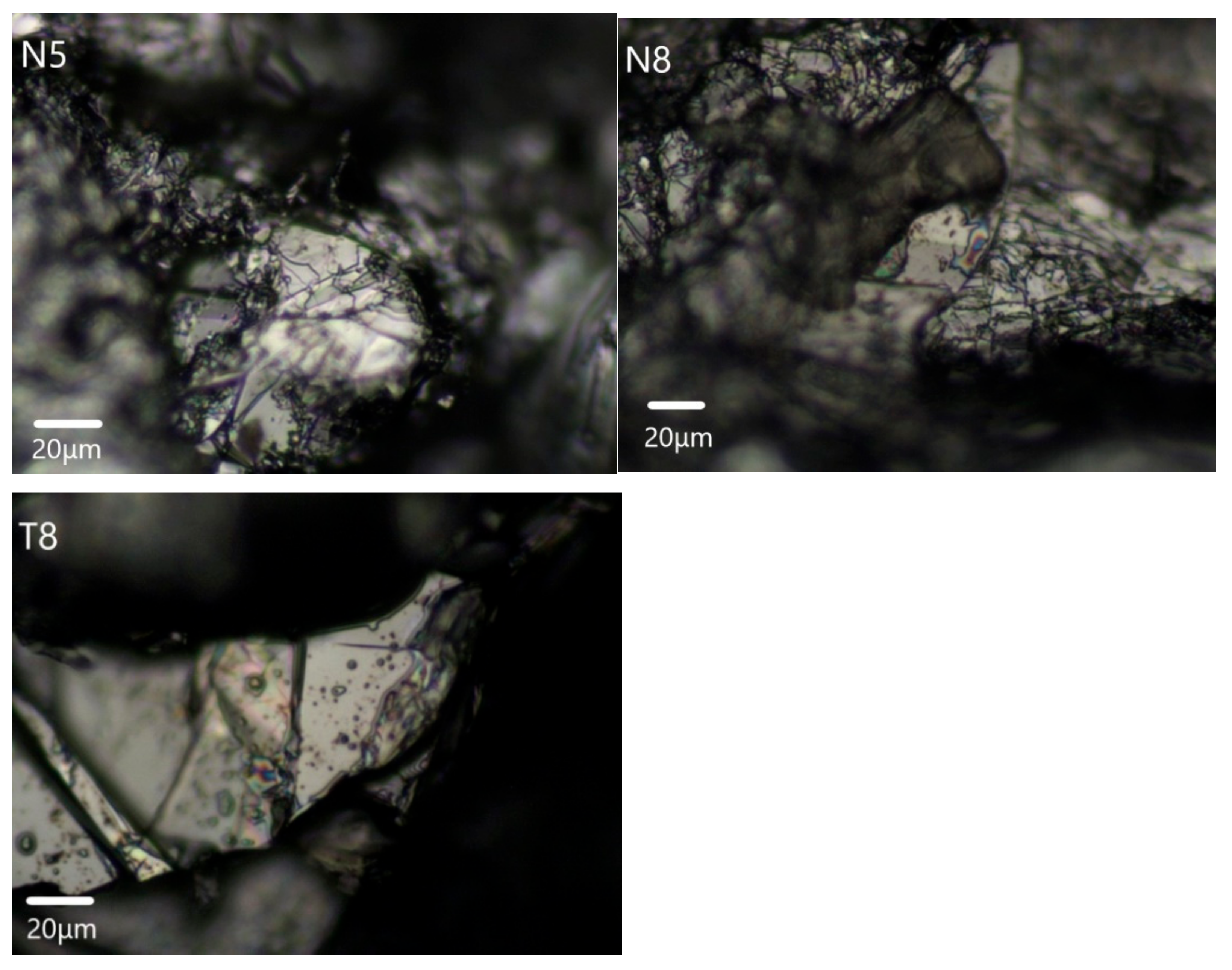
3.2.2. Anatase Inclusions
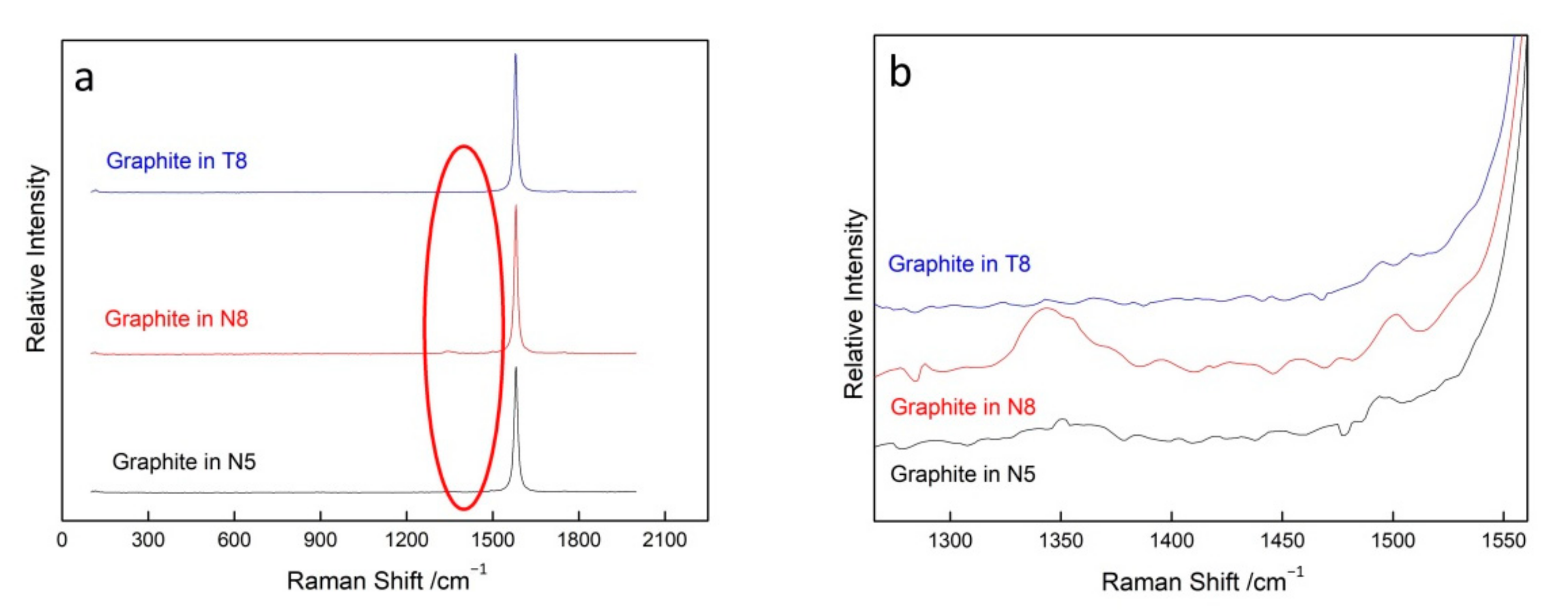
3.2.3. Graphite Inclusions
3.2.4. Zoisite Inclusions
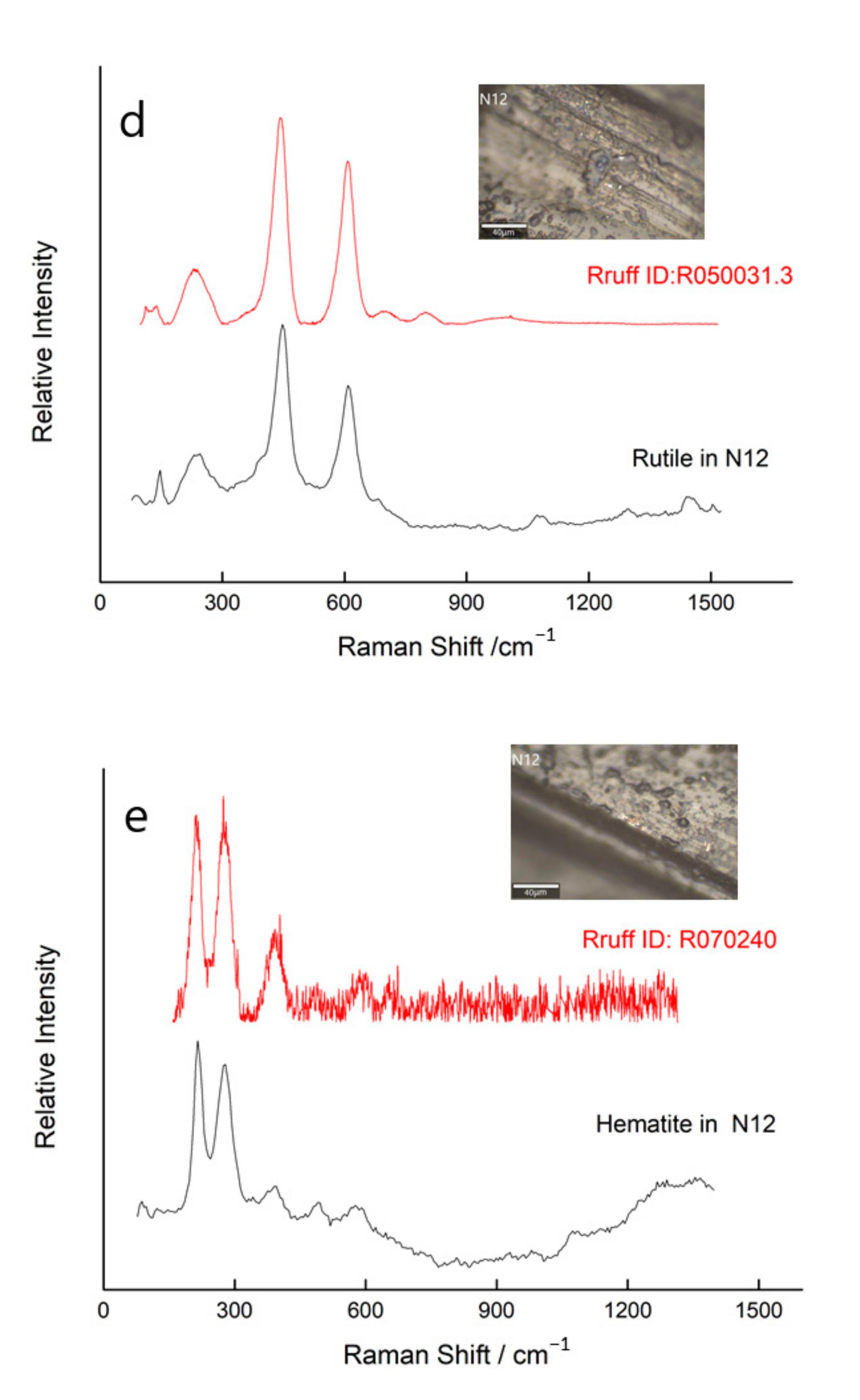
3.3. Other Characteristics Inclusions in Natural Unheated Tanzanite
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cairncross, B. The Where of Mineral Names: Tanzanite, A variety of Zoisite, Merelani Hills, Simanjiro District, Manyara Region, Tanzania. Rocks Miner. 2020, 95, 458–462. [Google Scholar] [CrossRef]
- Pluthametwisute, T.; Wanthanachaisaeng, B.; Saiyasombat, C.; Sutthirat, C. Cause of color modification in Tanzanite after heat treatment. Molecules 2020, 25, 4743. [Google Scholar] [CrossRef] [PubMed]
- He, X.M. Young magnificent blue “Heart of the Ocean”—Tanzanite. Sci. Cult. Pop. Land Resour. 2018, 02, 38–41. (In Chinese) [Google Scholar]
- Giuliani, G.; Feneyrol, J.; Ohnenstetter, D.; Marshall, D.; Walton, L.; Martelat, J.-E.; Fallick, A.E. Tsavorite and tanzanite deposits. Mineralogical association of canada short course series. In Geology of Gem Deposits, 2nd ed.; Groat, L.A., Ed.; The Mineralogical Association of Canada: Quebec, QC, Canada, 2014; pp. 217–246. [Google Scholar]
- Ccato, A.; Bersani, D.; Caggiani, M.C.; Mazzoleni, P.; Barone, G. Raman studies on zoisite and tanzanite for gemmological applications. J. Raman Spectrosc. 2021, 06, 1–13. [Google Scholar] [CrossRef]
- Olivier, B. The Geology and Petrology of the Merelani-Tanzanite Deposit, Northeast Tanzania. Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2006. [Google Scholar]
- Switzer, G.S. Composition of green garnet from Tanzania and Kenya. Gems Gemol. 1974, 14, 296–297. [Google Scholar]
- Wilson, W.E.; Sau, J.M.; Pardieu, V.; Hughes, R.W. The Merelani Tanzanite mines, Lelatema mountains, Arusha Region, Tanzania. Mineral. Rec. 2009, 40, 347–408. [Google Scholar]
- Naeser, C.W.; Saul, J.M. Fission track dating of tanzanite. Am. Mineral. 1974, 59, 613–614. [Google Scholar]
- Feneyrol, J.; Giuliani, G.; Demaiffe, D.; Ohnenstetter, D.; Fallick, A.E.; Dubessy, J.; Martelat, J.E.; Rakotondrazafy, A.F.M.; Omito, E.; IchanG’I, D.; et al. Age and origin of the Tsavorite and Tanzanite mineralizing fluids in the Neoproterozoic Mozambique Metamorphic Belt. Can. Mineral. 2017, 55, 763–786. [Google Scholar] [CrossRef]
- Jaszczak, J.A.; Trinchillo, D. Miracle at Merelani: A remarkable occurrence of graphite, diopside, and associated minerals from the Karo mine, Block D, Merelani Hills, Arusha region, Tanzania. Rocks Miner. 2013, 88, 154–165. [Google Scholar] [CrossRef]
- Thongnopkun, P.; Chanwanitsakun, P. Effect of heat treatment on spectroscopic properties of Tanzanite. J. Phys. Conf. Ser. 2018, 1144, 012183. [Google Scholar] [CrossRef]
- Malisa, E.; Kinnunen, K.; Koljonen, T. Notes on fluid inclusions in vanadiferous Zoisite (Tanzanite) of the Merelani area, Tanzania. Bull. Geol. Soc. Finl. 1986, 58, 53–58. [Google Scholar] [CrossRef]
- Gubelin, E.J.; Weibel, M. Green vanadium grossular garnet from Lualenyi, near Voi, Kenya. Lapidary J. 1975, 29, 402–426. [Google Scholar]
- Giarola, M.; Mariotto, G.; Ajò, D. Micro-Raman investigations on inclusions of unusual habit in a commercial Tanzanite gemstone. J. Raman Spectrosc. 2012, 43, 556–558. [Google Scholar] [CrossRef]
- Castillo, A.; Smith, T. Merelaniite inclusions in tanzanite. Gems Gemol. 2018, 52, 226–227. [Google Scholar]
- Jaszczak, J.A.; Rumsey, M.S.; Bindi, R.L.; Hackney, S.A.; Wise, M.A.; Stanley, C.J.; Spratt, J. Merelaniite, Mo4Pb4VSbS15, a new molybdenum-essential member of the Cylindrite Group, from the Merelani tanzanite Deposit, Lelatema Mountains, Manyara region, tanzania. Minerals 2016, 6, 115. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.D.; Lu, R. Color shift and pleochroism in tanzanite. Gemol. Technol.-China Int. Gems Jewel. Acad. Conf. 2015, 4, 130–133, (In Chinese with English Abstract). [Google Scholar]
- Barot, N.R.; Boehm, E.W. Gem-quality green zoisite. Gems Gemol. 1992, 28, 80–102. [Google Scholar] [CrossRef]
- Ye, H.J. Analysis and Research on Gemological and Mineralogical Characteristics of Tanzanite. Master’s Thesis, China University of Geosciences, Wuhan, China, 2020. (In Chinese). [Google Scholar]
- Du, G.P.; Fan, J.L. Characteristics of Raman spectral of calcite group minerals. Mineral. Petrol. 2010, 30, 32–35, (In Chinese with English Abstract). [Google Scholar]
- Fu, P.G.; Zheng, H.F. Raman spectra of aragonite and calcite at high temperature and high pressure. Spectrosc. Spectr. Anal. 2013, 33, 1557–1561, (In Chinese with English Abstract). [Google Scholar]
- Liu, C.J.; Zheng, H.F. In situ experimental study of phase transition of calcite by Raman spectroscopy at high temperature and high pressure. Spectrosc. Spectr. Anal. 2012, 32, 378–382, (In Chinese with English Abstract). [Google Scholar]
- Gillet, P.; Biellmann, C.; Reynard, B.; McMillan, P. Raman Spectroscopic Studies of Carbonates Part I: High-Pressure and High-Temperature Behaviour of Calcite, Magnesite, Dolomite and Aragonite. Phys. Chem. Miner. 1993, 20, 1–18. [Google Scholar] [CrossRef]
- Ding, X.; He, J.J.; Liu, Z.Y. Experimental studies on crystal growth of anatase under hydrothermal. Earth Sci. 2018, 43, 1763–1772, (In Chinese with English Abstract). [Google Scholar]
- Sekiya, T.; Ohta, S.; Kamei, S.; Hanakawa, M.; Kurita, S. Raman spectroscopy and phase transition of anatase TiO2 under high pressure. J. Phys. Chem. Solids 2011, 62, 717–721. [Google Scholar] [CrossRef]
- Pan, X.Y.; Ma, X.M.; You, J.L.; Zhu, L.H. Structural characterization of nanocrystalline TiO2 powders by X-ray diffractometer, transmission electron microscopy and high temperature Raman spectroscopy. J. Funct. Mater. 2003, 34, 192–193, (In Chinese with English Abstract). [Google Scholar]
- Chang, H.; Huang, P.J. Thermo-Raman studies on anatase and rutile. J. Raman Spectrosc. 1998, 29, 97–102. [Google Scholar] [CrossRef]
- Du, Y.L.; Deng, Y.; Zhang, M.S. Variable-temperature Raman scattering study on anatase titanium dioxide nanocrystals. J. Phys. Chem. Solids. 2006, 67, 2405–2408. [Google Scholar] [CrossRef]
- Dunn, P.J. Notes on inclusions in tanzanite and tourmalinated quartz. J. Gemmol. 1975, 14, 335–338. [Google Scholar] [CrossRef]
- Tan, P.H.; Yu, G.T.; Huang, F.M.; Zhang, S.L. A comparative Raman study of carbon nanotubes and the highly oriented pyrolytic graphite. Chin. J. Light Scatt. 1996, 8, 125–130, (In Chinese with English Abstract). [Google Scholar]
- Yang, J.G.; Wu, C.P. Raman spectra study on expanded graphite. Chin. J. Light Scatt. 2006, 17, 341–436, (In Chinese with English Abstract). [Google Scholar]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, D. Iridescent Inclusion in Tanzanite. Gems Gemol. 2016, 52, 312–313. [Google Scholar]
- Fan, J.L.; Guo, S.G.; Mao, J.; Shi, L.Y. Study on Raman Spectra of Zoisite and Its Similar Gemstones. Appl. Laser 2007, 27, 209–212, (In Chinese with English Abstract). [Google Scholar]
- Qiang, J.Y.; Chuan, X.Y. Oxidation modification of natural molybdenite using concentrated sulfuric acid and its electrocatalytic properties. Acta Sci. Nat. Univ. Pekin. 2018, 54, 579–584, (In Chinese with English Abstract). [Google Scholar]
- Chen, X.Y.; Dai, H.; Cao, S.Q.; Yu, L.; Jiang, X.P. Study on the spectral characteristics of yellow prehnite. Geol. Anhui 2018, 28, 154–156, (In Chinese with English Abstract). [Google Scholar]
- Balachandran, U.; Eror, N.G. Raman-spectra of titanium-dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]

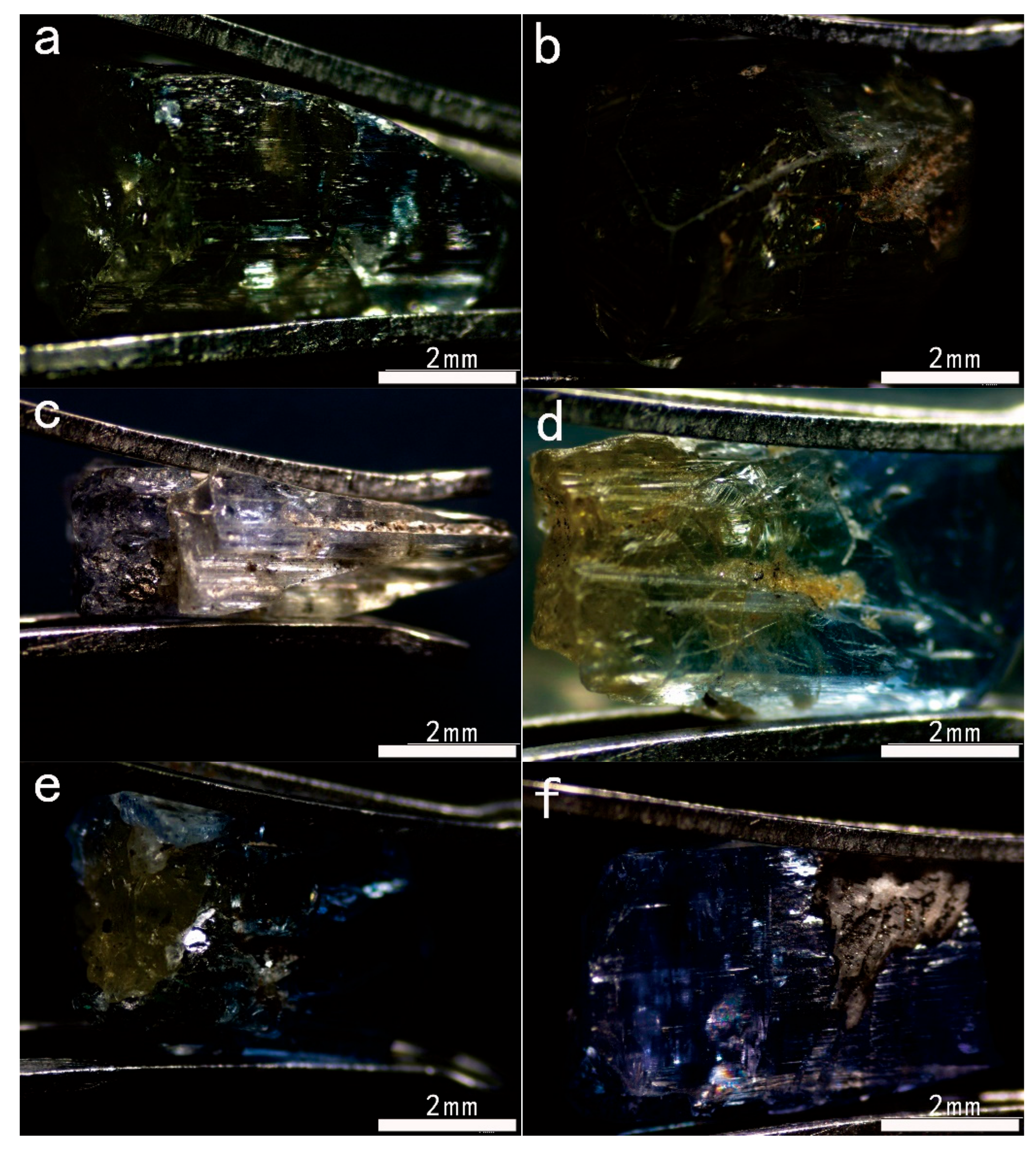

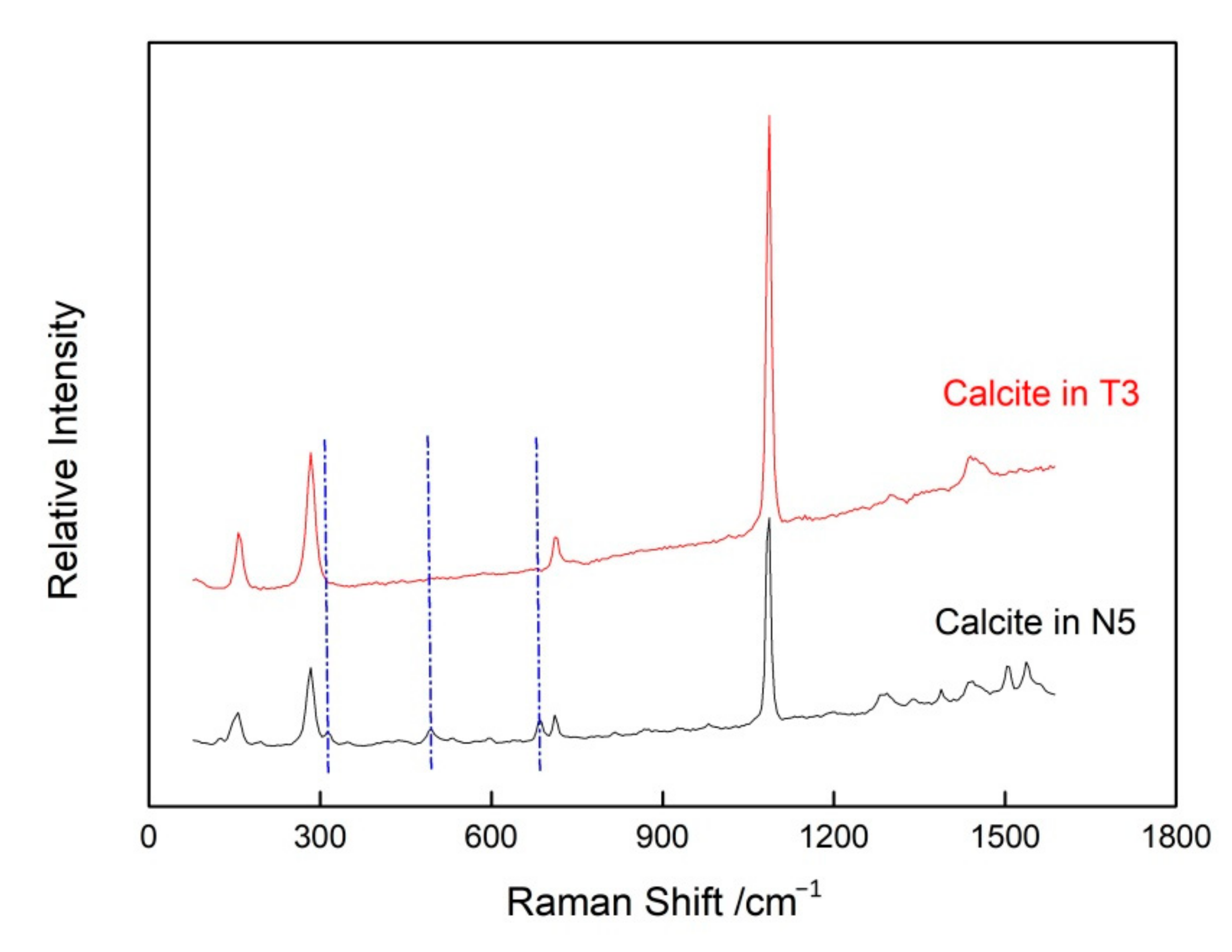
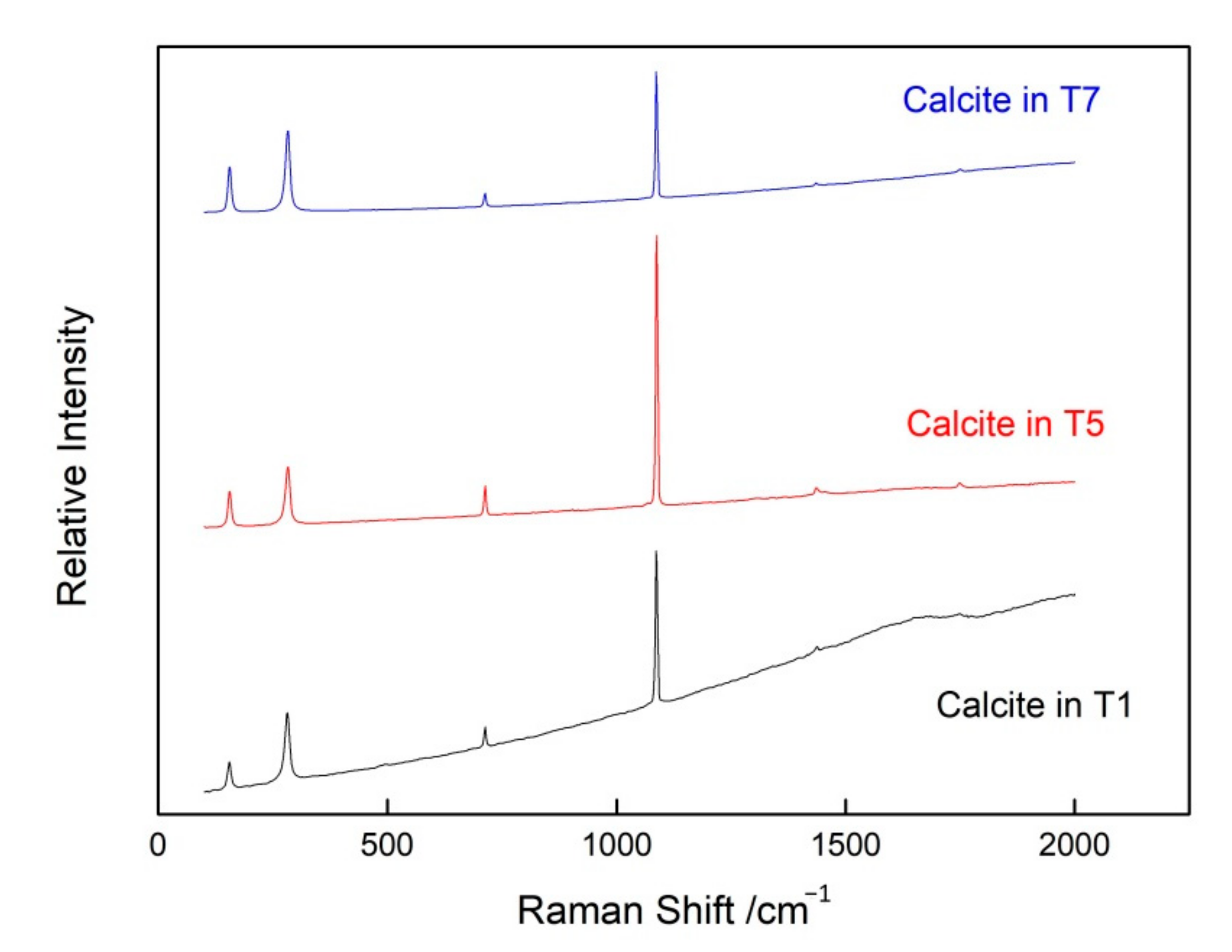
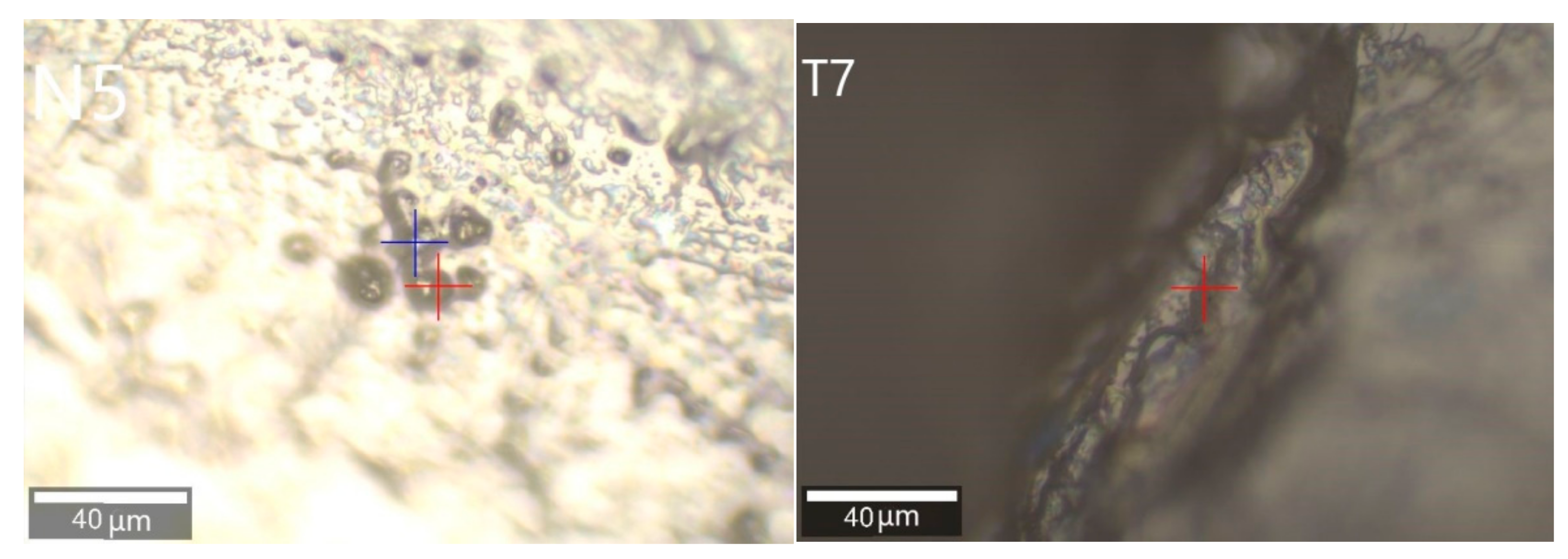



| Sample Number | Color | Transparency | Refractive Index | Specific Gravity | Pleochroism |
|---|---|---|---|---|---|
| A1 | blue-violet | translucent | 1.69 | 3.36 | blue, violet |
| N1 | yellow | transparent | 1.71 | 3.35 | yellow, light grey-blue, light violet |
| N2 | yellow-blue | transparent | 1.71 | 3.35 | dark blue, yellow, light violet |
| N3 | light yellow-blue | transparent | 1.71 | 3.35 | yellow-green, blue, light violet |
| N4 | brown-blue | translucent | 1.70 | 3.35 | yellow, light grey-blue, light violet |
| N5 | grey violet-blue | transparent | 1.70 | 3.35 | yellow, blue, light violet |
| N6 | brown-blue | translucent | 1.71 | 3.35 | yellow, dark blue, light violet |
| N7 | blue-yellow | transparent | 1.71 | 3.35 | yellow section: yellow, grey-blue, violet |
| N8 | Indigo-violet | transparent | 1.71 | 3.34 | grey-blue section light yellow, blue, light violet |
| N9 | light violet | transparent -opaque | 1.71 | 3.34 | yellow section: yellow, blue, light violet-pink |
| N10 | light violet | transparent-translucent | 1.71 | 3.35 | blue section light yellow, light blue, grey- violet |
| N11 | light violet | transparent | 1.70 | 3.35 | violet-pink, blue, light yellow-green |
| N12 | dark violet-blue | translucent | 1.70 | 3.35 | dark blue, violet, light yellow-green |
| N13 | light grey-violet | transparent-translucent | 1.70 | 3.35 | yellow-green, light blue, violet-pink |
| T1 | light violet | transparent-opaque | 1.70 | 3.33 | violet-pink, blue |
| T2 | grey-violet | transparent-opaque | 1.70 | 3.29 | violet-pink, blue |
| T3 | violet | opaque | 1.70 | 3.29 | violet, blue |
| T4 | grey-violet | opaque | 1.70 | 3.28 | violet, blue |
| T5 | grey-violet | opaque | 1.70 | 3.24 | not measurable |
| T6 | violet | opaque | 1.70 | 3.30 | blue, violet |
| T7 | light violet | opaque | 1.70 | 3.21 | blue, violet |
| T8 | light violet | opaque | 1.70 | 3.16 | dark blue, dark violet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Ye, H.; Liu, Y. The Different Inclusions’ Characteristics between Natural and Heat-Treated Tanzanite: Evidence from Raman Spectroscopy. Crystals 2021, 11, 1302. https://doi.org/10.3390/cryst11111302
Yang S, Ye H, Liu Y. The Different Inclusions’ Characteristics between Natural and Heat-Treated Tanzanite: Evidence from Raman Spectroscopy. Crystals. 2021; 11(11):1302. https://doi.org/10.3390/cryst11111302
Chicago/Turabian StyleYang, Shuqi, Huijuan Ye, and Yingxin Liu. 2021. "The Different Inclusions’ Characteristics between Natural and Heat-Treated Tanzanite: Evidence from Raman Spectroscopy" Crystals 11, no. 11: 1302. https://doi.org/10.3390/cryst11111302
APA StyleYang, S., Ye, H., & Liu, Y. (2021). The Different Inclusions’ Characteristics between Natural and Heat-Treated Tanzanite: Evidence from Raman Spectroscopy. Crystals, 11(11), 1302. https://doi.org/10.3390/cryst11111302






