Effect of Modification on Microstructure and Properties of AZ91 Magnesium Alloy
Abstract
1. Introduction
- overheating of the molten alloy.
- Elfinal process—modification with iron (III) chloride.
- modification with carbon.
2. Materials and Methods
- overheating of 10 min. at 850 °C with subsequent rapid cooling to pouring temperature,
- application of hexachlorethane (commercial name DEGASAL C1/A), 1 kg/100 kg of the alloy at temperature of ~740 °C,
- application of wax-CaF2-carbon powder (commercial name NUCLEANT 5000), 0.3 kg/100 kg of the alloy at temperature of approx. 730 °C.
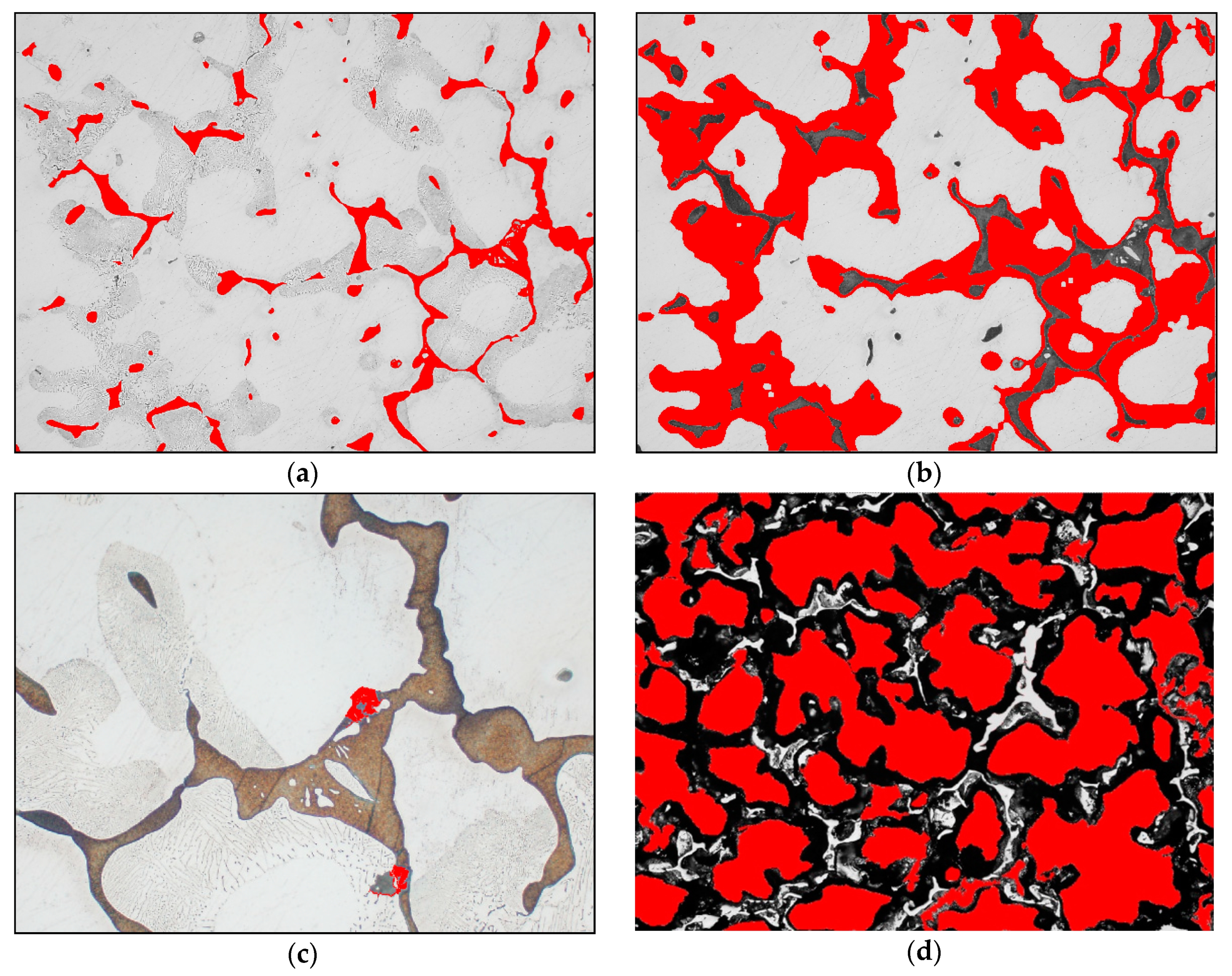
- volume fraction of intermetallic phases throughout the sample section, VV [%],
- average area of solid solution grain cross-section A [μm2].
3. Results and Discussion
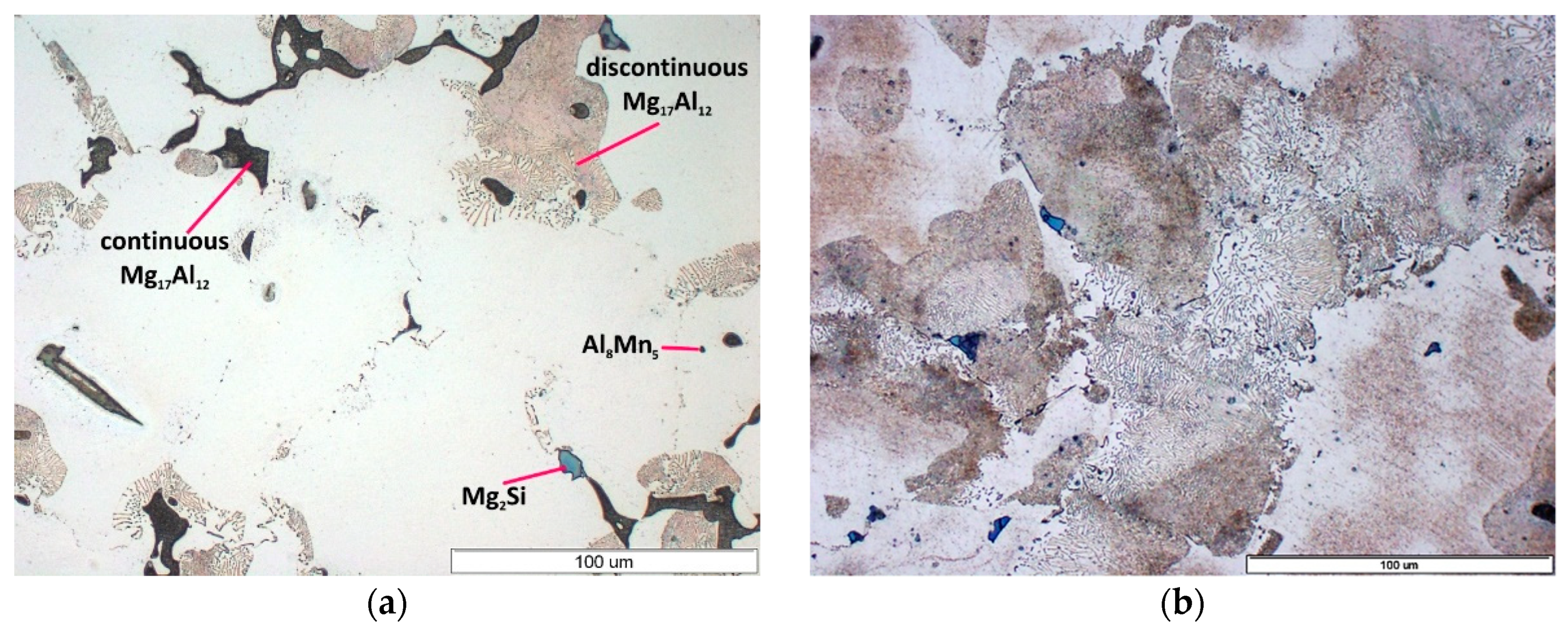
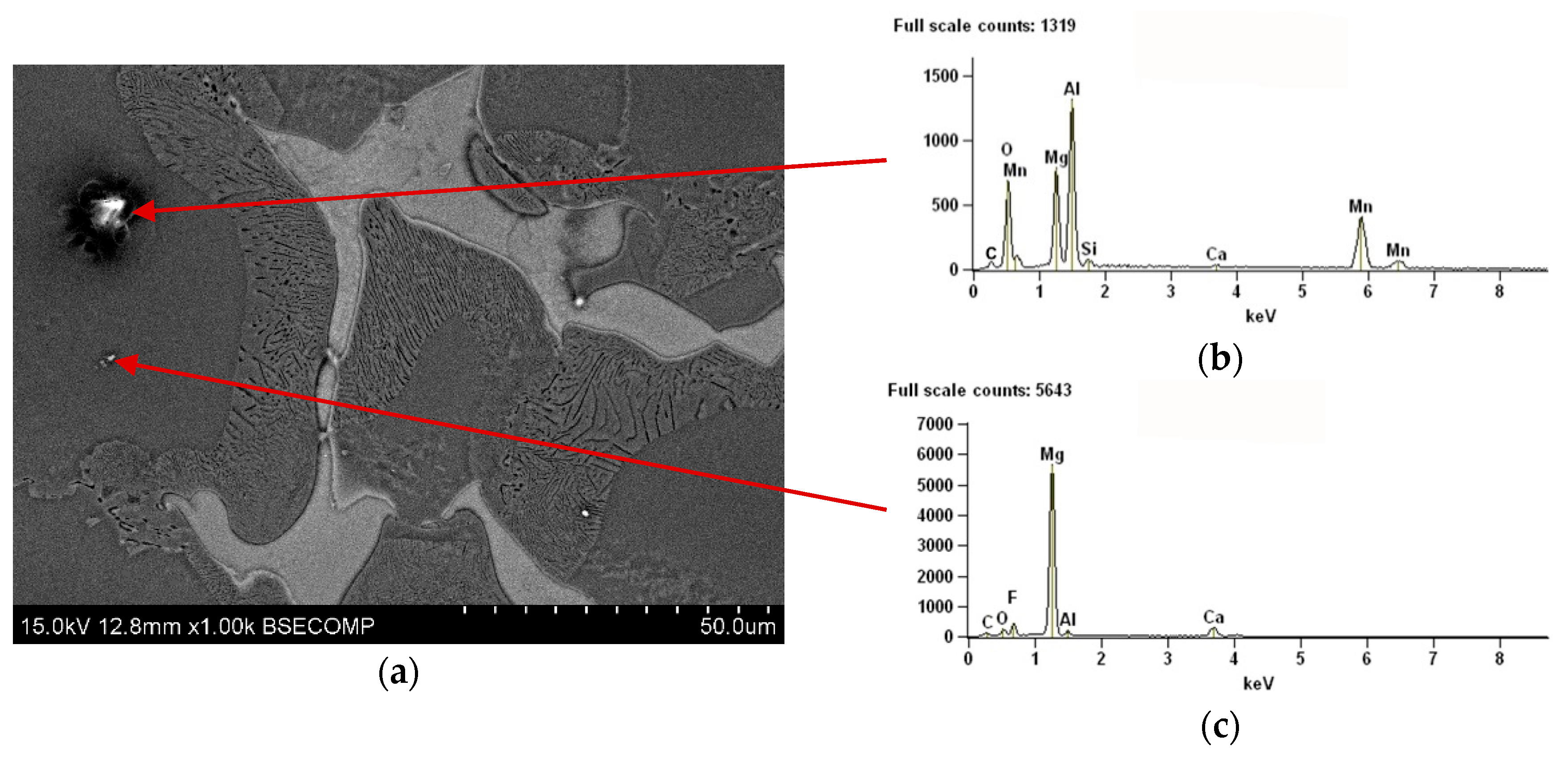
3.1. As-Cast Condition
- continuous precipitates located mainly on grain boundaries of α-Mg solid solution;
- plate-like precipitates (discontinuous precipitates) formed in discontinuous diffusive transition creating the mixture of α-Mg and discontinuous precipitates of Mg17Al12 phase.
3.2. Effect of Overheating
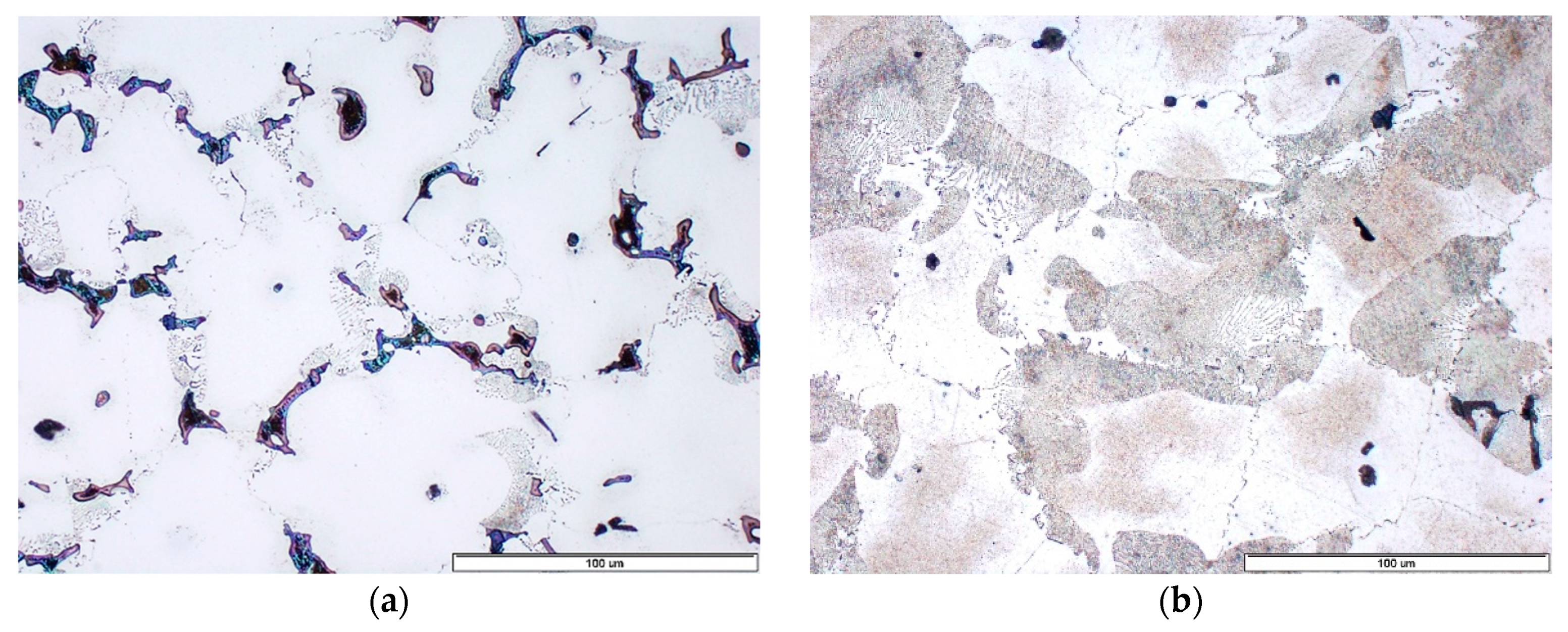
3.3. Effect of Hexachlorethane Refiner
3.4. Effect of Wax-CaF2-Carbon Refiner
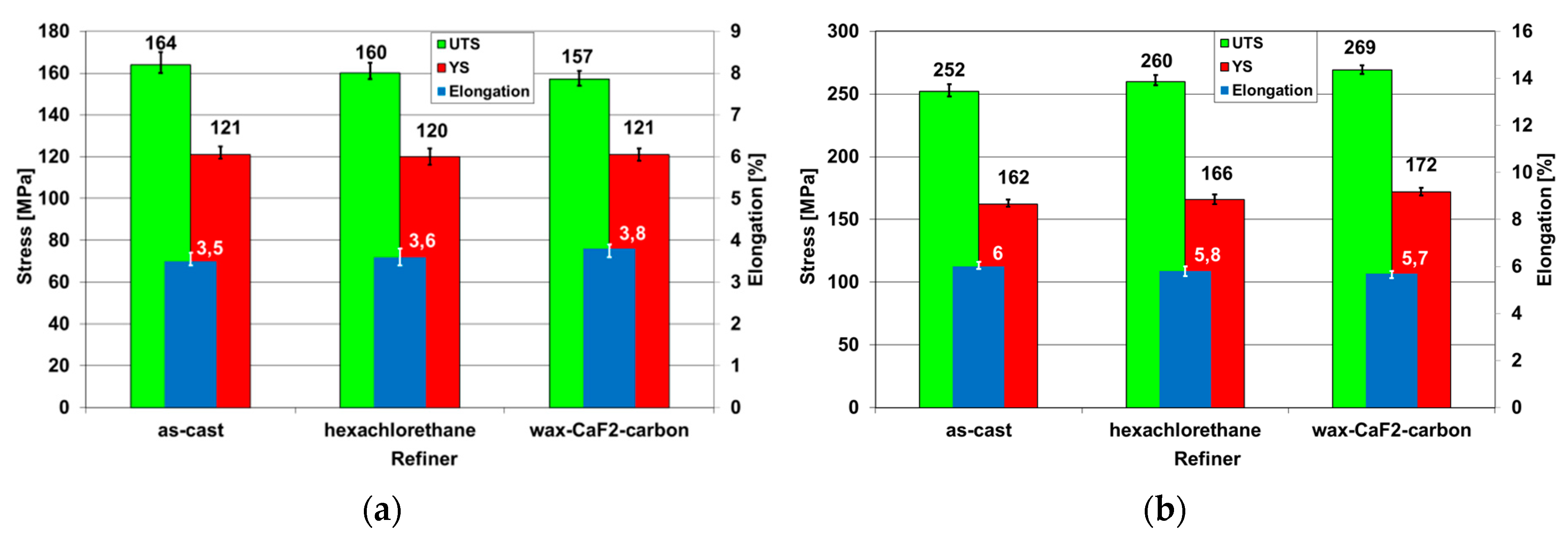
3.5. Influence of Modification on Mechanical Properties
4. Conclusions
- (1)
- Microstructure of AZ91 alloy in as-cast condition consists of α-Mg solid solution with continuous precipitates of Mg17Al12 phase, discontinuous precipitates of α-Mg+Mg17Al12 compound, and the particles of Mg2Si and Al8Mn5 phases.
- (2)
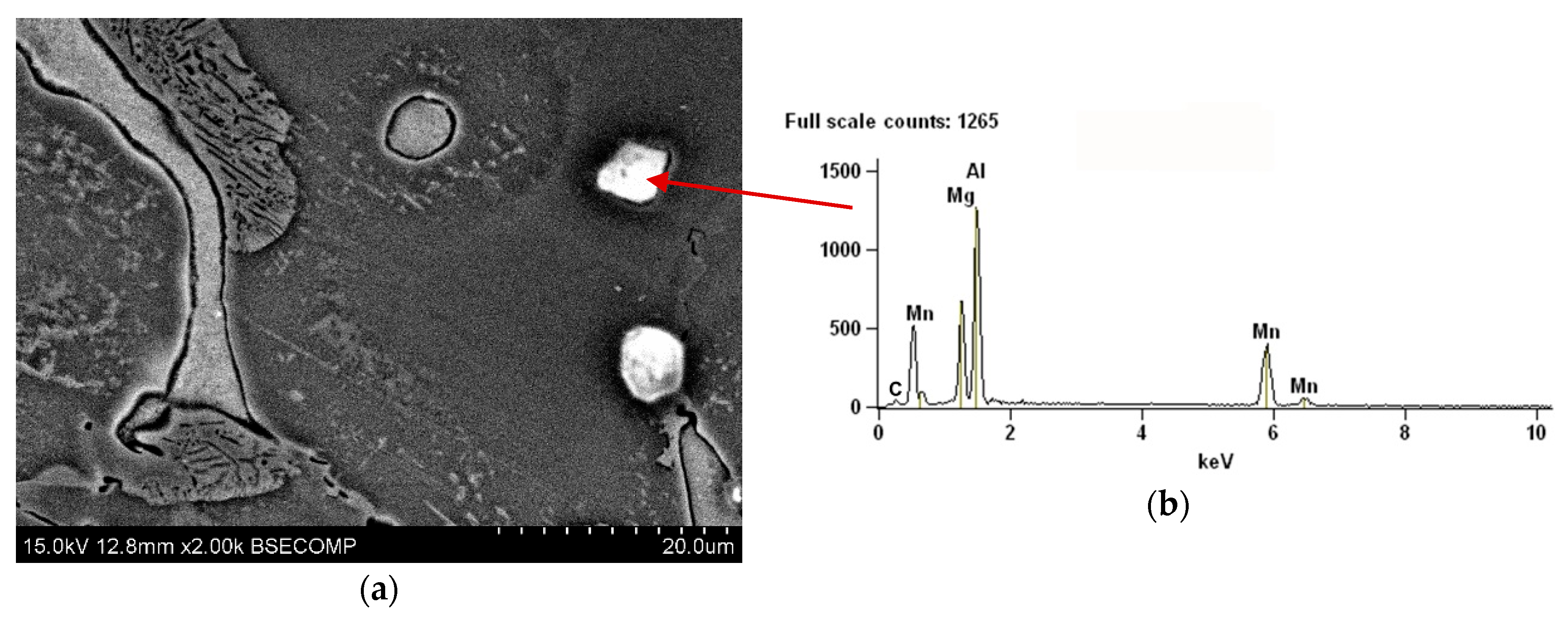
- Overheating of the alloy caused the decrease of the average area of α-Mg solid solution grain cross-section and the increase of the volume fraction of α-Mg+Mg17Al12 areas. Numerous aluminium oxides and iron diffused from the crucible acted as the nucleation sites. Their presence was effective in grain refinement but had a negative effect on the quality and mechanical properties of the castings.
- (3)
- Carbon hexachloride turned out to be highly effective both in grain refinement and degassing of the melt. Al4C3 phase and Al8Mn5 phase nucleating on the surface of Al4C3 acted as nucleation sites. Unfortunately, the application of carbon hexachloride is limited due to the emission of toxic gases, which cause severe environment pollution.
- (4)
- Wax-CaF2-carbon was the most effective modifier. The mechanism of grain refinement was complex. In the first stage, as in the case of carbon hexachloride, the precipitates of Al4C3 phase were formed, while the second stage comprised the formation of Al2Ca phase, which promotes nucleation and increases the supercooling.
- (5)
- The type of applied modifier does not influence the mechanical properties of AZ91 alloy in the as-cast condition. A slight decrease of ultimate tensile strength resulted from the decrease of the volume fraction of α-Mg+Mg17Al12 discontinuous precipitates’ areas. After age-hardening treatment, the increase of mechanical properties with the grain refinement was observed and was attributed to the nucleation of discontinuous precipitates of Mg17Al12 phase on the grain boundaries.
Author Contributions
Funding
Conflicts of Interest
References
- Das, S.K.; Chang, C.F.; Raybould, D.; King, J.F.; Thistlewaite, S.R. New developments in rapidly solidified magnesium alloys. In Magnesium Alloys and Their Applications; Mordike, B.L., Hehmann, F., Eds.; DGM Informationsgesellschaft: Oberursel, Germany, 1992; pp. 487–494. [Google Scholar]
- Bettles, C.J.; Forwood, C.T.; StJohn, D.H.; Frost, M.T.; Jones, D.S.; Qian, M.; Song, G.L.; Griffiths, J.R.; Nie, J.F. AMC-SC1: An elevated temperature magnesium alloy suitable for precision sand casting of powertrain components. In Magnesium Technology 2003; Kaplan, H.I., Ed.; TMS: San Diego, CA, USA, 2003; p. 223. [Google Scholar]
- Song, G.L.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Qian, M.; StJohn, D.H.; Frost, M.T. Zirconium alloying and grain refinement of magnesium alloys. Magnes. Technol. 2003, 2003, 209–214. [Google Scholar]
- Kondic, V. Metallurgical Principles of Founding, 1st ed.; Edward Arnold: London, UK, 1968. [Google Scholar]
- Changjiang, S.; Qingyou, H.; Qijie, Z. Review of grain refinement methods for as-cast microstructure of magnesium alloy. China Foundry 2019, 6, 93–103. [Google Scholar]
- Farbenindustrie, I.G. Procedure for Grain Refining Magnesium and Its Alloys. Belgian Patent 444,757, 1942. [Google Scholar]
- Wood, R.T. Grain refinement of magnesium castings. Mod. Met. 1952, 8, 65–68. [Google Scholar]
- Emley, E.F. Principles of Magnesium Technology, 1st ed.; Pergamon Press Ltd.: Oxford, UK, 1966. [Google Scholar]
- Lee, Y.C.; Dahle, A.K.; StJohn, D.H. The role of solute in grain refinement of magnesium. Meter. Mater. Trans. A 2000, 31A, 2895–2906. [Google Scholar] [CrossRef]
- Nelson, C.E. Grain size behavior in magnesium casting alloys. Trans. Am. Foundrymen’s Soc. 1948, 56, 1–23. [Google Scholar]
- StJohn, D.; Qian, M.; Easton, M.; Cao, P.; Hildebrand, Z. Grain refinement of magnesium alloys. Meter. Mater. Trans. A 2005, 36A, 1669–1680. [Google Scholar] [CrossRef]
- Qian, M.; Cao, P. Discussion on grain refinement of magnesium alloys by carbon inoculation. Scr. Mater. 2005, 52, 415–419. [Google Scholar] [CrossRef]
- Renger, K.; Simon, R. Grain refinement of AZ91 by Nucleant 5000. In Proceedings of the 1st International Light Metals Technology Conference 2003, Brisbane, Australia, 18–20 September 2003; Dahle, A.K., Ed.; CRC for Cast Metals Manufacturing (CAST): Brisbane, Australia, 2003; pp. 231–234. [Google Scholar]
- ASTM B557M—Standard Test Methods for Tension Testing Wrought and Cast Aluminum- and Magnesium-Alloy Products (Metric); ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Kiełbus, A.; Rzychoń, T. The intermetallic phases in sand casting magnesium alloys for elevated temperature. Mater. Sci. Forum 2011, 690, 214–217. [Google Scholar] [CrossRef]
- Karaluk, E. A review: Past, present, and future of grain refining of magnesium castings. J. Magnes. Alloys 2019, 7, 355–369. [Google Scholar] [CrossRef]
- StJohn, D.; Easton, M.; Qian, M.; Taylor, J. Grain Refinement of Magnesium Alloys: A Review of recent research, theoretical developments, and their application. Meter. Mater. Trans. A 2013, A44, 2935–2949. [Google Scholar] [CrossRef]
- Vinotha, D.; Raghukandan, K.; Pillai, U.; Pai, B. Grain refining mechanisms in magnesium alloys—An overview. Trans. Indian Inst. Met. 2009, 62, 521–532. [Google Scholar] [CrossRef]
- Li, S.; Tang, B.; Zeng, D. Effects and mechanism of Ca on refinement of AZ91D alloy. J. Alloys Compd. 2007, 437, 317–321. [Google Scholar] [CrossRef]



| Element | Al | Zn | Mn | Cu | Si | Fe | Ni | Mg |
|---|---|---|---|---|---|---|---|---|
| [%] | 8.9 | 0.6 | 0.21 | 0.02 | 0.05 | 0.006 | 0.001 | balance |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełbus, A.; Jarosz, R.; Gryc, A. Effect of Modification on Microstructure and Properties of AZ91 Magnesium Alloy. Crystals 2020, 10, 536. https://doi.org/10.3390/cryst10060536
Kiełbus A, Jarosz R, Gryc A. Effect of Modification on Microstructure and Properties of AZ91 Magnesium Alloy. Crystals. 2020; 10(6):536. https://doi.org/10.3390/cryst10060536
Chicago/Turabian StyleKiełbus, Andrzej, Robert Jarosz, and Adam Gryc. 2020. "Effect of Modification on Microstructure and Properties of AZ91 Magnesium Alloy" Crystals 10, no. 6: 536. https://doi.org/10.3390/cryst10060536
APA StyleKiełbus, A., Jarosz, R., & Gryc, A. (2020). Effect of Modification on Microstructure and Properties of AZ91 Magnesium Alloy. Crystals, 10(6), 536. https://doi.org/10.3390/cryst10060536






