Backbone Conformation Shifts in X-ray Structures of Human Acetylcholinesterase upon Covalent Organophosphate Inhibition
Abstract
:1. Introduction
2. Materials and Methods
3. Results
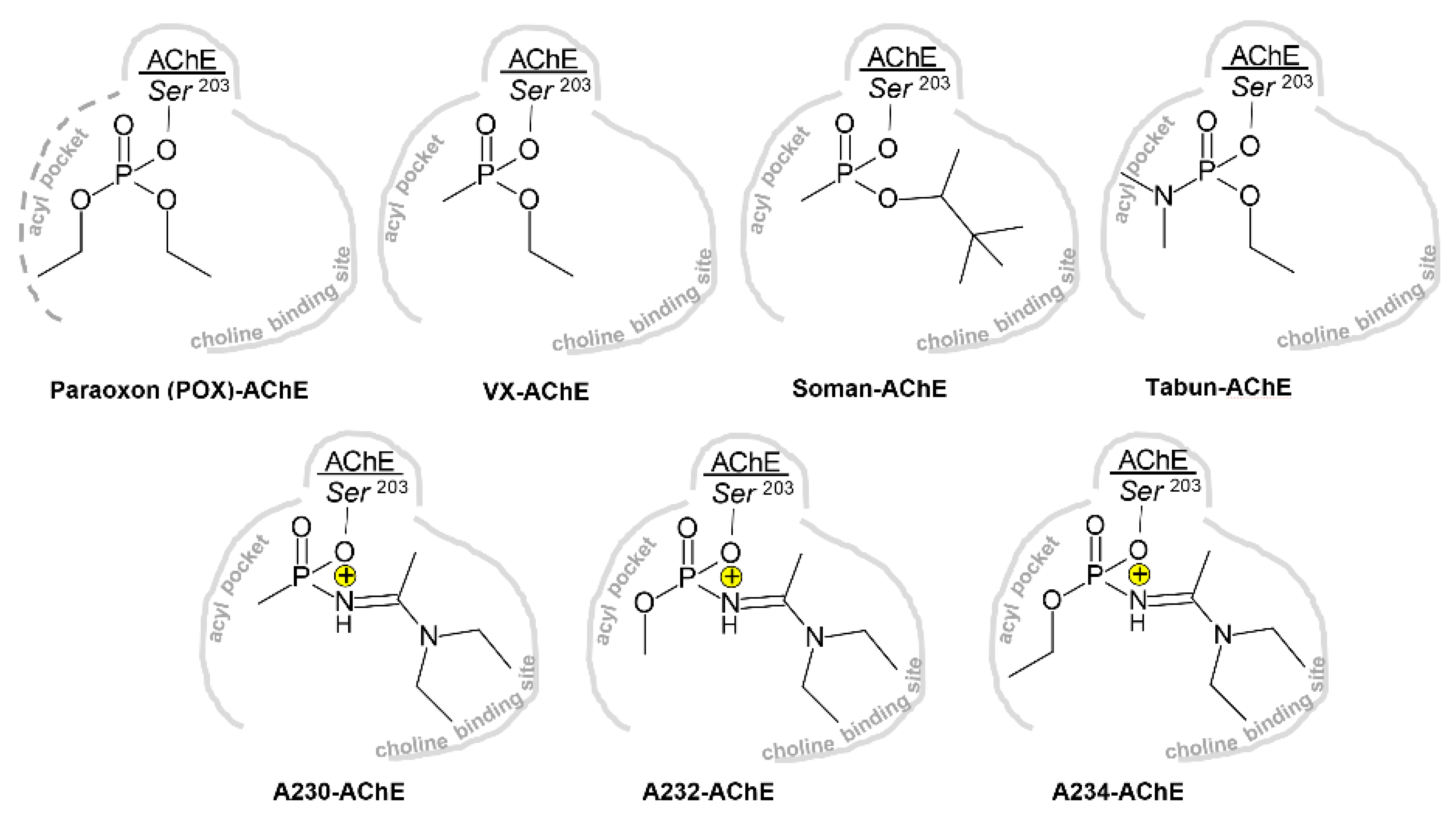
3.1. Effects of Conjugation of an OP Pesticide and “Classical” Nerve Agent OPs to hAChE
3.1.1. Conjugation of Paraoxon (POX)
3.1.2. Conjugation of “Classical” Nerve Agent OPs
3.2. Effects of Conjugation of the Novel, A-Type Nerve Agent OPs to hAChE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taylor, P. Anticholinesterase Agents. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollman, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2018; pp. 163–177. [Google Scholar]
- Aldridge, W.N.; Reiner, E. Enzyme Inhibitors as Substrates: Interactions of Esterases with Esters of Organophosphorus and Carbamic Acids, 1st ed.; North-Holland Pub. Co.: Amsterdam, Holland, 1972; 328p. [Google Scholar]
- Gerlits, O.; Blakeley, M.P.; Keen, D.A.; Radić, Z.; Kovalevsky, A. Room temperature crystallography of human acetylcholinesterase bound to a substrate analogue 4K-TMA: Towards a neutron structure. Curr. Res. Struct. Biol. 2021, 3, 206–215. [Google Scholar] [CrossRef]
- Harvey, S.P.; McMahon, L.R.; Berg, F.J. Hydrolysis and enzymatic degradation of Novichok nerve agents. Heliyon 2020, 6, 1–4, e03153. [Google Scholar] [CrossRef]
- OPCW. Decision: Recommendation for a Change to Schedule 1 of the Annex on Chemicals to the Chemical Weapons Convention. In Proceedings of the Sixty-Second Meeting of the Executive Council, The Hague, The Netherlands, 14 January 2019. EC-M-62/DEC.1*. [Google Scholar]
- OPCW. Opening Statement by the Director-General to the Executive Council at its Ninetieth Session. In Proceedings of the Ninetieth Meeting of the Executive Council, The Hague, The Netherlands, 12 March 2019. Notes by Director-General; EC-90/DG.16*. [Google Scholar]
- Dawson, A.H.; Eddleston, M.; Senarathna, L.; Mohamed, F.; Gawarammana, I.; Bowe, S.J.; Manuweera, G.; Buckley, N.A. Acute human lethal toxicity of agricultural pesticides: A prospective cohort study. PLoS Med. 2010, 7, e1000357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolgin, E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat. Med. 2013, 19, 1194−1195. [Google Scholar] [CrossRef]
- Stone, R.U.K. attack shines spotlight on deadly nerve agent developed by Soviet scientists. Science 2018, 359, 1314–1315. [Google Scholar] [CrossRef]
- Stone, R. How German military scientists likely identified the nerve agent used to attack Alexei Navalny. Science 2020. [Google Scholar] [CrossRef]
- Mirzayanov, V.S. State Secrets: An Insider’s Chronicle of the Russian Chemical Weapons Program; Outskirts Press: Denver, Colorado, 2009; ISBN 978-1-4327-2566-2. [Google Scholar]
- Sit, R.K.; Kovarik, Z.; Maček Hrvat, N.; Žunec, S.; Green, C.; Fokin, V.V.; Sharpless, K.B.; Radić, Z.; Taylor, P. Pharmacology, Pharmacokinetics, and Tissue Disposition of Zwitterionic Hydroxyiminoacetamido Alkylamines as Reactivating Antidotes for Organophosphate Exposure. J. Pharmacol. Exp. Ther. 2018, 367, 363–372. [Google Scholar] [CrossRef]
- Franklin, M.C.; Rudolph, M.J.; Ginter, C.; Cassidy, M.S.; Cheung, J. Structures of paraoxon-inhibited human acetylcholinesterase reveal perturbations of the acyl loop and the dimer interface. Proteins 2016, 84, 1246–1256. [Google Scholar] [CrossRef]
- Blumenthal, D.K.; Cheng, X.; Fajer, M.; Ho, K.Y.; Rohrer, J.; Gerlits, O.; Taylor, P.; Juneja, P.; Kovalevsky, A.; Radić, Z. Covalent inhibition of hAChE by organophosphates causes homodimer dissociation through long-range allosteric effects. J. Biol. Chem. 2021, 297, 1–14. [Google Scholar] [CrossRef]
- Bester, S.M.; Guelta, M.A.; Height, J.J.; Pegan, S.D. Crystal Structure of Recombinant Human Acetylcholinesterase Inhibited by A-234. 2020. Available online: https://www.rcsb.org/structure/6NTL (accessed on 20 September 2021).
- wwPDB Consortium. Protein Data Bank: The single global archive for 3D macromolecular structure data. Nucleic Acids Res. 2019, 47, D520–D528. [Google Scholar]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Grochulski, P.; Li, Y.; Schrag, J.D.; Bouthillier, F.; Smith, P.; Harrison, D.; Rubin, B.; Cygler, M. Insights into interfacial activation from an open structure of Candida rugosa lipase. J. Biol. Chem. 1993, 268, 12843–12847. [Google Scholar] [CrossRef]
- Radić, Z.; Pickering, N.A.; Vellom, D.C.; Camp, S.; Taylor, P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry 1993, 32, 12074–12084. [Google Scholar] [CrossRef]
- McGuire, J.R.; Bester, S.M.; Guelta, M.A.; Cheung, J.; Langley, C.; Winemiller, M.D.; Bae, S.Y.; Funk, V.; Myslinski, J.M.; Pegan, S.D.; et al. Structural and Biochemical Insights into the Inhibition of Human Acetylcholinesterase by G-Series Nerve Agents and Subsequent Reactivation by HI-6. Chem. Res. Toxicol. 2021, 34, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Sussman, J.L.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef]
- Cheng, Y.; Suen, J.K.; Radić, Z.; Bond, S.D.; Holst, M.J.; McCammon, J.A. Continuum simulations of acetylcholine diffusion with reaction-determined boundaries in neuromuscular junction models. Biophys. Chem. 2007, 127, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millard, C.B.; Kryger, G.; Ordentlich, A.; Greenblatt, H.M.; Harel, M.; Raves, M.L.; Segall, Y.; Barak, D.; Shafferman, A.; Silman, I.; et al. Crystal structures of aged phosphonylated acetylcholinesterase: Nerve agent reaction products at the atomic level. Biochemistry 1999, 38, 7032–7039. [Google Scholar] [CrossRef]
- Hörnberg, A.; Tunemalm, A.K.; Ekström, F. Crystal structures of acetylcholinesterase in complex with organophosphorus compounds suggest that the acyl pocket modulates the aging reaction by precluding the formation of the trigonal bipyramidal transition state. Biochemistry 2007, 46, 4815–4825. [Google Scholar] [CrossRef]
- Raves, M.L.; Harel, M.; Pang, Y.P.; Silman, I.; Kozikowski, A.P.; Sussman, J.L. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat. Struct. Biol. 1997, 4, 57–63. [Google Scholar] [CrossRef]
- Santoni, G.; de Sousa, J.; de la Mora, E.; Dias, J.; Jean, L.; Sussman, J.L.; Silman, I.; Renard, P.Y.; Brown, R.; Weik, M.; et al. Structure-Based Optimization of Nonquaternary Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. J. Med. Chem. 2018, 61, 7630–7639. [Google Scholar] [CrossRef] [Green Version]
- Gorecki, L.; Gerlits, O.; Kong, X.; Cheng, X.; Blumenthal, D.K.; Taylor, P.; Ballatore, C.; Kovalevsky, A.; Radić, Z. Rational design, synthesis, and evaluation of uncharged, “smart” bis-oxime antidotes of organophosphate-inhibited human acetylcholinesterase. J. Biol. Chem. 2020, 295, 4079–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colletier, J.P.; Fournier, D.; Greenblatt, H.M.; Stojan, J.; Sussman, J.L.; Zaccai, G.; Silman, I.; Weik, M. Structural insights into substrate traffic and inhibition in acetylcholinesterase. EMBO J. 2006, 25, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Radić, Z.; Sulzenbacher, G.; Kim, E.; Taylor, P.; Marchot, P. Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding. J. Biol. Chem. 2006, 281, 29256–29267. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Structure | PDB ID | Resolution (Å2) | AP Group | Affected Structural Element in hAChE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active Center | PAS | C-Terminus | Backside Surface | ||||||||||
| Ω Loop (69–96) | AP Loop (285–300) | α-Helix (280–288) | α-Helix I (335–352) | α-Helix II (356–380) | Loop (160–165) | α-Helix + Loop (180–200) | α-Helix (215–230) | Loop (300–310) | α-Helix (315–325) | ||||
| Apo hAChE | 4EY4 | 2.16 | - | - | - | - | - | - | - | - | - | - | - |
| POX-hAChE | 5HF5 | 2.15 | CH3CH2-O- | - | ++++ | - | + | ++++ | - | - | - | - | - |
| POX-hAChE *HI6 | 5HF9 | 2.20 | CH3CH2-O- | + | - | - | + | +++ | - | - | - | - | + |
| VX-hAChE | 6CQZ | 2.22 | CH3 - | - | - | - | - | + | + | + | + | + | + |
| VX-hAChE*HI6 | 6CQW | 2.28 | CH3 - | - | - | - | - | ++ | + | + | + | + | ++ |
| soman-hAChE | 6WVC | 2.60 | CH3 - | - | - | - | - | - | + | + | + | ++ | + |
| soman-hAChE*HI6 | 6WVO | 2.19 | CH3 - | + | - | - | + | ++ | - | + | + | - | - |
| tabun-hAChE | 6WUV | 2.63 | NH2 - | - | - | - | - | ++ | - | - | - | - | - |
| tabun-hAChE*HI6 | 6WUY | 2.46 | NH2 - | - | - | ++ | + | ++++ | + | - | - | - | + |
| A230-hAChE | 6NTO | 2.05 | CH3 - | - | - | - | - | + | - | + | + | + | - |
| A230-hAChE*HI6 | 6NTN | 2.70 | CH3 - | - | - | - | - | + | - | + | + | + | - |
| A232-hAChE | 6NTK | 2.41 | CH3-O- | - | - | - | + | - | + | - | - | + | - |
| A232-hAChE*HI6 | 6NTM | 2.55 | CH3-O- | - | - | - | - | - | + | - | - | + | - |
| A234-hAChE | 6NTL | 2.25 | CH3CH2-O- | - | - | - | + | ++ | + | + | + | + | ++ |
| A234-hAChE*HI6 | 6NTG | 2.65 | CH3CH2-O- | - | - | - | + | +++ | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luedtke, S.; Bojo, C.; Li, Y.; Luna, E.; Pomar, B.; Radić, Z. Backbone Conformation Shifts in X-ray Structures of Human Acetylcholinesterase upon Covalent Organophosphate Inhibition. Crystals 2021, 11, 1270. https://doi.org/10.3390/cryst11111270
Luedtke S, Bojo C, Li Y, Luna E, Pomar B, Radić Z. Backbone Conformation Shifts in X-ray Structures of Human Acetylcholinesterase upon Covalent Organophosphate Inhibition. Crystals. 2021; 11(11):1270. https://doi.org/10.3390/cryst11111270
Chicago/Turabian StyleLuedtke, Stephanie, Celine Bojo, Yunshen Li, Emilio Luna, Bianca Pomar, and Zoran Radić. 2021. "Backbone Conformation Shifts in X-ray Structures of Human Acetylcholinesterase upon Covalent Organophosphate Inhibition" Crystals 11, no. 11: 1270. https://doi.org/10.3390/cryst11111270








