Preparation of Superhydrophobic Surfaces Based on Rod-Shaped Micro-Structure Induced by Nanosecond Laser
Abstract
:1. Introduction
2. Experiment
2.1. Materials and Pretreatment
2.2. Fabrication of Superhydrophobic Surface
2.3. Surface Characterization
3. Results and Discussions
4. Conclusions
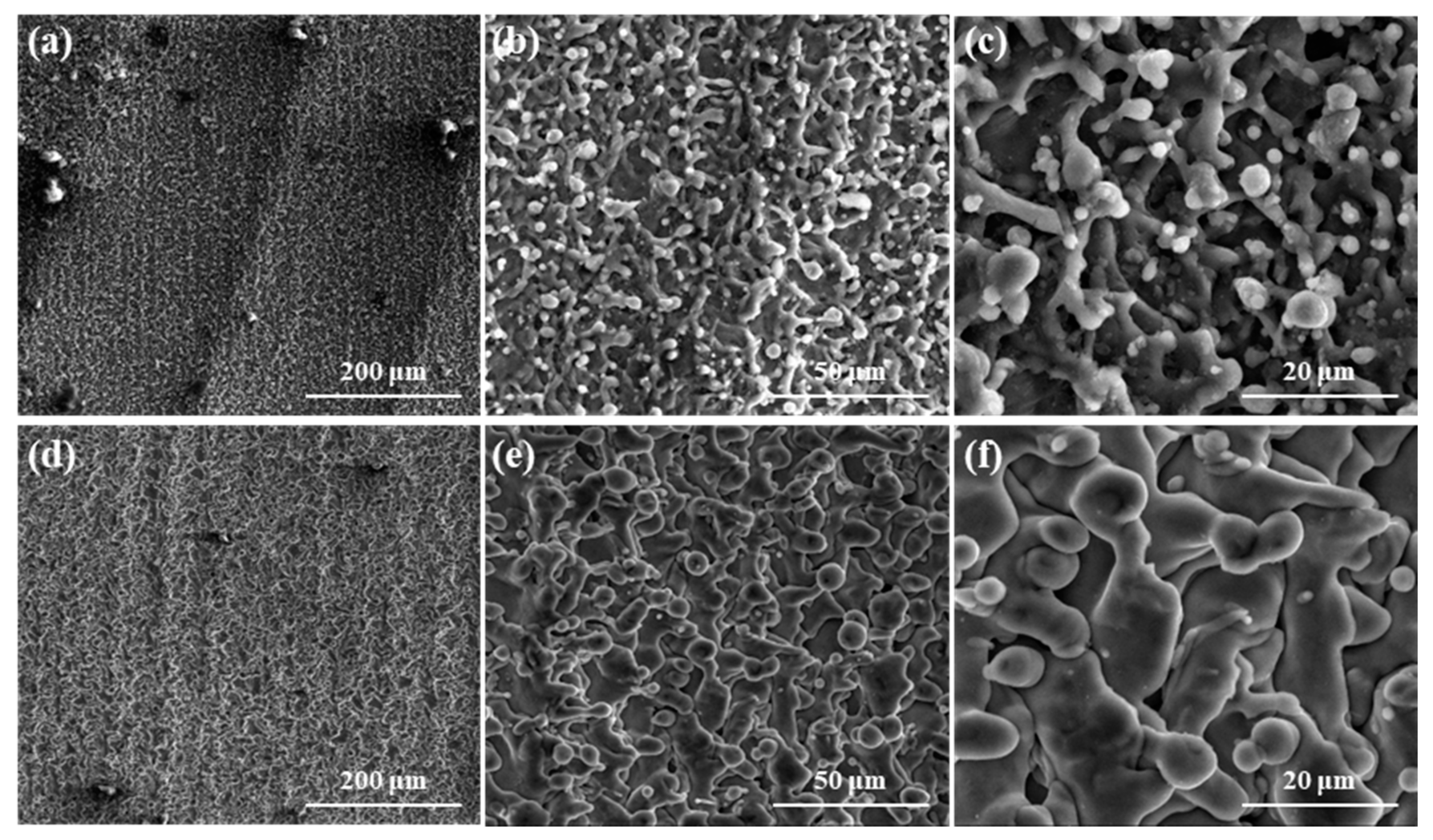
- After laser treatment, a large number of nanoscale particle structures were distributed on the surface of the microscale rod-shaped micro-structure, and the micro–nano composite structure was formed on the surfaces of both the Al alloy and Ti alloy. The size of the rod-shaped micro-structure on the surface of Al alloy was smaller and more fragmentary than that on the surface of Ti alloy, which was attributed to the higher coefficient of thermal conductivity (CTC), specific heat capacity (SHC), and the lower melting point (MP) of the Al alloy.
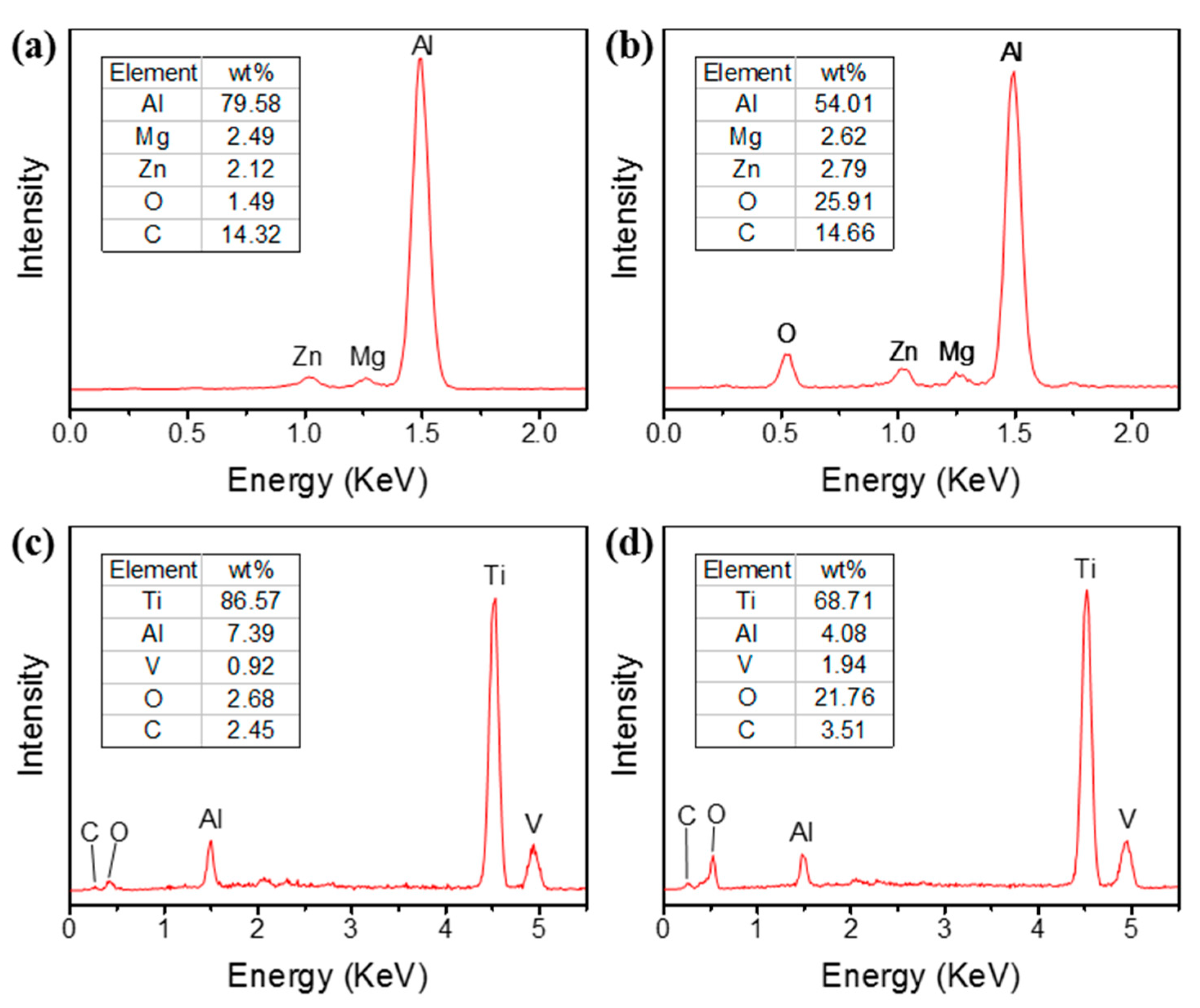
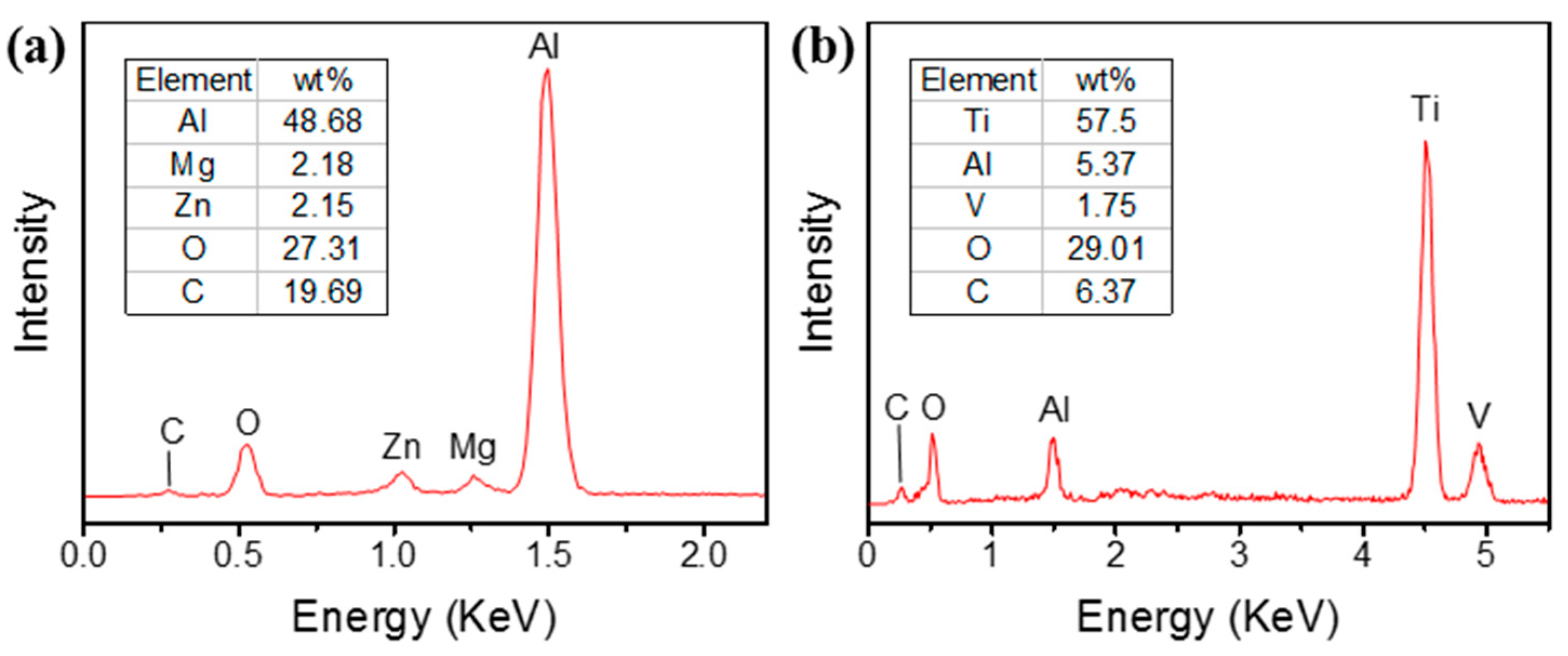
- The chemical composition changed at the same time as the laser treatment, which was verified by EDS. It was found that the ratio of oxygen on both metal surfaces showed a trend of significant increase, which was due to the ablative oxidation of the metal elements. The surface oxidation of the Al alloy was more intense due to its more fragmentary rod-shaped micro-structure.
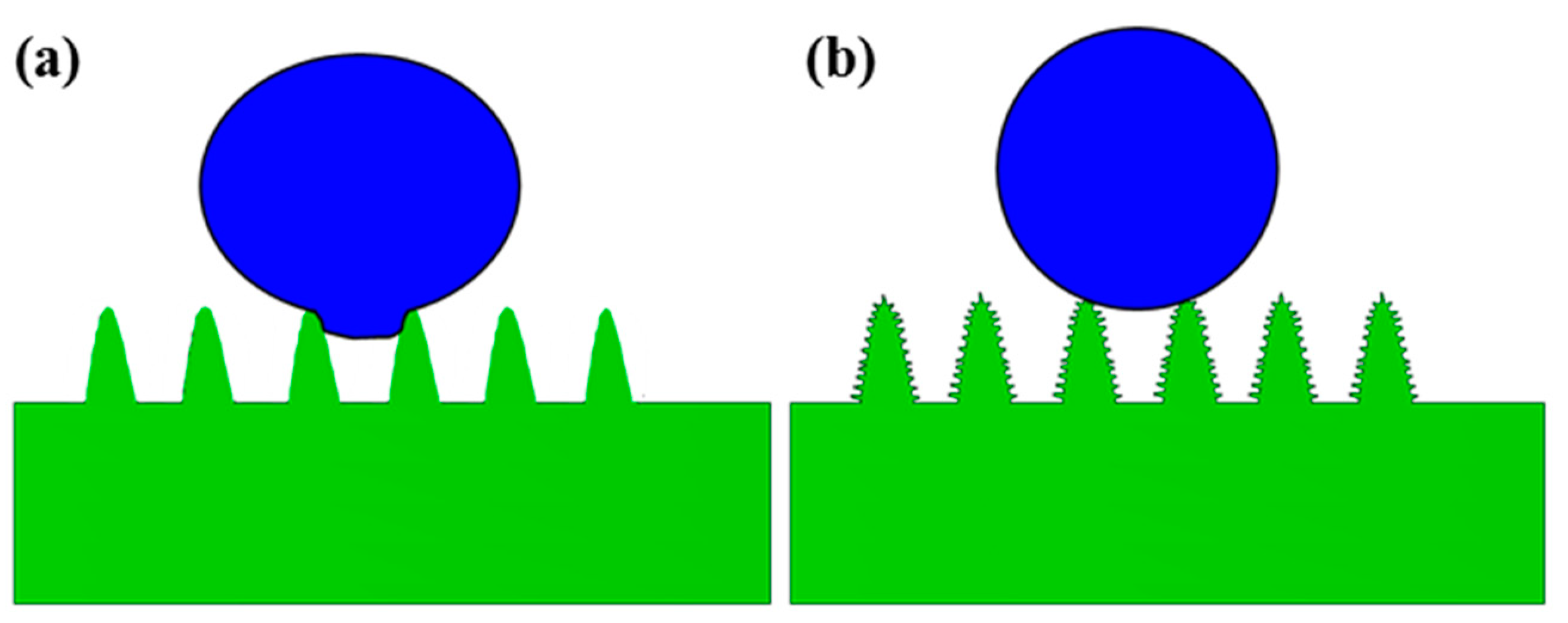
- The laser-treated surface became hydrophobic or even superhydrophobic from superhydrophilic after 20 days of being placed in the air due to the adsorption of organic matter. The height-to-width ratio of the micro–nano composite rod-shaped micro-structure on the surface of the Al alloy was larger in comparison to that of the Ti alloy, which leads to a larger contact angle (CA) and stronger hydrophobicity.
Author Contributions
Funding
Conflicts of Interest
References
- Mizine, I.; Amromin, E.; Crook, L.; Day, W.; Korpus, R. High-Speed Trimaran Drag: Numerical Analysis and Model Tests. J. Ship Res. 2004, 48, 248–259. [Google Scholar] [CrossRef]
- Solomon, B.R.; Khalil, K.S.; Varanasi, K.K. Drag Reduction using Lubricant-Impregnated Surfaces in Viscous Laminar Flow. Langmuir 2014, 30, 10970–10976. [Google Scholar] [CrossRef]
- Demirel, Y.K.; Khorasanchi, M.; Turan, O.; Incecik, A.; Schultz, M.P. A CFD model for the frictional resistance prediction of antifouling coatings. Ocean Eng. 2014, 89, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Lindholdt, A.; Dam-Johansen, K.; Yebra, D.M.; Olsen, S.M.; Kiil, S. Estimation of long-term drag performance of fouling control coatings using an ocean-placed raft with multiple dynamic rotors. J. Coat. Technol. Res. 2015, 12, 975–995. [Google Scholar] [CrossRef] [Green Version]
- Mohanarangam, K.; Cheung, S.C.P.; Tu, J.Y.; Chen, L. Numerical simulation of micro-bubble drag reduction using population balance model. Ocean Eng. 2009, 36, 863–872. [Google Scholar] [CrossRef]
- Hao, W.U.; Yongpeng, O.; Qing, Y.E. Experimental study of air layer drag reduction on a flat plate and bottom hull of a ship with cavity. Ocean Eng. 2019, 183, 236–248. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, Y.; Zhai, J. A Lotus-Leaf-like Superhydrophobic Surface: A Porous Microsphere/Nanofiber Composite Film Prepared by Electrohydrodynamics. Angew. Chem. 2004, 43, 4338–4341. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.; She, P.; Sun, H.; Liu, Z.N. A Photo-Curing Method to Prepare Biomimetic Micro-Nano Structure of Butterfly Wing Scale. Appl. Mech. Mater. 2015, 713–715, 2576–2580. [Google Scholar] [CrossRef]
- Feng, X.Q.; Gao, X.; Wu, Z.; Jiang, L.; Zheng, Q.S. Superior water repellency of water strider legs with hierarchical structures: Experiments and analysis. Langmuir 2007, 23, 4892. [Google Scholar] [CrossRef]
- Lin, J.; Tan, X.; Shi, T.; Tang, Z.; Liao, G. Leaf Vein-Inspired Hierarchical Wedge-Shaped Tracks on Flexible Substrate for Enhanced Directional Water Collection. ACS Appl. Mater. Interfaces 2018, 10, 44815–44824. [Google Scholar] [CrossRef]
- Jiang, Z.; Fang, S.; Wang, C.; Wang, H.; Ji, C. Durable polyorganosiloxane superhydrophobic films with a hierarchical structure by sol-gel and heat treatment method. Appl. Surf. Sci. 2016, 390, 993–1001. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.; Ling, H.; Li, M.; Mao, D. Super-hydrophobic nickel films with micro-nano hierarchical structure prepared by electrodeposition. Appl. Surf. Sci. 2010, 256, 2400–2404. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Chen, B.; Liu, C.; Zhang, M.; Li, C. Fabrication of superhydrophobic textured steel surface for anti-corrosion and tribological properties. Appl. Surf. Sci. 2015, 359, 905–910. [Google Scholar] [CrossRef]
- Xin, G.; Wu, C.; Cao, H.; Liu, W.; Zhang, G. Superhydrophobic TC4 alloy surface fabricated by laser micro-scanning to reduce adhesion and drag resistance. Surf. Coat. Technol. 2020, 391, 125707. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Guo, C.; Chen, F.; Hou, X. A review of femtosecond laser-structured superhydrophobic or underwater superoleophobic porous surfaces/materials for efficient oil/water separation. RSC Adv. 2019, 9, 12470–12495. [Google Scholar] [CrossRef]
- Lin, Y.; Han, J.; Cai, M.; Liu, W.; Luo, X.; Zhang, H.; Zhong, M. Durable and robust transparent superhydrophobic glass surfaces fabricated by a femtosecond laser with exceptional water repellency and thermostability. J. Mater. Chem. A 2018, 6, 9049–9056. [Google Scholar] [CrossRef]
- Xin, G.; Rong, Y.; Huang, Y.; Wu, C. Process and mechanism of cutting thermoplastic polyurethane (TPU) film by nanosecond ultraviolet laser. J. Mater. Sci. 2021, 56, 16167–16180. [Google Scholar] [CrossRef]
- Fang, Y.; Yong, J.; Chen, F.; Huo, J.; Yang, Q. Bioinspired Fabrication of Bi/Tridirectionally Anisotropic Sliding Superhydrophobic PDMS Surfaces by Femtosecond Laser. Adv. Mater. Interfaces 2018, 5, 1701245. [Google Scholar] [CrossRef]
- Höhm, S.; Herzlieb, M.; Rosenfeld, A.; Krüger, J.; Bonse, J. Femtosecond laser-induced periodic surface structures on silicon upon polarization controlled two-color double-pulse irradiation. Opt. Express 2015, 23, 61–71. [Google Scholar] [CrossRef]
- He, A.; Liu, W.; Xue, W.; Yang, H.; Cao, Y. Nanosecond laser ablated copper superhydrophobic surface with tunable ultrahigh adhesion and its renewability with low temperature annealing. Appl. Surf. Sci. 2018, 434, 120–125. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef]
- Xin, G.; Wu, C.; Liu, W.; Rong, Y.; Huang, Y. Anti-corrosion superhydrophobic surfaces of Al alloy based on micro-protrusion array structure fabricated by laser direct writing. J. Alloys Compd. 2021, 881, 160649. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of Functional Nanoengineering and Nanosecond Laser Texturing for Design of Superhydrophobic Aluminum Alloy with Exceptional Mechanical and Chemical Properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef]
- Liu, W.; Cai, M.; Luo, X.; Chen, C.; Pan, R.; Zhang, H.; Zhong, M. Wettability transition modes of aluminum surfaces with various micro/nanostructures produced by a femtosecond laser. J. Laser Appl. 2019, 31, 022503. [Google Scholar] [CrossRef]
- Long, J.; Zhong, M.; Fan, P.; Gong, D.; Zhang, H. Wettability conversion of ultrafast laser structured copper surface. J. Laser Appl. 2015, 27, S29107. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tian, Y.L.; Yang, C.J.; Wang, F.J.; Liu, X.P. Modification of wetting property of Inconel 718 surface by nanosecond laser texturing. Appl. Surf. Sci. 2017, 414, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Domantovsky, A.G.; Shiryaev, A.A. Comment on “Nanosecond laser textured superhydrophobic metallic surfaces and their chemical sensing applications” by Duong V. Ta, Andrew Dunn, Thomas J. Wasley, Robert W. Kay, Jonathan Stringer, Patrick J. Smith, Colm Connaughton, Jonathan D. Shephard (Appl. Surf. Sci. 357 (2015) 248C254). Appl. Surf. Sci. 2016, 379, 111–113. [Google Scholar]
- Kulinich, S.A.; Honda, M.; Zhu, A.L.; Rozhin, A.G.; Du, X.W. The icephobic performance of alkyl-grafted aluminum surfaces. Soft Matter 2015, 11, 856–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, J.; Chen, F.; Yang, Q.; Farooqa, U.; Hou, X. Photoinduced switchable underwater superoleophobicity–superoleophilicity on laser modified titanium surfaces. J. Mater. Chem. A 2015, 3, 10703–10709. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]


| Al Alloy | Ti Alloy | ||
|---|---|---|---|
| Original | 20 days after laser treatment | Original | 20 days after laser treatment |
| 39 ± 2° | 157 ± 1° | 53 ± 3° | 147 ± 2° |
| Roughness Characterization Parameter | Al Alloy | Ti Alloy | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Maximum peak height Rp/μm | 1.579 | 13.275 | 3.655 | 13.233 |
| Maximum sag height Rv/μm | 5.359 | 12.587 | 6.939 | 25.863 |
| Contour mean difference Ra/μm | 0.287 | 2.762 | 3.927 | 4.770 |
| Ratio of height to width Str/ | 0.186 | 0.511 | 0.243 | 0.710 |
| Void volume Vv/(mL/m2) | 0.441 | 5.502 | 0.732 | 5.536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xue, G.; Wu, Y.; Wang, X.; Pan, H. Preparation of Superhydrophobic Surfaces Based on Rod-Shaped Micro-Structure Induced by Nanosecond Laser. Crystals 2021, 11, 1274. https://doi.org/10.3390/cryst11111274
Li Z, Xue G, Wu Y, Wang X, Pan H. Preparation of Superhydrophobic Surfaces Based on Rod-Shaped Micro-Structure Induced by Nanosecond Laser. Crystals. 2021; 11(11):1274. https://doi.org/10.3390/cryst11111274
Chicago/Turabian StyleLi, Zhi, Gang Xue, Yanming Wu, Xinghua Wang, and Hengpei Pan. 2021. "Preparation of Superhydrophobic Surfaces Based on Rod-Shaped Micro-Structure Induced by Nanosecond Laser" Crystals 11, no. 11: 1274. https://doi.org/10.3390/cryst11111274
APA StyleLi, Z., Xue, G., Wu, Y., Wang, X., & Pan, H. (2021). Preparation of Superhydrophobic Surfaces Based on Rod-Shaped Micro-Structure Induced by Nanosecond Laser. Crystals, 11(11), 1274. https://doi.org/10.3390/cryst11111274





