A Literature Review on High-Performance Photocatalysts for Sustainable Cancer Therapy
Abstract
:1. Introduction
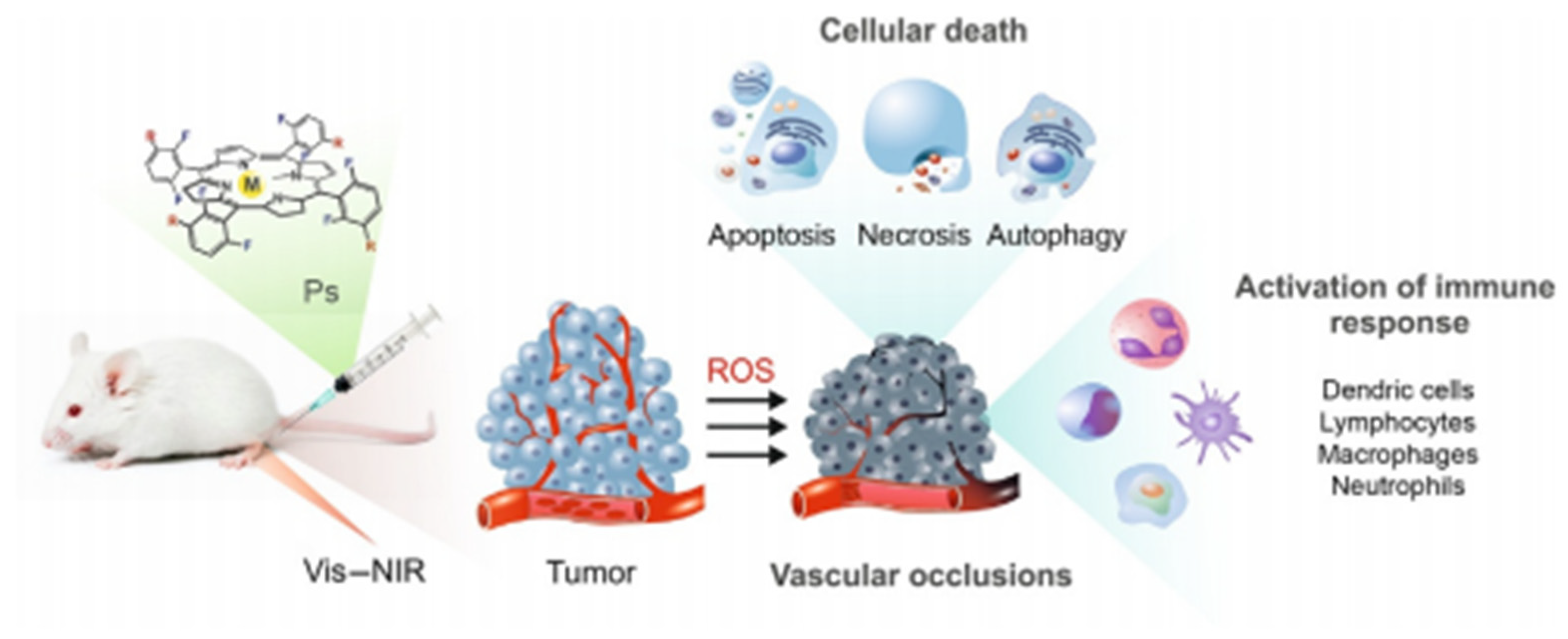
2. Photocatalysts for Cancer Therapy
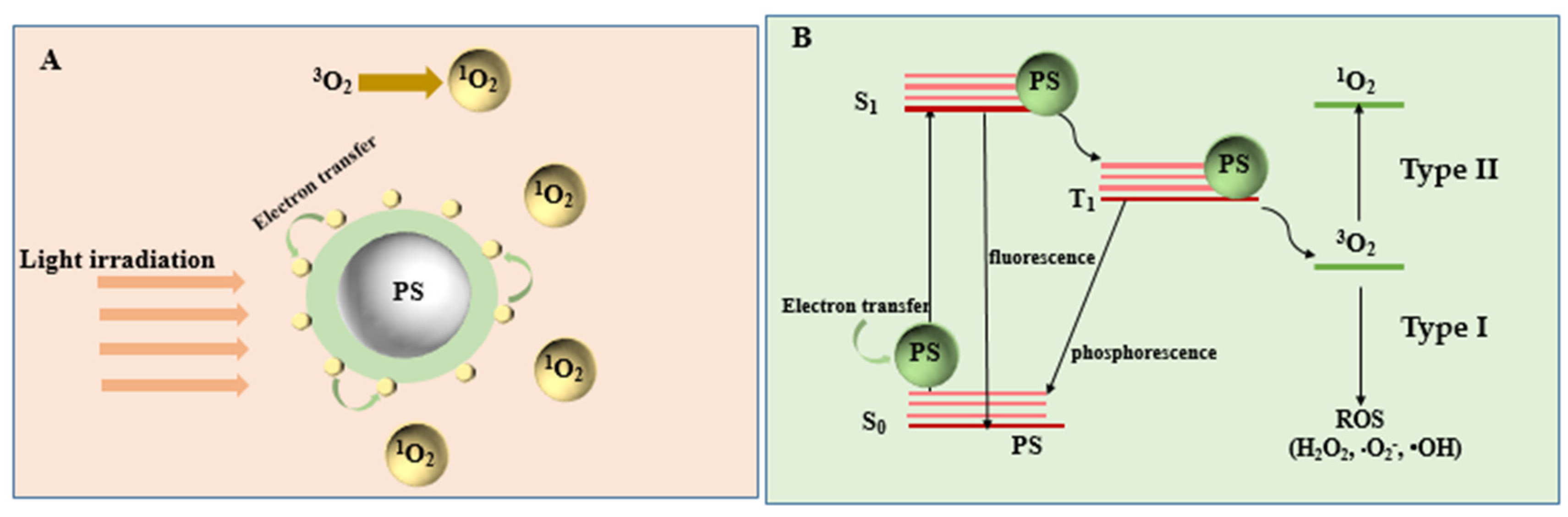
Mechanism of PDT
3. Common Photocatalysts for Cancer Therapy
3.1. Supermolecular Photocatalyst
3.2. Metal and Metal Oxide Photocatalysts for Cancer Therapy
3.2.1. IrIII Photocatalyst for Cancer Therapy
3.2.2. Cerium Oxide for Cancer Therapy
3.2.3. Titanium Dioxide for Cancer Therapy
3.2.4. Cuprous Oxide for Cancer Therapy
3.3. Z-Scheme Structure Photocatalyst
3.4. Piezocatalysis for Cancer Therapy
4. Up-Conversion Nanoparticles for Photocatalytic Anti-Cancer Therapy
5. Challenges and Prospects of Photocatalysts for Cancer Cell Treatment
- How to control the ultraviolet-visible light to trigger the photocatalytic reaction for cancer therapy, breaking the limitation of penetration depth.
- Whether it can break through the mechanism of conventional reactive oxygen species to avoid further deterioration of the hypoxic environment in the tumor area.
- Whether the abundant reactive oxygen species can be produced in reducing environment in vivo to attack the DNA molecules in tumor cells.
- How to control the toxicity of photocatalysts and drug consumption caused by metabolism or immunity.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N. Targeting Cancer Cells with Nanotherapeutics and Nanodiagnostics: Current Status and Future Perspectives; Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 52–68. [Google Scholar]
- Boehm, J.S.; Garnett, M.J.; Adams, D.J.; Francies, H.E.; Golub, T.R.; Hahn, W.C.; Iorio, F.; McFarland, J.M.; Parts, L.; Vazquez, F. Cancer Research Needs a Better Map; Nature Publishing Group: Cambridge, MA, USA, 2021. [Google Scholar]
- Hahn, W.C.; Bader, J.S.; Braun, T.P.; Califano, A.; Clemons, P.A.; Druker, B.J.; Ewald, A.J.; Fu, H.; Jagu, S.; Kemp, C.J. An expanded universe of cancer targets. Cell 2021, 184, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Li, K.; Hou, P.; Xiao, R.; Yuan, Y.; Sun, Z. Nanoscale metal–organic framework composites for phototherapy and synergistic therapy of cancer. Mater. Chem. Front. 2021, 5, 1632–1654. [Google Scholar] [CrossRef]
- Bown, S. Phototherapy of tumors. World J. Surg. 1983, 7, 700–709. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Deng, K.; Wang, M.; Liu, Y.; Chang, M.; Huang, S.; Li, C.; Wei, Y.; Cheng, Z.; Han, G. Hydrogenated titanium oxide decorated upconversion nanoparticles: Facile laser modified synthesis and 808 nm near-infrared light triggered phototherapy. Chem. Mater. 2019, 31, 774–784. [Google Scholar] [CrossRef]
- Barbeira, A.N.; Dickinson, S.P.; Bonazzola, R.; Zheng, J.; Wheeler, H.E.; Torres, J.M.; Torstenson, E.S.; Shah, K.P.; Garcia, T.; Edwards, T.L. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat. Commun. 2018, 9, 1–20. [Google Scholar] [CrossRef]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.H.; Chung, H.J.; Park, T.G. Nanomaterials for cancer therapy and imaging. Mol. Cells 2011, 31, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Szaciłowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic photochemistry: Frontiers and mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- de Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial effect of titanium dioxide nanoparticles. In Antimicrobial Resistance-A One Health Perspective; IntechOpen: London, UK, 2020. [Google Scholar]
- Yamaguchi, K.; Sugiyama, T.; Kato, S.; Kondo, Y.; Ageyama, N.; Kanekiyo, M.; Iwata, M.; Koyanagi, Y.; Yamamoto, N.; Honda, M. A novel CD4–conjugated ultraviolet light-activated photocatalyst inactivates HIV–1 and SIV efficiently. J. Med. Virol. 2008, 80, 1322–1331. [Google Scholar] [CrossRef]
- Seo, J.W.; Chung, H.; Kim, M.Y.; Lee, J.; Choi, I.H.; Cheon, J. Development of water–soluble single–crystalline TiO2 nanoparticles for photocatalytic cancer–cell treatment. Small 2007, 3, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kalbacova, M.; Macak, J.; Schmidt-Stein, F.; Mierke, C.; Schmuki, P. TiO2 nanotubes: Photocatalyst for cancer cell killing. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2008, 2, 194–196. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Huang, J.; Chen, C.; Liu, G.; Jiang, Y.; Zhao, Y.; Jiang, Z. Photokilling cancer cells using highly cell-specific antibody–TiO2 bioconjugates and electroporation. Bioelectrochemistry 2007, 71, 217–222. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@ AgCl/ZnO nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloy. Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Chen, X.; Zhang, H.; Wang, J. A full–spectrum metal–free porphyrin supramolecular photocatalyst for dual functions of highly efficient hydrogen and oxygen evolution. Adv. Mater. 2019, 31, 1806626. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Yao, Y.; Wu, C.; Luo, W.; Zou, Z. Photocatalytic Materials: An Apollo’s Arrow to Tumor Cells. Trends Chem. 2020, 2, 1126–1140. [Google Scholar] [CrossRef]
- Rozhkova, E.A.; Ulasov, I.; Lai, B.; Dimitrijevic, N.M.; Lesniak, M.S.; Rajh, T. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano Lett. 2009, 9, 3337–3342. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Henderson, B.W. Photodynamic Therapy: Basic Principles and Clinical Applications; CRC Press: New York, NY, USA, 2020. [Google Scholar]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Y.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Aggregation-Induced Generation of Reactive Oxygen Species: Mechanism and Photosensitizer Construction. Molecules 2021, 26, 268. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M. Reactive oxygen species in photodynamic therapy: Mechanisms of their generation and potentiation. Adv. Inorg. Chem. 2017, 70, 343–394. [Google Scholar]
- Wang, J.; Liu, D.; Zhu, Y.; Zhou, S.; Guan, S. Supramolecular packing dominant photocatalytic oxidation and anticancer performance of PDI. Appl. Catal. B Environ. 2018, 231, 251–261. [Google Scholar] [CrossRef]
- Zou, Q.; Abbas, M.; Zhao, L.; Li, S.; Shen, G.; Yan, X. Biological photothermal nanodots based on self-assembly of peptide–porphyrin conjugates for antitumor therapy. J. Am. Chem. Soc. 2017, 139, 1921–1927. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Liu, W.; Yan, Z.; Zhu, Y.; Zhou, S.; Guan, S. Photogenerated-hole-induced rapid elimination of solid tumors by the supramolecular porphyrin photocatalyst. Natl. Sci. Rev. 2021, 8, nwaa155. [Google Scholar] [CrossRef]
- Wang, Y.; Du, W.; Zhang, T.; Zhu, Y.; Ni, Y.; Wang, C.; Raya, F.M.S.; Zou, L.; Wang, L.; Liang, G. A Self-Evaluating Photothermal Therapeutic Nanoparticle. ACS Nano 2020, 14, 9585–9593. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lu, Y.; Mu, X.; Chen, Z.; Liu, S.; Zhou, X.; Liu, S.; Li, Z. Intriguing H-Aggregates of Heptamethine Cyanine for Imaging-Guided Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 32388–32396. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Dwivedi, S.; Umar, A.; Singh, S.; Hwang, I.; Shin, H.-S.; Musarrat, J.; Al-Khedhairy, A.A.; Kim, Y.-S. ZnO nanoparticles induce oxidative stress in Cloudman S91 melanoma cancer cells. J. Biomed. Nanotechnol. 2013, 9, 441–449. [Google Scholar] [CrossRef]
- Wahab, R.; Kaushik, N.K.; Kaushik, N.; Choi, E.H.; Umar, A.; Dwivedi, S.; Musarrat, J.; Al-Khedhairy, A.A. ZnO nanoparticles induces cell death in malignant human T98G gliomas, KB and non-malignant HEK cells. J. Biomed. Nanotechnol. 2013, 9, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.-S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B Biointerfaces 2014, 117, 267–276. [Google Scholar] [CrossRef]
- Wason, M.S.; Colon, J.; Das, S.; Seal, S.; Turkson, J.; Zhao, J.; Baker, C.H. Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of conventional chemotherapeutics with redox-active cerium oxide nanoparticles—A novel aspect in cancer therapy. Mol. Cancer Ther. 2014, 13, 1740–1749. [Google Scholar] [CrossRef] [Green Version]
- Alili, L.; Sack, M.; Karakoti, A.S.; Teuber, S.; Puschmann, K.; Hirst, S.M.; Reilly, C.M.; Zanger, K.; Stahl, W.; Das, S. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor–stroma interactions. Biomaterials 2011, 32, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Zhang, H.; Zi, X.; Pan, X.; Chen, F.; Luo, W.; Li, J.; Zhu, H.; Hu, Y. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis. 2013, 4, e783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; He, Y.; Bo, R.; Ma, Z.; Wang, Z.; Dong, L.; Lin, T.-Y.; Xue, X.; Li, Y. A facile approach to fabricate self-assembled magnetic nanotheranostics for drug delivery and imaging. Nanoscale 2018, 10, 21634–21639. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef]
- Jukapli, N.M.; Bagheri, S. Recent developments on titania nanoparticle as photocatalytic cancer cells treatment. J. Photochem. Photobiol. B Biol. 2016, 163, 421–430. [Google Scholar] [CrossRef]
- Kubota, Y.; Shuin, T.; Kawasaki, C.; Hosaka, M.; Kitamura, H.; Cai, R.; Sakai, H.; Hashimoto, K.; Fujishima, A. Photokilling of T-24 human bladder cancer cells with titanium dioxide. Br. J. Cancer 1994, 70, 1107–1111. [Google Scholar] [CrossRef]
- Huang, C.; Liang, C.; Sadhukhan, T.; Banerjee, S.; Fan, Z.; Li, T.; Zhu, Z.; Zhang, P.; Raghavachari, K.; Huang, H. In–vitro and In–vivo Photocatalytic Cancer Therapy with Biocompatible Iridium (III) Photocatalysts. Angew. Chem. 2021, 133, 9560–9565. [Google Scholar] [CrossRef]
- Tudor, D.; Nenu, I.; Filip, G.A.; Olteanu, D.; Cenariu, M.; Tabaran, F.; Ion, R.M.; Gligor, L.; Baldea, I. Combined regimen of photodynamic therapy mediated by Gallium phthalocyanine chloride and Metformin enhances anti-melanoma efficacy. PLoS ONE 2017, 12, e0173241. [Google Scholar] [CrossRef]
- Maji, S.K.; Kim, D.H. AgInS2-coated upconversion nanoparticle as a photocatalyst for near-infrared light-activated photodynamic therapy of cancer cells. ACS Appl. Bio Mater. 2018, 1, 1628–1638. [Google Scholar] [CrossRef]
- Wheate, N.J.; Collins, J.G. Multi-nuclear platinum complexes as anti-cancer drugs. Coord. Chem. Rev. 2003, 241, 133–145. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, Y.; Yao, X.; Chen, D.; Fan, M.; Jin, Z.; He, Q. Photocatalysis-mediated drug-free sustainable cancer therapy using nanocatalyst. Nat. Commun. 2021, 12, 1–11. [Google Scholar]
- Sang, D.; Wang, K.; Sun, X.; Wang, Y.; Lin, H.; Jia, R.; Qu, F. NIR-Driven Intracellular Photocatalytic O2 Evolution on Z-Scheme Ni3S2/Cu1. 8S@ HA for Hypoxic Tumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 9604–9619. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Chen, Y.; Shi, J. Piezocatalytic tumor therapy by ultrasound-triggered and BaTiO3-mediated piezoelectricity. Adv. Mater. 2020, 32, 2001976. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Shen, Z.; Pei, Y.; Lv, Y.; Chen, X.; Ren, J.; Yang, K.; Pei, Z. Pillar [5] arene-based supramolecular photosensitizer for enhanced hypoxic-tumor therapeutic effectiveness. Chem. Commun. 2021, 57, 7625–7628. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, D.; Wang, Y.; Wang, K.; Yi, X.; Kong, D.; Yang, Z.; Liu, Q.; Ding, D. Biocompatible fluorescent supramolecular nanofibrous hydrogel for long-term cell tracking and tumor imaging applications. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhao, J.; Wang, A.; Li, Q.; Cui, W. Supramolecular assembly of protein-based nanoparticles based on tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) for cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124486. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Banerjee, S.; Qiu, K.; Zhang, P.; Blacque, O.; Malcomson, T.; Paterson, M.J.; Clarkson, G.J.; Staniforth, M.; Stavros, V.G. Targeted photoredox catalysis in cancer cells. Nat. Chem. 2019, 11, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Romero-Canelón, I.; Qamar, B.; Hearn, J.M.; Habtemariam, A.; Barry, N.P.; Pizarro, A.M.; Clarkson, G.J.; Sadler, P.J. The potent oxidant anticancer activity of organoiridium catalysts. Angew. Chem. 2014, 126, 4022–4027. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Luan, Q.; Yang, D.; Yao, X.; Zhou, K. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J. Phys. Chem. C 2011, 115, 4433–4438. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase–like activity of polymer–coated cerium oxide nanoparticles. Angew. Chem. 2009, 121, 2344–2348. [Google Scholar] [CrossRef]
- Lin, W.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int. J. Toxicol. 2006, 25, 451–457. [Google Scholar] [CrossRef]
- Park, E.-J.; Choi, J.; Park, Y.-K.; Park, K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 2008, 245, 90–100. [Google Scholar] [CrossRef]
- Pešić, M.; Podolski-Renić, A.; Stojković, S.; Matović, B.; Zmejkoski, D.; Kojić, V.; Bogdanović, G.; Pavićević, A.; Mojović, M.; Savić, A. Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chem. Biol. Interact. 2015, 232, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Raja, G.; Cao, S.; Kim, D.-H.; Kim, T.-J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater. Sci. Eng. C 2020, 107, 110303. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Horikoshi, S.; Serpone, N.; Knowland, J. In vitro photochemical damage to DNA, RNA and their bases by an inorganic sunscreen agent on exposure to UVA and UVB radiation. J. Photochem. Photobiol. A Chem. 1997, 111, 205–213. [Google Scholar] [CrossRef]
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.-P.; Min-hua, X.; Yuan, C.-W.; Rui-rong, Y. The study of the photokilling effect and mechanism of ultrafine TiO2 particles on U937 cells. J. Photochem. Photobiol. A Chem. 1997, 108, 229–233. [Google Scholar] [CrossRef]
- Li, Z.; He, J.; Li, B.; Zhang, J.; He, K.; Duan, X.; Huang, R.; Wu, Z.; Xiang, G. Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated apoptotic cell death in liver cancer cells. J. Int. Med Res. 2020, 48, 0300060520903652. [Google Scholar] [CrossRef]
- Botelho, M.C.; Costa, C.; Silva, S.; Costa, S.; Dhawan, A.; Oliveira, P.A.; Teixeira, J.P. Effects of titanium dioxide nanoparticles in human gastric epithelial cells in vitro. Biomed. Pharmacother. 2014, 68, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Wang, Y.; Yang, Q.; Gao, Y.; Duan, X.; Fu, Q.; Chu, C.; Pan, X.; Cui, X.; Sun, Y. Cuprous oxide nanoparticles trigger ER stress-induced apoptosis by regulating copper trafficking and overcoming resistance to sunitinib therapy in renal cancer. Biomaterials 2017, 146, 72–85. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, B.; Yang, X.; Xiao, Y.; Wang, X.; Shao, C.; Tang, R. A drug-free tumor therapy strategy: Cancer-cell-targeting calcification. Angew. Chem. Int. Ed. 2016, 55, 5225–5229. [Google Scholar] [CrossRef]
- Huo, M.; Wang, L.; Chen, Y.; Shi, J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem. Soc. Rev. 2018, 47, 1938–1958. [Google Scholar] [CrossRef]
- Fan, K.; Xi, J.; Fan, L.; Wang, P.; Zhu, C.; Tang, Y.; Xu, X.; Liang, M.; Jiang, B.; Yan, X. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef]
- Pan, C.; Ou, M.; Cheng, Q.; Zhou, Y.; Yu, Y.; Li, Z.; Zhang, F.; Xia, D.; Mei, L.; Ji, X. Z-scheme Heterojunction functionalized pyrite Nanosheets for modulating tumor microenvironment and strengthening photo/Chemodynamic therapeutic effects. Adv. Funct. Mater. 2020, 30, 1906466. [Google Scholar] [CrossRef]
- Kang, Y.; Lei, L.; Zhu, C.; Zhang, H.; Mei, L.; Ji, X. Piezo-Photocatalytic Effect Mediating Reactive Oxygen Species Burst for Cancer Catalytic Therapy. Mater. Horiz. 2021, 8, 2273–2285. [Google Scholar] [CrossRef]
- Dou, Q.Q.; Rengaramchandran, A.; Selvan, S.T.; Paulmurugan, R.; Zhang, Y. Core–shell upconversion nanoparticle–semiconductor heterostructures for photodynamic therapy. Sci. Rep. 2015, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rimoldi, T.; Orsi, D.; Lagonegro, P.; Ghezzi, B.; Galli, C.; Rossi, F.; Salviati, G.; Cristofolini, L. CeF3-ZnO scintillating nanocomposite for self-lighted photodynamic therapy of cancer. J. Mater. Sci. Mater. Med. 2016, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.N.; Zhang, F.; Zhang, C.L.; Guo, Y.C.; Dai, W.; Qian, H.S. Fabrication of Zinc Oxide Composite Microfibers for Near-Infrared-Light-Mediated Photocatalysis. ChemCatChem 2017, 9, 3611–3617. [Google Scholar] [CrossRef]
- Gu, B.; Pliss, A.; Kuzmin, A.N.; Baev, A.; Ohulchanskyy, T.Y.; Damasco, J.A.; Yong, K.-T.; Wen, S.; Prasad, P.N. In-situ second harmonic generation by cancer cell targeting ZnO nanocrystals to effect photodynamic action in subcellular space. Biomaterials 2016, 104, 78–86. [Google Scholar] [CrossRef] [PubMed]
| Photocatalysts | The Wavelength of Exciting Lights, Irradiation Time | Properties and Application in Cancer Therapy | Mechanism | Performance/ Efficiency | Ref. | |
|---|---|---|---|---|---|---|
| Supramolecular photocatalyst | J-aggregated perylenetetracarboxylic diimide | Irradiated under 600 nm (220 mW/cm2) for 10 min at 12 h post-injection | Higher biocompatibility and lower cytotoxicity in the dark, exhibiting potential application in photocatalytic anti-cancer treatment for Hela cells. | High 1O2 quantum yields. | Have a 1O2 quantum yields of 0.66; phenol degradation reached more than 50% in 4 h. | [32] |
| Peptide−Porphyrin Conjugates | Irradiated with a laser (0.3 W) for 10 min at 24 h post-injection | High biocompatibility, and efficient inhibition of MCF-7 tumors. | Various light-absorbing molecules, especially possessing near-infrared absorbance. | The tumors can be successfully ablated via treating with peptide−porphyrin photothermal nanodots under light irradiation. | [33] | |
| Nano-tetra-carboxyphenyl porphyrin | Irradiated with a 600 nm light of 0.1 W cm−2 for 10 min after injection of photosensitize. | High biocompatibility, killing Hela cells, and had significant effects on MCF-7, HepG-2. | Excellent singlet oxygen evolution. | It can completely ablate the subcutaneous tumor cells of 100 mm3, and photocatalyst dispersion of 25 μg mL−1 can completely kill the Hela cells. | [34] | |
| Biotin-CystamineCys-Lys(Cypate)-CBT | 808 nm laser irradiation at 0.4 W cm−2 for 5 min. | Photosensitize nanoparticle was uptaken by HeLa cells mainly through the endocytosis pathway. | Increased the PTT efficiency of the tumors through simultaneous intra- and intermolecular fluorescence quenching of the Cypate fluorophore. | Had an excellent PTT effect on the cells with a half-inhibitory concentration of 24.4 ± 7.0 μM. | [35] | |
| Diketoole-triphenylamine | 808 nm laser irradiation for 15 min. | High light-to-heat conversion efficiency, better EPR effect, and better phototherapy efficacy. | - | Tumors with a volume of 50 mm3 were successfully ablated. | [36] | |
| Metal Oxide | ZnO nanoparticles | - | More efficacious on cancer cells T98 G, HepG2, MCF-7 and less toxic on normal human cells. | Killing cancer cells by increasing both mitotic and interphase death. | HepG2 and MCF-7 cells exhibited a significant cell viability reduction(95% and 96%; p < 0.05) when treated with 25 g/mL ZnO nanoparticles. | [37,38,39] |
| Cerium oxide | Irradiated with a light of 463 nm wavelength for 30 min. | Good performance in inhibition of 518A2, HT-29 etc. cancer cells. | Affecting the formation of myofibroblasts, exhibiting cytotoxicity, and invading tumor cells | Compared to no treatment group, cerium oxide induced cancer cell death (12.5%, p = 0.0055). | [40,41,42] | |
| Copper oxide | Killing B16-F10 and HeLa cells in a dose- and time-dependent manner. | Low toxicity and can be rapidly removed from the organs. | Targeting the mitochondria and induce apoptosis of cancer cells by initiating mitochondrion-mediated apoptosis signaling pathway. | The half-inhibitory concentration for the B16-F10 cells, HeLa cells were 1.992 mg/mL and 8.28 mg/mL respectively after 48 of copper oxide treatment. | [43] | |
| Iron oxide | - | Outstanding superparamagnetic properties to accumulate in a specific tissue under an external magnetic field. | Inhibiting cell proliferation and induce cell apoptosis and autophagy. | Almost half of the HT-29 cells with a concertration of 5 μM were killed. | [44,45] | |
| Titanium oxide | Irradiated with a 500 W high-pressure mercury lamp or irradiated with visible light for 1h to kill T-24 human bladder cancer cells. | Used for the treatment of superficial tumors like skin, oral cavity, gastrointestinal tract, trachea and urinary bladder. | Produced photogenerated holes on the surface, hydroxyl radicals and hydrogen peroxide inside or outside the cells, then killed the cancer cells. | About 80% of T-24 cancer cells were killed by the photo-excited titanium dioxide particles. | [46,47] | |
| Iridium (III) photocatalysts | Photo-irradiation with 525 nm green light (11.7 J cm−2, 30 min). | They can induce damage of intracellular proteins. | Iridium (III) compounds are good photocatalysts for the oxidation of NADH, NADPH and amino acids via a SET mechanism. | The IC50 reached 8.1 μM for Hep-G2 cells. | [48] | |
| GaPc-PDT | Irradiated with a wave length 630 nm, lamp power 11.83 mW/cm2 for melanoma cell (WM35 and M1/15) treatment. | Tumor killing was not influenced by the melanoma stage. | A better therapeutic response overcoming the melanoma activation of survival mechanisms. | Have significant increased cell death (28.1% for WM35, and 40.2% for M1-15). | [49] | |
| AgInS2-coated upconversion nanoparticle | Irradiated with a 980 nm contentious laser for 30 min. | Induced the formation of a cytotoxic reactive oxygen species by the hybrid material under NIR light irradiation | - | Induced in vitro cervical cancer cell death with ~27% efficiency. | [50] | |
| Platinum complexes | Cisplatin | Have wide application in oesophageal, colorectal, or prostate cancer. | Have curative effects in germ cell cancer. | - | - | [51] |
| Cycloplatam | Have low toxicity to organ. | - | - | |||
| Z-scheme structure photocatalyst | SnS1.68-WO2.41 | Irradiated with an 808 nm NIR laser (0.5 W/cm2) for 20min for 4T1 tumor and HeLa tumor therapy. | Without the need of any drug and therapeutic agent assistance. | Oxidizing or consuming intratumoral over-expressed glutathione (GSH) by holes and simultaneously generates hydrogen molecules in a lasting and controllable way under NIR irradiation. | SnS1.68-WO2.41 showed obvious cytotoxicity to 4T1 and HeLa cells with IC50 = 17.1 μg/mL and IC50 = 32.6 μg/mL at 0.2 W/cm2, respectively. | [52] |
| Ni3S2/Cu1.8S@HA | Irradiated with the near infrared (NIR) (808 nm) for 0.5 h to treat HepG2 tumor cells. | Exhibited new biodegradability, and can be metabolized and eliminated by feces and urine within 2 weeks. | Realizing intracellular photocatalytic O2 evolution to relieve hypoxia in tumor microenvironment and enhance PDT. | Had a NIR harvest and photothermal conversion efficiency of 49.5%. | [53] | |
| Piezocatalysis | BaTiO3 | Under the ultrasonic vibration, the electrons and holes are separated by the piezoelectricity. | Exhibited stable sensitizers and dynamical control of redox reaction outcomes. | Catalyzing the generation of ROS such as toxic hydroxyl (•OH), superoxide radicals (•O2−) in situ for tumor eradication. | The tumors can be completely eradiated by piezocatalysis therapy in five days after three treatments. Lifespans of all treated mice can be prolonged to over 40 days without reoccurrence. | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, H.; Cheng, Z. A Literature Review on High-Performance Photocatalysts for Sustainable Cancer Therapy. Crystals 2021, 11, 1241. https://doi.org/10.3390/cryst11101241
Yi H, Cheng Z. A Literature Review on High-Performance Photocatalysts for Sustainable Cancer Therapy. Crystals. 2021; 11(10):1241. https://doi.org/10.3390/cryst11101241
Chicago/Turabian StyleYi, Hanxi, and Zeneng Cheng. 2021. "A Literature Review on High-Performance Photocatalysts for Sustainable Cancer Therapy" Crystals 11, no. 10: 1241. https://doi.org/10.3390/cryst11101241
APA StyleYi, H., & Cheng, Z. (2021). A Literature Review on High-Performance Photocatalysts for Sustainable Cancer Therapy. Crystals, 11(10), 1241. https://doi.org/10.3390/cryst11101241





