1. Introduction
A membrane may be defined as a permeable or semi-permeable layer which controls the flow of compounds and hence delivers one product with less of another component, thus resulting in the product concentration in those components [
1]. This process is used in industry for the recovery of reactants and valuable gases, the isolation of products, and pollution control [
2]. The flow of gases is based on their differences in permeability, which is a function of membrane properties and the nature of gases [
3]. Generally, the performance of a membrane is defined by its permeability and selectivity and a good indication is with high values of both achieved for efficiency in commercial applications, but in practicality, there is a trade-off between these parameters. However, in any given process, there is an economic optimum in the combination of selectivity and permeance.
Permeance/Permeation is a measure of the amount of permeate that flows through the membrane and is evaluated by the flowrate at which a fluid passes through the area of the membrane. Selectivity refers to the ability of the membrane to separate gases and gives a measure of the purity of the permeate gas and losses. It is evaluated by the concentration of components passing through the membrane versus the concentration in the feed [
4]. For single gases, it is calculated by the ratio of their permeation rates.
2. Characterisation of Separation Properties
In order to make efficient use of resources and achieve effective membrane separation, high permeability and selectivity are required. There are several factors that have been identified by previous authors to affect gas flow in porous media [
5,
6,
7,
8,
9,
10,
11]. This study classified them into fluid properties and structural parameters.
2.1. Fluid Properties
These include the properties of the fluid that affect the movement or rate of flow of fluids through the membrane.
2.1.1. Molecular Weight
Molecular weight can be defined as a measure of the total atomic weight values of atoms present in each molecule. In relation to this work, we examine methane and carbon dioxide gases which have molecular weights of 16 g/gmol and 44 g/gmol, respectively, and try to describe how this fluid property affects the flow of gases through the membranes. In other words, how the weight of atoms present in each molecule of CH
4 and CO
2 changes the rate at which they flow through. Some authors have stated that flux is inversely proportional to the square root of the gas molecular weight [
12]; it is, therefore, expected that in a separation process, CH
4 (16 g/gmol) would be more selectively convened than CO
2 (44 g/gmol).
2.1.2. Kinetic Diameter
Kinetic diameter is an indication of the size of a molecule (3.3 and 3.8 for CO
2 and CH
4, respectively), which estimates the possibility of a molecule colliding with another. Thus, it is associated with the mean free path of molecules in a gas, that is, the average distance that a particle will travel without collision. This is imperative when discussing the behaviour of gases, as it plays an important role in the determination of the gas flow mechanism. For instance, in viscous flow, the pore diameter is large compared to the mean free path of the gas molecules, and there is frequent collision between the molecules, whilst in Knudsen flow, the pore size is larger than that of the gas molecules but smaller than its mean free path. Thus, there is elastic collision between the gas molecules and the pore wall but no interaction between the molecules. Hence, the molecule-to-wall collisions are frequent, thereby allowing lighter molecules to preferentially diffuse through the pores [
2].
2.1.3. Flowrate
Flowrate is a quantification of bulk fluid movement. In terms of this research, we consider volumetric flow rate to be the rate at which a volume of fluid moves per unit time. The rate at which CH4 and CO2 gases flow through the membrane is strongly related to invading pressure and permeability. The faster a gas flows, the higher the flux and permeance, which increases the selectivity of that gas in each membrane.
2.1.4. Pressure
Pressure is an important engineering quantity that affects many aspects of fluid mechanics. It has been identified that pressure significantly affects CH
4 diffusion at lower pressure rather than high pressure [
11]. This is confirmed in
Section 4.3; however, the authors did not relate this to the CO
2 diffusion rate and other system factors, which are crucial to a biogas separation unit. Hence, these experiments used up to eight isobars to investigate the effect of the coupling of pressure and other parameters on the separation process.
2.2. Structural Parameters
These are the inherent properties of the membrane matrix system that affect the mode at which fluids pass through based on its components, symmetry, and general make-up.
2.2.1. Pore Size
Pore size is the distance between two opposite walls of a pore; thus, it is a measure of how large or small each pore within a material is. Due to the nature and mode of membrane preparation, we generally have a non-uniform range of pores, and hence, the mean pore size is used. Pore size is characterised in the laboratory using nitrogen physisorption, where the sample is degassed by loading the insulated sample-containing cell into the degassing station (for 3 hours at 300 °C) of the physisorption equipment to remove any moisture and impurities. The degassed samples are re-weighed and moved to the analysis station where the sample-containing cell is immersed into liquid nitrogen dewar and analysed at 77 K to provide pore size measurements. The effect of pore size to flowrate has been studied extensively [
8,
9,
10,
11]. However, only limited studies have touched on it from the perspective of gas separation. From the studies [
8,
9], it can be deduced that displacement of CH
4 by CO
2 in coal bed reservoirs is affected by pore size. Another investigation [
10,
11] revealed that pore size significantly affects the diffusion and separation of N
2/CO
2. Drawing an analogy from these authors, it is expected that pore size would affect biogas separation. Thus, in this study, different pore sizes were experimentally tested to identify separation opportunities.
2.2.2. Surface Area
The effect of surface area is also examined to ascertain if and to what extent this improves the performance parameters (permeability and selectivity) of the membrane system. Generally, higher surface area leads to a higher rate of reaction due to more acting sites for reaction progression. With this in view, this work proceeded to utilise two membranes with the same mean pore size (15 nm) and similar characteristics but different surface area and thickness (3 mm and 5 mm) to confirm this in relation to fluid flow in porous media.
2.2.3. Operating Temperature
Consequently, the experiment included varying temperatures of the core sample to investigate the effect of the coupling of temperature and other parameters on the separation process.
3. Methodology
Alumina membranes were used in this study and supplied by Ceramiques Techniques et Industrielles (CTI SA) in France, with mean pore sizes ranging from 15 to 6000 nm. A shell and tube system was used wherein the membrane was fitted into the centre of the annulus, and both ends were sealed with graphite seal to avoid gas slip and to isolate the feed gases from the permeate. A snoop solution was also used as an indicator for leaks. The outer shell was covered with a fire-resistant glass fibre insulating material, and thermocouples were attached to various points in order to maintain the temperature of the unit. Gas permeation measurements were carried out using single gases; the gas to be analysed was fed in at a predetermined pressure of 0.2 bar and readings were recorded while operating at thermal stability of 20 °C, 50 °C, 70 °C, and 100 °C, respectively (with ±5 K error). The flow meter confirmed that a steady constant driving force was maintained. At the outlet, there was also a flow meter to measure the flowrate of the outgoing gas and the experiment was repeated at 0.6, 1.0, 1.4, 1.8, 2.2, 2.6, and 3.0 bar (with ±0.01 bar error). By comparing the flow characteristics of the different gases, the perm-selectivity of the membrane was found.
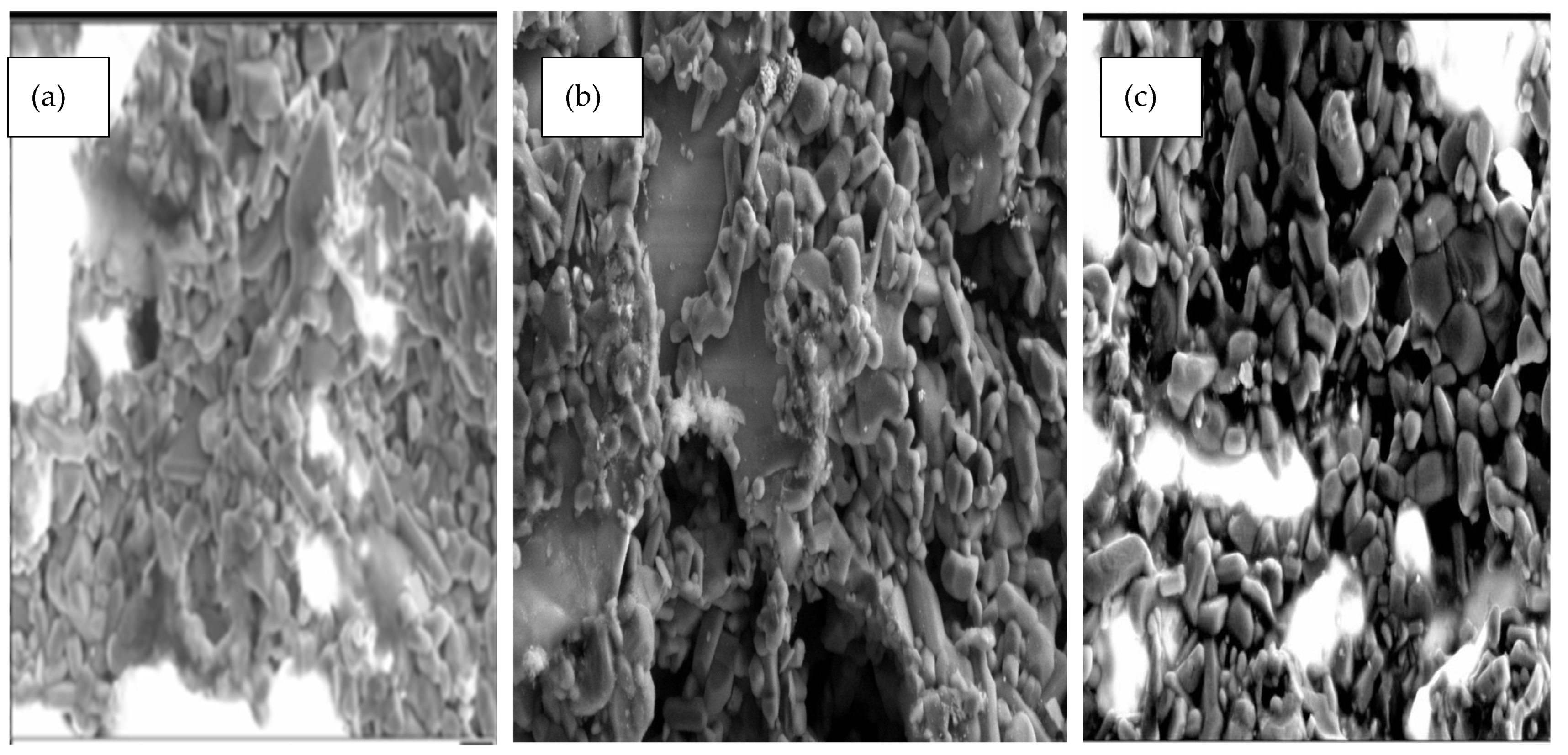
SEM analysis was carried out using a Zeiss (Evo LS10 S, Aberdeen, UK) with an Oxford Instruments INCA System Energy Dispersive X-ray Analyser (INCAx-act 51-ADD0020, Aberdeen, UK). Samples were prepared by freezing in liquid nitrogen and then fracturing to prevent deformation of the membrane structure. SEM micrographs were taken in the order of magnification 2.00 K for all membranes. The images show that the 6000 nm membranes have many large pores compared to the 15 and 200 nm membranes, which are characterised with smaller pores, although they still contain defects, which allow the unwanted gases to pass through and impact the transport mechanism of these membranes. From the results (see
Figure 1), the difference in surface morphology is very clear, with more defects shown in the 6000 nm (= 0.04%) sample compared with the more closely-knit structures of the 15 nm (= 0.13%) and 200 nm (= 0.20%) samples.
4. Results and Discussion
Understanding the transport behaviour of each gas in membranes under various operating conditions is the foundation of achieving the effective separation of mixed gases. Based on the results from laboratory experiments, it is evident that fluid flow through the membrane is governed by both fluid and structural properties, and this affects the transport mechanism within the membrane. Gas transport in membranes can take place through several mechanisms, including Hagen–Poiseuille flow, Knudsen diffusion, surface diffusion, capillary condensation, and molecular sieving. These mechanisms can occur depending on the membrane pore size, the molecular size of permeating species, the interactions between permeating molecules and membrane material, and other operating conditions.
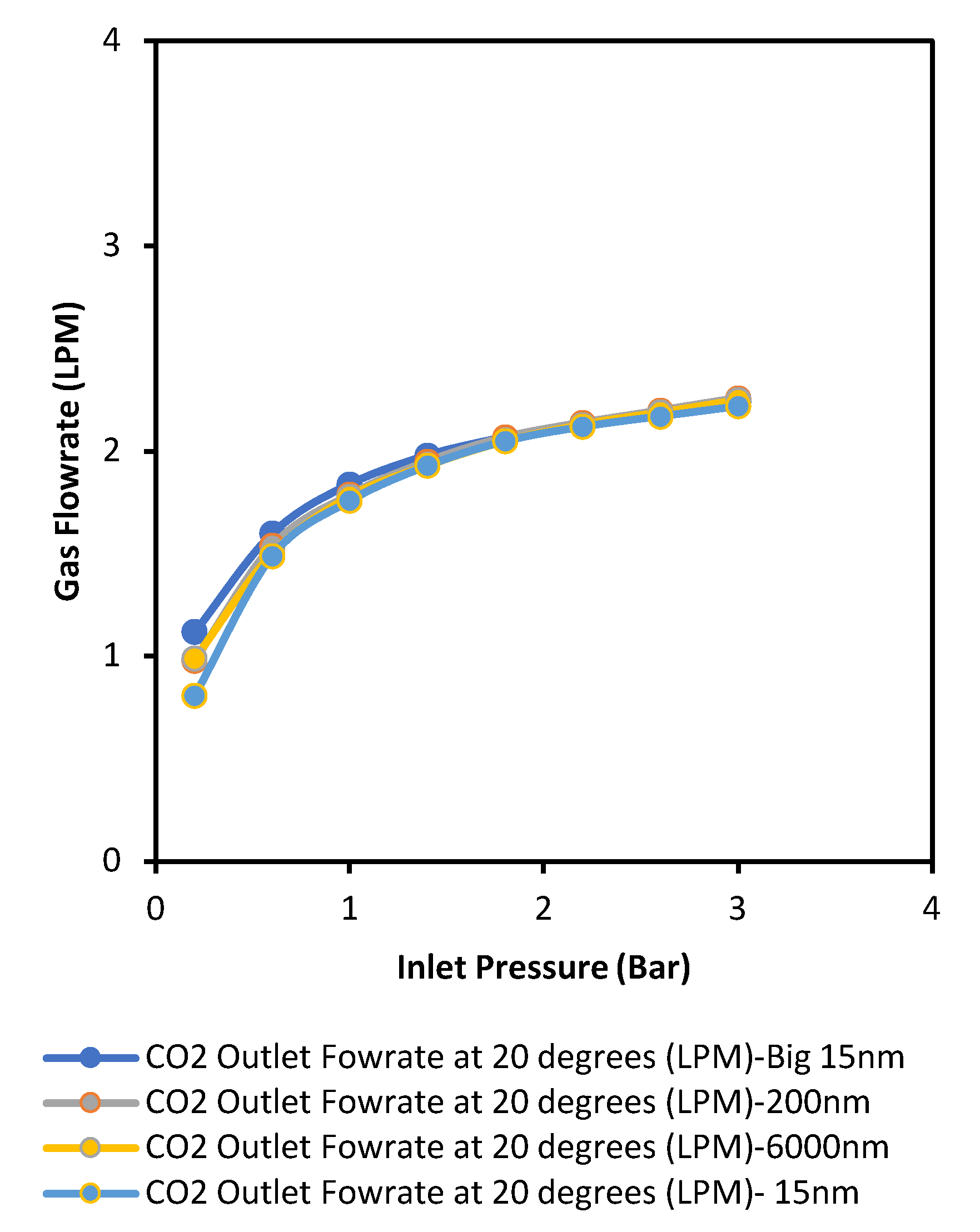
4.1. Pore Size
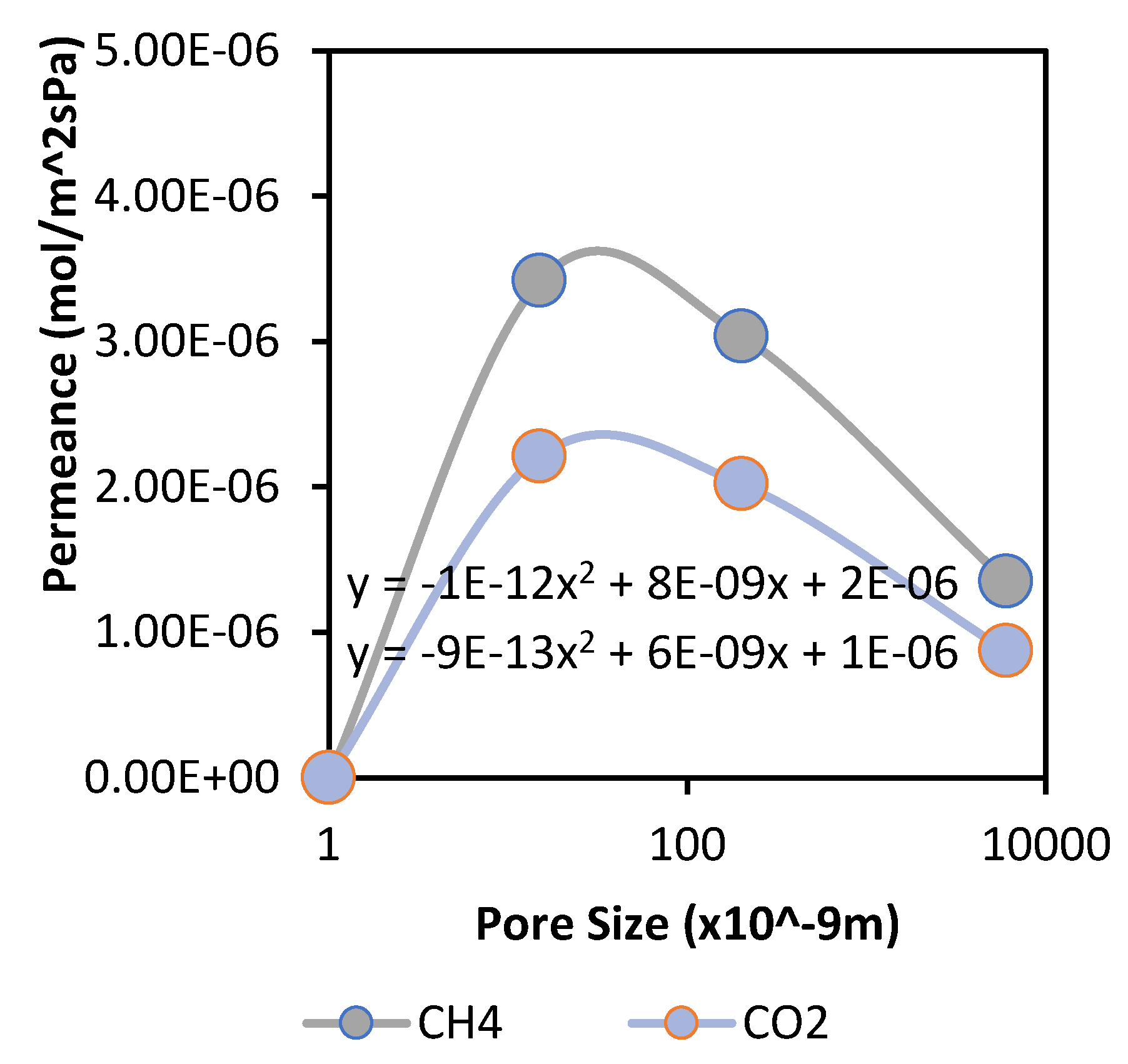
The membranes were made to specification by CTI France and had mean pore sizes of 15, 200, and 6000 nm. These pore sizes were selected because they cover the spectrum of meso and macro pores. It was confirmed that lower pore sizes improved the separation performance of the membrane. The 15 nm membrane offered greater permeance when compared with membranes of higher pore size, as shown in the following
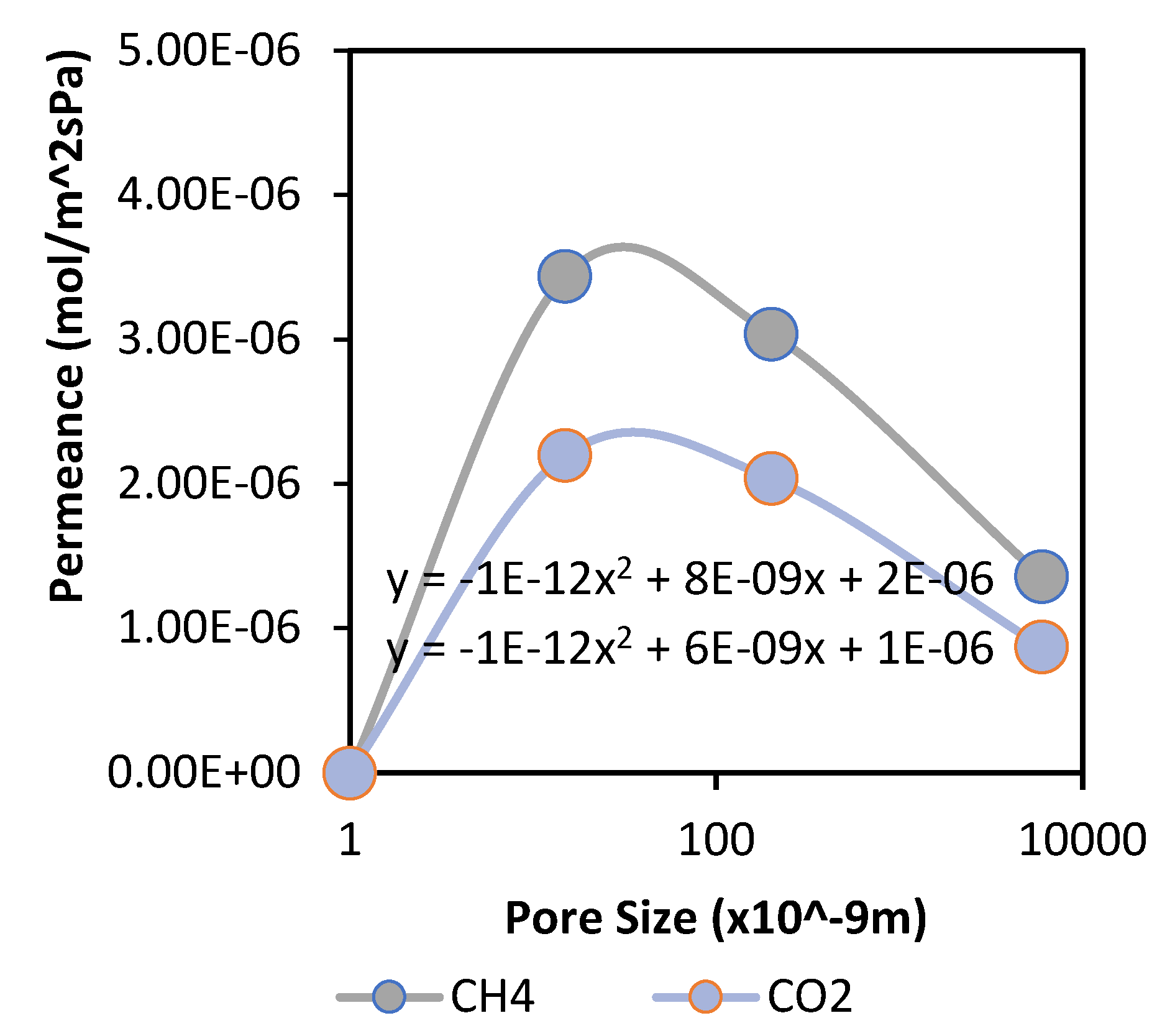
Figure 2 and
Figure 3. Nonetheless, with regard to preferential gas flow, methane had a higher flux than carbon dioxide; this was not to be expected as the kinetic diameter of CO
2 is less than that on CH
4. This means that molecular sieving (or size exclusion) is not the prevalent flow mechanism at play within the pressure and temperature ranges used. In this case, Knudsen diffusion is prevalent following the relation between fluid flow and molecular weight, as deduced from Graham’s law of diffusion.
A quadratic curve is noticed in
Figure 2 and
Figure 3, which is in the form y = a + bx + cx
2 and can be linked to the parallel flow model described by De Meis [
12], which confirms the interplay of viscous and Knudsen diffusion within these membranes. It appears that Knudsen diffusion occurs on the top membrane layer (having a lower average/mean pore size), while a combination of laminar flow and Knudsen diffusion takes place in other layers. This study, therefore, confirms that Knudsen diffusion can occur in the microporous range due to more molecule/pore wall collision depending on the type of gases used. In this case, the membrane selectivity values were in the ideal Knudsen selectivity range for CO
2 and CH
4 gases due to the differences in the molecular weight of the gases. However, the presence of viscous flow limits the separation properties initiated by Knudsen diffusion. Thus, for effective separation, we require the introduction of a dominant flow mechanism, such as surface diffusion, to cause significant interaction of the target gas molecules with the pore surfaces and further improve the membrane performance.
4.2. Surface Area
With respect to the thicker membrane (5 mm/Big15 nm), pressure was increased considerably to force the flow of gases through the pores. The results also reveal that the membrane with a larger surface area achieved higher performance compared with the other of smaller surface area. As expressed in the literature, this is an effect of more acting sites that maximise contact between the gases and the surface of the inorganic membrane, creating more active channels for gas permeation (
Figure 3 and
Figure 4 below).
4.3. Pressure
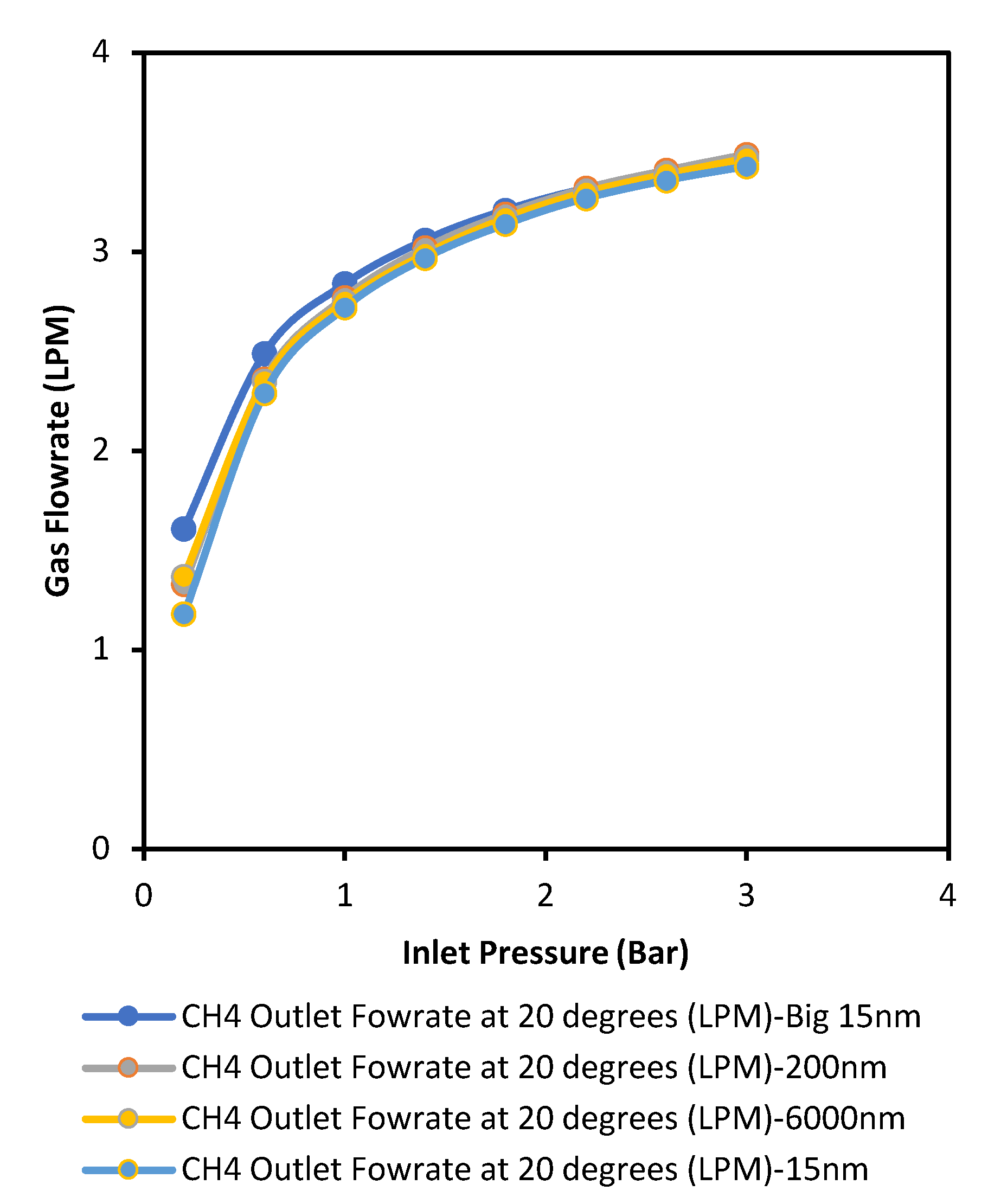
Figure 4 and
Figure 5 show that at low pressures, gas molecules flow through each membrane (regardless of pore size and surface area) at their standard rates based on the inherit gas properties, but a significant increase in pressure forces the gas molecules to travel through the membranes at the maximum velocities, causing them to overcome any barriers with supporting activation energies. This means there is a transition from Knudsen to viscous flow regime or fluids go through parallel flow regimes.
4.4. Temperature
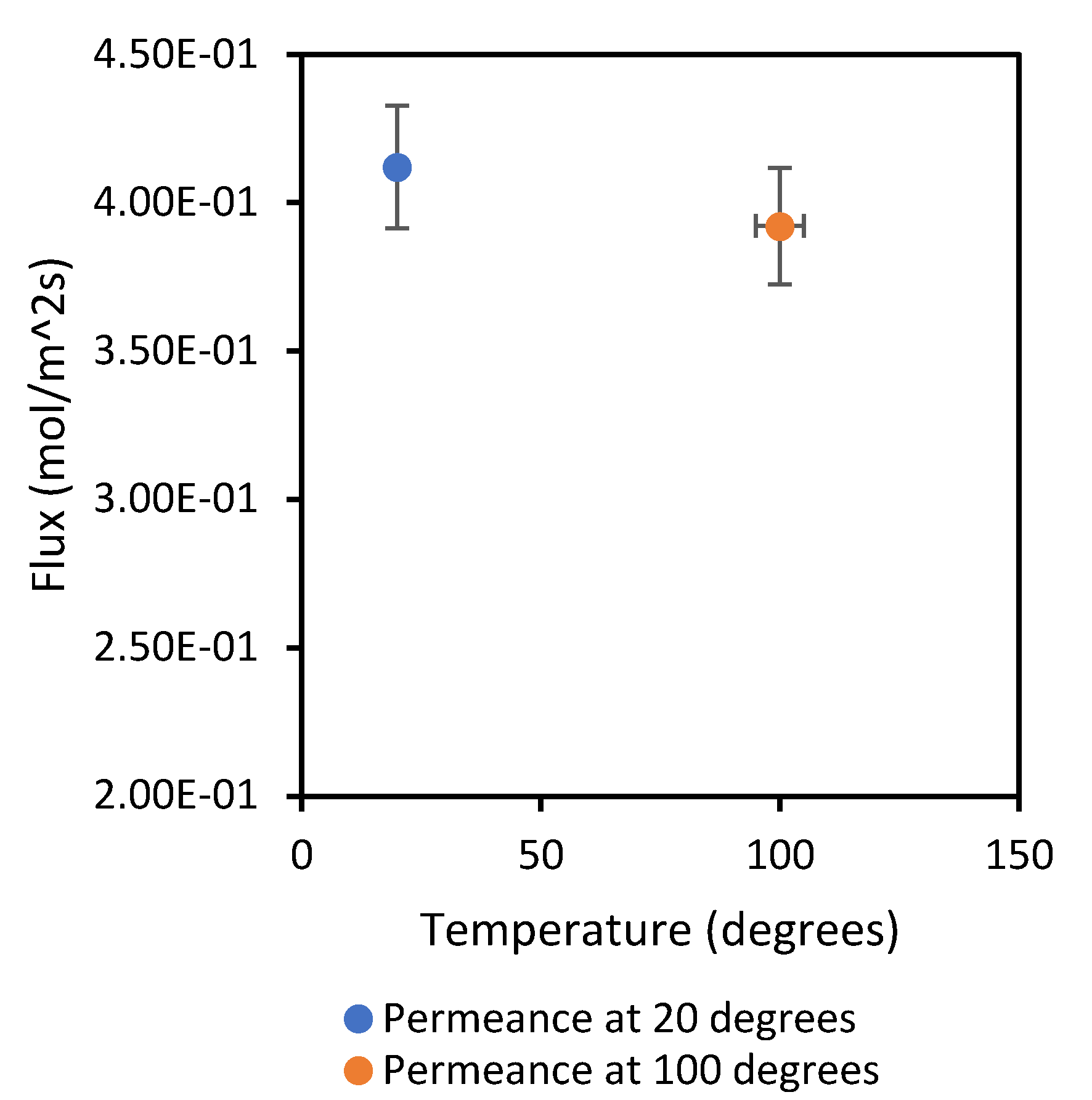
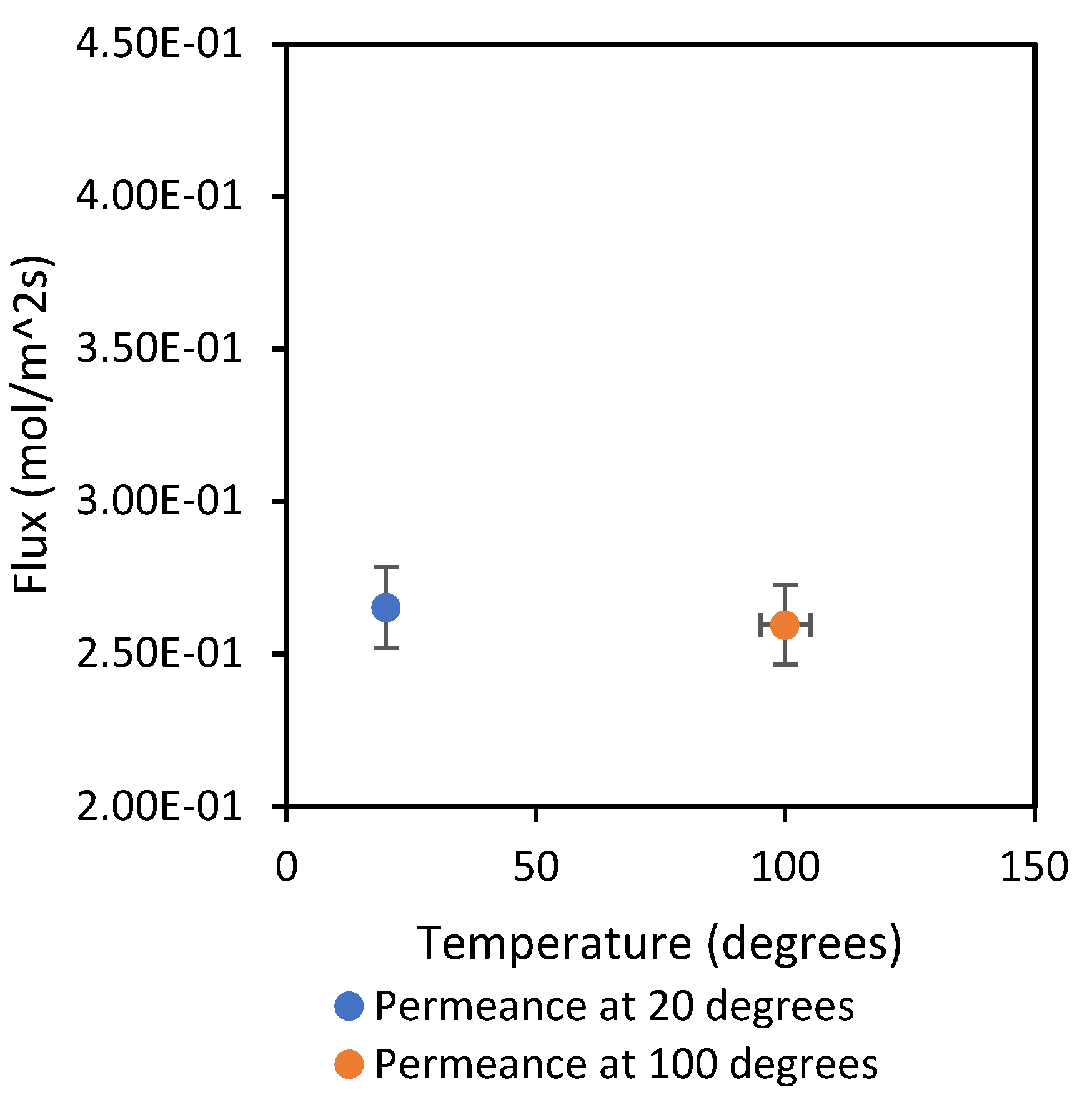
The results in
Figure 6 and
Figure 7 show an overall increase in gas flowrate as temperature increases from 20 °C to 100 °C. This can be explained by the influence of temperature on the kinetic energy of gas molecules, which makes the molecules move faster with an increase in temperature. On the other hand, the flow mechanism is confirmed. CH
4 flux was reduced from 0.4120 to 0.3922 mol/m
2s when the temperature was increased from 20 to 100 °C. Likewise, CO
2 sees a decline in flux from 0.2653 to 0.2595 mol/m
2s with temperature increase; these types of changes in flux are typical of the Knudsen regime. Furthermore, to confirm this trend, both gases were analysed at 400 °C, and the flux for CH
4 and CO
2 was 0.2181 and 0.1219, respectively. From the Knudsen equation, it is known that the permeating flux decreases as temperature increases in this regime, usually in mesoporous membranes, because the temperature increase causes the decrease in surface concentration to overcome the increased effect on diffusivity.
5. Conclusions
This study confirms that both fluid and structural properties play a vital role in determining the performance of methane and carbon dioxide gas separation with inorganic ceramic membranes. The results show that a combination of flow mechanisms is at play in gas transport through membranes owing to the inherent properties of the membrane. In this case, viscous and Knudsen flow occur in parallel due to the presence of defects in the pore structure which impact the mode of gas transport through the pores. The study also shows that depending on the properties of the gases, microporous membranes are sufficient to initiate a degree of selectivity sufficient for Knudsen separation. Further study is being carried out to investigate the effect of loading the membrane support with high affinity material that will not only improve adsorption properties but will also reduce pore size, and ultimately, improve the selectivity of the membrane. The effect of subsequent loadings on permeance and selectivity will be studied and compared with what was achieved from these initial support characteristics. This will provide more information on optimising membrane performance to enhance the mass transport of carbon dioxide through the membrane as the permeate leaves methane at the retentate side still possessing sufficient pressure for transport and storage [
13].
Author Contributions
Conceptualization, P.O. and E.G.; Data curation, P.O.; Formal analysis, P.O. and E.G.; Funding acquisition, F.M.-S.; Investigation, P.O. and O.A.; Methodology, P.O.; Supervision, F.M.-S. and E.G.; Validation, O.A. and E.G.; Visualization, P.O. and F.M.-S. Writing—original draft preparation, P.O.; Writing—review and editing, P.O. and F.M.-S.; All authors have read and agreed to the published version of the manuscript.
Funding
F.M.-S. would like to thank Edinburgh Napier University for funding his time under the Project 2706622.
Acknowledgments
The authors would like to thank Robert Gordon University for the use of its facility to complete the work. The authors would like to thank to MDPI for promoting open-access to knowledge, even more, providing discounts to reviewers that helps in publishing open-access research like this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Beil, M.; Beyrich, W. Biogas upgrading to biomethane. In The Biogas Handbook; Elsevier: Amsterdam, The Netherlands, 2013; pp. 342–377. [Google Scholar]
- Sridhar, S.; Bee, S.; Bhargava, S. Membrane-based gas separation: Principle, applications and future potential. Chem. Eng. Dig. 2014, 27, 77–87. [Google Scholar]
- Wiheeb, A.D.; Karim, A.M.A.; Mohammed, T.E.; Othman, M.R. Hydrogen purification using a microporous hydrotalcite-silica composite membrane. Diyala J. Eng. Sci. 2015, 8, 846–854. [Google Scholar]
- Havas, D.; Lin, H. Optimal membranes for biogas upgrade by removing CO2: High permeance or high selectivity? Sep. Sci. Technol. 2017, 52, 186–196. [Google Scholar] [CrossRef]
- Abunumah, O.; Ogunlude, P.; Gobina, E. Experimental Evaluation of the Mobility Profile of Enhanced Oil Recovery Gases. Adv. Chem. Eng. Sci. 2021, 11, 154. [Google Scholar] [CrossRef]
- Ogunlude, P.; Abunumah, O.; Gobina, E. A Study of Gas Diffusion Characteristics on Nano-Structured Ceramic Membranes. Eur. J. Eng. Form. Sci. 2020, 4, 21–23. [Google Scholar] [CrossRef]
- Ogunlude, P.; Abunumah, O.; Orakwe, I.; Shehu, H.; Muhammad-Sukki, F.; Gobina, E. Comparative evaluation of the effect of pore size and temperature on gas transport in nano-structured ceramic membranes for biogas upgrading. WEENTECH Proc. Energy 2019, 5. [Google Scholar] [CrossRef]
- Liu, X.; He, X. Effect of pore characteristics on coalbed methane adsorption in middle-high rank coals. Adsorption 2017, 23, 3–12. [Google Scholar] [CrossRef]
- Peng, Z.; Li, X. Improvements of the permeability experiment in coalbed methane. Arab. J. Geosci. 2018, 11, 259. [Google Scholar] [CrossRef]
- Chen, G.; An, Y.; Shen, Y.; Wang, Y.; Tang, Z.; Lu, B.; Zhang, D. Effect of pore size on CH4/N2 separation using activated carbon. Chin. J. Chem. Eng. 2020, 28, 1062–1068. [Google Scholar] [CrossRef]
- Pillalamarry, M.; Harpalani, S.; Liu, S. Gas diffusion behavior of coal and its impact on production from coalbed methane reservoirs. Int. J. Coal Geol. 2011, 86, 342–348. [Google Scholar] [CrossRef]
- De Meis, D. Gas-Transport-through-Porous-Membranes. ENEA 2017, 1–19. Available online: https://www.researchgate.net/publication/316473135_Gas_transport_through_porous_membranes (accessed on 20 August 2021).
- Xomeritakis, G.; Naik, S.; Braunbarth, C.; Cornelius, C.; Pardey, R.; Brinker, C. Organic-templated silica membranes: I. Gas and vapor transport properties. J. Membr. Sci. 2003, 215, 225–233. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).