Abstract
Triazolopyridines are a family of compounds that, owing to their biological activity, have many pharmaceutical applications. In this study, 3-(pyridine-4-yl)-[1,2,4]triazolo[4,3-a]pyridine and 6-bromo-3-(pyridine-4-yl)-[1,2,4]triazolo[4,3-a]pyridine were synthesized by using the chlorinated agent NCS for hydrazones under very mild conditions. The characterization of these compounds was achieved by 1H NMR, 13C NMR, FTIR, MS and X-ray diffraction. The compound 3-(pyridine-4-yl)-[1,2,4]triazolo[4,3-a]pyridine was crystallized in the monoclinic space group P 21/c with a = 15.1413(12), b = 6.9179(4), c = 13.0938(8) Å, β = 105.102(6)°, V = 1324.16(16)Å3, Z = 4, and R = 0.0337. Also compound 6-bromo-3-(pyridine-4-yl)-[1,2,4]triazolo[4,3-a]pyridine was crystallized in the monoclinic space group P 21/c with a = 14.3213(11), b = 6.9452(4) (4), c = 12.6860(8)Å, β = 100.265(6)°, V = 1241.62(14)Å3, Z = 4, and R = 0.0561.
1. Introduction
Triazolopyridines represent an important class of heterocycles with broad uses in the pharmaceutical area as well as medicinal chemistry [1,2,3,4,5,6,7,8,9,10,11]. This family of compounds comprises biologically active agents including antibacterial [1], antifungal [2] anxiolytic [3], herbicidal [4] and pesticidal [5], antithrombotic, anti-inflammatories, and antiproliferative agents [6,7]. Triazolopyridines act as inhibitors of mitogen-activated protein (MAP) kinases [6] or growth hormone secretagogues and antithrombotic agents [8,9]. Also, triazolopyridine derivatives bearing sulfonamide substituent are found to be a good antimalarial agent [10]. Recently some triazolopyridines have been described as potential anticancer agents, as well as selective TNKS1 inhibitors [11]. Therefore, versatile and widely applicable methods for their synthesis are of considerable interest. Several methods have been reported for the synthesis of Triazolopyridines. Most of these methods are furnished by the oxidative cyclization of heterocyclic substituted hydrazones; however, these have limitations and drawbacks [12,13,14,15,16,17,18,19,20,21,22,23,24]. 1,1-carbonyldiimidazole (CDI) is used as a mild and efficient reagent in the synthesis of triazolopyridines [25]. Recently, electrochemical synthesis of 1,2,4-Triazolepyridines and another fused heterocycle has been described [26]. Most of the protocols, however, still require expensive TM catalysts and superstoichiometric amounts of external oxidants under harsh conditions [27]. Limitations of the existing protocols include: (1) harsh reaction conditions; (2) the use of expensive catalysts or superstoichiometric amounts of oxidizing agents; (3) limited substrate scopes or scalability; (4) low chemo-selectivity. The harsh conditions utilized in the aforementioned methods can be problematic with substrates that are sensitive to high temperatures or oxidants.
Therefore, it is desirable to develop complementary approaches for the fast and efficient synthesis of valuable 1,2,4-triazole-fused heterocycles. Using N-Chlorosuccinimide (NCS) as an oxidative cyclizing agent of 2-pyridylhydrazones opens the door to the development of a method to furnish [1,2,4]triazolo[4,3-a]pyrazines and pyrimidines. To the best of our knowledge, the synthesis of the target compounds is not known in the literature by using the chlorinated agent NCS for hydrazones under very smooth conditions.
2. Materials and Methods
2.1. Materials and Physical Measurements
All commercially available reagents and solvents were used without further purification. Melting points were measured in the open capillary tubes on a Boetius melting point apparatus. NMR spectra (400/100 MHz) were acquired on a Bruker Avance 600 spectrometer (Bruker, Billerica, MA, USA). The spectra were recorded for 1H and 13C NMR at room temperature. Chemical shifts were reported in ppm (ν) and J values in Hz. Multiplicity was designated as the singlet (s), doublet (d), triplet (t), and multiplet (m). Infrared spectra (IR) were registered using the Bruker Tensor-27 FT-IR Spectrometer. All spectra were recorded in the range of 400–4000 cm−1 at room temperature. TLC was carried out on silica gel plates (Merck, Darmstadt, Germany) using a mixture of dichloromethane and methanol as an eluent; visualization was accomplished with UV light.
2.2. Chemistry
2.2.1. General Procedure for Synthesis of Hydrazones
Compounds were prepared via condensation reaction of 4-pyridinecarboxaldehyde with corresponding hydrazines in ethanol, following a previously reported procedure for related systems [28]. Further, 0.05 mol of pyridine-4-aldehyde was added to a solution of 0.05 mol of the appropriate hydrazine in ethanol (20 mL) at room temperature. The reaction mixture was stirred until the completion of the reaction (by TLC). A pale yellow solid precipitated and was collected by filtration and recrystallized from hot ethanol.
2.2.2. General Procedure for Synthesis of [1,2,4]Triazolo[4,3-a]pyridines Derivatives
Synthesis of 3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1 and 6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 2 was as follows (Scheme 1): 10 mmol of the appropriate hydrazone was dissolved in a minimum amount of dry DMF (20 mL), the mixture was cooled in an ice bath, then 11 mmol of N-chlorosuccinimide (NCS) was added portion-wise to the reaction mixture. It is worth noting that the reaction is highly exothermic and should be handled with care [29,30]. The reaction mixture was stirred at 0 °C for about 1 h, then the reaction mixture was allowed to warm up to room temperature. After the completion of the reaction, as indicated by TLC, the yellow solid was collected by filtration and washed twice with petroleum ether. The resulting solid was dissolved in 50 mL of hot water and 10 mmol of Et3N was added drop-wise while cooling. Pale yellow plates were formed, filtered, and washed with cooled water to afford more than 90% product.
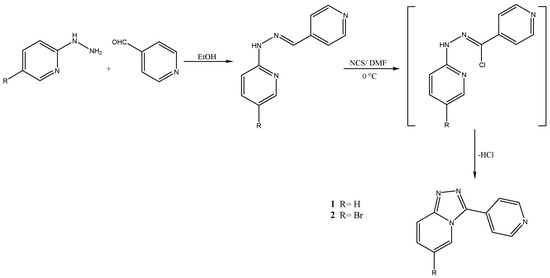
Scheme 1.
Synthesis of 3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1 and 6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 2.
3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1. Off pale yellow solid; Yield 92%; mp 188–189 °C; 1H NMR (200 MHz, DMSO) δ 8.63–8.48 (m, 2H), 8.15 (ddt, J = 5.0, 1.7, 0.7 Hz, 1H), 7.98 (s, 1H), 7.69 (ddd, J = 8.6, 7.1, 1.8 Hz, 1H), 7.64–7.59 (m, 2H), 7.32 (dt, J = 8.6, 1.1 Hz, 1H), 6.83 (ddt, J = 6.5, 5.0, 0.8 Hz, 1H); 13C NMR (50 MHz, DMSO) δ 156.96, 151.02, 150.15, 148.19, 143.32, 138.61, 136.25, 122.18, 120.53, 116.36, 107.29; IR(ATR) 1634, 1605, 1493, 1465, 1414, 1376, 1305, 1284, 1212, 1143, 1087, 1005, 992, 841,750, 737, 693 cm−1; EI-MS: 197.2 [M + H] +.
6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 2. Off pale green crystals; Yield: 93%; mp 203–205 °C; 1H NMR (200 MHz, DMSO) δ 8.92 (dd, J = 1.7, 0.9 Hz, 1H), 8.88–8.72 (m, 2H), 8.03–7.94 (m, 2H), 7.91 (dd, J = 9.7, 1.0 Hz, 1H), 7.61 (dd, J = 9.7, 1.6 Hz, 1H); 13C NMR (50 MHz, DMSO) δ 150.50, 149.27, 144.15, 133.49, 131.64, 124.32, 121.92, 116.70, 109.24, 38.93; IR(ATR) 1600, 1523, 1417, 1336, 1296, 1209, 1091, 992, 825, 789, 727 cm−1; EI-MS: 275.2/277.2 [M + H]+.
2.3. Crystal Structural Determination
Crystals of compounds 1 and 2 were obtained via recrystallization from a hot aqueous solution. The diffraction data were collected using MoKα radiation (λ = 0.71073 Å) at 193.00(10) K using a STOE IPDS2T-diffractometer. The structure was solved using the SHELXT crystallographic software package and refined through full-matrix, least-squares techniques on F2 by the SHELXL-2018 crystallographic software package [31]. Selected crystallographic data of compounds 1 and 2 are listed in Table 1. The supplementary crystallographic data for 1 and 2 were deposited at the Cambridge Crystallographic Data Center (CCDC) as 2049251 and 2049252, respectively.

Table 1.
Crystal parameters, data collection, and structure refinement details for compounds 1 and 2.
3. Results and Discussion
3.1. Chemistry
Herein we describe the use of N-chlorosuccinimid (NCS) as an efficient reagent for the synthesis of [1,2,4] triazolo[4,3-a]pyridine derivatives. While NCS is well known as a chlorinating agent of hydrazones and this is the first time we have explored its new function as a cyclizing agent for 2-pyridylhydrazones to achieve the target depicted in Scheme 1. It is worth mentioning that we use NCS as a chlorinating agent for hydrazones to furnish the corresponding hydrazonoyl chlorides, which usually react with arylacetonitriles to afford aminopyrazoles. However, in this case, the use of 2-hydrazinopyridin for preparing hydrazones and their treatment with NCS as a chlorinating agent did not yield the corresponding hydrazonoyl chloride. In fact, the compound isolated was 3-(pyridine-4-yl)[1,2,4]triazolo[4,3-a]pyridines obtained via oxidative cyclization. This can be explained by the initial formation of the chlorohydrazone and by the subsequent loss of HCl and nitrilimine generation. Due to the presence of the nitrogen of the pyridine moiety in a suitable position, the intermediate cyclizes to give the unprecedented [1,2,4]triazolo[4,3-a]pyridine.
3.2. Crystal Structure and Formation of Hydrogen Bond
The 3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1 and 6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 2 crystallized in monoclinic space group P21/c. Figure 1 shows molecular structures and atom numbers of the compounds 1 and 2.
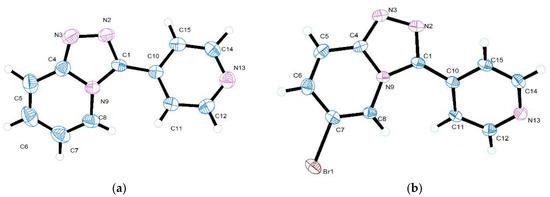
Figure 1.
Molecular structures with atom numbering of (a) 3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1; (b) 6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo-[4,3-a]pyridine 2.
The selected values of bond distances and angles are presented in Table 2. The analogous bond lengths and angles are almost equal in both compounds. In general, the average bond lengths and bond angles of these rings are within the normal ranges [22,24,25,26,27,28,30,31,32,33,34,35,36,37].

Table 2.
Selected bond lengths [Å], angles [°] for compounds 1 and 2.
The unit cells of both 1 and 2 contain four molecules (Z = 4), and the 1,2,4-triazolo[4,3-a]pyridine ring system in both structures accomplish a planar structure in accordance with similar systems previously reported. [22,25] An angle between the plane of 1,2,4-triazolo[4,3-a]pyridine ring system (C4, C5, C6, C7, C8, N9, C1, N2, N3) and the plane of pyridine ring (C10, C15, C14, N13, C12, C11) is equal to 26.79o and 30.41o in 1 and 2, respectively. However, it is observed that in the 1,2,4-triazolo[4,3-a]pyridine ring system, the C8–N9, C4–N9, and C1–N2 bonds are significantly longer than the C=N bond (1.28 Å) [38], which indicates a significant conjugation effect in the fused ring system.
The title compounds 1 and 2 have an extensive network of hydrogen bonds. The parameters of H-bonds are given in Table 3.

Table 3.
Hydrogen-bond parameters (Ǻ) for compounds 1 and 2.
There are three water molecules per unit cell with an extensive network of hydrogen bonds between water molecules, and also the molecule linked to water by O1W—H2W⋅⋅⋅N13 and O1W—H1W⋅⋅⋅N13 hydrogen bonds in 1 and 2, respectively, as shown in Figure 2.
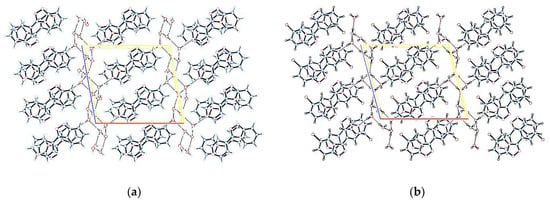
Figure 2.
Crystal structure and hydrogen bonds for (a) 3-(pyridin-4-yl)-[1,2,4]triazolo[4,3-a]pyridine 1; (b) 6-bromo-3-(pyridin-4-yl)-[1,2,4]triazolo-[4,3-a]pyridine 2.
4. Conclusions
In summary, we have developed an efficient procedure for the oxidative cyclization of 2-pyridylhydrazones to achieve triazolopyridines. Synthesis of the desired products proceeds under very mild conditions and includes dehydrative cyclization upon treating with NCS in DMF at 0 °C. Access to the unprecedented cyclized product under the conditions applied makes this reaction an operationally very convenient and high yielding step for the synthesis of [1,2,4]triazolo[4,3-a] pyridines. To the best of our knowledge, usage of NCS as a cyclizing agent was not mentioned before in this context, the reaction is robust and the products can be isolated in excellent yields.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11101156/s1, Figure S1: 1H NMR of 1, Figure S2: 13C NMR of 1, Figure S3: 1H NMR of 2, Figure S4: 13C NMR of 2, Figure S5: FTIR-Spectrum of 1, Figure S6: FTIR-Spectrum of 2, Figure S7: Fragment Ion Scan of 1, Figure S8: Fragment Ion Scan of 2, Figure S9: Fragment Ion Scan of 2, Table S1: Crystal data and structure refinement for 1, Table S2: Atomic coordinates and equivalent isotropic displacement parameters (Å2) for 1. U(eq) is defined as one-third of the trace of the orthogonalized Uij tensor, Table S3: Anisotropic displacement parameters (Å2) for 1. The anisotropic displacement factor exponent takes the form: -2π2[ h2 a*2U11 + … + 2 h k a* b* U12 ], Table S4: Hydrogen coordinates and isotropic displacement parameters (Å2) for 1, Table S5: Bond lengths [Å] and angles [°] for 1, Table S6: Torsion angles [°] for 1, Table S7: Hydrogen bonds for 1 [Å and °], Table S8: Crystal data and structure refinement for 2, Table S9: Atomic coordinates and equivalent isotropic displacement parameters (Å2) for 2. U(eq) is defined as one-third of the trace of the orthogonalized Uij tensor, Table S10: Anisotropic displacement parameters (Å2) for 2. The anisotropic displacement factor exponent takes the form: -2π2[ h2 a*2U11 + … + 2 h k a* b* U12 ], Table S11: Hydrogen coordinates and isotropic displacement parameters (Å2) for 2, Table S12: Bond lengths [Å] and angles [°] for 2, Table S13: Torsion angles [°] for 2, Table S14: Hydrogen bonds for 2 [Å and °].
Author Contributions
Conceptualization, S.E.-K. and B.A.T.; Data curation, S.E.-K. and L.N.; Investigation, B.A.T., D.S. and L.N.; Methodology, S.E.-K.; Project administration, H.-P.D.; Resources, H.-P.D.; Software, D.S.; Supervision, H.-P.D.; Validation, S.E.-K., B.A.T., K.W., D.S., L.N., O.R. and H.-P.D.; Visualization, L.N.; Writing—original draft, S.E.-K.; Writing—review and editing, S.E.-K., B.A.T., K.W., D.S., L.N., O.R. and H.-P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in supplementary materials.
Acknowledgments
Said El-Kurdi would like to thank DAAD for funding his short visit (2018), Bassam Abu Thaher would like also to thank AvH Foundation for funding his short visit (2018). The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research, and Culture and the Furtwangen University in the funding program Open Access Publishing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sadana, A.K.; Mirza, Y.; Aneja, K.R.; Prakash, O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar] [CrossRef]
- Prakash, O.; Hussain, K.; Aneja, D.K.; Sharma, C.; Aneja, K.R. A facile iodine(III)-mediated synthesis of 3-(3-aryl-1-phenyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[4,3-a]pyridines via oxidation of 2-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)-1-(pyridin-2-yl)hydrazines and their antimicrobial evaluations. Org. Med. Chem. Lett. 2011, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cid-Núñez, J.M.; Trabanco-Suárez, A.A.; Lavreysen, H.; Ceusters, M. 1,2,4-triazolo[4,3-a]pyridine Compounds and Their Use as Positive Allosteric Modulators of mGluR2 Receptors. WO 2015032790 A1, 12 March 2015. [Google Scholar]
- Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo[4,3-a]pyridine derivatives. Pest Manag. Sci. 2015, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Maehata, R.; Shimomura, M. Preparation of Fused Heterocyclic Compounds as Pesticides against Harmful Arthropods. WO 2018139436 A1, 2 August 2018. [Google Scholar]
- Kalgutkar, A.S.; Hatch, H.L.; Kosea, F.; Nguyen, H.T.; Choo, E.F.; McClure, K.F.; Taylor, T.J.; Henne, K.R.; Kuperman, A.V.; Dombroski, M.A.; et al. Preclinical pharmacokinetics and metabolism of 6-(4-(2,5-difluorophenyl)oxazol-5-yl)-3-isopropyl-[1,2,4]-triazolo[4,3-a]pyridine, a novel and selective p38α inhibitor: Identification of an active metabolite in preclinical species and human liver microsomes. Biopharm. Drug Dispos. 2006, 27, 371–386. [Google Scholar] [CrossRef] [PubMed]
- McClure, K.F.; Abramov, Y.A.; Laird, E.R.; Barberia, J.T.; Cai, W.; Carty, T.J.; Cotina, S.R.; Danley, D.E.; Dipesa, A.J.; Donahue, K.M.; et al. Theoretical and Experimental Design of Atypical Kinase Inhibitors: Application to p38 MAP Kinase. J. Med. Chem. 2005, 48, 5728–5737. [Google Scholar] [CrossRef]
- Bektas, H.; Karaali, N.; Sahin, D.; Demirbas, A.; Karaoglu, S.A.; Demirbas, N. Synthesis and Antimicrobial Activities of Some New 1,2,4-Triazole Derivatives. Molecules 2010, 15, 2427–2438. [Google Scholar] [CrossRef] [Green Version]
- Lawson, E.C.; Hoekstra, W.J.; Addo, M.F.; Andrade-Gordon, P.; Damiano, B.P.; Kauffman, J.A.; Mitchell, J.A.; Maryanoff, B.E. 1,2,4-triazolo[3,4-a]pyridine as a novel, constrained template for fibrinogen receptor (GPIIb/IIIa) antagonists. Bioorg. Med. Chem. Lett. 2001, 11, 2619–2622. [Google Scholar] [CrossRef]
- Karpina, V.R.; Kovalenko, S.S.; Kovalenko, S.M.; Drushlyak, O.G.; Bunyatyan, N.D.; Georgiyants, V.A.; Ivanov, V.V.; Langer, T.; Maes, L. A Novel Series of [1,2,4]Triazolo[4,3-a]Pyridine Sulfonamides as Potential Antimalarial Agents: In Silico Studies, Synthesis and In Vitro Evaluation. Molecules 2020, 25, 4485. [Google Scholar] [CrossRef]
- Ryu, H.; Nam, K.-Y.; Kim, H.J.; Song, J.-Y.; Hwang, S.-G.; Kim, J.S.; Kim, J.; Ahn, J. Discovery of a Novel Triazolopyridine Derivative as a Tankyrase Inhibitor. Int. J. Mol. Sci. 2021, 22, 7330. [Google Scholar] [CrossRef]
- Moreau, S.; Coudert, P.; Rubat, C.; Vallee-Goyet, D.; Gardette, D.; Jean-Claude Gramain, J.-C.; Couquelet, J. Synthesis and anticonvulsant properties of triazolo- and imidazopyridazinyl carboxamides and carboxylic acids. Bioorg. Med. Chem. 1998, 6, 983–991. [Google Scholar] [CrossRef]
- Nitlikar, L.H.; Darandale, S.N.; Shinde, D.B. Exploring the Unexplored Practical and Alternative Synthesis of 3-(Trifluoromethyl)-triazolopiperazine the Key Intermediate for Sitagliptin. Lett. Org. Chem. 2013, 10, 348–352. [Google Scholar] [CrossRef]
- Al-Issa, S.A.R. Synthesis of a New Series of Pyridine and Fused Pyridine Derivatives. Molecules 2012, 17, 10902–10915. [Google Scholar] [CrossRef] [Green Version]
- Lankau, H.-J.; Langen, B.; Grunwald, C.; Hoefgen, N.; Stange, H.; Dost, R.; Egerland, U. (1,2,4)Triazolo[4,3-a]quinoxaline Derivatives as Inhibitors of Phosphodiesterases. WO 2012/104293, 9 August 2012. (A1) English. [Google Scholar]
- Nelson, P.J.; Potts, K.T. A New One-Step Synthesis of Substituted Coumarins. J. Org. Chem. 1962, 27, 3243–3247. [Google Scholar] [CrossRef]
- El Khadem, H.S.; Kawai, J.; Swartz, D.L. Synthesis and Rearrangements of Imidazolo- and Triazolo-Diazines. Heterocycles 1989, 28, 239–248. [Google Scholar] [CrossRef]
- Zavodskaya, A.V.; Bakharev, V.V.; Parfenov, V.E.; Gidaspov, A.A.; Slepukhin, P.A.; Isenov, M.L.; Eltsov, O.S. Synthesis of new 5-aza-isosteres of guanine containing aryl and hetaryl substituents on the 1,2,4-triazole ring. Tetrahedron Lett. 2015, 56, 1103–1106. [Google Scholar] [CrossRef]
- Butler, R.N.; O’Sullivan, P.; Scott, F.L. The reactions of lead tetra-acetate with substituted benzothiazolylhydrazones. J. Chem. Soc. C 1971, 2265–2268. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G.; Kumar, V.; Mittal, A. Copper(II) chloride mediated synthesis and DNA photocleavage activity of 1-aryl/heteroaryl-4-substituted-1,2,4-triazolo[4,3-a]quinoxalines. Eur. J. Med. Chem. 2011, 46, 6083–6088. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.; Thiel, O.R.; Achmatowicz, M.M.; Reichelt, A.; Larsen, R.D. Palladium-Catalyzed Coupling of Aldehyde-Derived Hydrazones: Practical Synthesis of Triazolopyridines and Related Heterocycles. Angew. Chem. Int. Ed. 2010, 49, 8395–8398. [Google Scholar] [CrossRef]
- Shawali, A.S. Tandem in situ generation and 1,5-electrocyclization of N-hetaryl nitrilimines. A facile methodology for synthesis of annulated 1,2,4-triazoles and their acyclo C-nucleosides. Arkivoc 2010, 33–97. [Google Scholar] [CrossRef]
- Chen, H.; Shang, Z.; Chang, J. Novel Synthesis of 7-β-D-Ribofuranosyl-7H-1,2,4-triazolo[3,4-i]purines with Use of NBS. Synthetic Commun. 2006, 36, 445–450. [Google Scholar] [CrossRef]
- Sun, X.; Yu, M.; Mu, X.; Zhou, Z.; Wang, L.; Liu, J.; Liu, X. A Facile Approach to [1,2,3]Triazolo[3,4-i]Purine via PIDA Oxidation Ring-closing Reaction. J. Heterocyclic Chem. 2021. [Google Scholar] [CrossRef]
- Andreas, R.; James, R.F.; Robert, M.R.; Oliver, R.T.; Michal, M.A.; Robert, D.L.; Dawei, Z. Palladium-Catalyzed Chemoselective Monoarylation of Hydrazides for the Synthesis of [1,2,4]Triazolo[4,3-a]pyridines. Org. Lett. 2010, 12, 792–795. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, S. Transition Metal-Catalyzed C–H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.C.P.; Zheng, N.; Buchwald, S.L. Combined C−H Functionalization/C−N Bond Formation Route to Carbazoles. J. Am. Chem. Soc. 2005, 127, 14560–14561. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Shimizu, S.; Imamura, Y.; Ueki, T. Incompatibilities between N-Bromosuccinimide and Solvents. Org. Process Res. Dev. 2014, 18, 354–358. [Google Scholar] [CrossRef]
- Process Wednesday: 10% NBS in DMF will Exotherm--Who Knew? Available online: http://chemjobber.blogspot.com/2014/02/process-wednesday-10-nbs-in-dmf-will.html (accessed on 26 August 2021).
- Butler, R.N.; Johnston, S.M. Stereoisomerization in heterocyclic hydrazones derived from 2-acylpyridines and their oxidative cyclization with mercury(II) acetate and lead tetra-acetate to fused 1,2,4-triazoles and 1,2,3-triazolium systems. J. Chem. Soc. Perkin Trans. 1984, 1, 2109–2116. [Google Scholar] [CrossRef]
- Mu, J.-X.; Yang, M.-Y.; Sun, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Wu, H.-K.; Liu, X.-H. Synthesis, Crystal Structure and DFT Studies of 8-chloro-3-((3-chlorobenzyl)thio)-[1,2,4]triazolo[4,3-a]pyridine. Crystals 2015, 5, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.H.; Pan, L.; Tan, C.X.; Weng, J.Q.; Wang, B.L.; Li, Z.M. Synthesis, crystal structure, bioactivity and DFT calculation of new oxime ester derivatives containing cyclopropane moiety. Pestic. Biochem. Phys. 2011, 101, 143–147. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhao, W.; Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, crystal structure and antifungal activity of 4-(5-((2,4-Dichlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine. Chin. J. Struct. Chem. 2015, 34, 203–207. [Google Scholar] [CrossRef]
- Weng, J.Q.; Wang, L.; Liu, X.H. Synthesis, Crystal structure and herbicidal activity of a 1,2,4-triazol-5(4H)-one derivative. J. Chem. Soc. Pak. 2012, 34, 1248–1252. [Google Scholar]
- Wang, Z.X.; Jian, F.F.; Duan, C.Y.; Bai, Z.P.; You, X.Z. 2-(2-Hydroxybenzylidene)-1-(2-picoloyl)hydrazine Hemihydrate. Acta Cryst. 1998, C54, 1927–1929. [Google Scholar] [CrossRef]
- Tong, J.Y.; Wu, H.K.; Sun, N.B.; Liu, X.H. Synthesis, crystal structure and biological activity of a new 1,2,4-triazole derivative. Chin. J. Struct. Chem. 2013, 32, 607–611. [Google Scholar]
- Shao, X.; Xu, Z.; Zhao, X.; Xu, X.; Tao, L.; Zhong, L.; Xuhong, Q. Synthesis, crystal structure, and insecticidal activities of highly congested hexahydroimidazo[1,2-a] pyridine derivatives: Effect of conformation on activities. J. Agric. Food Chem. 2010, 58, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).