CO2 Capture in A Bubble-Column Scrubber Using MEA/CaCl2/H2O Solution—Absorption and Precipitation
Abstract
1. Introduction
2. Absorption Rate and Mass Transfer Coefficient
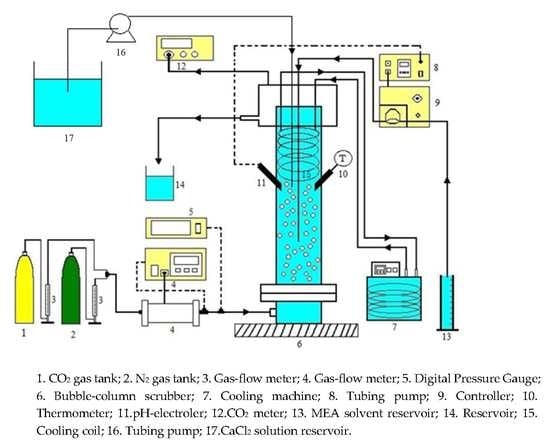
3. Experiment
4. Results and Discussions
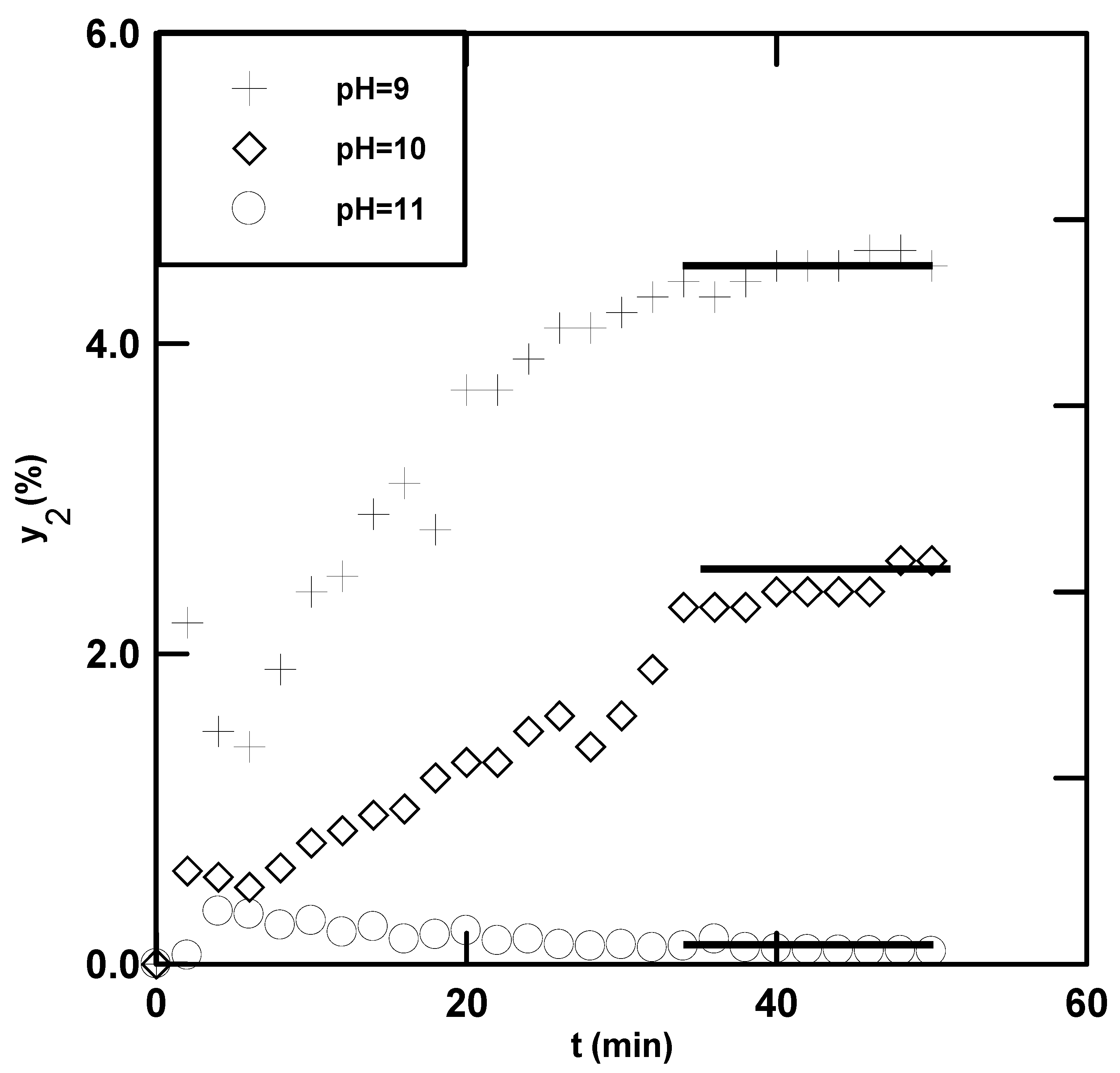
4.1. Experimental Data
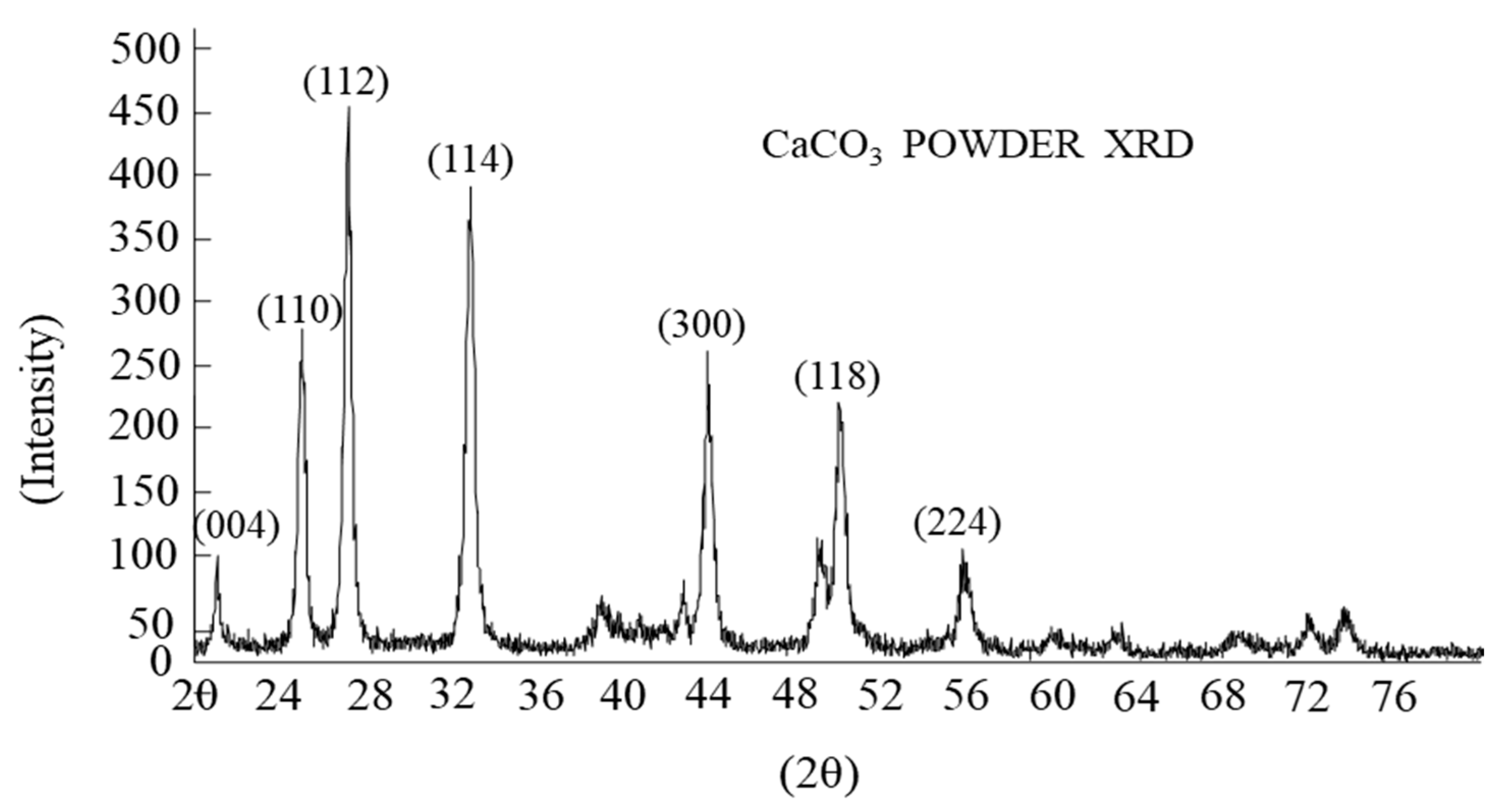
4.2. Identification of Solid Components
4.3. Effects of Variables on Vaterite Morphology and Size
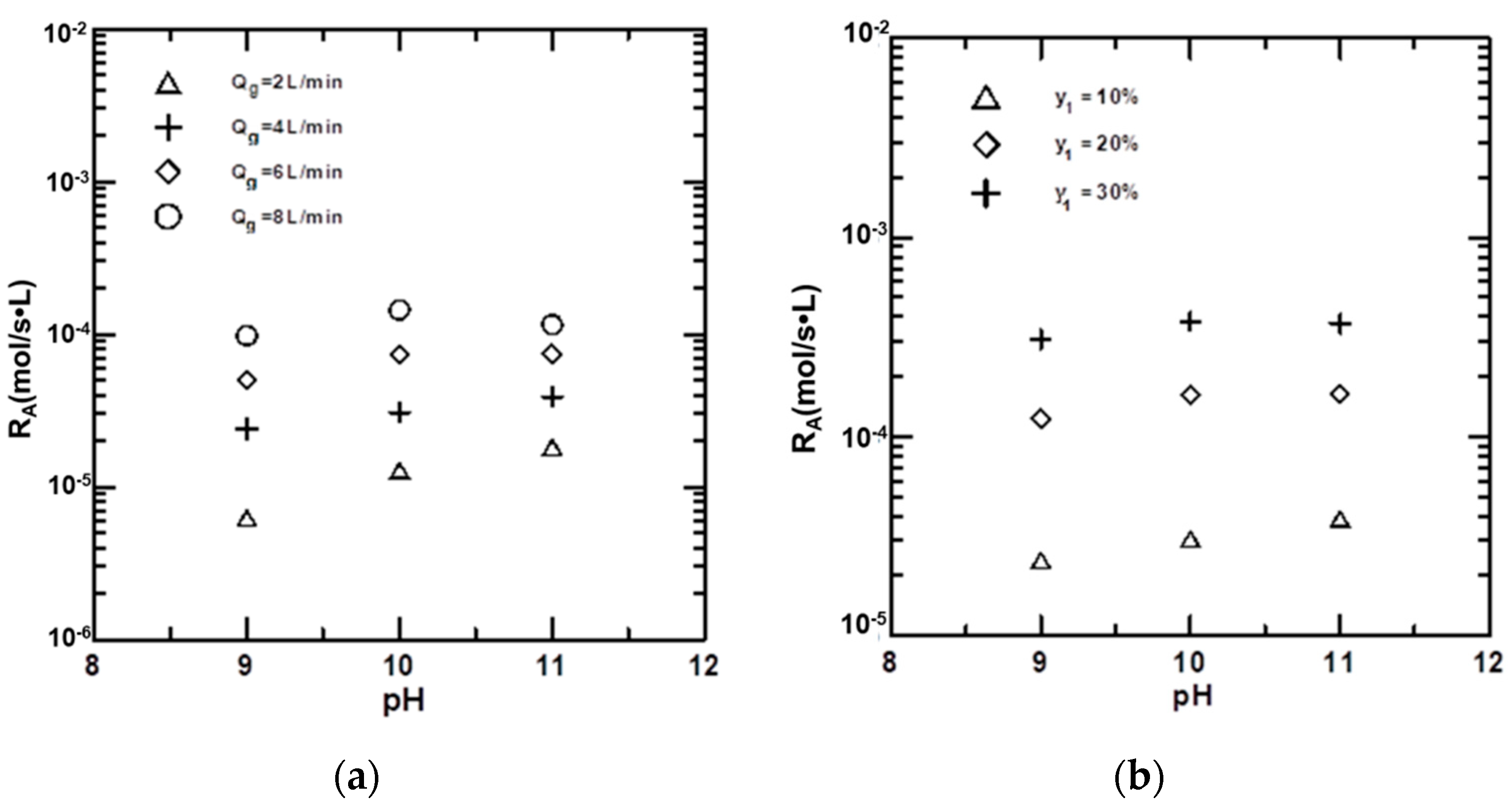
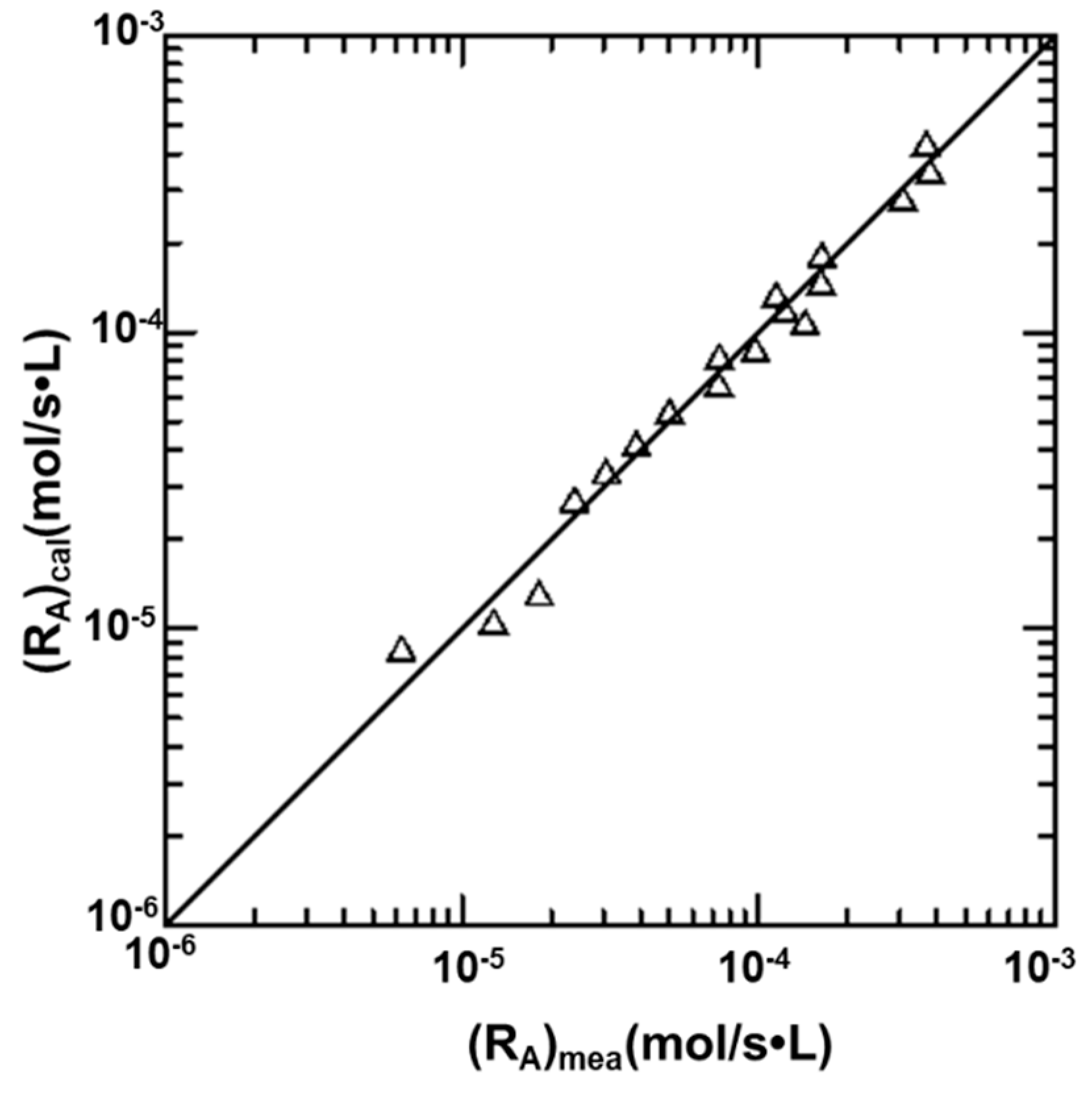
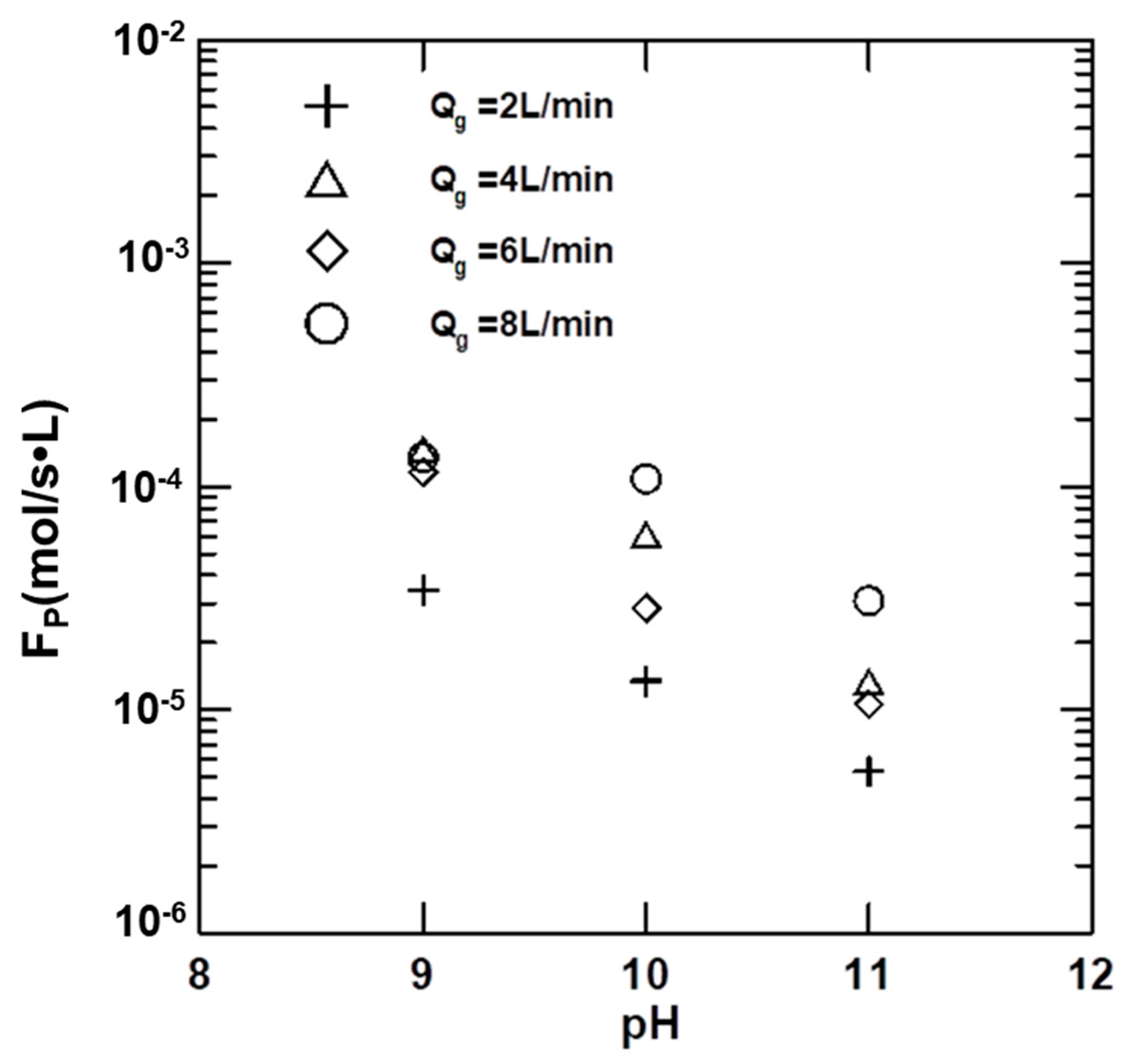
4.4. Effects of Variables on the Absorption Rate of CO2
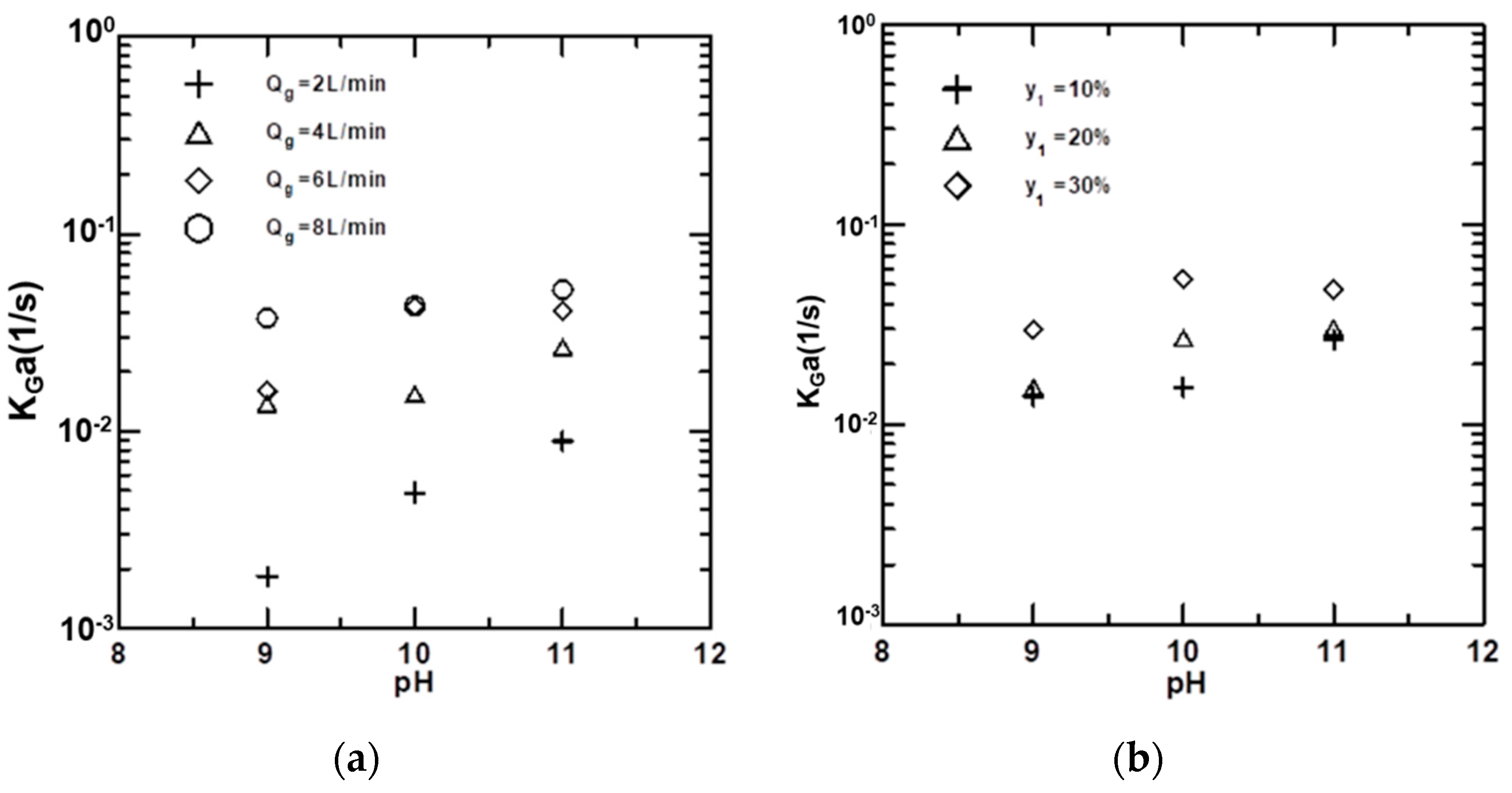
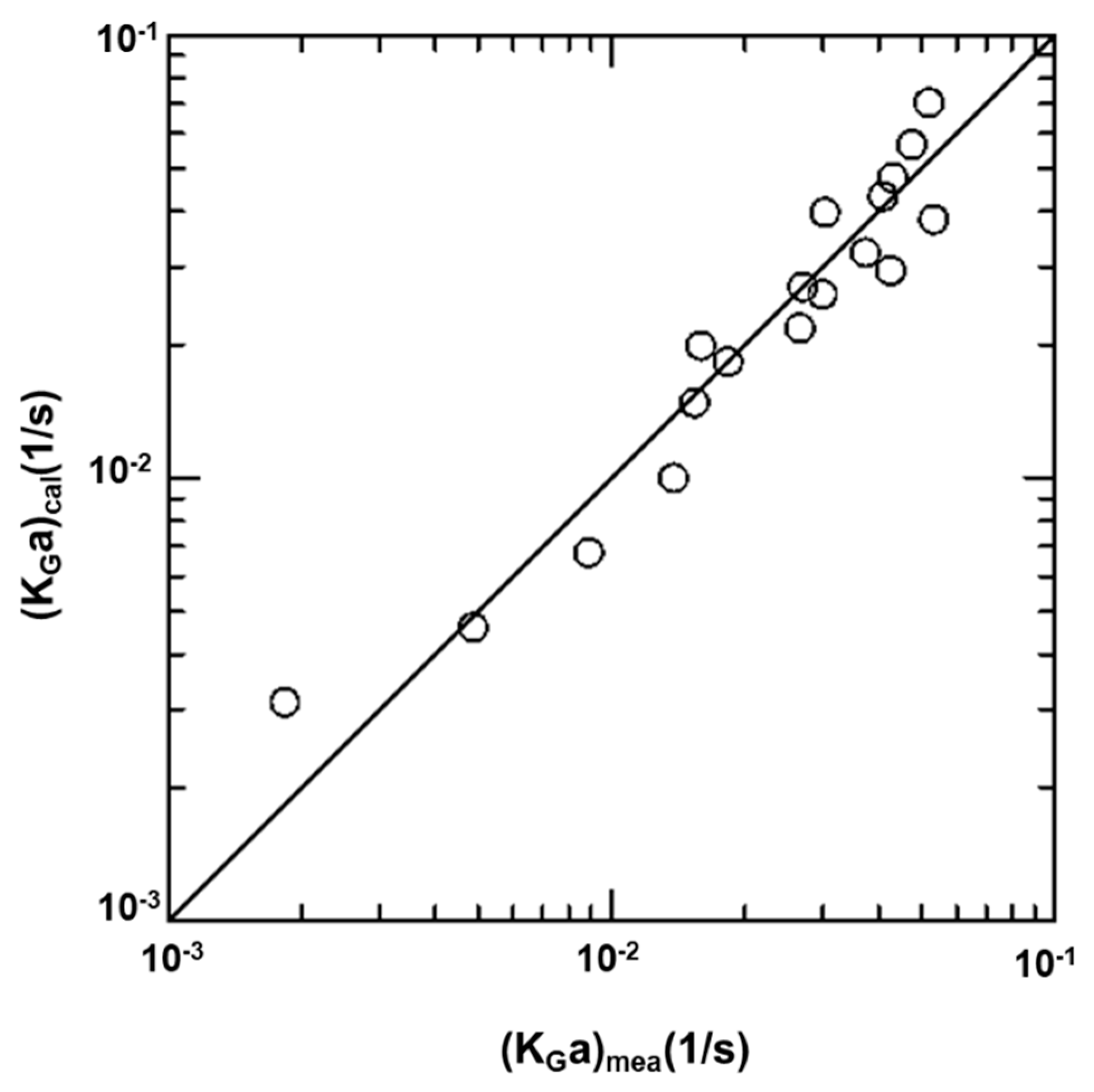
4.5. Effects of Variables on KGa
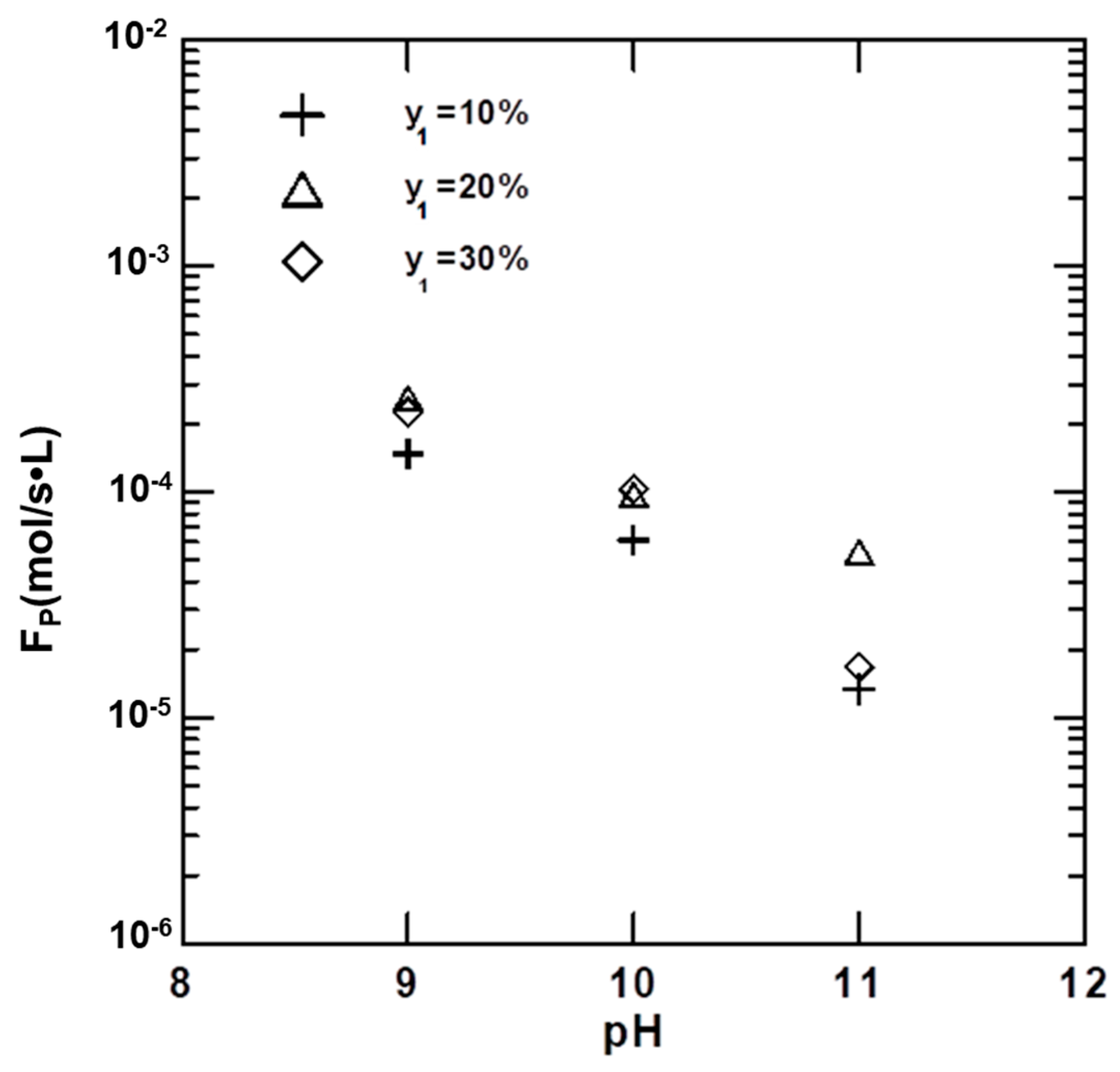
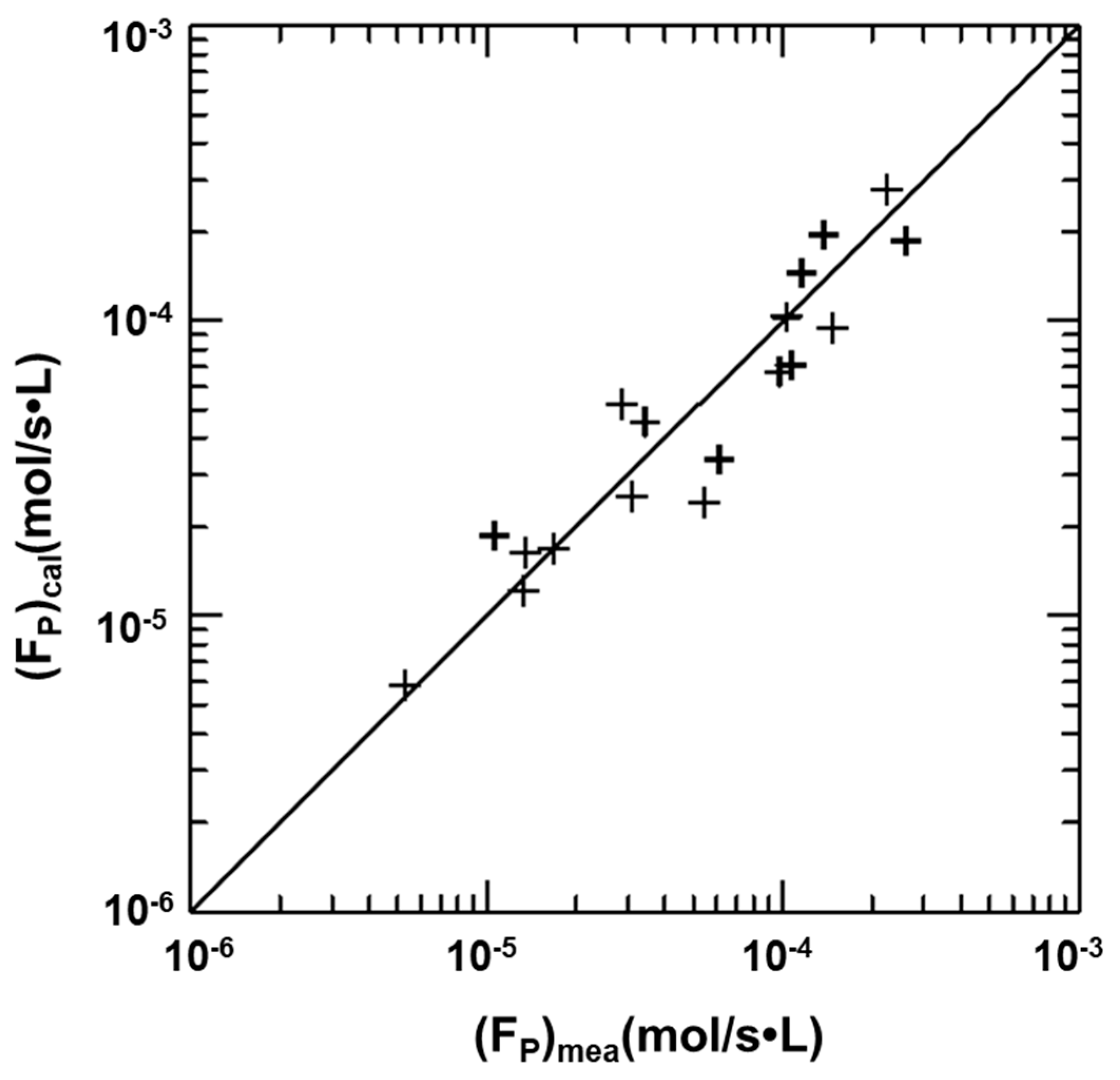
4.6. Precipitation Rates of Vaterites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Streedhar, I.; Nahar, T.; Venugopal, A.; Strinivas, B. Carbon capture by absorption---path covered and ahead. Renew. Sustain. Energy Rev. 2017, 76, 1080–1107. [Google Scholar] [CrossRef]
- Ali Khan, A.; Hader, G.N.; Saha, A.K. Carbon dioxide capture characteristics from flue gas using aqueous 2-amino-2-methyl-1-propanal (AMP) and monoethanolamine (MEA) solutions in packed bed absorption and regeneration columns. Int. J. Greenh. Gas Control. 2015, 32, 15–23. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, C.Y. A review of solar collectors and thermal energy storage in solar thermal Applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, C.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 Capture by Absorption an Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar]
- Mangalapally, H.P.; Notz, R.; Hoch, S.; Asprion, N.; Mangalapally, H.P.; Notz, R.; Hoch, S.; Asprion, N.; Sieder, G.; Garcia, H.; et al. Pilot plant experimental studies of post combustion CO2 capture by reactive absorption with MEA and new solvent. Energy Procedia. 2009, 1, 963–970. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. Absorption of CO2 into aqueous blends of alkanolamines prepared from renewable resources. Chem. Eng. Sci. 2007, 62, 7344–7350. [Google Scholar] [CrossRef]
- Kwak, N.S.; Lee, J.H.; Lee, I.Y.; Jang, K.R.; Shim, J.G. A study of new absorbent for post-combustion CO2 capture test bed. J. Taiwan Inst. Chem. Eng. 2014, 45, 2549–2556. [Google Scholar] [CrossRef]
- Hwang, K.S.; Park, S.W.; Park, D.W.; Oh, K.J.; Kim, S.S. Absorption of carbon dioxide into diisopropanolamine solutions of polar organic solvents. J. Taiwan Inst. Chem. Eng. 2010, 41, 16–21. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, B.; Lee, J.H.; You, J.K.; Park, K.T.; Baek, I.H.; Hur, N.H. Aqueous hydrazine as a promising candidate for capturing carbon dioxide. Inter. J. Greenh. Gas Control 2014, 29, 256–262. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y.; Peng, Y.C. Effect of reactor geometry on aqueous ammonia-based carbon dioxide capture in bubble column reactors. Inter. J. Greenh. Gas Control 2013, 17, 481–487. [Google Scholar] [CrossRef]
- Andersson, V.; Franck, P.A.; Berntsson, T. Industrial excess heat driven post-combustion CCS: The effect of stripper temperature level. Inter. J. Greenh. Gas Control 2014, 21, 1–10. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.C.; Li, M.H. Kinetics of absorption of carbon dioxide into aqueous solutions of 2-amino-2-methyl-1-propanol + monoethanolamin. Chem. Eng. Sci. 2000, 55, 161–175. [Google Scholar] [CrossRef]
- Lin, C.Y.; Soriano, A.N.; Li, M.H. Kinetics study of carbon dioxide absorption into aqueous solution containing N-methyldiethanolamine + Diethanolamine. J. Taiwan Inst. Chem. Eng. 2009, 40, 403–412. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Azarpour, A.; Akbari, V.; Wan Alwi, S.R.; Manan, Z.A. CO2 capture with potassium carbonate solutions: A state-of-the-art review. Inter. J. Greenh. Gas Control 2015, 41, 142–162. [Google Scholar] [CrossRef]
- Chen, P.C.; Shi, W.; Du, R.; Chen, V. Scrubbing of CO2 greenhouse gases, accompanied by precipitation in a continuous bubble-column scrubber. Ind. Eng. Chem. Res. 2008, 47, 6336–6343. [Google Scholar] [CrossRef]
- Chen, P.C.; Shi, W.; Du, R.; Chen, V. Crystallization kinetics of barium carbonate crystals in a lab-scale bubble-column scrubber. J. Taiwan Inst. Chem. Eng. 2014, 45, 2418–2426. [Google Scholar] [CrossRef]
- Chen, P.C. Absorption of Carbon Dioxide in a Bubble Column Scrubber. Greenhouse Gases; Liu, G., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 5; pp. 95–116. [Google Scholar]
- Petrov, P.; Ewert, G.; Rohm, H.J. Chemisorptive removal of carbon dioxide from process streams using a reactive bubble column with simultaneous production of usable materials. Chem. Eng. Technol. 2006, 29, 1084–1089. [Google Scholar] [CrossRef]
- Sánchez, O.; Michaud, S.; Escudié, R.; Delgenès, J.-P.; Bernet, N. Liquid mixing and gas–liquid mass transfer in a three-phase inverse turbulent bed reactor. Chem. Eng. J. 2005, 114, 1–7. [Google Scholar] [CrossRef]
- Chen, P.C.; Luo, Y.X.; Cai, P.W. CO2 Capture using monoethanolamine in a bubble-column scrubber. Chem. Eng. Technol. 2015, 38, 274–282. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. CO2-aklanolamine reaction kinetics: A review of recent studies. Chem. Eng. Technol. 2007, 11, 1467–1474. [Google Scholar] [CrossRef]
- Chen, P.C.; Yu, S.C. CO2 capture and crystallization of ammonia biocarbonate in a lab-scale scrubber. Crystals 2018, 8, 39. [Google Scholar] [CrossRef]
- Li, L.; Maeder, M.; Burns, R.; Puxty, G.; Clifford, S.; Yu, H. The Henry coefficient of CO2 in the MEA-CO2-H2O system. Energy Procedia 2017, 114, 1841–1847. [Google Scholar] [CrossRef]
- Tong, D.; Martin Trusler, J.P. Solubility of carbon dioxide in aqueous solution on monoethanolamine or 2-amino-2-methyl-1-propanal: Experimental measurements and modeling. Inter. J. Greenh. Gas Control 2012, 6, 37–47. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Li, F.; Xie, B.; Qiana, Y. Solvothermal growth of vaterite in the presence of ethylene glycol, 1,2-propanediol and glycerin. J. Crystal Growth 2002, 236, 357–362. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2014, 45, 644–658. [Google Scholar] [CrossRef]
- Brecevic, L.; Kralj, D. On calcium carbonates: From fundamental research to application. Crocatia Chem. Acta 2007, 80, 467–484. [Google Scholar]
- Al-Naimi, S.A.; Salih, S.A.J.; Al-Atabi, H. Simulation study of mass transfer coefficient in slurry bubble column reactor using neural network. Al-Khwarizmi Eng. J. 2013, 9, 60–70. [Google Scholar]
- Lau, R.; Lee, P.H.V.; Chen, T. Mass transfer studies in shallow bubble column reactors. Chem. Eng. Process. Process Intensif. 2012, 62, 18–25. [Google Scholar] [CrossRef]
- Vandu, C.O.; Koop, K.; Krishna, R. Volumetric mass transfer coefficient in a slurry bubble column operating in the heterogeneous flow regime. Chem. Eng. Sci. 2004, 59, 5417–5423. [Google Scholar] [CrossRef]
- Dhaouadi, H.; Poncin, S.; Hornut, J.M.; Midoux, N. Gas–liquid mass transfer in bubble column reactor: Analytical solution and experimental confirmation. Chem. Eng. Process 2008, 47, 548–556. [Google Scholar] [CrossRef]
- Chen, P.C.; Chou, P.H.; Lin, S.Z.; Chen, H.W. Capturing CO2 by using a microalgae culture recycle solution. Chem. Eng. Technol. 2017, 40, 2274–2282. [Google Scholar] [CrossRef]
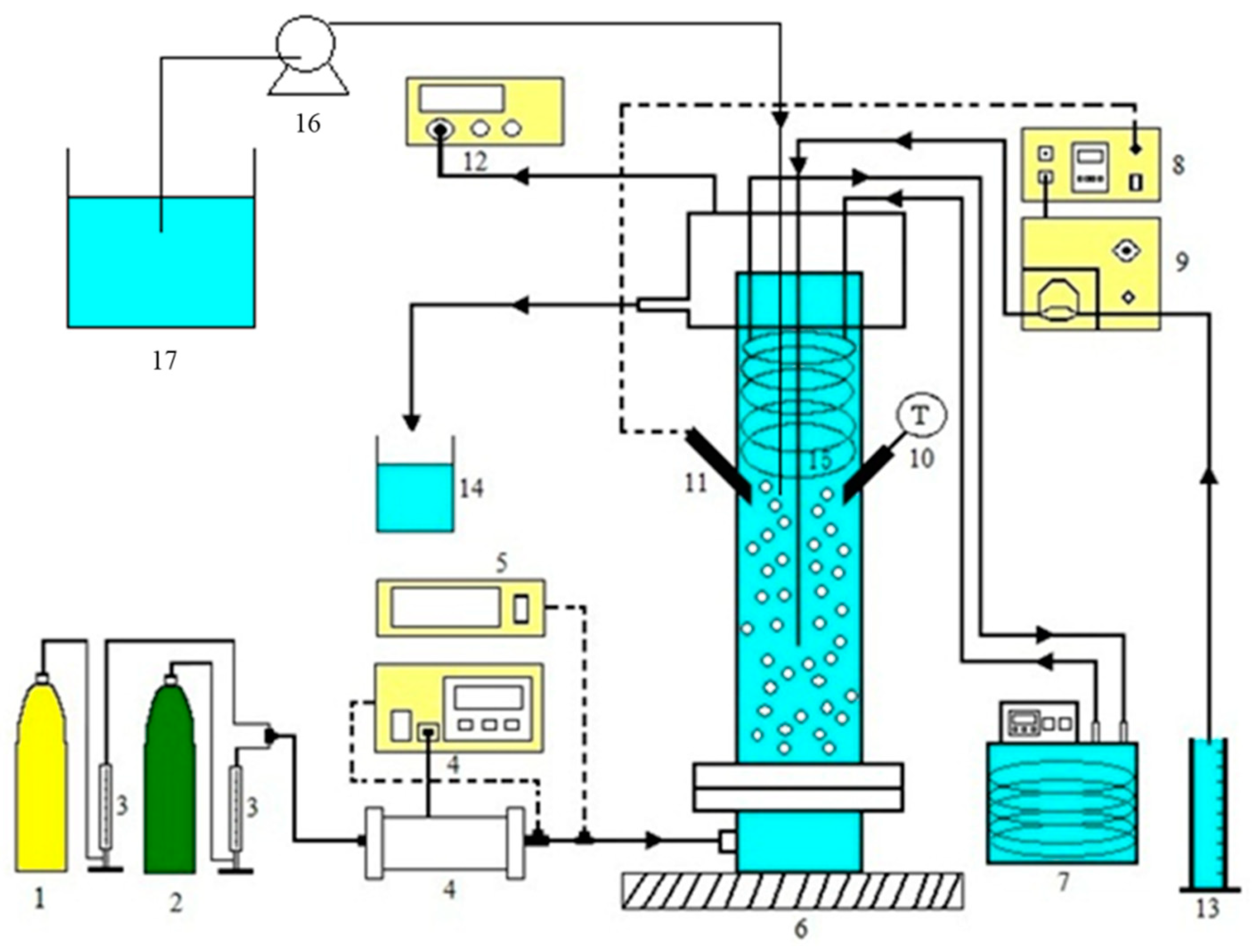
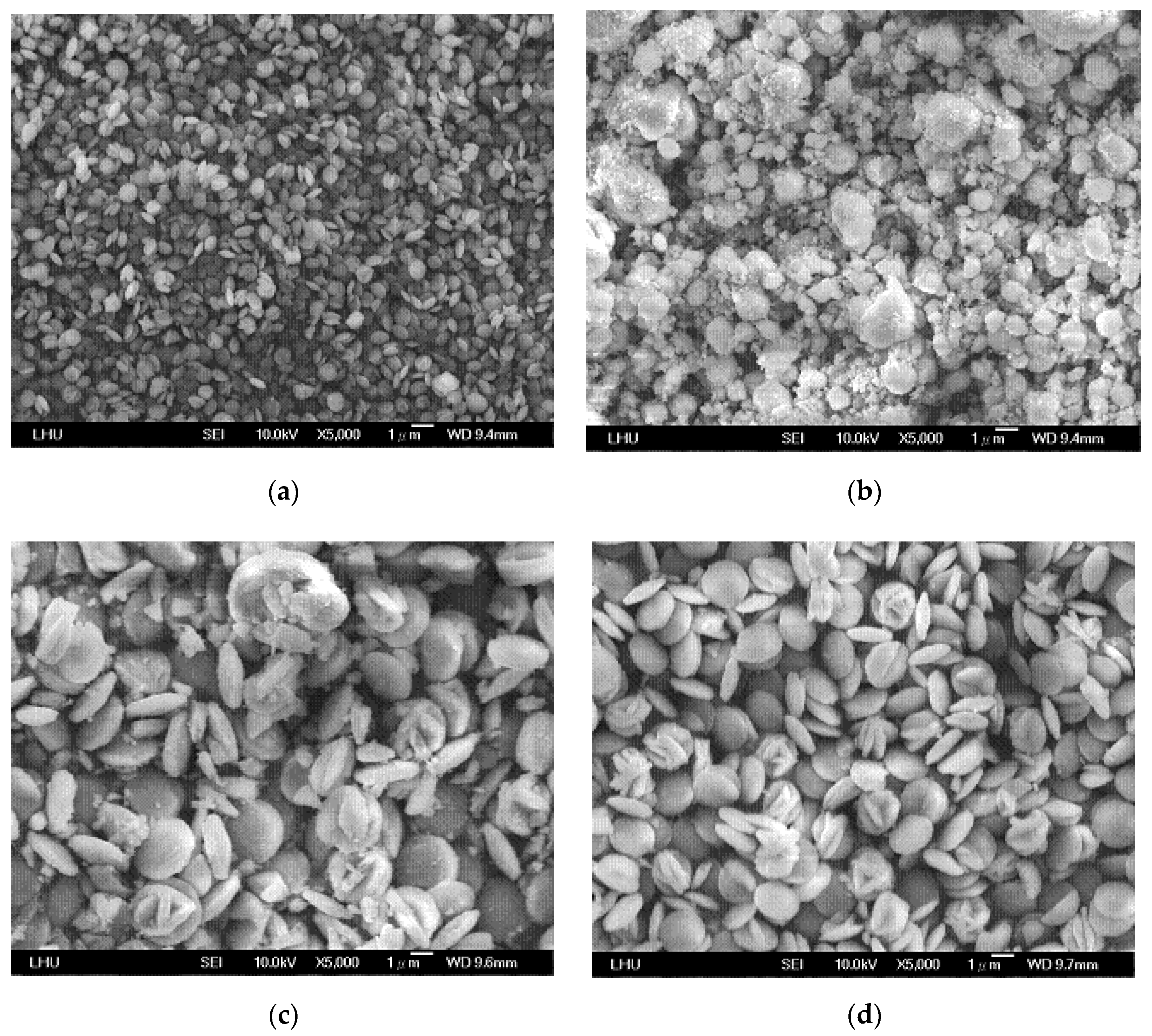
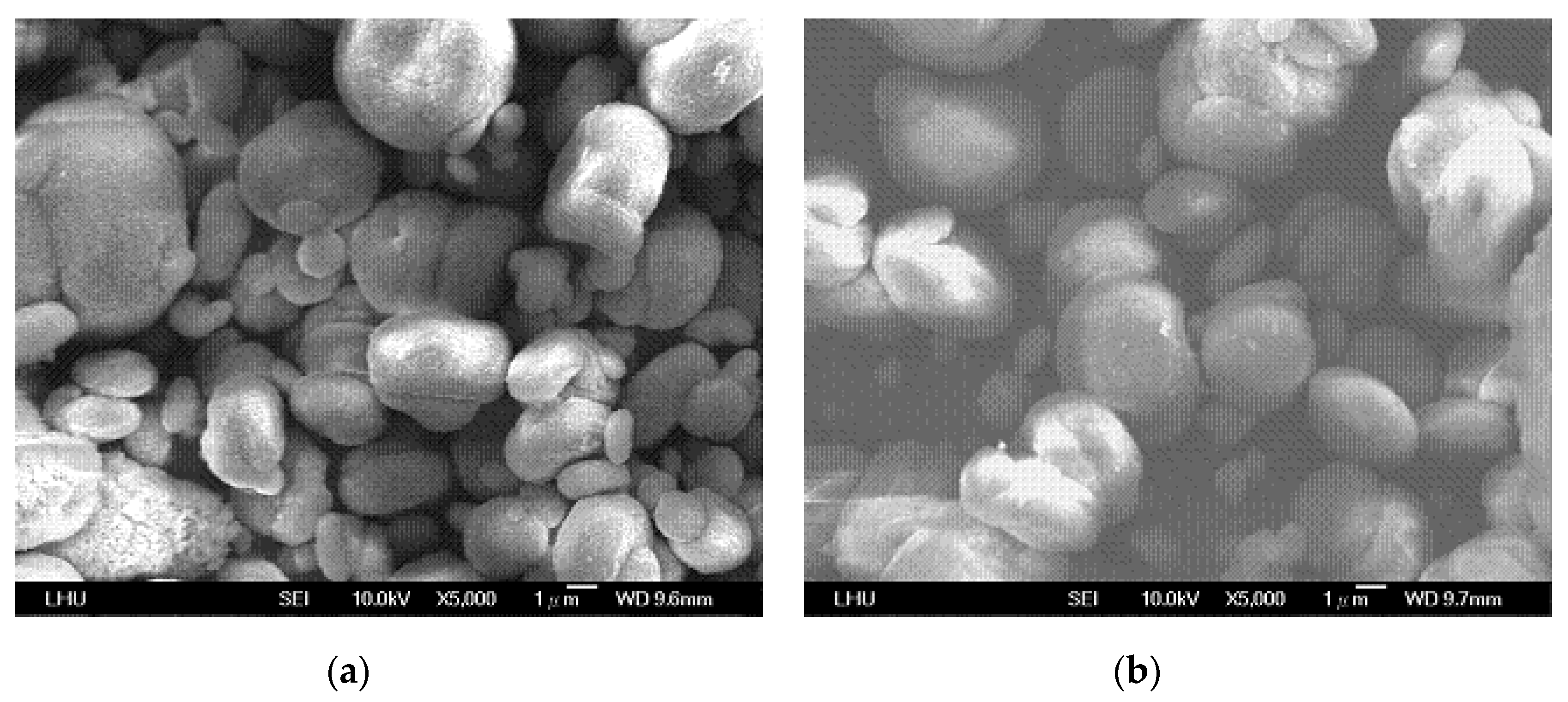
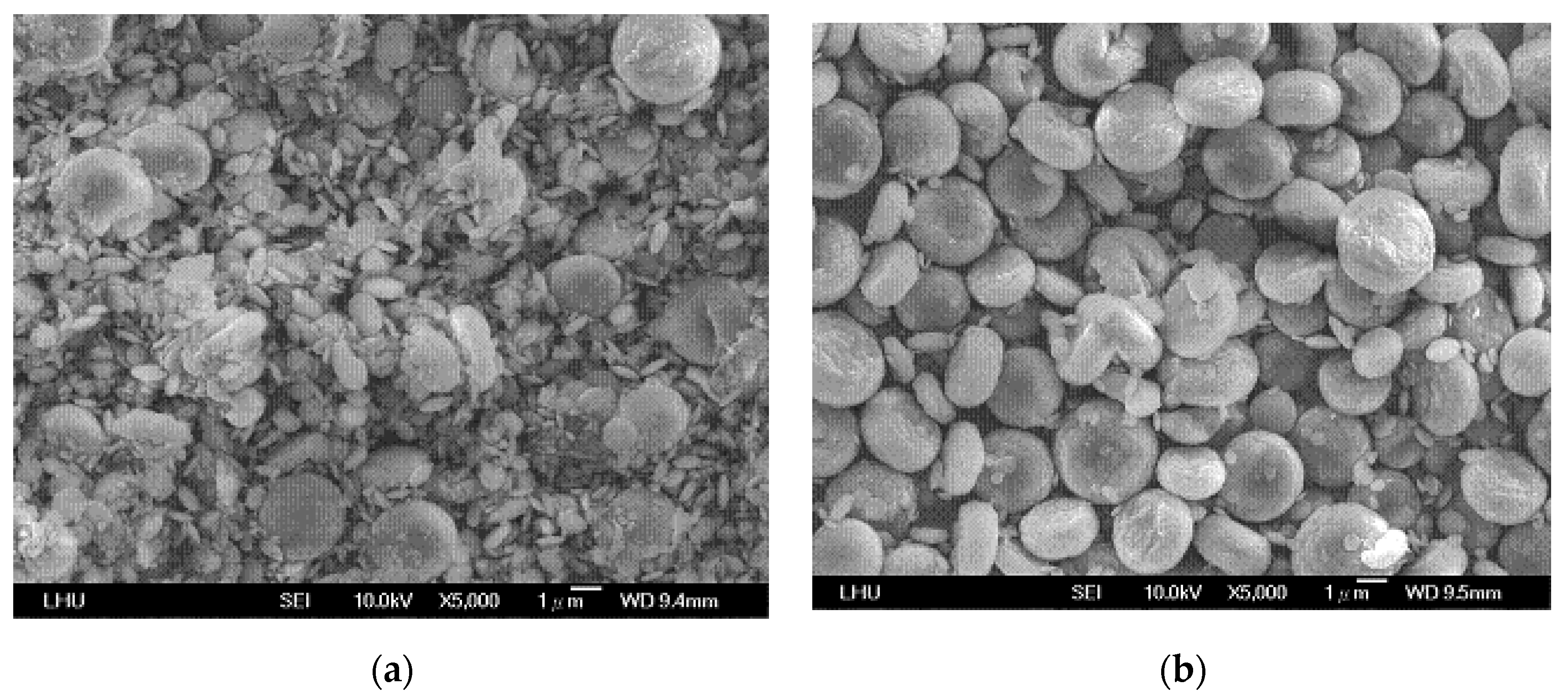
| Concentration of CO2 Gas (%) | 10–30 |
|---|---|
| Concentration of CaCl2 (M) | 0.2 |
| Concentration of MEA (M) | 4 |
| Gas-flow rate (L/min) | 2–8 |
| Flow rate of CaCl2 (mL/min) | 50 |
| Gas inlet temperature (°C) | 30 |
| Temperature in the column (°C) | 35 |
| pH | 9–11 |
| No. | pH | y1 (%) | u (cm/s) | RA × 105 (mol/s·L) | KGa × 102 1/s | τ min | Fp × 105 (mol/s·L) | E (%) | dp μm | Qadd (mL/min) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 10 | 1.7 | 0.63 | 0.183 | 17.00 | 3.42 | 32.8 | 1.60 | 2.0 |
| 2 | 10 | 10 | 1.7 | 1.28 | 0.487 | 16.71 | 1.35 | 71.5 | 1.37 | 2.9 |
| 3 | 11 | 10 | 1.7 | 1.82 | 0.889 | 16.01 | 0.53 | 100 | 1.45 | 2.6 |
| 4 | 9 | 10 | 3.4 | 2.40 | 1.38 | 14.98 | 14.71 | 55 | 1.33 | 9.0 |
| 5 | 10 | 10 | 3.4 | 3.07 | 1.54 | 13.92 | 6.11 | 75 | 1.41 | 13.5 |
| 6 | 11 | 10 | 3.4 | 3.88 | 2.66 | 13.56 | 1.34 | 99.2 | 1.83 | 15.2 |
| 7 | 9 | 10 | 5.1 | 5.02 | 1.59 | 9.57 | 11.59 | 68 | 1.14 | 12.4 |
| 8 | 10 | 10 | 5.1 | 7.36 | 4.26 | 9.51 | 2.85 | 100 | 0.64 | 25.3 |
| 9 | 11 | 10 | 5.1 | 7.40 | 4.10 | 4.57 | 1.06 | 100 | 1.78 | 176.6 |
| 10 | 9 | 10 | 6.8 | 9.78 | 3.74 | 11.88 | 13.76 | 84.1 | 1.01 | 20.2 |
| 11 | 10 | 10 | 6.8 | 14.4 | 4.31 | 7.70 | 10.68 | 100 | 0.94 | 44.3 |
| 12 | 11 | 10 | 6.8 | 11.5 | 5.20 | 4.95 | 3.08 | 100 | 1.28 | 228.4 |
| 13 | 9 | 20 | 3.4 | 12.3 | 1.53 | 14.17 | 26.08 | 68.7 | 1.63 | 42.4 |
| 14 | 10 | 20 | 3.4 | 16.3 | 2.70 | 11.74 | 9.77 | 97.5 | 1.44 | 43.0 |
| 15 | 11 | 20 | 3.4 | 16.4 | 3.03 | 3.90 | 5.43 | 100 | 0.72 | 143.6 |
| 16 | 9 | 30 | 3.4 | 30.8 | 2.99 | 12.60 | 22.50 | 73.4 | 1.65 | 24.4 |
| 17 | 10 | 30 | 3.4 | 38.0 | 5.33 | 9.37 | 10.30 | 98.3 | 1.31 | 64.8 |
| 18 | 11 | 30 | 3.4 | 36.9 | 4.76 | 3.18 | 1.68 | 100 | 0.75 | 128.6 |
| KGa(1/s) | RA × 104(mol/s×L) | Remarks and References |
|---|---|---|
| 0.01–0.14 | - | O2-Liquid(water/glycerin/alcohol)-Solid(PVC) [29] |
| 0.045–0.085 | - | O2(N2+O2)-H2O [30] |
| 0.02–0.14 | - | Air-Paraffin oil-Porous catalyst [31] |
| 0.01–0.15 | - | O2-H2O [32] |
| 0.0377–0.8881 | 3.68–56.8 | CO2-MEA-H2O [21] |
| 0.021–0.109 | 1.36–6.61 | CO2-NaOH-H2O [33] |
| 0.0143–0.3302 | 3.21–9.03 | CO2-NH3-H2O [23] |
| 0.0183–0.0520 | 0.063–3.80 | CO2-MEA-CaCl2-H2O [This work] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.C.; Zhuo, S.H. CO2 Capture in A Bubble-Column Scrubber Using MEA/CaCl2/H2O Solution—Absorption and Precipitation. Crystals 2020, 10, 694. https://doi.org/10.3390/cryst10080694
Chen PC, Zhuo SH. CO2 Capture in A Bubble-Column Scrubber Using MEA/CaCl2/H2O Solution—Absorption and Precipitation. Crystals. 2020; 10(8):694. https://doi.org/10.3390/cryst10080694
Chicago/Turabian StyleChen, Pao Chi, and Shiun Huang Zhuo. 2020. "CO2 Capture in A Bubble-Column Scrubber Using MEA/CaCl2/H2O Solution—Absorption and Precipitation" Crystals 10, no. 8: 694. https://doi.org/10.3390/cryst10080694
APA StyleChen, P. C., & Zhuo, S. H. (2020). CO2 Capture in A Bubble-Column Scrubber Using MEA/CaCl2/H2O Solution—Absorption and Precipitation. Crystals, 10(8), 694. https://doi.org/10.3390/cryst10080694