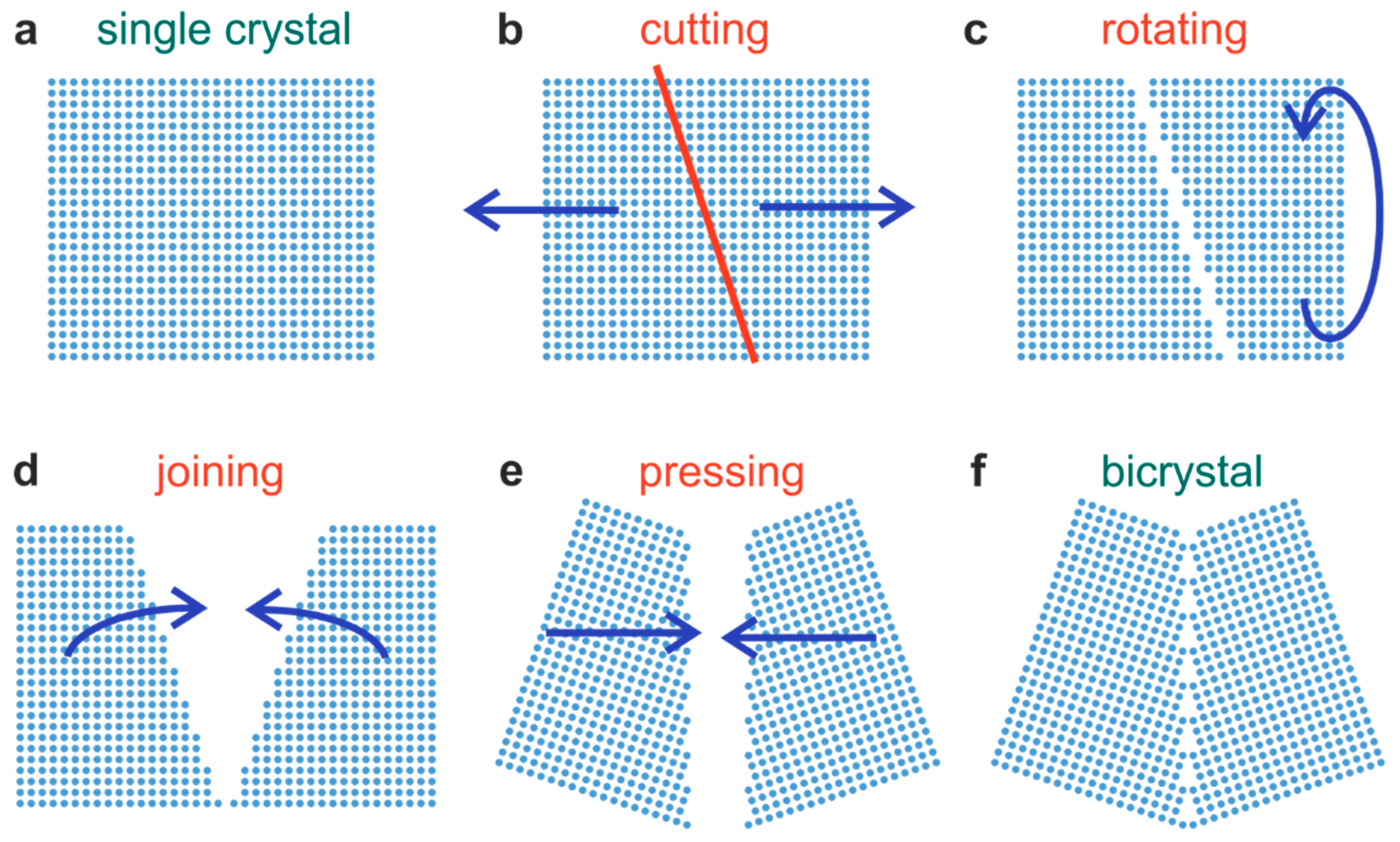
3.1. Structure of the Bicrystal Boundary
For this study, we selected bicrystals with a large tilt angle of 36.8°, providing a grain boundary with a high dislocation density. In order to produce such tilt bicrystals, a single crystal is cut at an angle of 18.4° with respect to the <100> axes. The two pieces are then joined together by pressing at high temperature [
26,
27,
28]. In our study, this preparation was performed by the manufacturers. In the ideal case, this approach should result in an energetically favorable, symmetric Σ5 boundary that has a regular arrangement of dislocations although this is, however, difficult to achieve in reality [
29,
30].
Figure 1 displays the simplified scheme of constructing a Σ5 boundary for a cubic system. The detailed structure of the Σ5 boundary for SrTiO
3 was theoretically calculated and also experimentally investigated by using high-resolution transmission electron microscopy (HR-TEM) [
31,
32].
Before focusing on the investigation of the electrical and optical properties of the SrTiO
3 bicrystals, we would like to comment on the quality of commercially available bicrystals. As elaborated in detail in our previous review [
14], a well-ordered arrangement of dislocations at the Σ5 boundary is not always observed. Using the etch-pit technique to mark the exits of dislocations, it has been noted that commercially available SrTiO
3 bicrystals are far from perfect [
14]. An example is shown in
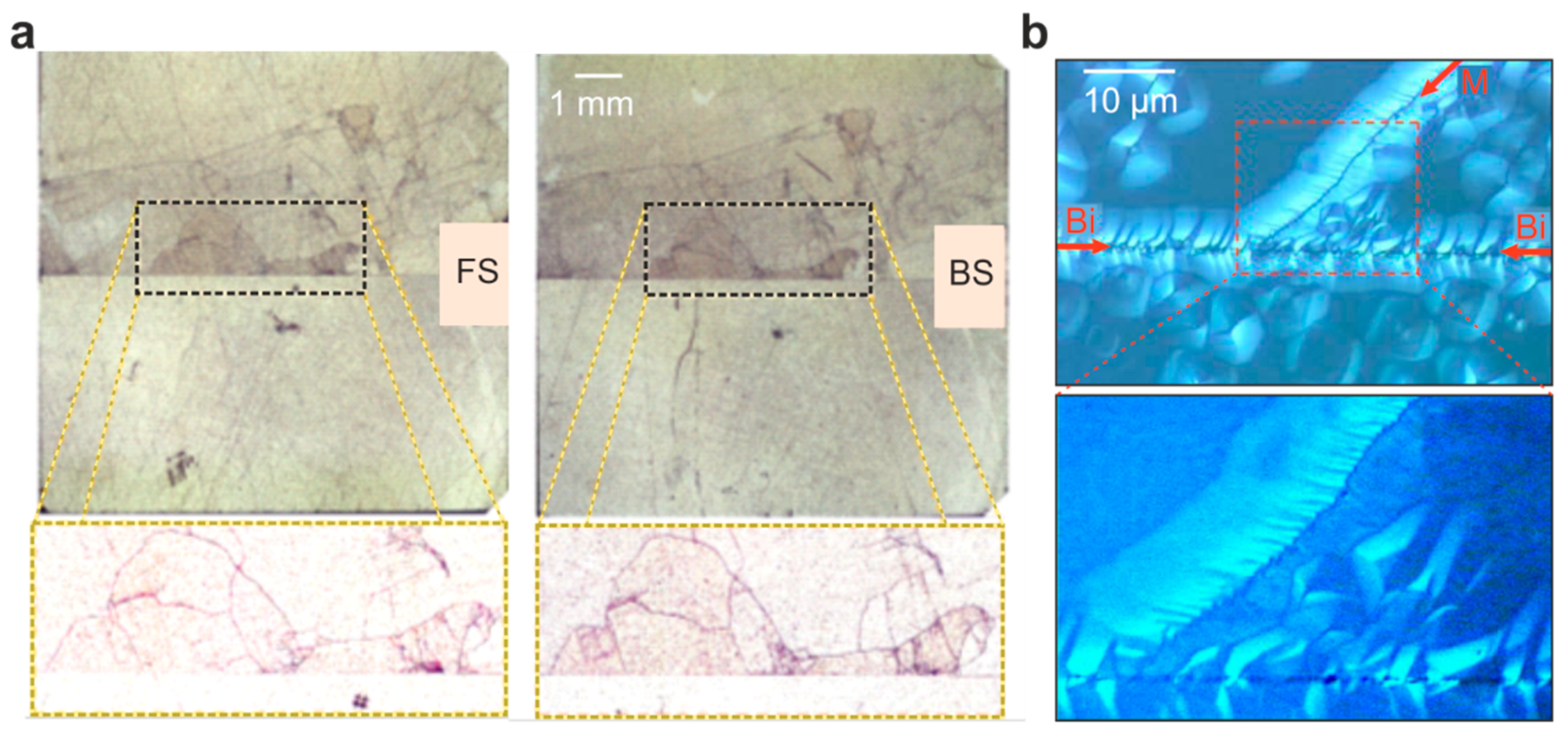
Figure 2a. After short etching of the crystal using HF, one can observe that there is not only one line of dislocations at the bicrystal boundary, but a mosaic structure of many small grains emerging from the junction can also be identified, each grain itself possessing dislocation-rich grain boundaries. As both the front- and backside of the bicrystal show a comparable pattern of etch pits.
We can conclude that the corresponding dislocations are not only near-surface dislocations but run through the entire crystal, thus marking real grain boundaries. Furthermore, it can also be observed that the dislocation density of the upper part of the bicrystal is much higher than in the lower part. This could relate to the fact that the two parts of the original crystal, which were joint after rotating and polishing (
Figure 1), had slightly different properties. Taking a closer look at the structure of the boundary (
Figure 2b), one can at first detect a high degree of irregularities at the bicrystal boundary (marked “Bi” in
Figure 2b), indicating that the arrangement of dislocations is not as regular as would be theoretically expected. In contrast to this, the distribution of additional etch pits that were found at grain boundaries, forming a mosaic structure (marked by “M” in
Figure 2b), appears to be much more regular. As we have observed such inhomogeneous dislocation arrangements in a variety of Verneuil-grown bicrystals purchased from different suppliers, we can conclude that dislocations have a strong tendency towards clustering and bundling. Hence, care should be taken when comparing results obtained from such bicrystals with theoretical predictions. To emphasize that the investigated bicrystals do not exhibit a well-defined Σ5 boundary but rather a broad, inhomogeneous, macroscopic dislocation network around the junction, we refer to them as “real” bicrystals. However, although the boundary structure of the investigated bicrystals was not ideal, we were still able to use them as model systems for the investigation of the impact of dislocations on the properties of SrTiO
3. Due to the presence of a mosaic structure generating a complex network of dislocations, the structure of the bicrystal was even closer to practical applications than originally intended, e.g., solid oxide fuel cells with a variety of different types of grain boundary [
33].
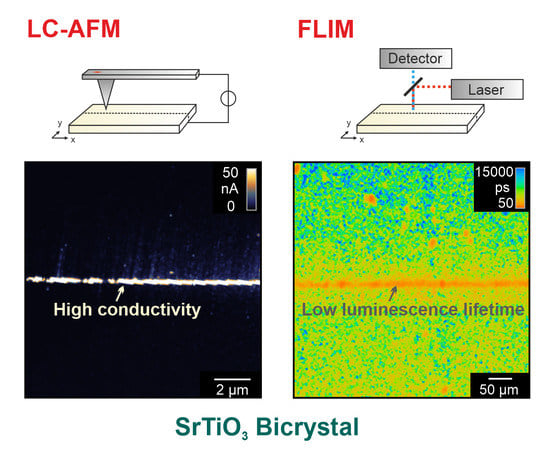
3.2. Investigation of Local Conductivity
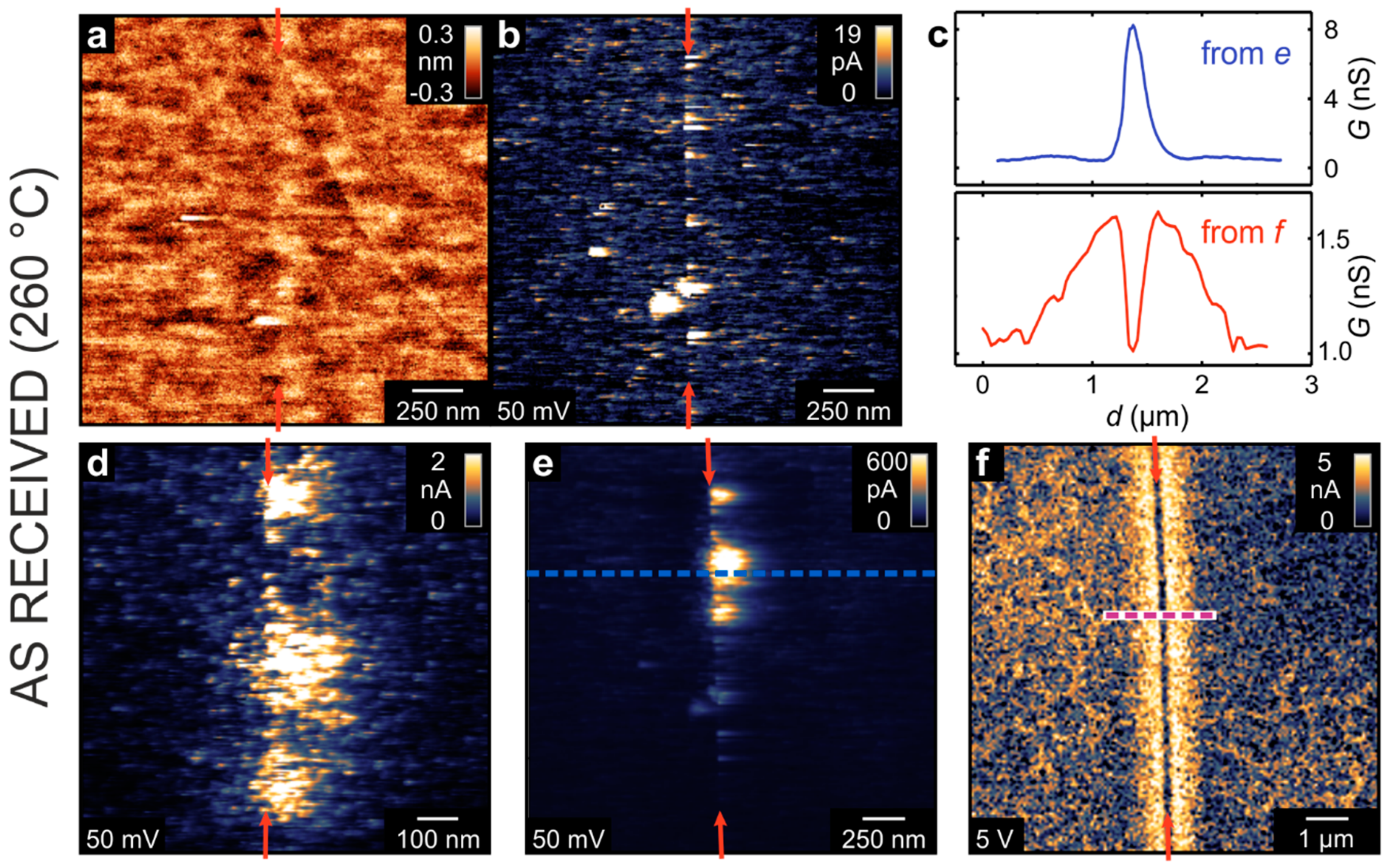
Using LC-AFM, the electrical properties of the as-received bicrystal junctions were investigated as presented in
Figure 3. In order to increase the conductivity of the solid oxide by means of thermal activation, the measurements were performed at a temperature of 260 °C under vacuum conditions. As the bicrystals had been epi-polished by the manufacturer, the surface in the vicinity of the junction was atomically flat. Hence, the position of the boundary is hard to identify in the AFM topography map (
Figure 3a). However, in the corresponding current map, recorded simultaneously with the topography by means of a voltage of 50 mV (
Figure 3b), an irregular arrangement of conducting spots along the junction is present. Well-conducting spots cannot only be seen directly at the junction but also several 100 nm away from the junction. In other regions along the boundary, spots with even higher conductivities have been recorded (
Figure 3e). Zooming in on the well-conducting spots (
Figure 3d), it can be seen that they consist of smaller spots with diameters in the range of 10–100nm. This could indicate that there is an agglomeration and bundling of conducting filaments formed along of the dislocation network. In some parts of the bicrystal junction, however, the picture was found to be completely different. The map shown in
Figure 3f was recorded in a region that did not show any detectable current at a voltage of 50 mV. However, after increasing the voltage to 5 V, a current in the nA range was observed. Directly at the boundary, the current was relatively low, whereas next to the boundary was a broad zone of 500 nm-width with a higher current.
Figure 3c shows a comparison of line profiles obtained from the current maps in
Figure 3e,f that illustrate the opposing behavior. Whereas, in the latter case, the boundary appears to have an increased but inhomogeneous conductivity, in the other case, the boundary itself appears to be insulating but with a zone of increased conductivity formed beside it. This illustrates that in a “real” bicrystal, a variety of electrically conducting paths can be present, which most likely relate to the imperfections in bicrystal fabrication, as described above.
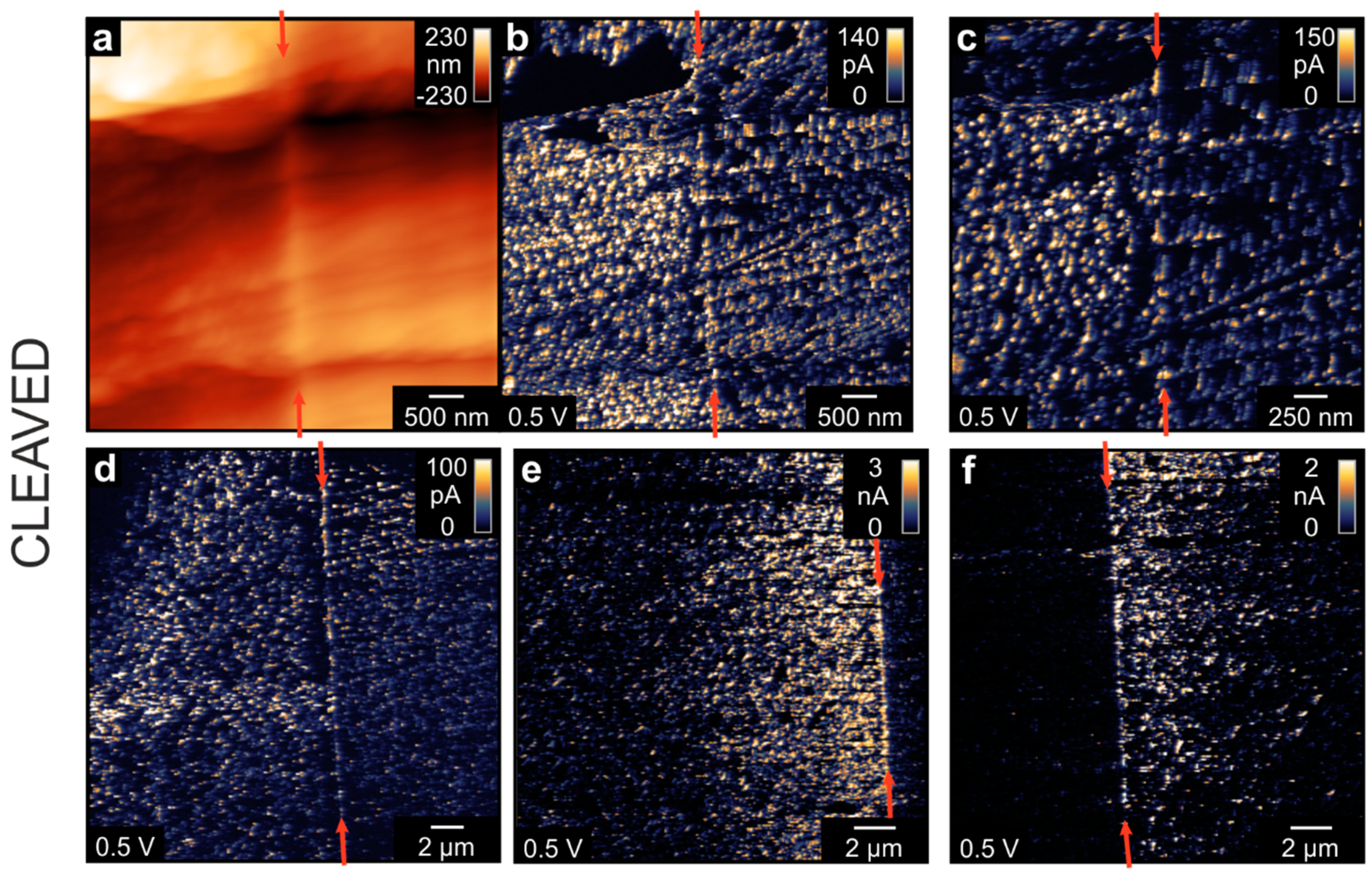
In order to obtain an insight into the conductivity in all dimensions, we investigated the bicrystal after cleaving it perpendicular to the boundary. As SrTiO
3 crystallizes in the cubic perovskite structure, it does not possess a defined cleavage plane, resulting in a shell-like fracturing [
34]. Hence, the topography map of the cleaved face (
Figure 4a) is relatively rough and different facets can be observed. The corresponding current maps at the same position (
Figure 4b,c) show an irregular spot-like conductivity pattern typical for cleaved SrTiO
3. Although the bicrystal boundary can be readily observed as a line of conducting spots in the center of the image, the current level of these boundary spots was the same as that of spots several 100 nm away from the boundary. This again indicates that a complex filamentary network in the junction zone had evolved. Comparing the topography image with the current map, it can be observed that the distribution of current spots follows the edges and kinks of the facets that had evolved during the cleaving procedure. This could also be an indication for the correlation between dislocations, which tend to agglomerate around edges and conducting filaments. In other parts of the cleavage plane, the distribution of conducting spots showed remarkable differences. In some positions, a relatively uniform distribution of spots on both sides of the boundary could be observed (
Figure 4d). Nevertheless, in other positions, there was a distinct difference between the two parts of the bicrystal. In
Figure 4e,f, one side of the bicrystal had a significantly lower conductivity than the other part with the boundary being a sharp separation line between the two regions. This could again be related to the fabrication process, whereby two parts of the original SrTiO
3 crystal, which might have had a slightly different content of point defects or extended defects, had been joined together to form the bicrystal.
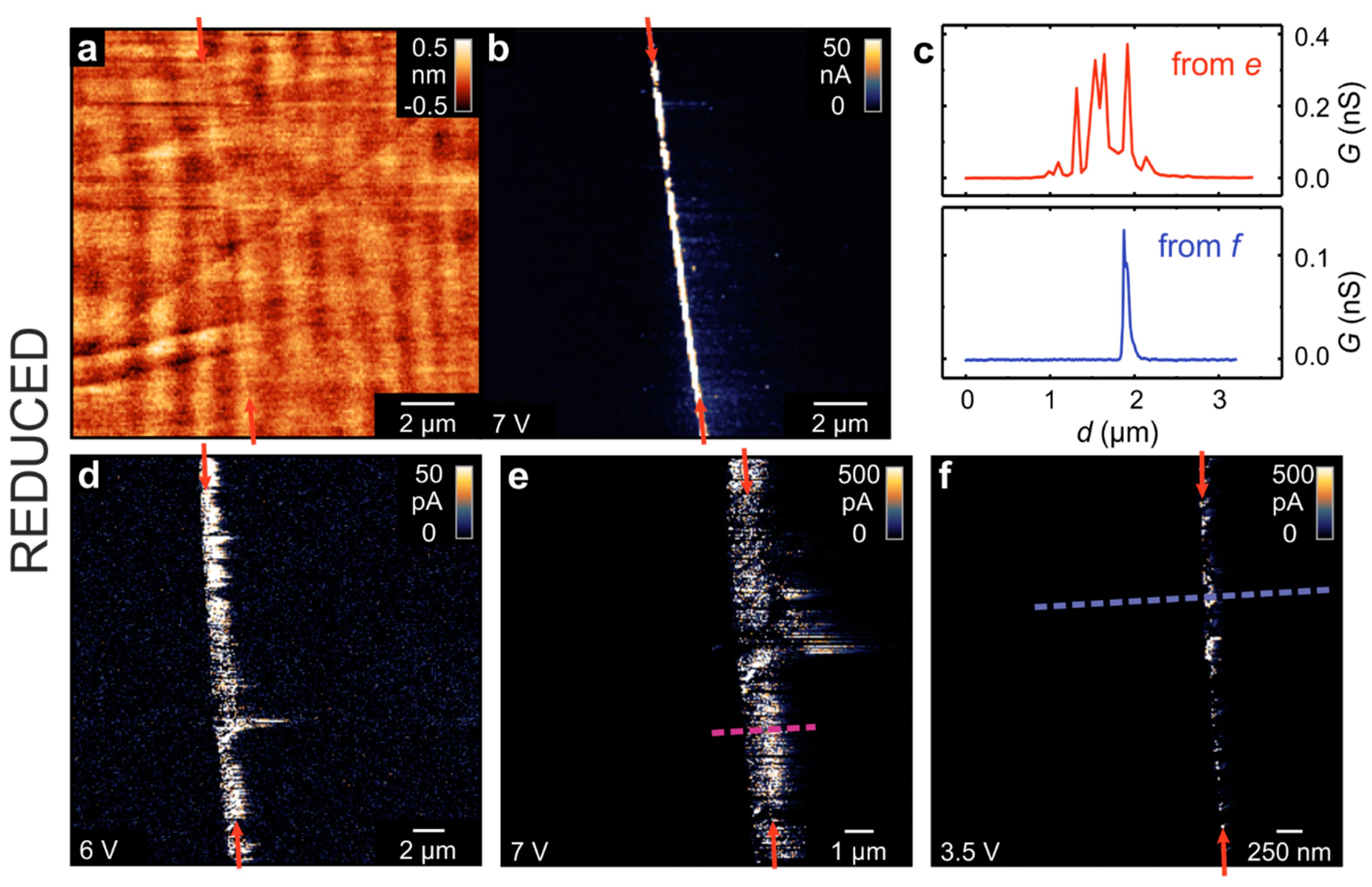
The impact of the thermally induced reduction on the local electrical properties of the bicrystal was investigated by repeating the LC-AFM experiment of the polished surface after annealing the sample in a vacuum in order to generate oxygen vacancies and charge carriers. The results are presented in
Figure 5. In the topography map, it can be seen that the surface of the crystal remained flat and no holes or islands are observed. In the conductivity maps, which were obtained at room temperature, the bicrystal junction can be easily identified as a line with increased conductivity. This general behavior has been observed for all of the investigated spots along the junction, but differences in the distribution of conducting spots on the nanoscale have also been found. In some regions, conducting spots could only be found directly at the boundary but with an irregular distribution along it (
Figure 5f). In other regions, a broad band of conducing spots can be observed to follow the boundary line (
Figure 5d,e). By extracting line profiles perpendicular to the boundary, these differences are illustrated (
Figure 5c). In the first case, the width of the well-conducting area at the boundary was in the range of 100 nm, while in the second, the inhomogeneously conducting band had a width of more than 1 µm. This difference might again relate to the imperfections in bicrystal production; however, the main result from this measurement is that the region of increased con-ductivity is much more confined to the boundary after thermal reduction than prior (
Figure 3). It must be noted that the measurements shown in
Figure 5 were performed ex situ after the exposure of the sample to ambient conditions. It has been shown previously that the thermally reduced SrTiO
3 surface is highly conductive under in situ conditions [
18] but that the majority of the surface becomes passivated upon exposure to oxygen at room temperature, thus becoming insulating again [
16]. Only a few conducting spots were found to remain highly conductive. Hence, we can conclude that in the measurements shown in
Figure 5, the remaining conductive filaments were preferentially formed in the dislocation-rich zone upon reduction. A similar correlation between the dislocations and the formation of conducting paths resistant to re-oxidation has been found in TiO
2 [
35].
3.3. Investigation of Macroscopic Conductivity
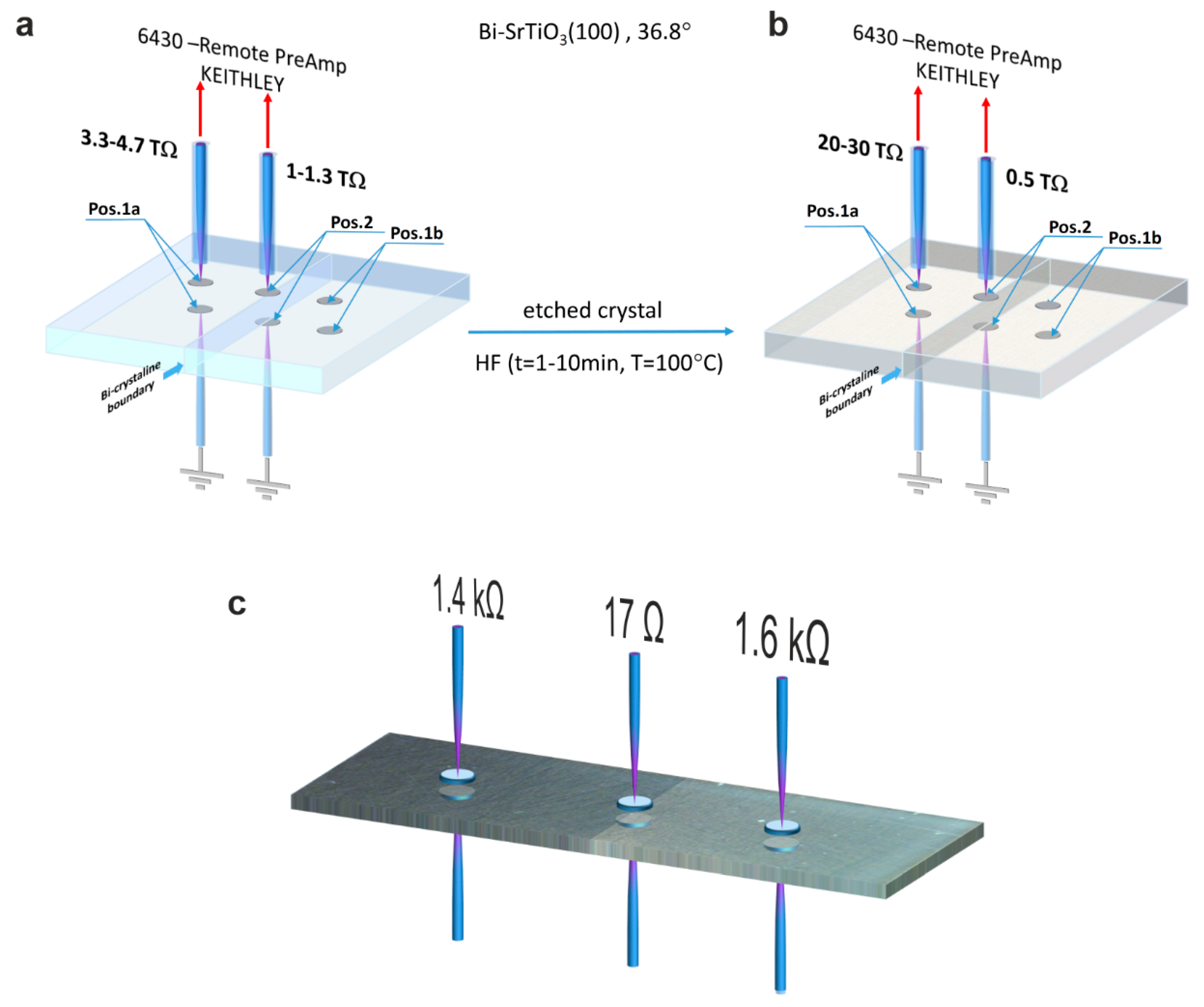
In order to illustrate the significance of the bicrystal boundary on even macroscopic electronic transport, resistance measurements in the out-of-plane geometry were performed, as illustrated in
Figure 6. In order to create good electrical contact with the crystal, In electrodes were used. The measurements were conducted at room temperature using direct current (DC) voltages below 1 V. The electrical resistance of the crystal was in the TΩ range, which requires the use of a highly sensitive and sufficiently screened measurement setup. The resistance measured at two positions away from the boundary (position 1a,b) was higher by a factor of 3–4 than that measured exactly at the boundary (position 2), which proves that the enhanced conductivity at the dislocation-rich boundary also has consequences at the macroscale. In a second step, we removed the surface layer by means of HF etching and repeated the measurement of the resistance after depositing fresh In electrodes. Now, the resistance measured away from the boundary was higher than before, while the resistance measured at the boundary was slightly lower (
Figure 6b). In the same way, we investigated another bicrystal, which was thermally reduced (
Figure 6c). In comparison to the as-received crystal, the resistance had dropped by many orders of magnitude into the kΩ range. A resistance of 17 Ω was measured directly at the boundary, which is lower by a factor of more than 80 compared to the surrounding. This further supports the conclusion that dislocations serve as easy reduction sites, thus providing a template for the formation of highly conducting filaments within the poorly conductive bulk.
When trying to understand the electric conductivity of SrTiO
3, the impact of dislocations must be taken into account, not only at the boundary itself but also in the surface layer of the polished crystal. We have described this effect in detail in Szot et al. [
9,
13,
14], but we will briefly recall the main conclusions here, as they will be useful for the analysis of the photoluminescence in the last section of this paper. The crystal preparation by the manufacturers includes cutting and polishing of the crystals. This induces a high density of dislocations, whose density decreases from the surface towards the bulk as shown in
Figure 7a. This graph shows the dislocation density in the surface layer measured by TEM analysis [
36]. It can be seen that the density decreases significantly towards the bulk, where densities below 10
8/cm
2 are typically found for Verneuil-grown SrTiO
3 crystals [
23,
37]. Additionally, the density of the conducting filaments of the reduced SrTiO
3 surface as a function of the depth, which was obtained via the LC-AFM analysis of a cleaved sample, is shown [
14]. It can be seen that the density of dislocations measured by TEM and the density of the conducting filaments both follow the same trend. This indicates that the filaments were formed along the dislocations. According to TEM analysis, one can distinguish two types of dislocations. Type “A” has a core consisting of αSrO + βTiO
2 + γTi
2O
3, which causes flexoelectric polarization, while type “B” has a structure of βTiO
2 + γTi
2O
3 + δTiO, causing ferroelectric polarization [
38,
39,
40].
We built a layered structure, wherein each layer represents a region of constant filament (dislocation) density
m. In the model of the hierarchical tree, the filament density gets reduced by a factor of two between subsequent layers.
Using this formula, we estimated the thickness
l of each layer from the measured depth-dependence of the filament density in
Figure 7a.
To transform this model with varying layer thicknesses into a quadratic grid of resistors, we scaled the single resistor elements accordingly.
As the starting value of the resistor elements at the surface, we chose
R1 = 1 TΩ, which was derived from LC-AFM data [
9]. Thereby, we constructed five layers of different resistors that were connected as a regular network, as shown in
Figure 8a. In the center of the simulation grid, we modelled the bicrystal boundary as a slab of low-resistance layer having the same configurations as those on the surface. In order to simulate the electrical measurements shown in
Figure 6, we modelled the connection to the voltage source either at the sides of the regular grid (position 1a) or directly above the bicrystal boundary (position 2). The results of this simulation for the as-received crystal are shown in
Figure 8b. The graphic illustrates the situation after back transformation into the configuration of the hierarchic tree with varying layer thickness. It can be seen that the majority of the current flows along the surface layers and along the bicrystal boundary reproducing the behavior observed by LC-AFM. The simulated total resistance present between the electrodes was 11.5 TΩ, which is on the same order of magnitude as the measured resistance, showing that the setup of the simulation was reasonable. When moving the electrode above the bicrystal boundary, the simulation results in a significantly lower total resistance of 3.8 TΩ, thus also reproducing the experiment.
For the simulation of the etched crystal, we removed the topmost layer of resistors, whose thickness corresponds to 1–2 µm. This was justified because we had previously determined the etching rate using an AFM analysis of the etch pits [
14]. The rest of the simulation grid remained the same as for the simulation of the as-received crystal. The results in
Figure 8c show that the total resistance was increased to 23.1 TΩ in the undistorted region, while the resistance for the electrode configuration directly above the bicrystal boundary was 3.84 TΩ and almost the same as for the as-received crystal.
In summary, the electrical circuit simulation shows that the model of a three-dimensional network of dislocations in the shape of a hierarchical tree is plausible and can qualitatively explain the observed conductivity behavior. A graphical illustration of our model is shown in
Figure 9. Already in the as-received crystal (center panel), the network of filaments has a higher conductivity than the surrounding SrTiO
3 matrix and results in an overall semiconducting behavior. Upon etching (left panel), the surface layer is removed resulting in a lower conductivity, as fewer filaments for transport in the surface layer are available. Additionally, an incorporation of fluorine from hydrofluoric acid into the crystal occurs [
41]. During thermal reduction under vacuum conditions (right panel), oxygen is removed from the crystal, typically in the range of 10
14–10
17O ions/cm
3, depending on the conditions (oxygen partial pressure, temperature, time) [
14]. Due to the preferential reduction limited to the core of the dislocations, these are transformed into metallic filaments [
16]. The local concentration of the oxygen vacancies close to the dislocations is 10
20–10
21/cm
3 and the Mott criterion is locally fulfilled [
42,
43]. This mechanism is further supported by a rearrangement and bundling of dislocations occurring due to the high-temperature annealing [
14]. In consequence, this implies that the formation of conducting filaments can be influenced by the introduction of dislocations e.g., by forming a bicrystal boundary or by mechanical polishing of the surface. This could offer promising opportunities to tune the electrical properties of SrTiO
3 by mechanical methods.
3.4. Investigation of Photoluminescence
As a complementary method for gaining insight into the electronic properties of the boundary region, FLIM was employed. To generate lifetime maps, the pulsed laser was scanned over the surface while at each point, a photoluminescence decay trace was recorded. Before showing the maps, we briefly discuss the lifetime calculation by fitting the decay traces to exponential functions. We investigated SrTiO
3 bicrystals following different preparation steps, as is shown in
Figure 10. Starting from an as-received crystal, we etched the crystal with HF analogously to the electrical measurements. This process simultaneously led to the evolution of etch pits marking the dislocations, as we will later show. Finally, we conducted thermal reduction, which resulted in sufficient self-doping to transform the crystal into the metallic state [
14].
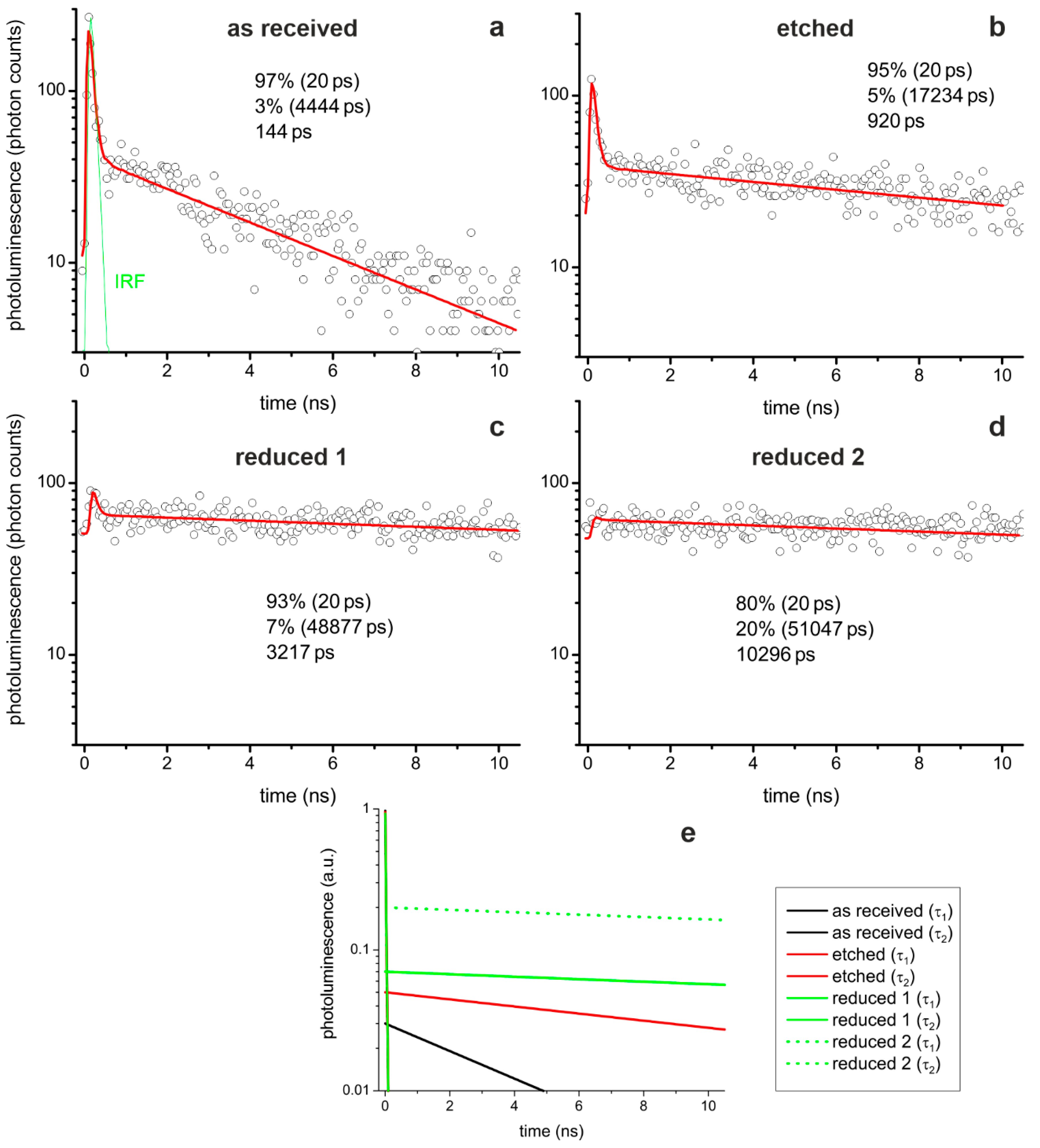
The photoluminescence traces for all three sample conditions are best described by a fitting model consisting of two exponential decays using a fast (≤20 ps) and a slow (>1 ns) photoluminescence process. The lifetime of the fast fit component was fixed at 20 ps. It is presumably even shorter, as the fast decay component and descending flank of the instrument response function (IRF) overlap. As we used dedicated dichroic filters, no scattered light reached the detector and we can conclude that the fast component is a real decay component. This indicates that at least two different decay channels exist in the investigated SrTiO3 samples.
A similar conclusion has been derived by Rubano et al. from single-photon excitation photoluminescence experiments, who ascribed a fast decay channel to the direct recombination of free electron–hole pairs and a slow decay channel to the recombination of bound electron–hole pair [
44,
45,
46]. These bound electron–hole pairs could be correlated with longer-lived deep trap states within the bandgap of SrTiO
3, as was already suggested in the case of hydrogenated TiO
2 [
47]. Presumably, these states originate from intrinsic defects, such as oxygen vacancies [
48]. Recently, Crespillo et al. [
49] conducted an in-depth study of the recombination mechanisms in SrTiO
3 and attributed the blue emission at 2.8 eV to a transition from conduction band states to the ground state level of the self-trapped exciton center, while the spontaneous annihilation of a self-trapped exciton center resulted in a green emission at 2.5 eV. However, one must also take into account that the presence of dislocations in the surface layer has an impact on the local electronic structure. It should be considered that vacancies might remain isolated only below a certain concentration. With increasing vacancy concentration, also the defect–defect interactions play a more important role, which could lead to agglomeration and clustering of vacancies, preferentially at dislocations. Hence, the models presented in the literature, which were developed for ideal crystals with statistical distributions of point defects, must be extended.
Comparing the three reduction states (
Figure 10a–d), it can be seen that the lifetime of the slow component increases with reduction. After etching, the lifetime of the slow component increased by a factor of four and, after thermal reduction, even by a factor of ten. This also led to an increase in the average lifetime, which was calculated as the amplitude-weighted mean of the two lifetimes. With regard to the two exemplarily shown decay traces of the thermally reduced crystal, it can be seen that the fit of the fast component becomes difficult. In some pixel photoluminescence decays, the fast component can clearly be identified (
Figure 10c) but is barely visible in others (
Figure 10d). Hence, the average lifetime is much shorter in the first case due to the large amplitude of the fast component than in the latter case, which results in a larger variation in the lifetime maps of the reduced crystal compared to the other two conditions (cf.
Figure 11 and
Figure 12).
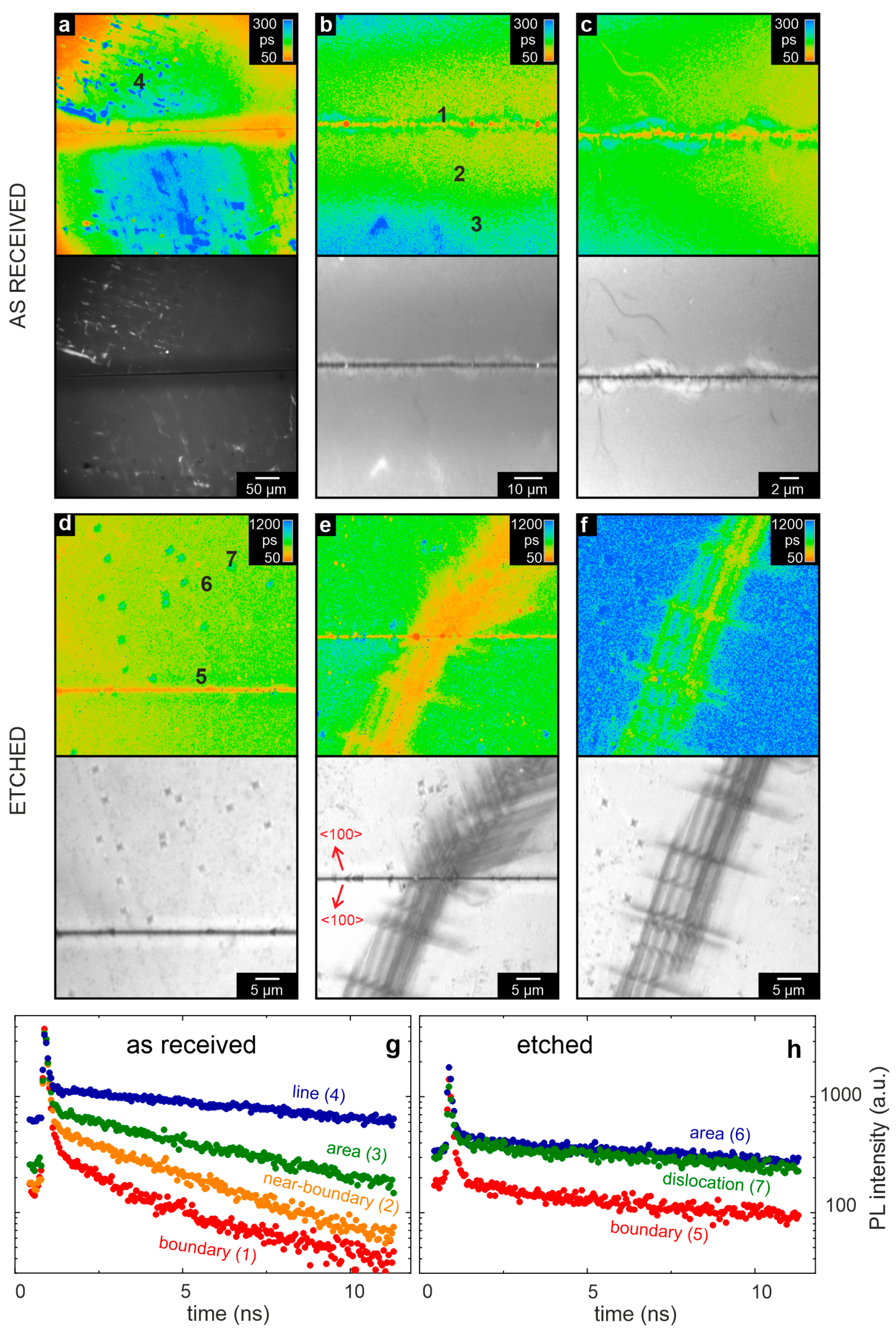
In the following, we focus on an investigation of the photoluminescent properties of the bicrystal, as observed initially in the as-received state. From each measurement region, the photoluminescence lifetime and intensity maps are depicted in
Figure 11a–c, together with the decay traces of selected spots in
Figure 11g. In the photoluminescence maps, the bicrystal junction can clearly be identified as a narrow, straight region of lower photoluminescence intensity. Along the boundary, an arrangement of spots can be observed that has a shorter lifetime than the surrounding. Those spots were occasionally embedded in structures with a meander-like shape and longer lifetime. An explanation for these observations could be that the mobility of the charge carriers along the dislocations is enhanced. Hence, the excited carriers can be effectively transported to recombination centers, resulting in shorter exciton lifetimes.
Additionally, further from the junction, inhomogeneous features with even longer lifetimes can be observed. Comparing the intensity and lifetime maps, the same structures can be detected around the boundary region. A shorter lifetime corresponds to a lower intensity and vice versa.
Furthermore, it must be considered that the crystallographic structure of dislocations can significantly alter from that of the matrix, as discussed above. In consequence, the electronic structure of dislocations differs from that of SrTiO
3, mainly in relation to a surplus of the Ti
3+ valence state providing different decay mechanisms, e.g., it might be possible that radiation-free Auger recombination becomes more important at the boundary, decreasing the intensity and lifetime. Additionally, the dislocations are surrounded by a stress field that leads to local flexoelectricity, which is known to have an impact on the luminescence properties [
38,
39,
50].
In order to more directly investigate a potential correlation between the position of the dislocations and photoluminescence lifetime, the bicrystal boundary was investigated by FLIM after etching the surface of the bicrystal by means of HF. As the etching process preferentially starts at the dislocation points, this method allows the position of the dislocations to be marked by the etch pits (
Figure 2). The photoluminescence and intensity maps shown in
Figure 11d–f at different positions on the bicrystal clearly depict the position of the etch pits. In comparison to the measurement of the as-received bicrystal, the average lifetime of the etched bicrystal is significantly higher. A possible reason for this finding could lay in the removal of the upper surface layer of the polished bicrystals upon etching. As discussed above, the dislocation density of the surface layer is much higher than that of the bulk [
36]. Hence, this could indicate that the presence of dislocation correlates to a smaller luminescence lifetime, which would be in agreement with the conclusions derived from the lifetime analysis of the boundary. Furthermore, secondary effects such as a modification of the chemical composition of the surface layer by fluorine, leading to the evolution of SrTiF
xO
y, may contribute [
41]. Despite these uncertainties, the position of the etch pits can be identified in the photoluminescence maps. As expected, a high concentration of etch pits can be observed close to the boundary. Although the average lifetime is longer compared to the as-received bicrystal, the relative differences between the boundary and the rest of the surface were preserved. At the boundary itself, the lifetime was shorter and the intensity lower than in the surrounding area. Additionally, a regular arrangement of stripes with enhanced etch pit density aligned along the main crystal axes can be observed (
Figure 11e,f). This dislocation-rich zone spreads from the bicrystal boundary in both parts of the bicrystal, indicating that the presence of mechanical stress leads to the generation and ordering of dislocations. The same holds true for the zone of arranged dislocations, indicating that this network of dislocations had similar properties as the bicrystal boundary. On the contrary, isolated etch pits separated from the boundary can be identified, which also show a lower photoluminescence intensity but an increased photoluminescence lifetime (see detailed analysis in the
Supplementary Materials). In order to explain the differences between the isolated and bundled dislocations, in our opinion, two scenarios must be considered. It is known that i) in SrTiO
3, different types of dislocations are present [
51]. Hence, it is conceivable that the details of structure and chemical composition of the core of the dislocations differ between isolated dislocations and dislocations forming boundaries. An alternative explanation would be ii) that the isolated dislocations serve as electron traps, thus resulting in longer photoluminescence lifetimes. In contrast, a dislocation network would provide a variety of conducting paths, thus allowing for the easy transport of the exited electron to a lattice site where efficient relaxation into the ground state is possible, resulting in shorter photoluminescence decay times. Such an effect would also be in accordance with the conclusions derived from our LC-AFM and macroscopic resistance investigations, which show that the electronic conductivity along a network of dislocations is significantly enhanced. A similar phenomenon has been observed by Wang et al. using cathodoluminescence [
52]. They found a higher luminescence at naturally occurring dislocations whereas on slip lines, which were induced by mechanical scratching, a lower luminescence was recorded.
Finally, we investigated the effect of thermally induced reduction on the photoluminescence properties of the bicrystal. After heating the bicrystal under reducing conditions at 850 °C for one hour, the measurements were conducted at room temperature in accordance with the procedure of the LC-AFM investigations in
Figure 5. In the photoluminescence maps shown in
Figure 12a, the position of the boundary can still be clearly identified as having a lower intensity and lifetime than the surrounding surface. This follows the same trend as prior to the reduction (
Figure 11). The photoluminescence average lifetime at the bicrystal boundary but even more so the lifetime measured apart from the boundary had changed significantly (see the very different photoluminescence lifetime scales in
Figure 11 and
Figure 12) and became slower and more inhomogeneous. In some regions on the sub-micrometer scale, the lifetime had increased by a factor of 100, whereas in other regions, it was almost the same or even slightly lower compared to the as-received sample. This effect is strongly influenced by variations in the presence of the fast decay component, as discussed above. The observed inhomogeneities could relate to local variations in the generation of oxygen vacancies and a change in the Ti/Sr ratio leading to variations in the electronic structure and thus to the dominant decay channel. For instance, oxygen vacancies can serve as localized traps for electrons, contributing to the observed extension of photoluminescence lifetimes [
48]. This effect could also be related to self-trapping of polarons, e.g., at the Ti site next to an isolated oxygen vacancy, which was found to have a significant impact on the electronic structure and the luminescence [
53]. Nevertheless, in our data, not only the magnitude of the fast decay component but also the lifetime of the slow decay component has changed dramatically, being about 10 times longer compared to the as-received SrTiO
3 crystal.
It is also known that with the thermally induced reduction of SrTiO
3, the surface region becomes Ti-rich and eventually, titanium suboxides form [
54,
55]. Hence, the incipient phase transformation could lead to clustering effects on the nanoscale, resulting in the observed inhomogeneous photoluminescence lifetime distribution. Regarding the decay curves shown for two selected spots at the boundary and surrounding surface (
Figure 12b), it can be seen that the fast component at the boundary had a much higher amplitude than in the surrounding surface. In consequence, the amplitude-weighted average lifetime at the boundary was also much shorter than in the surrounding surface.
Summing up the time-resolved photoluminescence investigation, we can state that in all measurements, two decay channels can be observed. The slow component appears to relate to the SrTiO3 matrix and depends strongly on the crystal preparation. It is significantly suppressed close to the bicrystal boundary and dislocation-rich slip bands, where the short component is dominant. One potential explanation for this behavior could be that the network of filaments formed along the dislocations provides an easy migration path for charge carriers. Hence, generated electron–hole pairs can easily be transported to recombination centers located at the dislocations. Our results show that the detailed physical nature of the photoluminescence decay close to dislocations is complex, as one has to consider the electronic structure of SrTiO3, the electronic structure of the dislocations, and furthermore potential easy migration paths of the excited charge carriers.