Preparation and Electrochemical Properties of Co3O4 Supercapacitor Electrode Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis and Preparation
3. Results and Discussion
3.1. Structural and Morphological Characterization
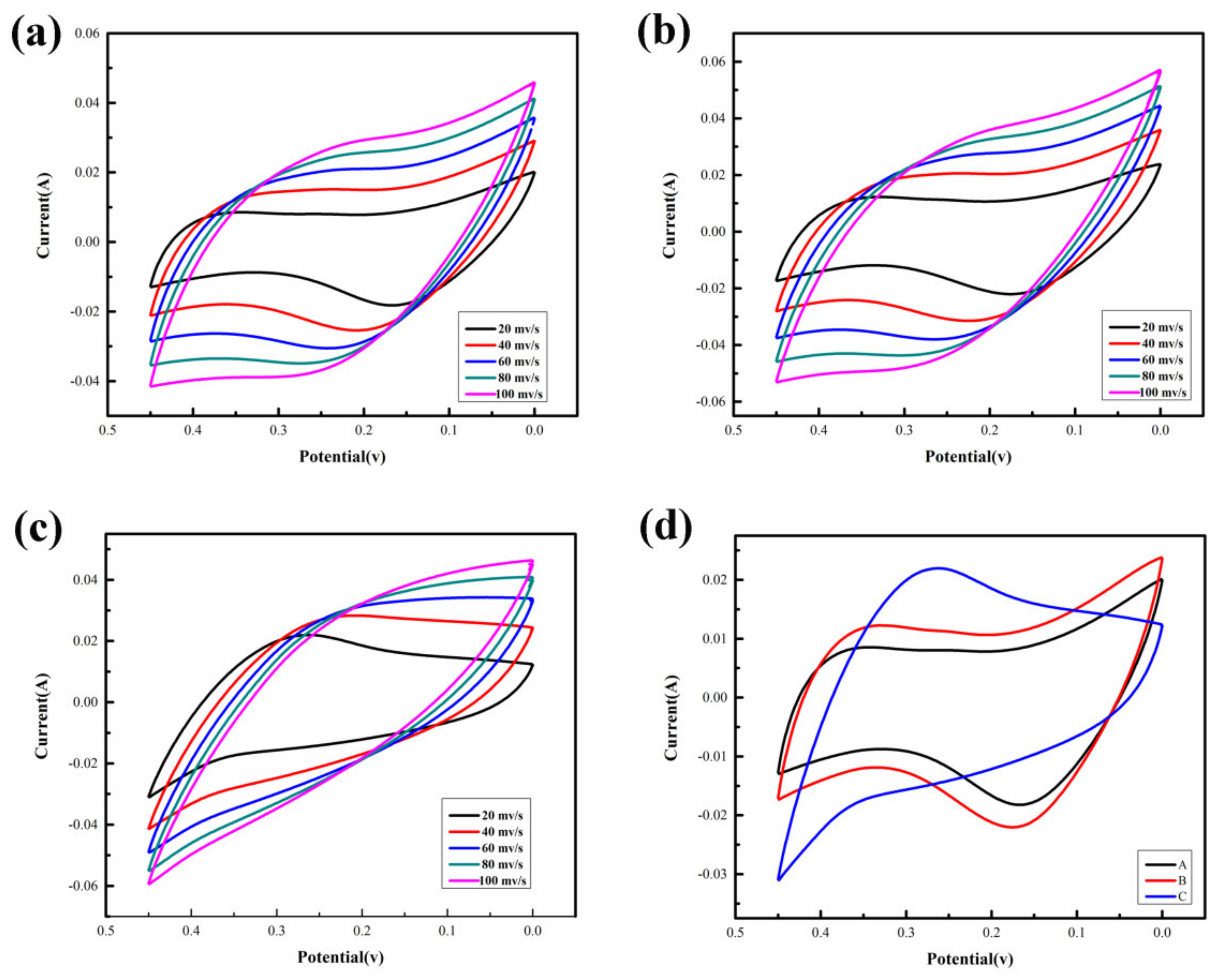
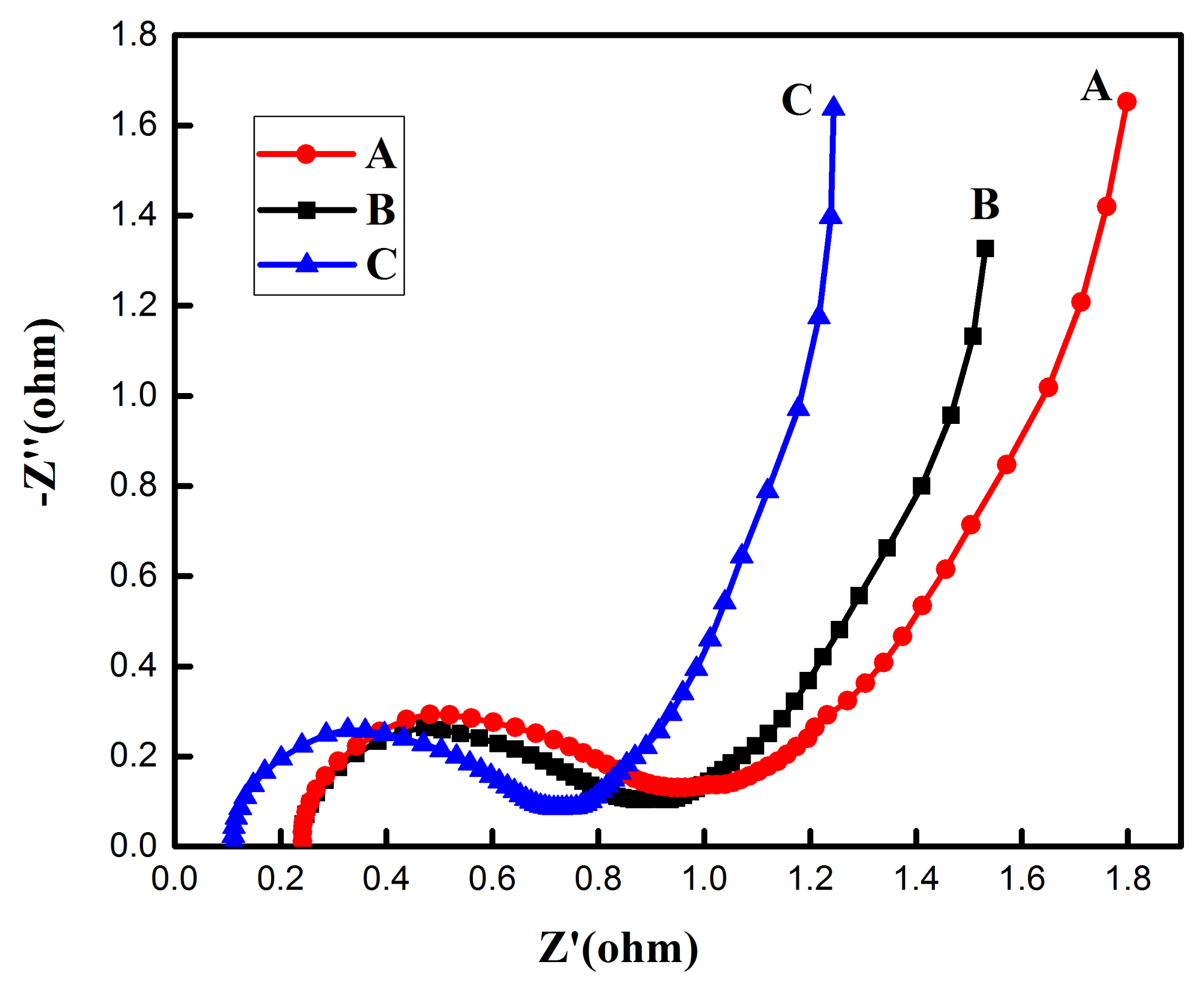
3.2. Electrochemical Performances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salunkhe, R.R.; Tang, J.; Kamachi, Y.; Nakato, T.; Kim, J.H.; Yamauchi, Y. Asymmetric Supercapacitors Using 3D Nanoporous Carbon and Cobalt Oxide Electrodes Synthesized from a Single Metal-Organic Framework. ACS Nano 2015, 9, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Pendashteh, A.; Moosavifard, S.E.; Rahmanifar, M.S.; Wang, Y.; El-Kady, M.F.; Kaner, R.B.; Mousavi, M.F. Highly Ordered Mesoporous CuCo2O4 Nanowires, a Promising Solution for High-Performance Supercapacitors. Chem. Mater. 2015, 27, 3919–3926. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, B.; Liu, R.; Wang, Q.F.; Hou, X.J.; Chen, D.; Wang, R.M.; Shen, G.Z. Fiber-Based Flexible All-Solid-State Asymmetric Supercapacitors for Integrated Photodetecting System. Angew. Chem. Int. Ed. 2014, 53, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Zhang, J.Q.; Wu, W.J.; Guo, X.; Xiong, P.; Liu, H.; Wang, G.X. Cobalt-doped MnO2 ultrathin nanosheets with abundant oxygen vacancies supported on functionalized carbon nanofibers for efficient oxygen evolution. Nano Energy 2018, 54, 129–137. [Google Scholar] [CrossRef]
- Seok, J.Y.; Lee, J.; Yang, M. Self-Generated Nanoporous Silver Framework for High-Performance Iron Oxide Pseudocapacitor Anodes. ACS Appl. Mater. Interfaces 2018, 10, 17223–17231. [Google Scholar] [CrossRef]
- Dong, Y.; Xing, L.; Chen, K.; Wu, X. Porous α-Fe2O3@C Nanowire Arrays as Flexible Supercapacitors Electrode Materials with Excellent Electrochemical Performances. Nanomaterials 2018, 8, 487. [Google Scholar] [CrossRef]
- Wu, Z.S.; Sun, Y.; Tan, Y.Z.; Yang, S.B.; Feng, X.L.; Mullen, K. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef]
- Eskusson, J.; Rauwel, P.; Nerut, J.; Jänes, A. A Hybrid Capacitor Based on Fe3O4-Graphene Nanocomposite/Few-Layer Graphene in Different Aqueous Electrolytes. J. Electrochem. Soc. 2016, 163, 2768–2775. [Google Scholar] [CrossRef]
- Wang, X.; Hu, A.; Meng, C.; Wu, C.; Yang, S.; Hong, X. Recent Advance in Co3O4 and Co3O4-Containing Electrode Materials for High-Performance Supercapacitors. Molecules 2020, 25, 269. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, L.; Zhang, H.; Zhang, Q.; Chen, W.; Zhu, J.; Song, D. Two-dimensional Co3O4 thin sheets assembled by 3D interconnected nanoflake array framework structures with enhanced supercapacitor performance derived from coordination complexes. Chem. Eng. J. 2016, 292, 1–12. [Google Scholar] [CrossRef]
- Gong, X.F.; Cheng, J.P.; Liu, F.; Zhang, L.; Zhang, X.B. Nickel-Cobalt hydroxide microspheres electrodepositioned on nickel cobaltite nanowires grown on Ni foam for high-performance pseudocapacitors. J. Power Sources 2014, 267, 610–616. [Google Scholar] [CrossRef]
- Xiao, Z.; Fan, L.; Xu, B.; Zhang, S.; Kang, W.; Kang, Z.; Lin, H.; Liu, X.; Zhang, S.; Sun, D. Green Fabrication of Ultrathin Co3O4 Nanosheets from Metal–Organic Framework for Robust High-Rate Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 41827–41836. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Su, H.; Jin, L.; Zhang, H.; Chu, X.; Yang, W. Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 2017, 505, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, V.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Shape controlled synthesis of rod-like Co3O4 nanostructures as high-performance electrodes for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2018, 29, 6059–6067. [Google Scholar] [CrossRef]
- Guo, X.G.; Li, X.M.; Xiong, Z.S.; Lai, C.; Li, Y.; Huang, X.Y.; Bao, H.B.; Yin, Y.J.; Zhu, Y.H.; Zhang, D.X. A comprehensive investigation on electrophoretic self-assembled nano-Co3O4 films in aqueous solution as electrode materials for supercapacitors. J. Nanopart. Res. 2016, 18, 144. [Google Scholar] [CrossRef]
- Wang, C.; Meng, Y.S.; Wang, L.; Zhu, F.L.; Zhang, Y. One Step Hydrothermal Synthesis of Flower-shaped Co3O4 Nanorods on Nickel Foam as Supercapacitor Materials and Their Excellent Electrochemical Performance. Chem. Res. Chin. Univ. 2018, 34, 882–886. [Google Scholar] [CrossRef]
- Deori, K.; Ujjain, S.K.; Sharma, R.K.; Deka, S. Morphology controlled synthesis of nanoporous Co3O4 nanostructures and their charge storage characteristics in supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10665–10672. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, J.; He, Y.; Zhong, C.; Hu, W.; Liu, L.; Deng, Y. Sub-3 nm Co3O4 Nanofilms with Enhanced Supercapacitor Properties. ACS Nano 2015, 9, 1730–1739. [Google Scholar] [CrossRef]
- Meng, T.; Xu, Q.-Q.; Wang, Z.-H.; Li, Y.-T.; Gao, Z.-M.; Xing, X.-Y.; Ren, T.-Z. Co3O4 Nanorods with Self-assembled Nanoparticles in Queue for Supercapacitor. Electrochim. Acta 2015, 180, 104–111. [Google Scholar] [CrossRef]
- Song, F.M.; Zan, G.T.; Chen, Y.; Wu, Q.S.; Xu, Y.Y. In situ transformation of iron-group ternary metal oxides nanocubes from Co/Ni-PBA for high-performance supercapacitors. J. Alloys Compd. 2018, 741, 633–641. [Google Scholar] [CrossRef]
- Xu, Y.N.; Ding, Q.; Li, L.; Xie, Z.J.; Jiang, G.X. Facile fabrication of porous Co3O4 nanowires for high performance supercapacitors. New J. Chem. 2018, 42, 20069–20073. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Sol-gel synthesis, characterisation, and photocatalytic activity of porous spinel Co3O4 nanosheets. Chem. Pap. 2014, 68, 1087–1096. [Google Scholar] [CrossRef]
- Wei, G.; Zhou, Z.; Zhao, X.; Zhang, W.; An, C. Ultrathin Metal–Organic Framework Nanosheet-Derived Ultrathin Co3O4 Nanomeshes with Robust Oxygen-Evolving Performance and Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 23721–23730. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.R.; Guo, Y.K.; Ju, L.L.; Xiao, H.; Hu, A.M.; Li, M. Facile synthesis of petal-like nanocrystalline Co3O4 film using direct high-temperature oxidation. J. Mater. Sci. 2019, 54, 7922–7930. [Google Scholar] [CrossRef]
- Duan, Y.; Hu, T.; Yang, L.; Gao, J.; Guo, S.; Hou, M.; Ye, X. Facile fabrication of electroactive microporous Co3O4 through microwave plasma etching for supercapacitors. J. Alloys Compd. 2019, 771, 156–161. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kulkarni, S.B. Chemical synthesis of Co3O4 nanowires for symmetric supercapacitor device. J. Mater. Sci. Mater. Electron. 2018, 29, 16401–16409. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, S.; Bai, Z.; Dai, P.; Yu, X.; Wu, M.; Hu, H. Facile fabrication and configuration design of Co3O4 porous acicular nanorod arrays on Ni foam for supercapacitors. Nanotechnology 2018, 29, 315402. [Google Scholar] [CrossRef]
- Ambare, R.C.; Lokhande, B.J. Ru incorporation enhanced electrochemical performance of spray deposited Mn: Co3O4 nano-composite: Electrochemical approach. J. Anal. Appl. Pyrolysis 2018, 132, 245–253. [Google Scholar] [CrossRef]
- You, Y.; Zheng, M.; Ma, L.; Yuan, X.; Zhang, B.; Li, Q.; Wang, F.; Song, J.; Jiang, D.; Liu, P.; et al. Galvanic displacement assembly of ultrathin Co3O4 nanosheet arrays on nickel foam for a high-performance supercapacitor. Nanotechnology 2017, 28, 105604. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, Y.Q.; Yang, G.W. A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 2016, 8, 4227–4235. [Google Scholar] [CrossRef]
- Kumar, M.; Subramania, A.; Balakrishnan, K. Preparation of electrospun Co3O4 nanofibers as electrode material for high performance asymmetric supercapacitors. Electrochim. Acta 2014, 149, 152–158. [Google Scholar] [CrossRef]
- Peterson, G.R.; Hung-Low, F.; Gumeci, C.; Bassett, W.P.; Korzeniewski, C.; Hope-Weeks, L.J. Preparation–Morphology–Performance Relationships in Cobalt Aerogels as Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Wan, L.; Sun, S.; Chen, Q.; Sun, J.; Xia, Q.; Xia, H. Phosphate Ion Functionalized Co3O4 Ultrathin Nanosheets with Greatly Improved Surface Reactivity for High Performance Pseudocapacitors. Adv. Mater. 2017, 29, 1604167. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, L.; Cao, J.; Wang, W.; Chen, Z. Preparation and electrochemical capacitance of cobalt oxide (Co3O4) nanotubes as supercapacitor material. Electrochim. Acta 2010, 56, 732–736. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Wang, J.; Kang, L.; Liu, Z.-H. Phosphate ion functionalized Co3O4 nanosheets/RGO with improved electrochemical performance. Colloids Surf. A 2020, 586, 124232. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, A.; Zhu, Q.; Nie, Z.; Zhang, Y.; Tang, Y.; Liang, S.; Cao, G. Facile synthesis of nanorod-assembled multi-shelled Co3O4 hollow microspheres for high-performance supercapacitors. J. Power Sources 2014, 272, 107–112. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Xiong, D.-B.; Qiao, Y.; Tang, Y.; Gao, F. Hybridized Phosphate with Ultrathin Nanoslices and Single Crystal Microplatelets for High Performance Supercapacitors. Sci. Rep. 2016, 6, 17613. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-Nanonet Hollow Structured Co3O4 for High Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef]
- Kong, S.Y.; Yang, F.; Cheng, K.; Ouyang, T.; Ye, K.; Wang, G.L.; Cao, D.X. In-situ growth of cobalt oxide nanoflakes from cobalt nanosheet on nickel foam for battery-type supercapacitors with high specific capacity. J. Electroanal. Chem. 2017, 785, 103–108. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ahmadi, R.; Gharailou, D.; Ganjali, M.R.; Norouzi, P. A facile route to preparation of Co3O4 nanoplates and investigation of their charge storage ability as electrode material for supercapacitors. J. Mater. Sci. Mater. Electron. 2016, 27, 8623–8632. [Google Scholar] [CrossRef]
- Yadav, A.A.; Chavan, U.J. Electrochemical supercapacitive performance of spray deposited Co3O4 thin film nanostructures. Electrochim. Acta 2017, 232, 370–376. [Google Scholar] [CrossRef]
- Wu, C.; Lu, X.; Peng, L.; Xu, K.; Peng, X.; Huang, J.; Yu, G.; Xie, Y. Two-dimensional vanadyl phosphate ultrathin nanosheets for high energy density and flexible pseudocapacitors. Nat. Commun. 2013, 4, 2431. [Google Scholar] [CrossRef] [PubMed]
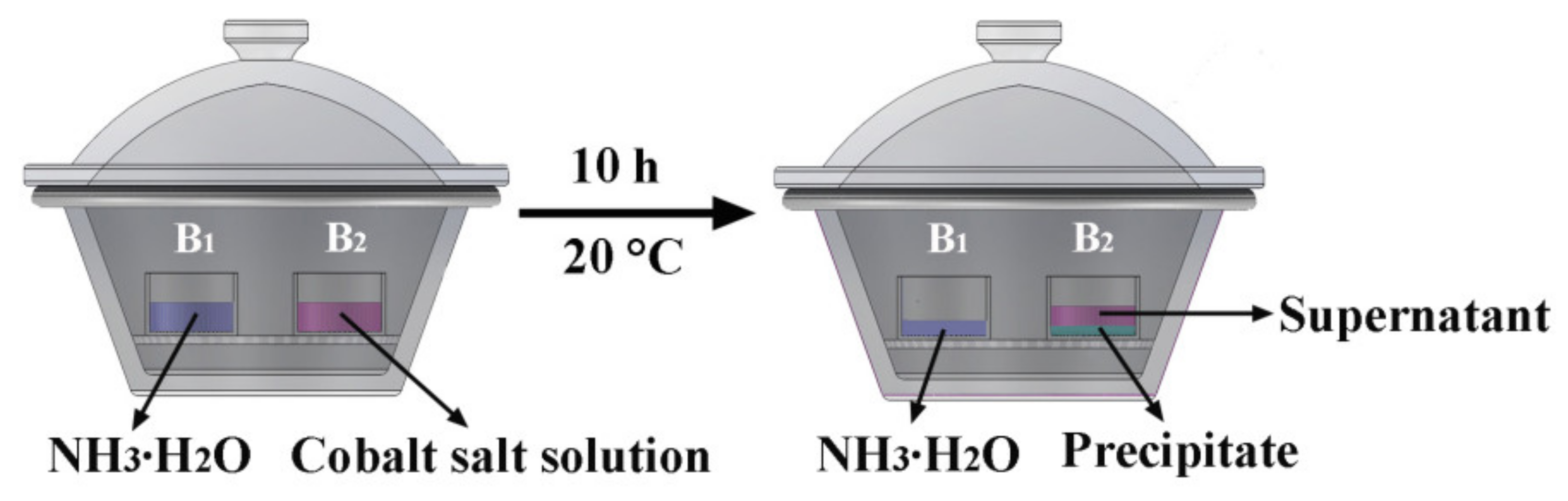
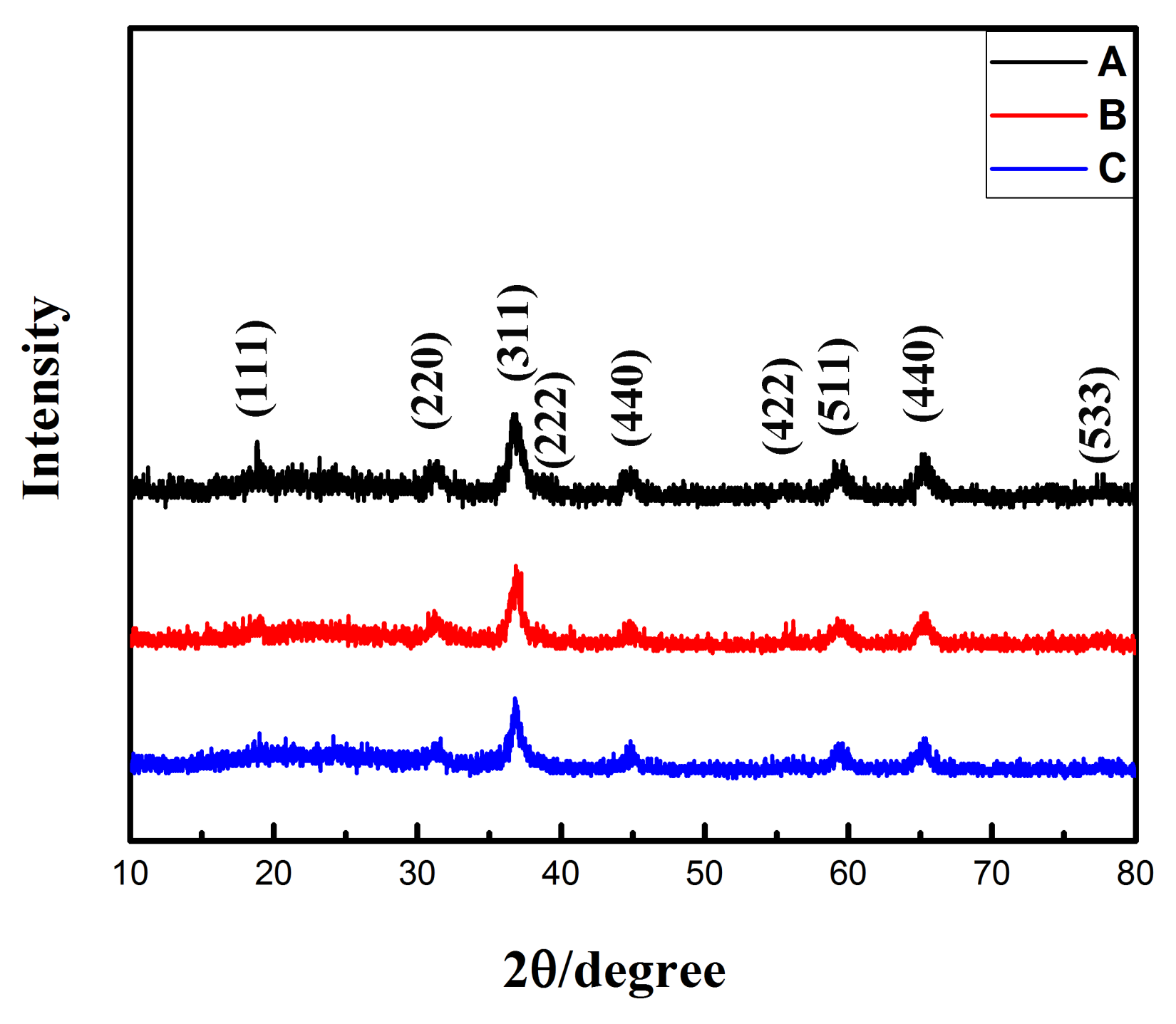
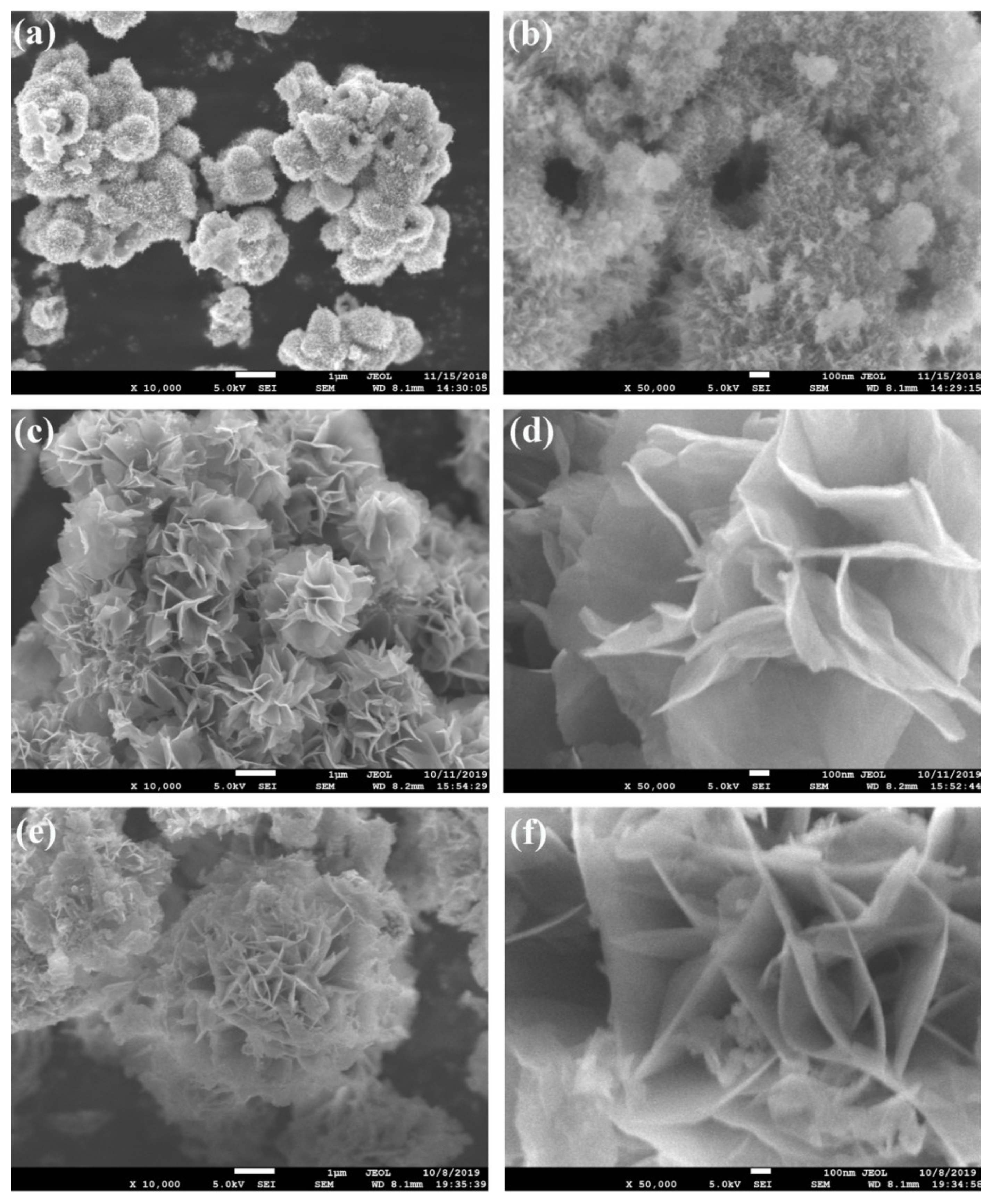
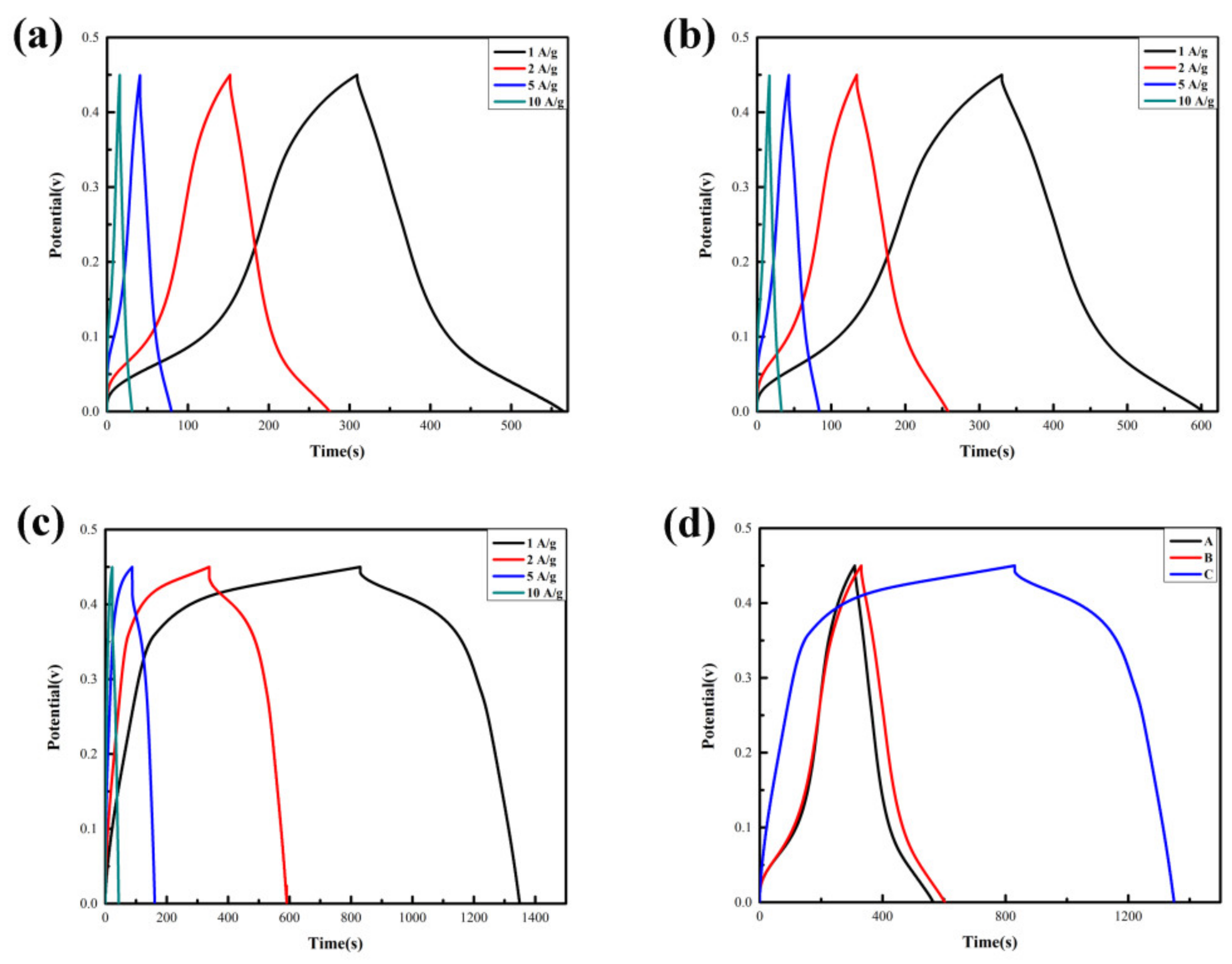

| Sample | Cobalt Source | Solvent | Precipitator | NaH2PO4·2H2O |
|---|---|---|---|---|
| A | Co(OAc)2·4H2O | H2O | NH3·H2O | Unmodified |
| B | CoCl2·6H2O | H2O | NH3·H2O | Unmodified |
| C | CoCl2·6H2O | H2O | NH3·H2O | Modified |
| Method | Structure | Specific Capacitance | Rate | Ref. |
|---|---|---|---|---|
| Hydrothermal | Nanoflake | 1500 F·g−1 at 1 A·g−1 | 55.2% at 10 A·g−1 | [10] |
| Template | Nanosheet | 1121 F·g−1 at 1 A·g−1 | 77.9% at 25 A·g−1 | [12] |
| Solvothermal | Nanoparticle | 523.0 F·g−1 at 0.5 A·g−1 | 66.9% at 5 A·g−1 | [13] |
| CVD method | Nanosphere | 128 F·g−1 at 10 A·g−1 | 90% at 20 A·g−1 | [25] |
| Chemical bath deposition | Nanowire | 850 F·g−1 at 5 mV·s−1 | 85% at 100 mV·s−1 | [26] |
| In-site self-organization | Nanorods | 1486 F·g−1 at 1 A·g−1 | 72.9% at 15 A·g−1 | [27] |
| Galvanic displacement | Nanosheet | 1095 F·g−1 at 1 A·g−1 | 61.9% at 15 A·g−1 | [29] |
| Laser ablation | Nanosheet | 762 F·g−1 at 6 A·g−1 | 82.7% at 36 A·g−1 | [30] |
| Electrospinning technique | Nanofiber | 340 F·g−1 at 1 A·g−1 | 87.1% at 10 A·g−1 | [31] |
| Sol-gel | Netlike | 708 F·g−1 at 5 A·g−1 | 71.9% at 50 A·g−1 | [32] |
| Chemical precipitation | Nanonet | 739 F·g−1 at 1 A·g−1 | 72.1% at 15 A·g−1 | [38] |
| Thermal decomposition | Nanoflake | 576.8 F·g−1 at 1 A·g−1 | 49.2% at 50 A·g−1 | [39] |
| Electrodeposition | Nanoplate | 517 F·g−1 at 1 A·g−1 | 39.1% at 20 A·g−1 | [40] |
| Spray pyrolysis | Thin film | 412 F·g−1 at 1 A·g−1 | 93% at 4 A·g−1 | [41] |
| Gas-phase diffusion precipitation | Nanofloc | 564 F·g−1 at 1 A·g−1 | 58.7% at 10 A·g−1 | A |
| Gas-phase diffusion precipitation | Nanosheet | 640 F·g−1 at 1 A·g−1 | 54.7% at 10 A·g−1 | B |
| Gas-phase diffusion precipitation | Nanosheet | 1140 F·g−1 at 1 A·g−1 | 41.8% at 10 A·g−1 | C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fu, J.; Wang, Q.; Dong, Z.; Wang, X.; Hu, A.; Wang, W.; Yang, S. Preparation and Electrochemical Properties of Co3O4 Supercapacitor Electrode Materials. Crystals 2020, 10, 720. https://doi.org/10.3390/cryst10090720
Wang X, Fu J, Wang Q, Dong Z, Wang X, Hu A, Wang W, Yang S. Preparation and Electrochemical Properties of Co3O4 Supercapacitor Electrode Materials. Crystals. 2020; 10(9):720. https://doi.org/10.3390/cryst10090720
Chicago/Turabian StyleWang, Xuelei, Jiawei Fu, Qiufeng Wang, Zhaojun Dong, Xiaoliang Wang, Anyu Hu, Wei Wang, and Shaobin Yang. 2020. "Preparation and Electrochemical Properties of Co3O4 Supercapacitor Electrode Materials" Crystals 10, no. 9: 720. https://doi.org/10.3390/cryst10090720
APA StyleWang, X., Fu, J., Wang, Q., Dong, Z., Wang, X., Hu, A., Wang, W., & Yang, S. (2020). Preparation and Electrochemical Properties of Co3O4 Supercapacitor Electrode Materials. Crystals, 10(9), 720. https://doi.org/10.3390/cryst10090720




