Thermodynamic Study of Adsorption Capacity between Metal Film and Optical Crystal: Adsorption Energy of Ni Films on LiNbO3 Substrates
Abstract
:1. Introduction
2. Model Building and Simulation Method
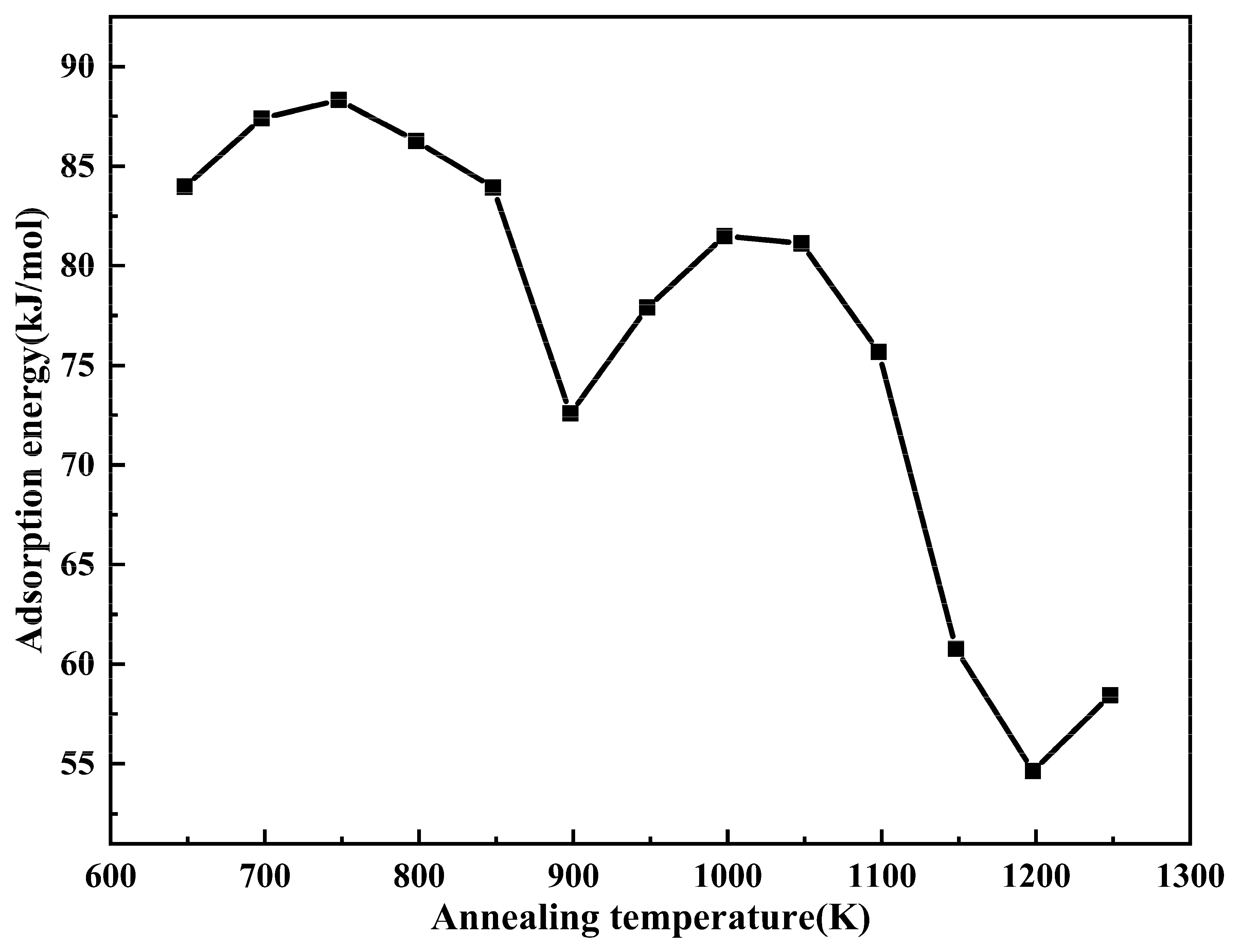
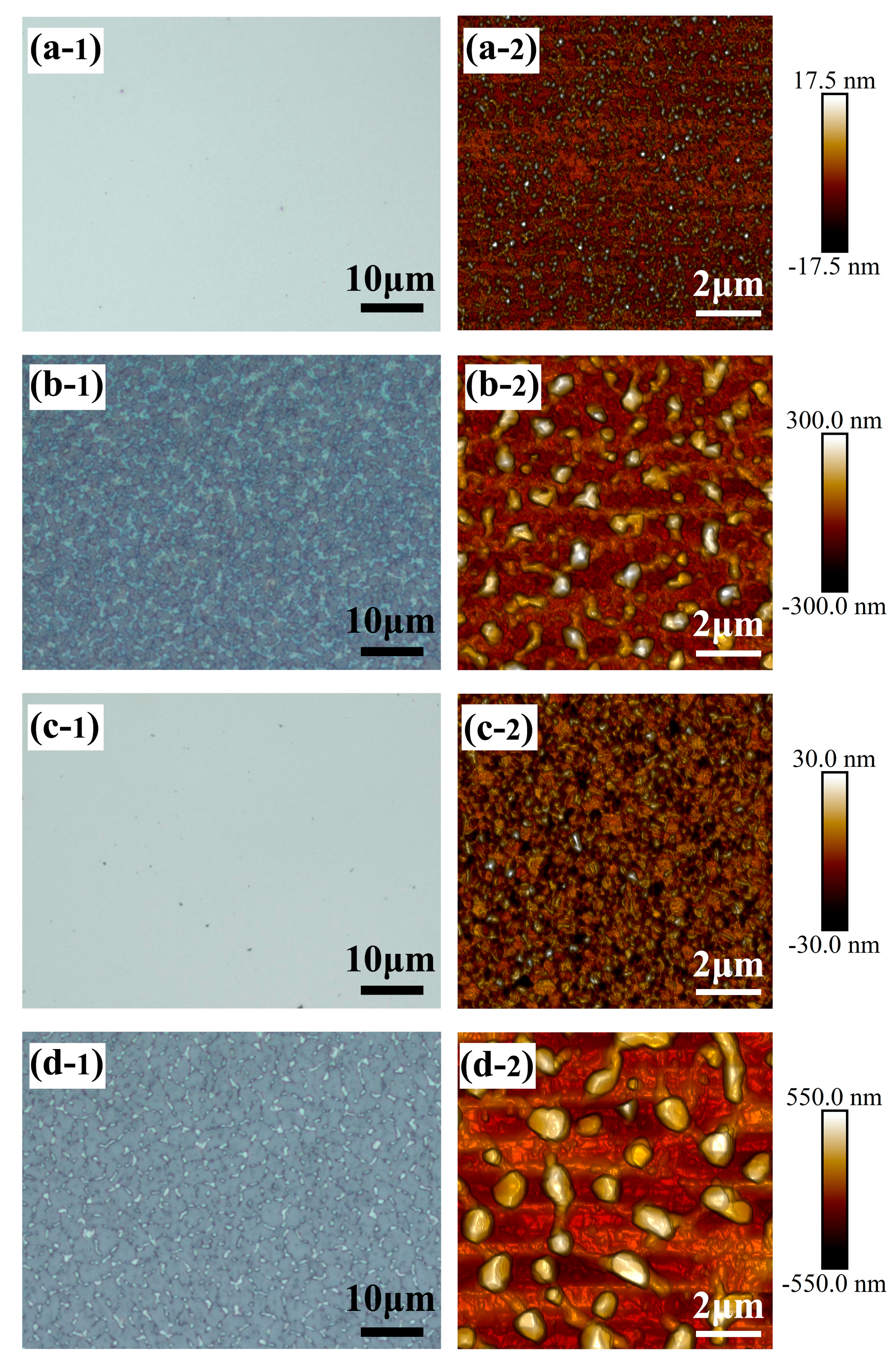
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Surface Chemistry and Macroscopic Assembly of Graphene for Application in Energy Storage. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. [Google Scholar]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Sun, M. Graphene, hexagonal boron nitride, and their heterostructures: Properties and applications. RSC Adv. 2017, 7, 16801–16822. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wang, Z.S.; Zhang, Z.D.; Dai, Z.G.; Wang, L.L.; Li, H.; Zhou, L.; Shang, Y.X.; He, J.; Fu, D.J.; et al. Direct graphene synthesis on SiO2/Si substrate by ion implantation. Appl. Phys. Lett. 2013, 102, 193102. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Z.; Sun, H.; Dai, D.; Cui, J.; Li, M.; Xu, Y.; Xu, M.; Du, Y.; Jiang, N.; et al. Direct formation of wafer-scale single-layer graphene films on the rough surface substrate by PECVD. Carbon 2018, 129, 456–461. [Google Scholar] [CrossRef]
- Kato, T.; Hatakeyama, R. Direct Growth of Doping-Density-Controlled Hexagonal Graphene on SiO2 Substrate by Rapid-Heating Plasma CVD. ACS Nano 2012, 6, 8508–8515. [Google Scholar] [CrossRef]
- Baraton, L.; He, Z.; Lee, C.S.; Maurice, J.; Cojocaru, C.S.; Gourgues-Lorenzon, A.; Lee, Y.H.; Pribat, D. Synthesis of few-layered graphene by ion implantation of carbon in nickel thin films. Nanotechnology 2011, 22, 085601. [Google Scholar] [CrossRef] [Green Version]
- Mun, J.H.; Lim, S.K.; Cho, B.J. Local growth of graphene by ion implantation of carbon in a nickel thin film followed by rapid thermal annealing. J. Electrochem. Soc. 2012, 159, G89–G92. [Google Scholar] [CrossRef]
- Kim, J.; Lee, G.; Kim, J. Wafer-scale synthesis of multi-layer graphene by high-temperature carbon ion implantation. Appl. Phys. Lett. 2015, 107, 033104. [Google Scholar] [CrossRef]
- Bautista-Flores, C.; Sato-Berrú, R.Y.; Mendoza, D. Raman spectroscopy of CVD graphene during transfer process from copper to SiO2/Si substrates. Mater. Res. Express 2019, 6, 015601. [Google Scholar] [CrossRef]
- Sedlovets, D.M.; Knyazev, M.A.; Trofimov, O.V.; Koveshnikov, S.V. Graphene-like film CVD on e-beam exposed SiO2/Si by the pyrolysis of different oxygen-containing precursors. Fullerness Nanotub. Carbon Nanostruct. 2020, 28, 309–312. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhang, H.; Song, L.; Ye, Q.; Cai, J. Synthesize monolayer graphene on SiO2/Si substrate with copper-vapor-assisted CVD method. Mater. Res. Express 2018, 5, 125601. [Google Scholar] [CrossRef]
- Shrestha, D.; Kolar, G.; Jiao, J. Characterization of Graphene Directly Grown at Ni/SiO2 Interface Using Inductively Coupled Chemical Vapor Deposition (ICP-CVD) at a Low Temperature. Microsc. Microanal. 2020, 26, 2336–2337. [Google Scholar] [CrossRef]
- Entani, S.; Takizawa, M.; Li, S.; Naramoto, H.; Sakai, S. Growth of graphene on SiO2 with hexagonal boron nitride buffer layer. Appl. Surf. Sci. 2019, 475, 6–11. [Google Scholar] [CrossRef]
- Xu, M.; Guo, X.; Chen, L.; Yu, A.; Zhou, X.; Wang, H.; Gu, Y.; Wang, F.; Zhu, Y. Gate-polarity-dependent doping effects of H2O adsorption on graphene/SiO2 field-effect transistors. J. Phys. D Appl. Phys. 2020, 53, 455301. [Google Scholar] [CrossRef]
- Woo, H.J.; Kim, S.; Choi, Y.; Cho, J.H.; Kim, S.H.; Song, Y.J. Inhomogeneous work-function hysteresis in chemical vapor deposition-grown graphene field-effect devices. Carbon 2021, 173, 594–599. [Google Scholar] [CrossRef]
- Inanc, D.O.; Celebi, C.; Yildiz, U.H. Lipid bilayer on wrinkled-interfaced graphene field effect transistor. Mater. Lett. 2021, 284, 128998. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, T.; Zhou, X.; Liu, X.; Li, X.; Zhou, K.; Dong, L.; Wei, D. Three-Dimensional Graphene Composite Containing Graphene-SiO2 Nanoballs and Its Potential Application in Stress Sensors. Nanomaterials 2019, 9, 438. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Qian, L.; Yang, B. Surface acoustic wave humidity sensor based on three-dimensional architecture graphene/PVA/SiO2 and its application for respiration monitoring. Sensor. Actuators B Chem. 2020, 308, 127693. [Google Scholar] [CrossRef]
- Li, J.; Zheng, P.; Dong, L.; Yang, W.; Liu, C.; Yang, Y.; Xue, Z.; Liu, G.; Li, P.; Di, Z. Centimeter-Scale Ge-Assisted Grown Graphene Directly on SiO2/Si for NO2 Gas Sensors. IEEE Sens. J. 2021, 21, 5164–5172. [Google Scholar] [CrossRef]
- Ansari, Z.A.; Singh, T.J.; Islam, S.M.; Singh, S.; Mahala, P.; Khan, A.; Singh, K.J. Photovoltaic solar cells based on graphene/gallium arsenide Schottky junction. Optik 2019, 182, 500–506. [Google Scholar] [CrossRef]
- Liu, J.; Xie, X.; Du, P.; Liu, Y.; Yang, H. Effect of Graphene on the Sunlight Absorption Rate of Silicon Thin Film Solar Cells. Plasmonics 2019, 14, 353–357. [Google Scholar] [CrossRef]
- Shin, D.H.; Kwak, G.Y.; Kim, J.M.; Jang, C.W.; Choi, S.; Kim, K.J. Remarkable enhancement of stability in high-efficiency Si-quantum-dot heterojunction solar cells by employing bis(trifluoromethanesulfonyl)-amide as a dopant for graphene transparent conductive electrodes. J. Alloy. Compd. 2019, 773, 913–918. [Google Scholar] [CrossRef]
- Sánchez-Dena, O.; Villagómez, C.J.; Fierro-Ruíz, C.D.; Padilla-Robles, A.S.; Farías, R.; Vigueras-Santiago, E.; Hernández-López, S.; Reyes-Esqueda, J.-A. Determination of the Chemical Composition of Lithium Niobate Powders. Crystals 2019, 9, 340. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.A.; Szaller, Z. Recent Progress in Lithium Niobate. Crystals 2020, 10, 780. [Google Scholar] [CrossRef]
- Sánchez-Dena, O.; Villalobos-Mendoza, S.D.; Farías, R.; Fierro-Ruiz, C.D. Lithium Niobate Single Crystals and Powders Reviewed—Part II. Crystals 2020, 10, 990. [Google Scholar] [CrossRef]
- Bazzan, M.; Fontana, M. Preface to Special Topic: Lithium Niobate Properties and Applications: Reviews of Emerging Trends. Appl. Phys. Rev. 2015, 2, 040501. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhao, H.; Hu, G.; Li, S.; Su, H.; Zhang, J. Graphene based surface plasmon polariton modulator controlled by ferroelectric domains in lithium niobate. Sci. Rep. 2015, 5, 18258. [Google Scholar] [CrossRef] [Green Version]
- Salas, O.; Garcés, E.; Castillo, F.L.; Magaña, L.F. Changes in the reflectivity of a lithium niobate crystal decorated with a graphene layer. J. Phys. Conf. Ser. 2017, 792, 012069. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Lu, F.; Li, K.; Xu, Y.; Ma, C. Synthesis of turbostratic graphene by direct carbon ions implantation on LiNbO3. Appl. Surf. Sci. 2019, 493, 1255–1259. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, F.; Liu, K.; Ma, C. Direct Graphene Synthesis on Lithium Niobate Substrate by Carbon Ion Implantation. Front. Mater. 2020, 7, 572280. [Google Scholar] [CrossRef]
- Weigel, T.; Funke, C.; Zschornak, M.; Behm, T.; Stocker, H.; Leisegang, T.; Meyer, D.C. X-ray diffraction using focused-ion-beam-prepared single crystals. J. Appl. Cryst. 2020, 53, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard III, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Salas, O.; Garcés, E.; Castillo, F.L.; Magaña, L.F. Absorption and reflectivity of the lithium niobate surface masked with a graphene layer. AIP Adv. 2017, 7, 015305. [Google Scholar] [CrossRef] [Green Version]
- Salas, O.; Garces, E.; Magana, L.F. Optical Absorption and Reflectivity of a Molecular Cluster of Lithium Niobate Adsorbed on a Graphene Layer. Crystals 2018, 8, 208. [Google Scholar] [CrossRef] [Green Version]

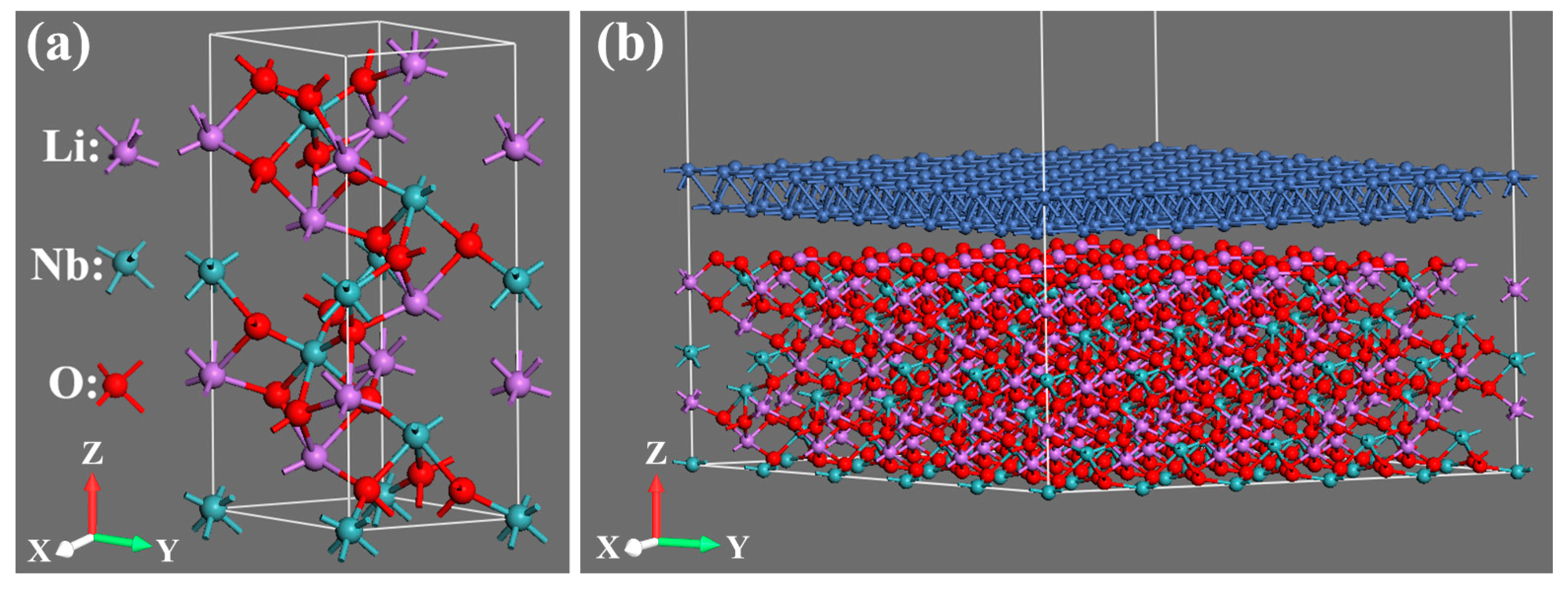
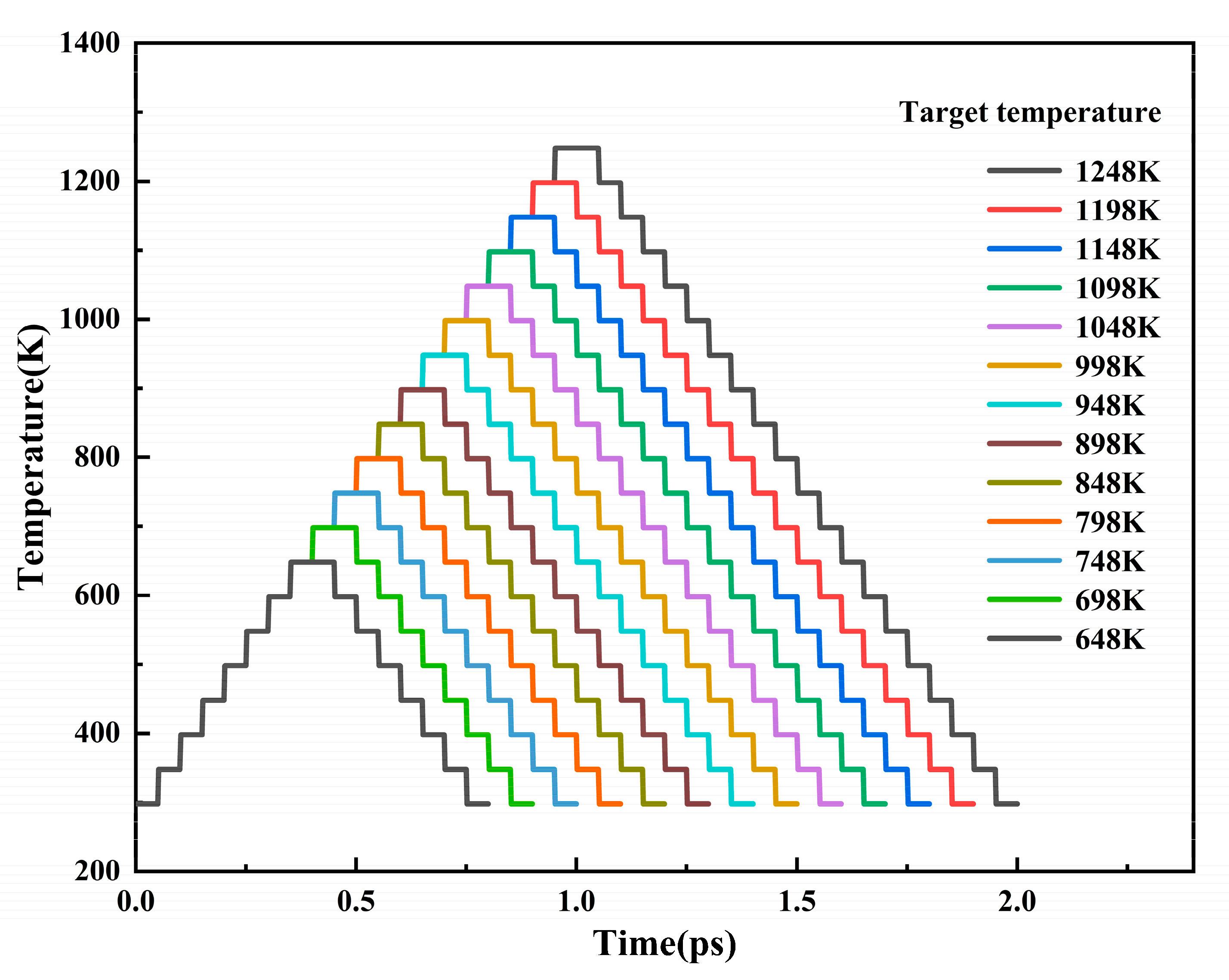


| Atom | x (Å) | y (Å) | z (Å) |
|---|---|---|---|
| Li | 0 | 0 | 0.2815 |
| Nb | 0 | 0 | 0.000055 |
| O | 0.0489 | 0.3438 | 0.06342 |
| Parameters | a (Å) | c (Å) | α | γ | V (Å3) |
|---|---|---|---|---|---|
| Initial | 5.1516 | 13.869 | 90° | 120° | 318.757 |
| Optimized | 5.19209 | 14.0134 | 90° | 120° | 327.159 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Lu, F.; Liu, K.; Ma, C. Thermodynamic Study of Adsorption Capacity between Metal Film and Optical Crystal: Adsorption Energy of Ni Films on LiNbO3 Substrates. Crystals 2021, 11, 1273. https://doi.org/10.3390/cryst11111273
Xu Y, Lu F, Liu K, Ma C. Thermodynamic Study of Adsorption Capacity between Metal Film and Optical Crystal: Adsorption Energy of Ni Films on LiNbO3 Substrates. Crystals. 2021; 11(11):1273. https://doi.org/10.3390/cryst11111273
Chicago/Turabian StyleXu, Yuhang, Fei Lu, Kaijing Liu, and Changdong Ma. 2021. "Thermodynamic Study of Adsorption Capacity between Metal Film and Optical Crystal: Adsorption Energy of Ni Films on LiNbO3 Substrates" Crystals 11, no. 11: 1273. https://doi.org/10.3390/cryst11111273
APA StyleXu, Y., Lu, F., Liu, K., & Ma, C. (2021). Thermodynamic Study of Adsorption Capacity between Metal Film and Optical Crystal: Adsorption Energy of Ni Films on LiNbO3 Substrates. Crystals, 11(11), 1273. https://doi.org/10.3390/cryst11111273





