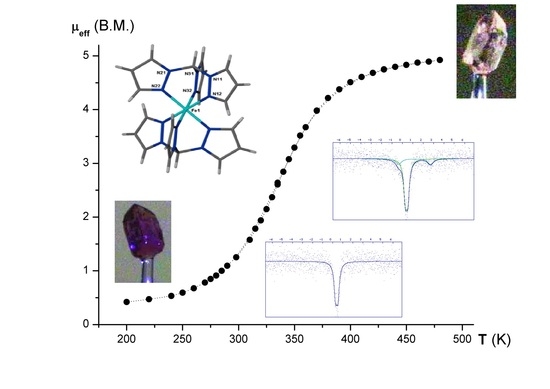
Spin Crossover in New Iron(II) Coordination Compounds with Tris(pyrazol-1-yl)Methane
Abstract
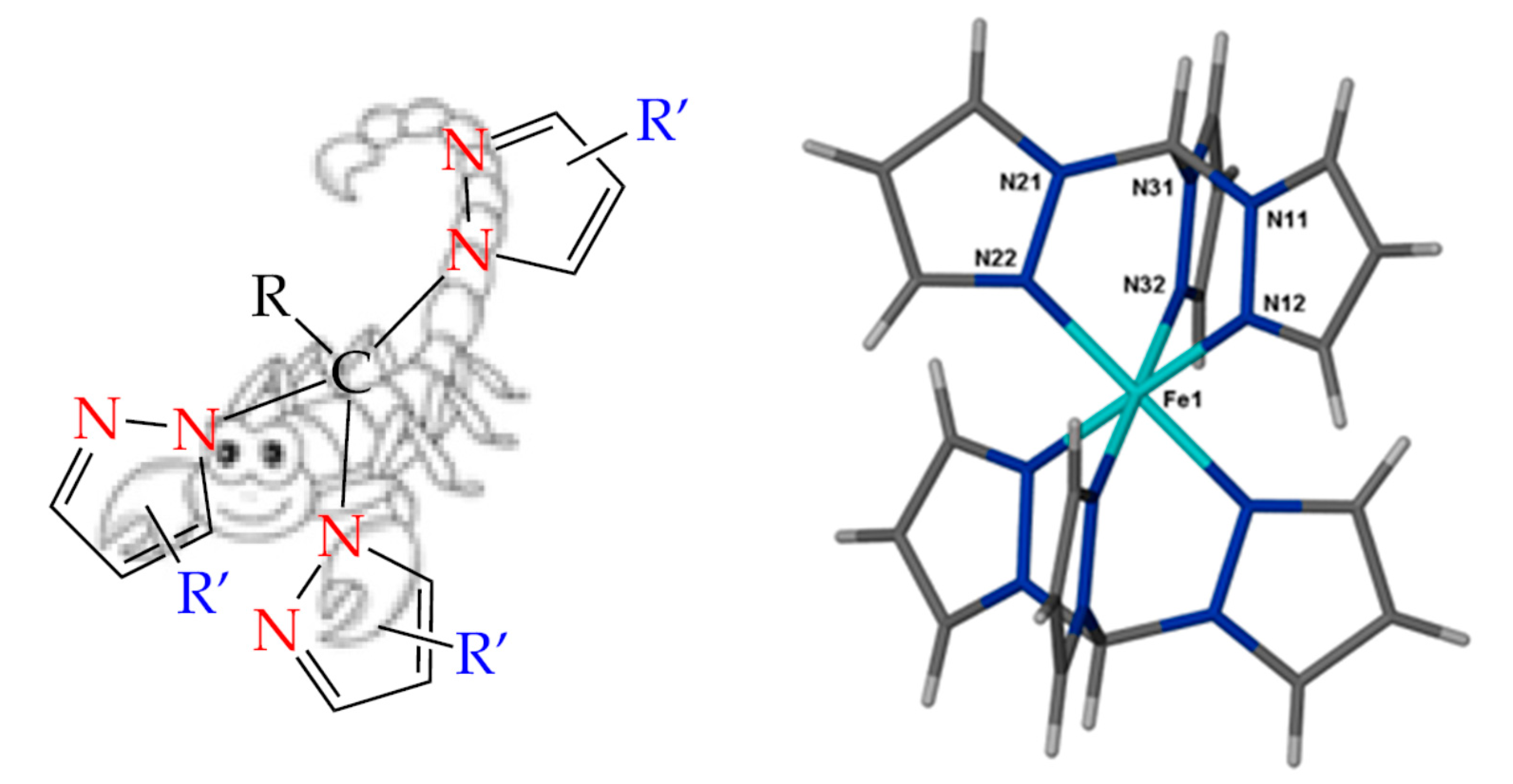
1. Introduction
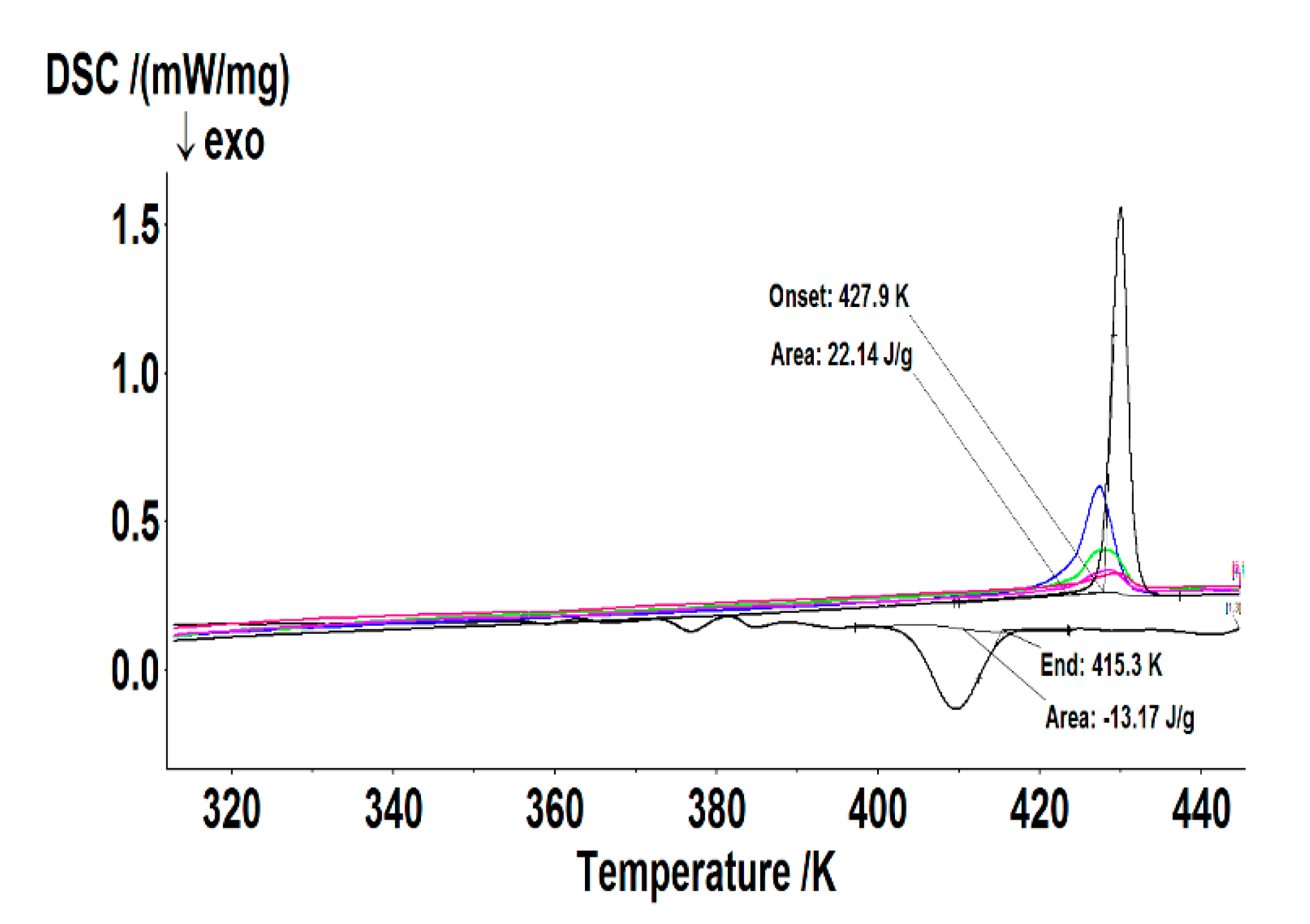
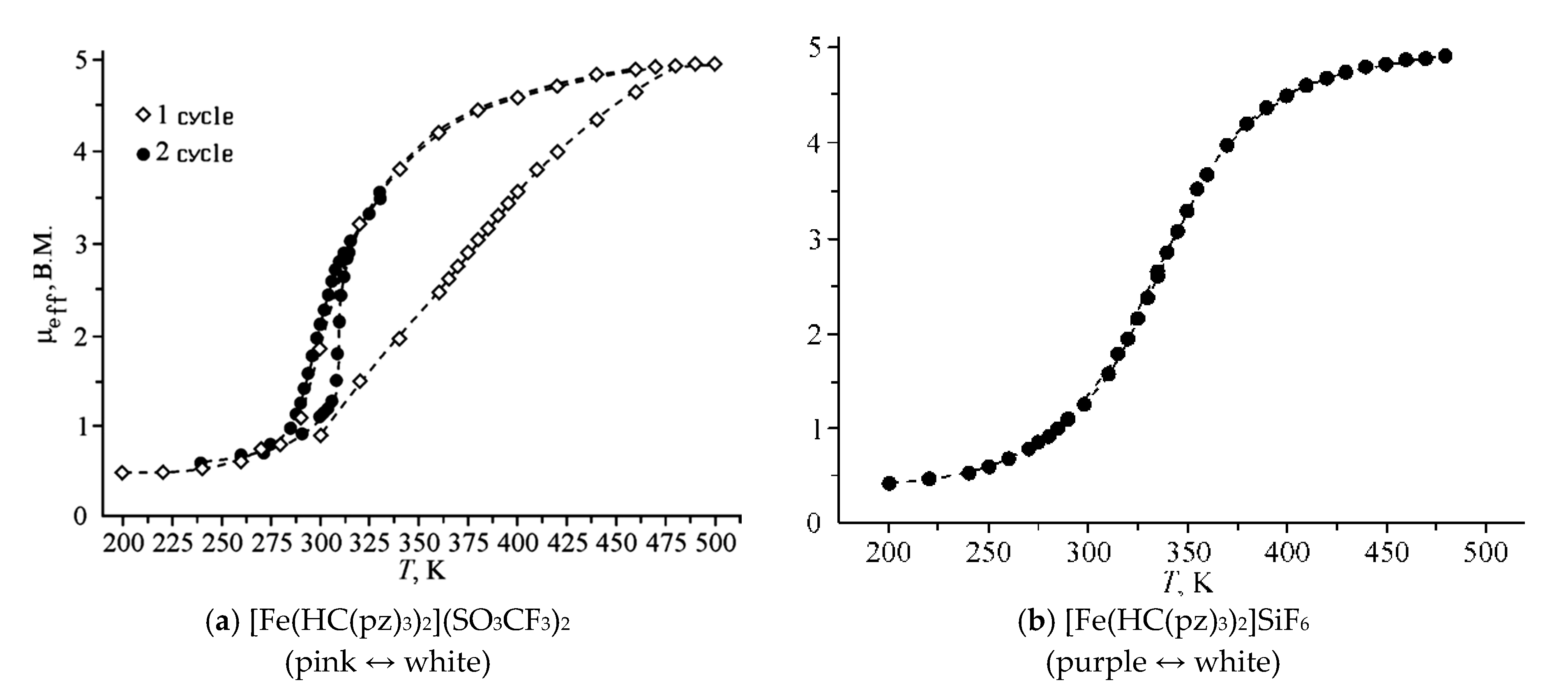
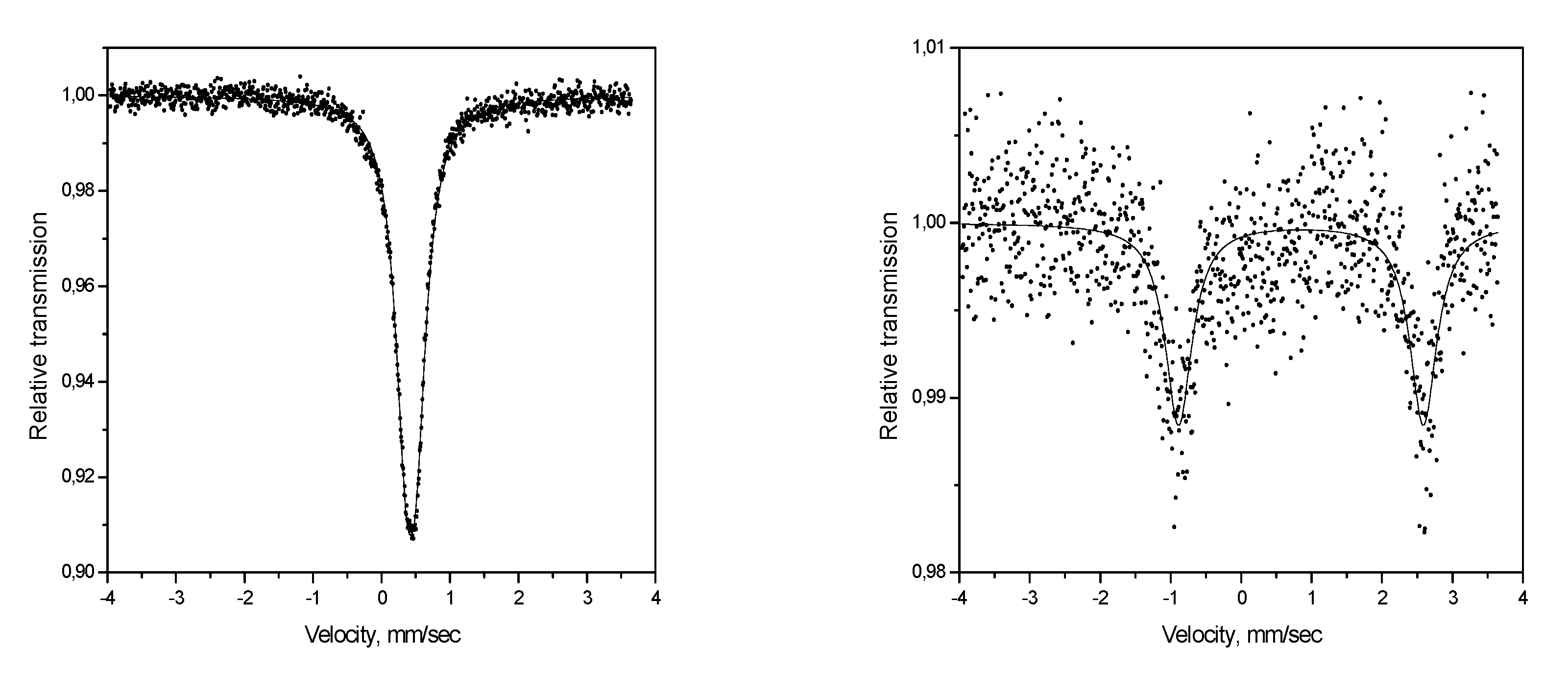
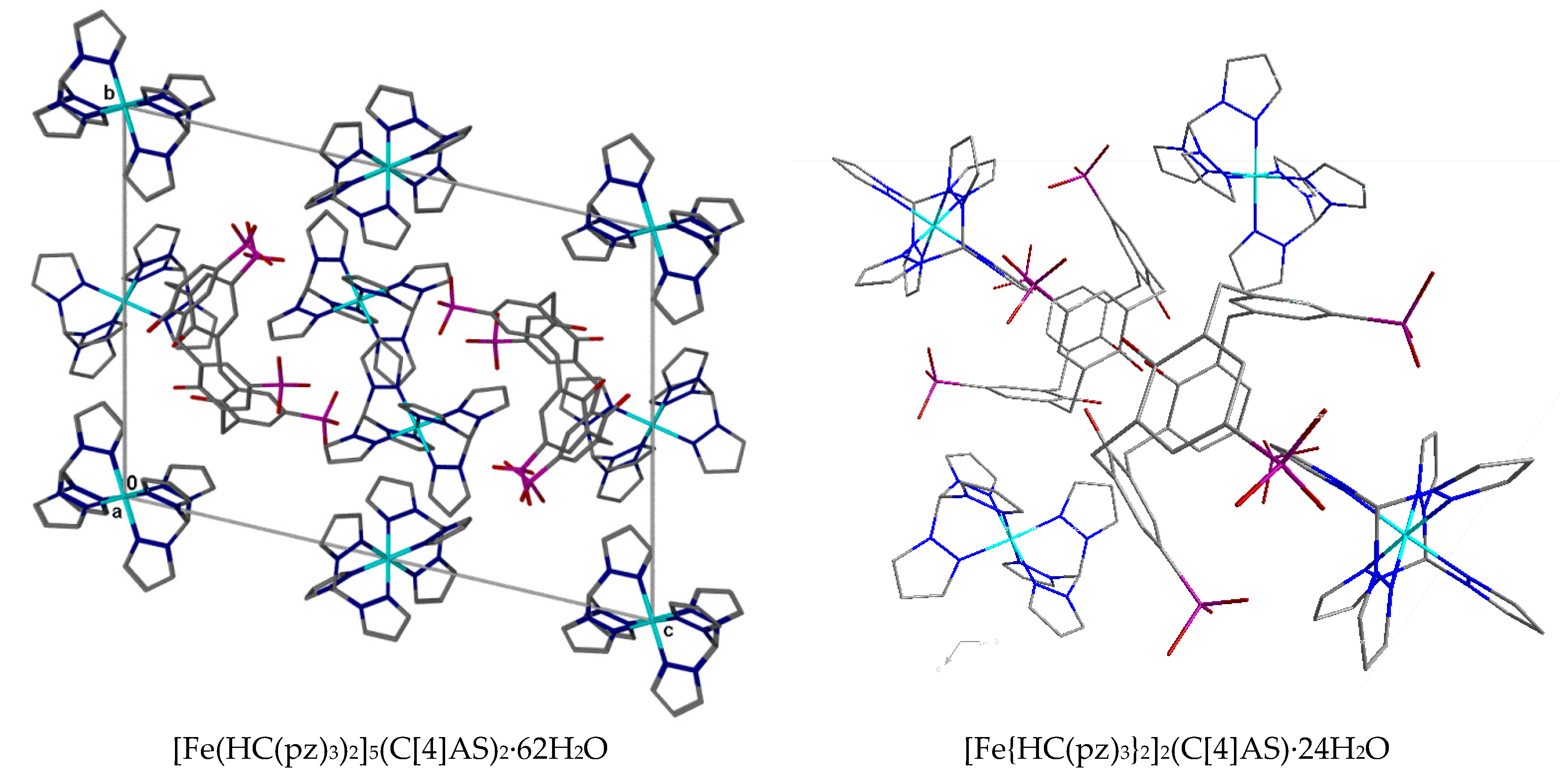
2. Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds: I–III. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2004; pp. 233–235. [Google Scholar]
- Bousseksou, A.; Molnar, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. (Ed.) Spin-Сrossover Materials: Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; 568p, ISBN 978-1-118-51931-8. [Google Scholar]
- Levchenko, G.G.; Khristov, A.V.; Varyukhin, V.N. Spin crossover in iron(II)-containing complex compounds under a pressure. Low Temp. Phys. 2014, 40, 571–585. [Google Scholar] [CrossRef]
- Gütlich, P.; Goodwin, H.A. Spin crossover-an overall perspective. In Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233, pp. 1–47. [Google Scholar]
- Gütlich, P. Photoswitchable coordination compound. Coord. Chem. Rev. 2001, 219–221, 839–879. [Google Scholar] [CrossRef]
- Boillot, M.-L.; Zarembowitch, J.; Sour, A. Ligand-Driven Light-Induced Spin Change (LD-LISC): A promising photomagnetic effect. In Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany, 2004; Volume 23, pp. 261–276. [Google Scholar]
- Miller, R.G.; Brooker, S. Reversible quantitative guest sensing via spin crossover of an iron(II) triazole. Chem. Sci. 2016, 7, 2501–2505. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, H.A. Spin transitions in six-coordinate iron(II) complexes. Coord. Chem. Rev. 1976, 18, 293–325. [Google Scholar] [CrossRef]
- Gütlich, P. Spin crossover in iron(II) complexes. In Metal Complexes; Bau, R., Gütlich, P., Teller, R.G., Eds.; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 1981; Volume 44, pp. 83–195. [Google Scholar] [CrossRef]
- König, E.; Ritter, G.; Kulshreshtha, S.K. The nature of spin-state transitions in solid complexes of iron(II) and the interpretation of some associated phenomena. Chem. Rev. 1985, 85, 219–234. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Ikorskii, V.N.; Varnek, V.A.; Oglezneva, I.M.; Lavrionov, S.V. High-temperature spin transition in iron(II) coordination compounds with triazoles. Soviet J. Coord. Chem. 1986, 12, 207–215. [Google Scholar]
- Olguín, J. Unusual metal centres/coordination spheres in spin crossover compounds. Coord. Chem. Rev. 2020, 407, 213148. [Google Scholar] [CrossRef]
- Gutlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Kahn, O.; Martinez, C.J. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Bousseksou, A.; Vieu, C.; Letard, J.-F.; Demont, P.; Tuchagues, J.-P.; Malaquin, L.; Menegotto, J.; Salmon, L. Molecular Memory and Method for Making Same. European Patent 1430552A1, 23 June 2004. [Google Scholar]
- Matsuda, M.; Isozaki, H.; Tajima, H. Electroluminescence quenching caused by a spin-crossover transition. Chem. Lett. 2008, 37, 374–375. [Google Scholar] [CrossRef]
- Muller, R.N.; Elst, V.; Laurent, S. Spin transition molecular materials: Intelligent contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 2003, 125, 8405–8407. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O.; Jay, C.; Garcia, Y.; Codjovi, E.; Philips Electronics, NV. Chemical Spin Transition Compounds Comprising Metal-Ligand Molecules Assotiated with Anion—Useful as Indicators of Temperature Increase Above a Threshold and Incorporated as Anti-Falsifiable Markers. European Patent 842988, 4 November 1996. [Google Scholar]
- Lavrenova, L.G.; Shakirova, O.G. Spin crossover and thermochromism of iron(II) coordination compounds with 1,2,4-triazoles and tris(pyrazol-1-yl)methanes. Eur. J. Inorg. Chem. 2013, 5–6, 670–682. [Google Scholar] [CrossRef]
- Hamon, P.; Thépot, J.-Y.; Le Floch, M.; Boulon, M.-E.; Cador, O.; Golhen, S.; Ouahab, L.; Fadel, L.; Saillard, J.-Y.; Hamon, J.-R. Dramatic Remote Substitutent Effects on the Electronic Spin State of Bis(scorpionate) Iron(II) Complexes. Angew. Chem. Int. Ed. 2008, 47, 8687–8691. [Google Scholar] [CrossRef]
- Vitze, H.; Bolte, M.; Lerner, H.-W.; Wagner, M. Third-Generation Scorpionates [RBpz3]−—How Influential Is the Nondonor Substituent R? Eur. J. Inorg. Chem. 2016, 15–16, 2443–2454. [Google Scholar] [CrossRef]
- Gruden-Pavlovic, M.; Stepanovic, S.; Peric, M.; Güell, M.; Swart, M. A density functional study of the spin state energetics of polypyrazolylborato complexes of first-row transition metals. Phys. Chem. Chem. Phys. 2014, 16, 14514–14522. [Google Scholar] [CrossRef]
- Trofimenko, S. Geminal poly(1-pyrazolyl)alkanes and their coordination chemistry. J. Am. Chem. Soc. 1970, 92, 5118–5126. [Google Scholar] [CrossRef]
- Mani, F. Four-, five-, and six-co-ordinated iron(II) complexes with poly(1-pyrazolyl)methanes. Inorg. Nucl. Chem. Lett. 1979, 15, 297–302. [Google Scholar] [CrossRef]
- Bigmore, H.R.; Lawrence, S.C.; Mountford, P.; Tredget, C.S. Coordination, organometallic and related chemistry of tris(pyrazolyl)methane ligands. Dalton Trans. 2005, 635–651. [Google Scholar] [CrossRef]
- Jesson, J.P.; Trofimenko, S.; Eaton, D.R. Spin equilibria in octahedral iron(II) poly(1-pyrazolyl)borates. J. Am. Chem. Soc. 1967, 89, 3158–3164. [Google Scholar] [CrossRef]
- Buchen, Th.; Gütlich, P. Substituent effects on the spin equilibrium in iron(II) pyrazolylborate complexes. Inorg. Chim. Acta 1995, 231, 221–223. [Google Scholar] [CrossRef]
- Halcrow, M.A. The effect of ligand design on metal ion spin state—Lessons from spin crossover complexes. Crystals. 2016, 6, 58. [Google Scholar] [CrossRef]
- Ikorskii, V.N. Effect of water on spin transitions in Fe(II) complexes with triazoles. Dokl. Phys. Chem. 2001, 377, 77–79. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Kirillova, E.V.; Ikorskii, V.N.; Shvedenkov, Y.G.; Varnek, V.A.; Sheludyakova, L.A.; Larionov, S.V. Iron(II) complexes with 4-R-1,2,4-triazoles (r = ethyl, propyl) exhibiting 1A1↔5T2 spin transition. Russ. J. Coord. Chem. 2001, 27, 46–50. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Yang, L.-J.; Liu, Q.-L.; Ling, Y.; Wang, W.; Sun, B.-W. Lattice water molecules tuned spin-crossover for an iron(II) complex with thermal hysteresis. Dalton Trans. 2014, 43, 16937–16942. [Google Scholar] [CrossRef]
- Bushuev, M.B.; Pishchur, D.P.; Korolkov, I.V.; Vinogradova, K.A. Prototypical iron(II) complex with 4-amino-1,2,4-triazole reinvestigated: An unexpected impact of water on spin transition. Phys. Chem. Chem. Phys. 2017, 19, 4056–4068. [Google Scholar] [CrossRef]
- McGarvey, J.J.; Toftlund, H.; Al-Obaidi, A.H.R.; Taylor, K.P.; Bell, S.E.J. Photoperturbation of the 1A⇔5T spin equilibrium in an iron(II) complex in solution via ligand field excitation. Inorg. Chem. 1993, 32, 2469–2472. [Google Scholar] [CrossRef]
- Long, G.J.; Grandjean, F.; Reger, D.L. Spin crossover in pyrazolylborate and pyrazolylmethane complexes. In Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233, pp. 91–122. [Google Scholar]
- Reger, D.L.; Little, C.A.; Rheingold, A.L.; Lam, M.; Liable-Sands, L.M.; Rhagitan, B.; Concolino, T.; Mohan, A.; Long, G.J.; Briois, V.; et al. A Synthetic, structural, magnetic, and spectral study of several {Fe[tris(pyrazolyl)methane]2}(BF4)2 complexes: observation of an unusual spin-state crossover. Inorg. Chem. 2001, 40, 1508–1520. [Google Scholar] [CrossRef]
- Anderson, P.A.; Astley, T.; Hitchman, M.A.; Keene, F.R.; Moubaraki, B.; Murray, K.S.; Skelton, B.W.; Tiekink, E.R.T.; Toftlund, H.; White, A.H. Structures and spectra of bis-tripodal iron(II) chelates, [FeL2]2+, where L=tris(pyrazol-1-yl)methane, tris(pyridin-2-yl)methane, bis(pyrazol-1-yl)(pyridin-2-yl)methane and tris(pyridin-2-yl)phosphine oxide. Magnetism and spin crossover in the (pz)3CH case. Dalton Trans. 2000, 20, 3505–3512. [Google Scholar] [CrossRef]
- Kuzu, I.; Krummenacher, I.; Hewitt, I.J.; Lan, Y.; Mereacre, V.; Powell, A.K.; Hofer, P.; Harmer, J.; Breher, F. Syntheses, structures and electronic properties of zwitterionic iron(II) and cobalt(II) complexes featuring ambidentate tris(pyrazolyl)methanide ligands. Chem. Eur. J. 2009, 15, 4350–4365. [Google Scholar] [CrossRef]
- Reger, D.L.; Little, C.A.; Smith, M.D.; Rheingold, A.L.; Lam, K.-C.; Concolino, T.L.; Long, G.J.; Hermann, R.P.; Grandjean, F. Synthetic, structural, magnetic, and mössbauer spectral study of {Fe[HC(3,5-Me2pz)3]2}I2 and its spin-state crossover behavior. Eur. J. Inorg. Chem. 2002, 5, 1190–1197. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Virovets, A.V.; Peresypkina, E.V.; Strekalova, A.D.; Piryazev, D.A.; Daletsky, V.A.; Sheludyakova, L.A.; Vasilevsky, S.F. Spin-crossover in the complex of iron(II) nitrate with tris(3,5-dimethylpyrazol-1-yl)methane. Inorg. Chim. Acta 2012, 382, 1–5. [Google Scholar] [CrossRef]
- Reger, D.L.; Little, C.A.; Rheingold, A.L.; Lam, M.; Concolino, T.; Mohan, A.; Long, G.J. Structural, electronic, and magnetic properties of {Fe[HC(3,5-Me2pz)3]2}(BF4)2 (pz = pyrazolyl): Observation of unusual spin-crossover behavior. Inorg. Chem. 2000, 39, 4674–4675. [Google Scholar] [CrossRef] [PubMed]
- Lavrenova, L.G.; Strekalova, A.D.; Virovets, A.V.; Piryazev, D.A.; Daletskii, V.A.; Sheludyakova, L.A.; Mikhailovskaya, T.F.; Vasilevskii, S.F. Spin crossover in the coordination compounds of iron(II) with tris(3,5-dimethylpyrazol-1-yl)methane. Russ. J. Coord. Chem. 2012, 38, 507–514. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Lavrenova, L.G.; Kuratieva, N.V.; Naumov, D.Yu.; Daletsky, V.A.; Sheludyakova, L.A.; Logvinenko, V.A.; Vasilevsky, S.F. Spin crossover in iron(II) complexes with tris(pyrazol-1-yl)methane. Russ. J. Coord. Chem. 2010, 36, 275–283. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Lavrenova, L.G.; Daletsky, V.A.; Shusharina, E.A.; Griaznova, T.P.; Katsyuba, S.A.; Syakaev, V.V.; Skripacheva, V.V.; Mustafina, A.R.; Solovieva, S.E. High-temperature spin-crossover in coordination compounds of iron(II) with tris(pyrazol-1-yl)methane. Inorg. Chim. Acta 2010, 363, 4059–4064. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Daletsky, V.A.; Lavrenova, L.G.; Kuratieva, N.V.; Shusharina, E.A.; Sheludyakova, L.A.; Vasilevsky, S.F. High-temperature spin transition in the iron(II) trifluoromethylsulfonate, perrhenate, and tetraphenylborate complexes with tris(pyrazol-1-yl)methane. Russ. J. Coord. Chem. 2011, 37, 511–517. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Daletskii, V.A.; Lavrenova, L.G.; Trubina, S.V.; Erenburg, S.B.; Zhizhin, K.Y.; Kuzhetsov, N.T. Iron(II) closo-borate complexes with 1,2,4-triazole derivatives: Spin crossover in the iron(II) closo-borate complexes with tris(pyrazol-1-yl)methane. Russ. J. Inorg. Chem. 2013, 58, 650–656. [Google Scholar] [CrossRef]
- Shakirova, О.G.; Kuratieva, N.V.; Korotaev, E.V.; Lavrenova, L.G. New iron(II) complexes [Fe(HC(pz)3)2]A2 with spin-crossover. Solid State Phenomena. 2015, 233–234, 534–537. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Lavrenova, L.G.; Korotaev, E.V.; Sheludyakova, L.A.; Varnek, V.A.; Shestopalov, M.A.; Mironov, Y.V. Spin-crossover in coordination compounds of iron(II) with tris(pyrazol-1-yl)methane and cluster anions. J. Struct. Chem. 2015, 56, 1520–1526. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Korotaev, E.V.; Evtushok, D.V.; Kuratieva, N.V.; Sheludyakova, L.A.; Shestopalov, M.A.; Lavrenova, L.G. Spin-crossover in iron(II) complexes with tris(pyrazol-1-yl)methane and сluster anions [{W6X8}X6]2− (X = Cl, Br, I). J. Mol. Struct. 2019, 1193, 1–6. [Google Scholar] [CrossRef]
- Moubaraki, B.; Leita, B.A.; Halder, G.J.; Betten, S.R.; Jensen, P.; Smith, J.P.; Cashion, J.D.; Kepert, C.J.; Letard, J.-F.; Murray, K.S. Structure, magnetism and photomagnetism of mixed-ligand tris(pyrazolyl)methane iron(II) spin crossover compounds. Dalton Trans. 2007, 39, 4413–4426. [Google Scholar] [CrossRef] [PubMed]
- Guionneau, P.; Marchivie, M.; Bravic, G.; L’etard, J.-F.; Chasseau, D. Structural aspects of spin crossover. Example of the [(FeLn)(NCS)2] complexes. In Spin Crossover in Transition Metal Compounds II; Springer: Berlin/Heidelberg, Germany, 2004; Volume 234, pp. 97–128. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1985; 864p, ISBN 978-0-444-42389-4. [Google Scholar]
- Griaznova, T.P.; Katsyuba, S.A.; Shakirova, O.G.; Lavrenova, L.G. Variable temperature ir spectroscopy and quantum chemistry as the tool for diagnostics of metal spin state. Chem. Phys. Lett. 2010, 495, 50–54. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Larionov, S.V. Spin Transition in Iron(II) Complexes with 1,2,4-Triazoles and Tetrazoles. Russ. J. Coord. Chem. 1998, 24, 379–395. [Google Scholar]
- Hauser, A. Ligand field theoretical considerations. In Spin Crossover in Transition Metal Compounds I; Springer: Berlin/Heidelberg, Germany, 2004; Volume 233, pp. 49–58. [Google Scholar]
- Sugano, S.; Tanabe, Y.; Kamimura, H. Multiplets of Transition Metal Ions in Crystals; Pure and Applied Physics; Academic Press: Cambridge, MA, USA, 1970; Volume 33, 333p, ISBN 978-0-126-76050-7. [Google Scholar]
- Larionov, S.V. Crystal-field parameters for the low-spin nitrous-heterocycle-containing iron(II) complexes possessing spin transition. Russ. J. Coord. Chem. 1999, 25, 408–410. [Google Scholar]
- Sugiyarto, K.H.; Goodwin, H.A. Cooperative Spin Transitions in Iron(II) Derivatives of 1,2,4-Triazole. Aust. J. Chem. 1994, 47, 263–277. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Shakirova, O.G.; Ikorskii, V.N.; Varnek, V.A.; Sheludyakova, L.A.; Larionov, S.V. 1A1 ↔ 5T2 spin transition in new thermochromic iron(II) complexes with 1,2,4-triazole and 4-amino-1,2,4-triazole. Russ. J. Coord. Chem. 2003, 29, 22–27. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Munoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005, 2062–2079. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Lavrenova, L.G.; Bogomyakov, A.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis and Magnetic Properties of Iron(II) closo-Borate Complexes with Tris(3,5-Dimethylpyrazol-1-yl)Methane. Russ. J. Inorg. Chem. 2015, 60, 786–789. [Google Scholar] [CrossRef]
- Shakirova, O.G.; Lavrenova, L.G.; Korotaev, E.V.; Kuratieva, N.V.; Burdukov, A.B.; Kolokolov, F.A. Structure and spin crossover in an iron(II) compound with tris(pyrazol-1-yl)methane and the complex [Eu(dipic)2(Hdipic)]2− anion. J. Struct. Chem. 2016, 57, 471–477. [Google Scholar] [CrossRef]
- Shvachko, Y.N.; Starichenko, D.V.; Korolyov, A.V.; Yagubskii, E.B.; Kotov, A.I.; Buravov, L.I.; Lyssenko, K.A.; Zverev, V.N.; Simonov, S.V.; Zorina, L.V.; et al. The Conducting Spin-Crossover Compound Combining Fe(II) Cation Complex with TCNQ in a Fractional Reduction State. Inorg. Chem. 2016, 55, 9121–9130. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Tahira, H.; Takahashi, N.; Otake, Yu.; Yamamura, Y.; Saito, K.; Oshio, H. Multiple bi-stability and tristability with dual spin-state conversions in [Fe(dpp)2][Ni(mnt)2]2·MeNO2. J. Am. Chem. Soc. 2010, 132, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Szafranowska, B.; Landvogt, C.; Beck, J. Structural Chemistry of Tetracyanopyrrolide (Part 2): Spin Crossover in a Bis-Tripodal Iron(II) Complex. Z. Für Anorg. Und Allg. Chem. 2015, 641, 176–179. [Google Scholar] [CrossRef]
- Karlin, R. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986; 328p, ISBN 978-3-642-70735-3. [Google Scholar]
- Shakirova, O.G.; Lavrenova, L.G.; Naumov, D.Y.; Daletsky, V.A.; Sheludyakova, L.A. High-temperature spin crossover in the [FeL2][FeL(NCS)3](NCS)·2H2O complex, L = tris(pyrazol-1-yl)methane. Polyhedron 2012, 31, 64–68. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Strekalova, A.D.; Smolentsev, A.I.; Naumov, D.Yu.; Bogomyakov, A.S.; Sheludyakova, L.A.; Vasilevskii, S.F. Mono- and heteroligand iron(II) complexes with tris(3,5-dimethylpyrazol-1-yl)methane. Russ. J. Coord. Chem. 2016, 42, 711–718. [Google Scholar] [CrossRef]
- Varnek, V.A.; Lavrenova, L.G. Исследование методом мессбауэровской спектроскопии природы влияния лиганда и внешнесферного аниона в комплексах железа (II) с 1,2,4-триазолом и 4-амино-1,2,4-триазолом на температуру спинового перехода 1A1↔5T2. Zhurn. Struktur. Khimii. 1995, 36, 120–127. (In Russian). Available online: https://jsc.niic.nsc.ru/article/16017/ (accessed on 21 September 2020).
- Varnek, V.A. Упрощенная формула для температуры спинового перехода 1A1 ⇔ 5Т2 в комплексах Fe(II). Zhurn. Struktur. Khimii. 1994, 35, 94–102. (In Russian). Available online: https://jsc.niic.nsc.ru/article/24469/ (accessed on 21 September 2020).
- Shakirova, O.; Kuratieva, N.; Korotaev, E.; Lavrenova, L.; Ovsyannikov, A.; Antipin, I.; Solovieva, S. Synthesis, crystal structures and high-temperature spin-crossover of new inclusion compounds of iron(II) tris(pyrazol-1-yl)methane complex with p-sulfonatocalix[4]arene. Inorg. Chim. Acta 2018, 476, 129–135. [Google Scholar] [CrossRef]
- Salaudeen, A.A.; Kilner, C.A.; Halcrow, M.A. Mononuclear and dinuclear iron thiocyanate and selenocyanate complexes of tris-pyrazolylmethane ligands. Polyhedron 2008, 27, 2569–2576. [Google Scholar] [CrossRef]
- Lavrenova, L.G. Spin crossover in homo- and heteroligand iron(II) complexes with tris(pyrazol-1-yl)methane derivatives. Russ. Chem. Bull. Int. Ed. 2018, 67, 1142–1152. [Google Scholar] [CrossRef]

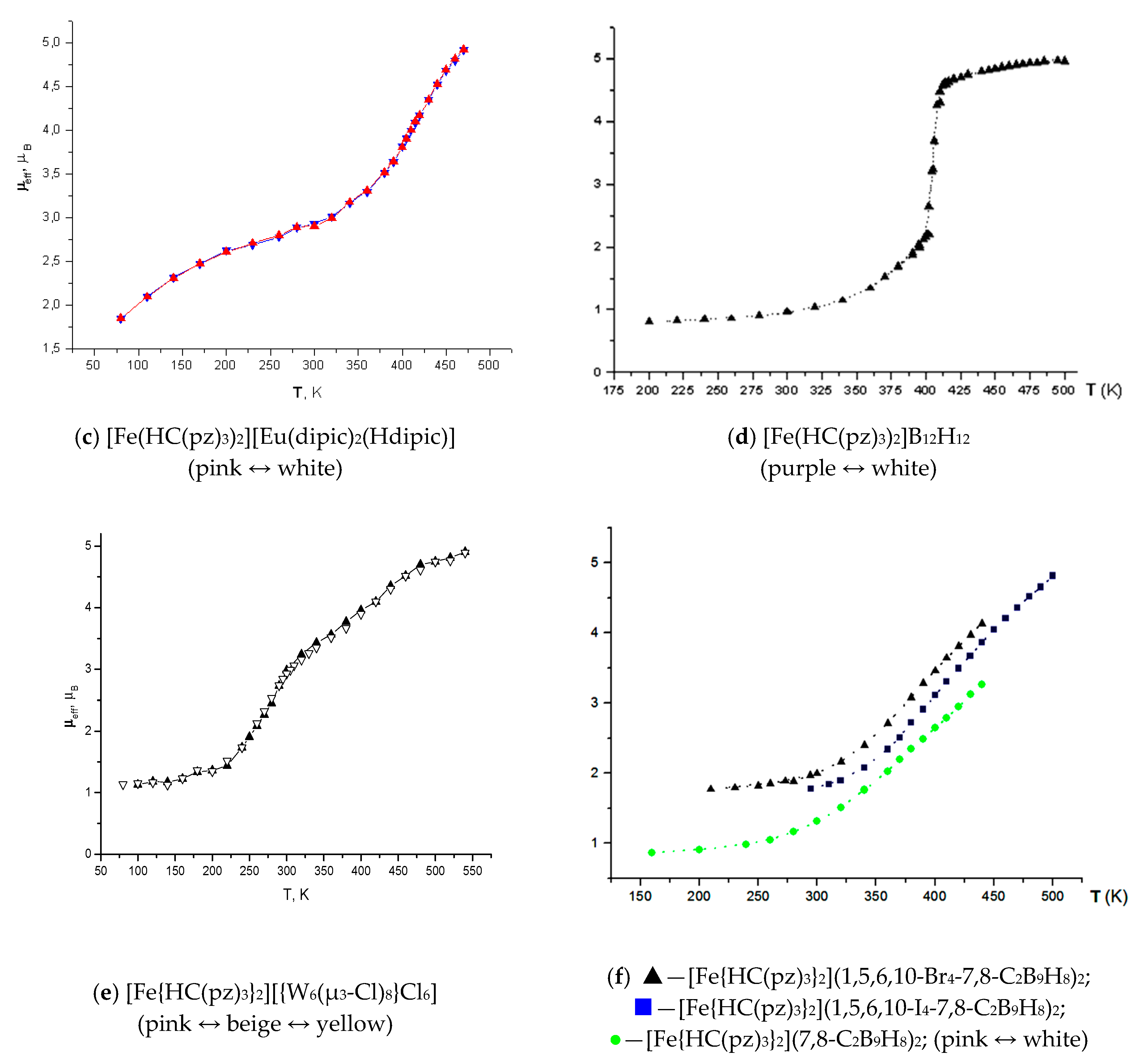

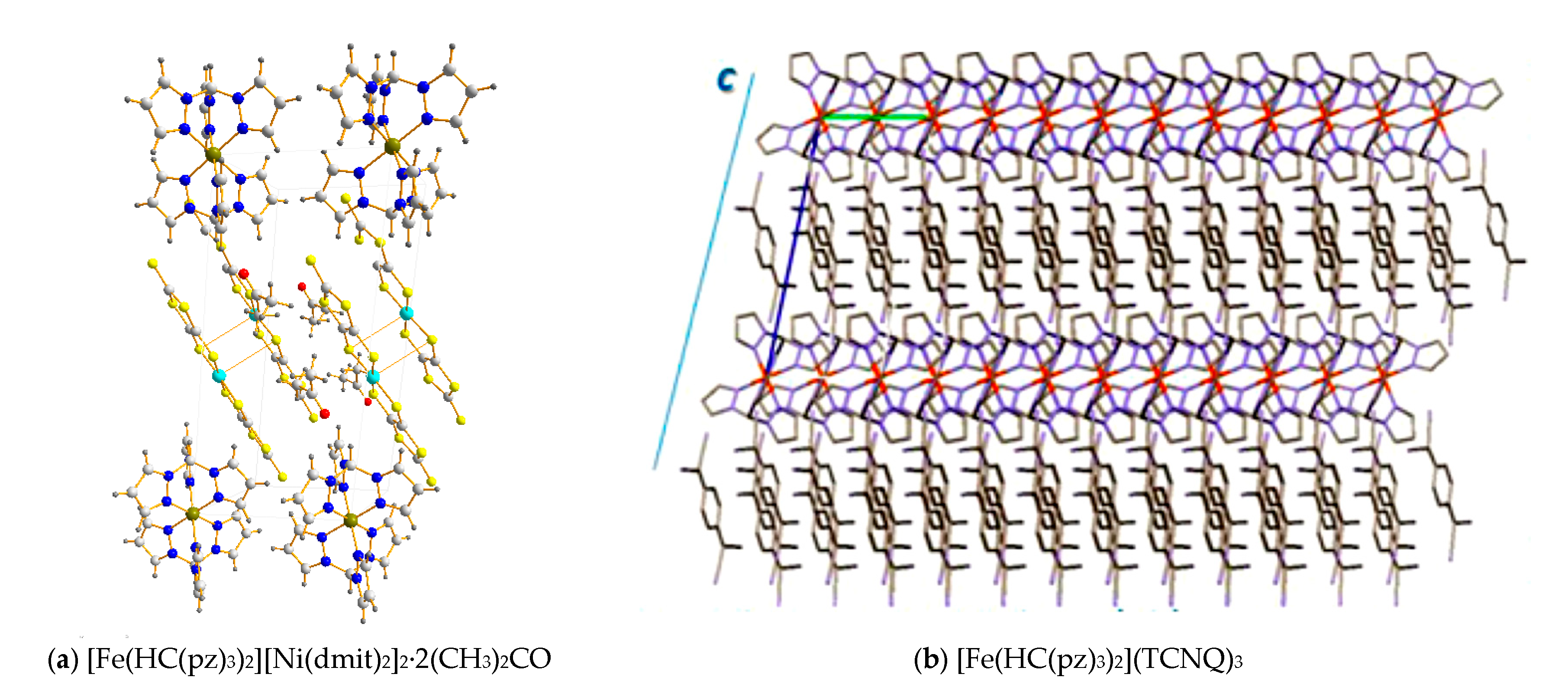

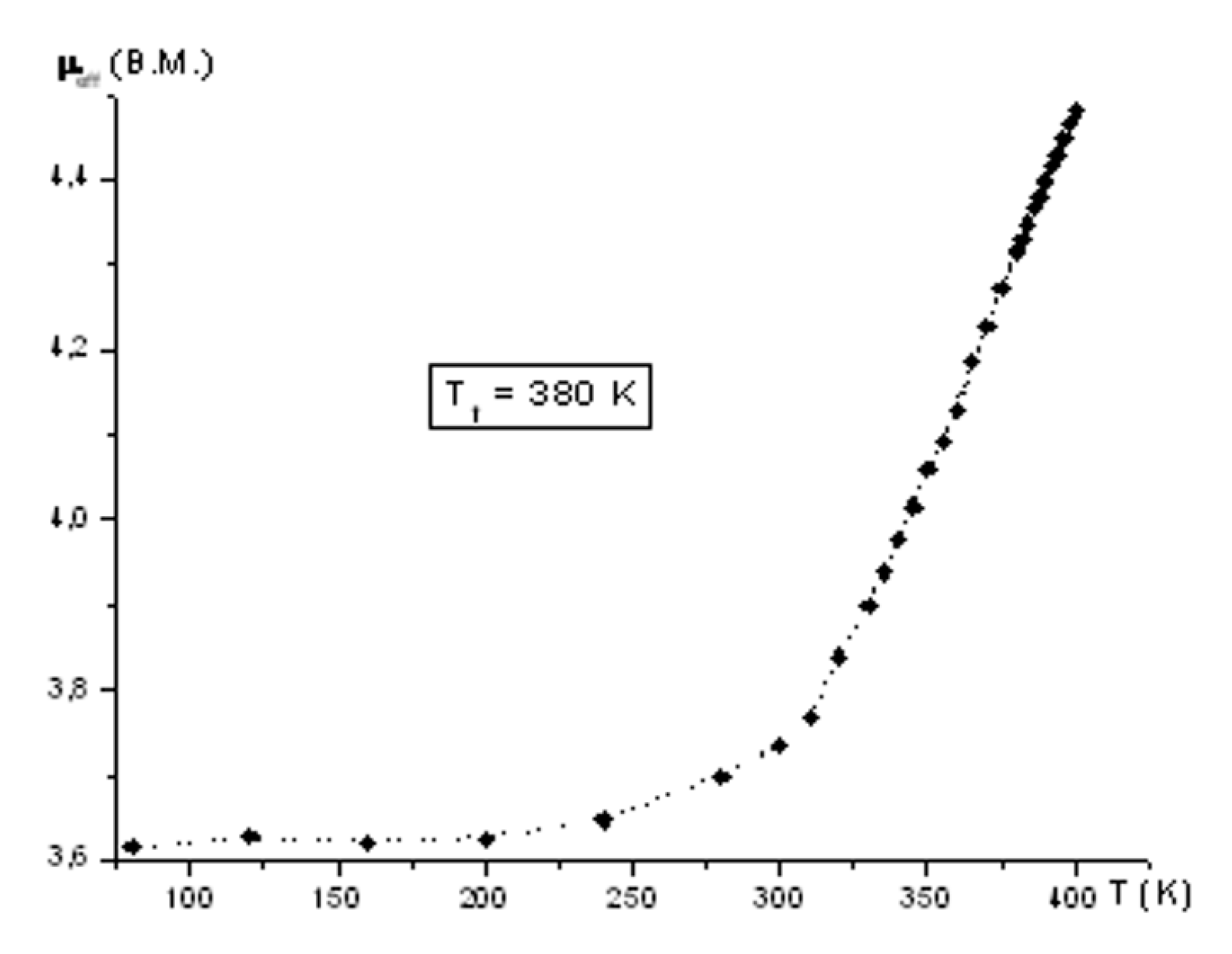

| Complex | d(Fe–N)average | ||
|---|---|---|---|
| [Fe(HC(pz)3)2](CF3SO3)2 | 1.9645 | 30.948 | 1.497 |
| [Fe(HC(pz)3)2]SiF6 | 1.9732 | 27.06 | 1.269 |
| [Fe(HC(pz)3)2](C8H5O4)2.C8H6O4 | 1.9682 | 31.36 | 1.513 |
| [Fe(HC(pz)3)2][Fe(HC(pz)3)(NCS)3](NCS).2H2O | 1.9697 | 29.745 | 1.43 |
| [Fe(HC(pz)3)2]5(C[4]AS)2.62H2O | 1.9632 | 31.472 | 1.576 |
| [Fe(HC(pz)3)2]I2 | 1.9743 | 27.24 | 1.402 |
| [Fe(HC(pz)3)2]SO4.7H2O | 1.9578 | 28.228 | 1.375 |
| [Fe(HC(pz)3)2](ReO4)2 | 1.9643 | 30.76 | 1.544 |
| [Fe(HC(pz)3)2]Cl2.2H2O | 1.9687 | 35.264 | 1.697 |
| [Fe(HC(pz)3)2][Eu(dipic)2(Hdipic)]·4H2O | 1.9682 | 31.666 | 1.66 |
| Fe(HC(pz)3)2](C12H25SO4)2 | 1.9617 | 29.488 | 1.475 |
| [Fe(HC(pz)3)2](NO3)2.H2O | 1.9616 | 28.016 | 1.372 |
| [Fe(HC(pz)3)2](C10H7SO3)2.2H2O | 1.9661 | 28.19 | 1.359 |
| [Fe(HC(pz)3)2]Br2 | 1.9663 | 25.166 | 1.28 |
| Т, K | 300 | 400 |
|---|---|---|
| Colour of crystal |  |  |
| d(Fe–N), Å | 1.9825 | 2.1216 |
| F, ° | 1.968 | 3.865 |
| Σ, ° | 31.596 | 63.912 |
| Spin state | LS | HS |
| a = b, Å | 12.9805(5) | 13.0341(16) |
| c, Å | 13.4503(4) | 13.8143(14) |
| V, Å3 | V = 1962.66(12) | 2032.46(41) |
| Z | 3 | 3 |
| ρ, g/cm3 | 2.01269 | 1.94358 |
| Complex | 300 K (LS) before Heating | 475 K (HS) | 300 K (LS) after Cooling | |||
|---|---|---|---|---|---|---|
| R, Å ± 0.01 Fe–N1 | R, Å ± 0.01 Fe–N2 | R, Å ± 0.01 Fe–N1 | R, Å ± 0.01 Fe–N2 | R, Å ± 0.01 Fe–N1 | R, Å ± 0.01 Fe–N2 | |
| [Fe(HC(pz)3)2]B10Cl10 | 1.96 | 2.89 | 2.09 | 2.98 | 1.96 | 2.89 |
| [Fe(HC(pz)3)2]B10H10 | 1.96 | 2.89 | 2.13 | 3.01 | 1.96 | 2.89 |
| [Fe(HC(pz)3)2]B12H12.2H2O | 1.96 | 2.91 | 2.15 | 3.03 | 1.96 | 2.91 |
| Complex | Spin State | ΔE, cm−1 | В, cm−1 | C, cm−1 | cm−1 | |
|---|---|---|---|---|---|---|
| Fe(Htrz)3A2 | HS | 11,100–11,770 | 600 | 2650 | 11,100–11,770 | 19,240–20,360 (21,120–21,230 [57]) |
| LS | 18,200–19,230 | |||||
| Fe(NH2trz)3A2 | HS | 10,870–12,990 | 630 | 2780 | 10,870–12,990 | 18,480–19,740 (20,450 [57]) |
| LS | 17,550–18,690 | |||||
| Fe(4-C2H5-trz)3A2 | HS | 11,630–11,770 | 620 | 2730 | 11,630–11,770 | 18,830 |
| LS | 17,860 | |||||
| Fe(4-C3H7-trz)3A2 | HS | 11,400–11,800 | 610 | 2690 | 11,400–11,800 | 18,460 |
| LS | 17,500 | |||||
| [Fe(HC(pz)3)2]A2 | HS | 18,690–20,830 | 690 | 3040 | 19,650–22,010 | |
| [Fe(HC(3,5-(CH3)2pz)3)2]A2 | LS | 12,900–14,600 | 720 | 3180 | 12,900–14,600 |
| Complex | Т, K | δ, mm/sec | ε, mm/sec |
|---|---|---|---|
| [Fe(HC(pz)3)2]I2 | 295 | 0.404 | 0.24 |
| [Fe(HC(pz)3)2]Br2 | 293 | 0.406 | 0.23 |
| [Fe(HC(pz)3)2](SO3CF3)2 | 293 | 0.407 | 0.28 |
| [Fe(HC(pz)3)2](ReO4)2 | 293 | 0.416 | 0.22 |
| [Fe(HC(pz)3)2]SO4·2H2O | 293 | 0.394 | 0.24 |
| [Fe(HC(pz)3)2]SiF6 | 295 | 0.427 | 0.16 |
| 473 | 0.849 | 3.47 | |
| [Fe(HC(pz)3)2](7,8-C2B9H12)2·2H2O | 295 | 0.39 | 0.28 |
| [Fe(HC(pz)3)2](1,5,6,10-Br4-7,8-С2B9H8)2·2H2O | 295 | 0.37 | 0.25 |
| [Fe(HC(pz)3)2](1,5,6,10-I4-7,8-С2B9H8)2 | 295 | 0.37 | 0.38 |
| [Fe(HC(pz)3)2][Mo6Cl14]·2H2O | 295 | 0.500 | 0.341 |
| 1.395 | 3.570 | ||
| [Fe(HC(pz)3)2][Mo6Br14]·H2O | 295 | 0.476 | 0.279 |
| 1.363 | 3.383 | ||
| [Fe(HC(pz)3)2]2[Re6S8(CN)6]·2H2O | 295 | 0.486 | 0.323 |
| 1.197 | 3.201 | ||
| for comparison | |||
| Fe(NH2trz)3SiF6 | 293 | 1.04 | 2.78 |
| 473 | (>0.90) | (<2.69) | |
| Fe(Htrz)3SiF6 | 293 | 0.42 | 0.26 |
| Fe(Htrz)3I2 | 295 | 0.42 | 0.25 |
| δ | ±0.003 | ±0.01 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakirova, O.G.; Lavrenova, L.G. Spin Crossover in New Iron(II) Coordination Compounds with Tris(pyrazol-1-yl)Methane. Crystals 2020, 10, 843. https://doi.org/10.3390/cryst10090843
Shakirova OG, Lavrenova LG. Spin Crossover in New Iron(II) Coordination Compounds with Tris(pyrazol-1-yl)Methane. Crystals. 2020; 10(9):843. https://doi.org/10.3390/cryst10090843
Chicago/Turabian StyleShakirova, Olga G., and Ludmila G. Lavrenova. 2020. "Spin Crossover in New Iron(II) Coordination Compounds with Tris(pyrazol-1-yl)Methane" Crystals 10, no. 9: 843. https://doi.org/10.3390/cryst10090843
APA StyleShakirova, O. G., & Lavrenova, L. G. (2020). Spin Crossover in New Iron(II) Coordination Compounds with Tris(pyrazol-1-yl)Methane. Crystals, 10(9), 843. https://doi.org/10.3390/cryst10090843