Liquid Phase Separation in Ag-Co-Cr-Fe-Mn-Ni, Co Cr-Cu-Fe-Mn-Ni and Co-Cr-Cu-Fe-Mn-Ni-B High Entropy Alloys for Biomedical Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- (1)
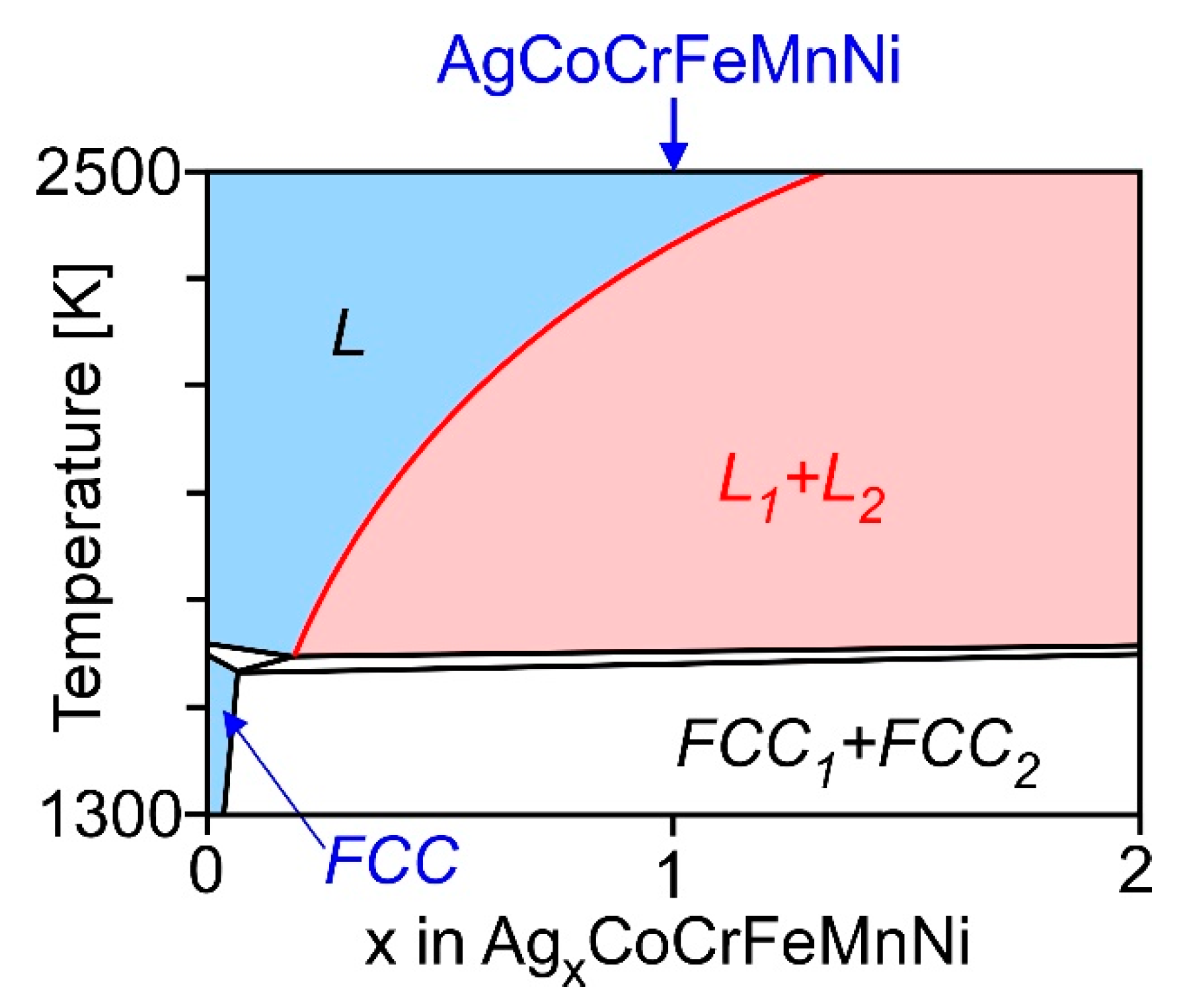
- LPS was observed in the arc-melted ingots of the equiatomic AgCoCrFeMnNi HEAs;
- (2)
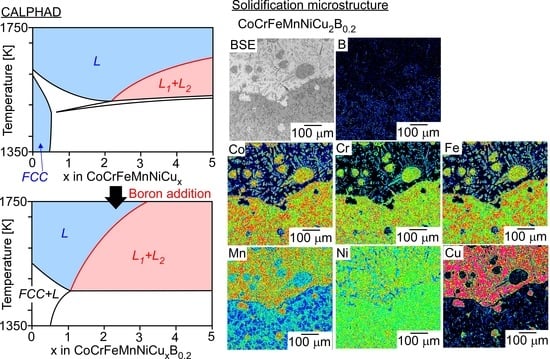
- A macroscopically phase-separated structure formed via LPS was not observed in the arc-melted ingots of the CoCrCuxFeMnNi (x = 1, 2, 3) HEAs, whereas LPS was observed in the arc-melted ingots of the B-added CoCrCuxFeMnNiB0.2 (x = 2, 3) HEAs. The addition of B enhanced the LPS tendency in the CoCrFeMnNiCux HEAs;
- (3)
- The occurrence of LPS in the arc-melted ingots of the Ag-Co-Cr-Fe-Mn-Ni and Co-Cr-Cu-Fe-Mn-Ni-B HEAs corresponded to the positive values of Hi-j (i = Ag)(j = Co, Cr, Fe, Mn, Ni) and Hi-j (i = Cu)(j = Co, Cr, Fe, Mn, Ni), the absence of Ag-containing and Cu-containing intermetallic compounds in the predicted phase diagrams, and the liquid miscibility gap in the calculated phase diagrams.
Author Contributions
Funding
Conflicts of Interest
References
- Devine, T.M.; Kummer, F.J.; Wulff, J. Wrought cobalt-chromium surgical implant alloys. J. Mater. Sci. 1972, 7, 126–128. [Google Scholar] [CrossRef]
- Devine, T.M.; Wulff, J. Cast vs. wrought cobalt-chromium surgical implant alloys. J. Biomed. Mater. Res. 1975, 9, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Rose, R.M.; Wulff, J. Recommended heat treatment and alloy additions for cast Co-Cr surgical implants. J. Biomed. Mater. Res. 1978, 12, 935–937. [Google Scholar] [CrossRef] [PubMed]
- Kilner, T.; Pilliar, R.M.; Weatherly, G.C.; Alibert, C. Phase identification and incipient melting in a cast Co-Cr surgical implant alloy. J. Biomed. Mater. Res. 1982, 16, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Niinomi, M. Design and development of metallic biomaterials with biological and mechanical biocompatibility. J. Biomed. Mater. Res. A 2019, 107, 944–954. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liew, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High-entropy Alloys, 1st ed.; Elsevier: Amsterdam, Nederland, 2014. [Google Scholar]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys, Fundamentals and Applications, 1st ed.; Springer: Berlin, Germany, 2016. [Google Scholar]
- Takeuchi, A. Alloy designs for high-entropy alloys, bulk metallic glasses and high-entropy bulk metallic glasses. J. Jpn. Inst. Met. 2015, 79, 157–168. [Google Scholar] [CrossRef]
- Takechi, A. Recent progress in alloy designs for high-entropy crystalline and glassy alloys. J. Jpn. Soc. Powder. Met. 2016, 63, 209–216. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Zhang, W.; Liew, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Miracle, D.B. High entropy alloys as a bold step forward in alloy development. Nat. Commun. 2019, 10, 1805. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Nakano, T. Development of Co-Cr-Mo-Fe-Mn-W and Co-Cr-Mo-Fe-Mn-W-Ag high-entropy alloys based on Co-Cr-Mo alloys. Mater. Trans. 2020, 61, 567–576. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef]
- Wang, S.P.; Xu, J. TiZrNbTaMo high-entropy alloy designed for orthopedic implants: As-cast microstructure and mechanical properties. Mater. Sci. Eng. C 2017, 73, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Mizuuchi, K.; Nakano, T. Solidification microstructures of the ingots obtained by arc melting and cold crucible levitation melting in TiNbTaZr medium-entropy alloy and TiNbTaZrX (X = V, Mo, W) high-entropy alloys. Entropy 2019, 21, 483. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Hori, T.; Nakano, T. Microstructure of equiatomic and non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. J. Alloy. Compd. 2018, 753, 412–421. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of Non-equiatomic Ti-Nb-Ta-Zr-Mo High-Entropy Alloys for Metallic Biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Yang, Z.; Liang, X.; Lei, Z.; Huang, H.; Wang, H.; Liu, X.; An, K.; Wu, W.; et al. Formation, structure and properties of biocompatible TiZrHfNbTa high-entropy alloys. Mater. Res. Lett. 2019, 7, 225–231. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of TiZrTaHfNb and Ti1.5ZrTa0.5Hf0.5Nb0.5 refractory high-entropy alloys for biomedical applications. Intermet. 2019, 113, 106572. [Google Scholar] [CrossRef]
- Popescu, G.; Ghiban, B.; Popescu, C.A.; Rosu, L.; Trusca, R.; Carcea, I.; Soare, V.; Dumitrescu, D.; Constantin, I.; Olaru, M.T.; et al. New TiZrNbTaFe high entropy alloy used for medical applications. IOP Conf. Ser. 2018, 400, 022049. [Google Scholar]
- Nagase, T.; Iijima, Y.; Matsugaki, A.; Ameyama, K.; Nakano, T. Design and fabrication of Ti-Zr-Hf-Cr-Mo and Ti-Zr-Hf-Co-Cr-Mo high-entropy alloys as metallic biomaterials. Mater. Sci. Eng. C 2020, 107, 110322. [Google Scholar] [CrossRef]
- Hsu, U.S.; Hung, U.D.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Yang, C.C. Alloying behavior of iron, gold and silver in AlCoCrCuNi-based equimolar high-entropy alloys. Mater. Sci. Eng. A 2007, 460, 403–408. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Liu, C.T. Anomalous solidification microstructures in Co-free AlxCrCuFeNi2 high-entropy alloy. J. Alloy. Compd. 2013, 557, 77–81. [Google Scholar] [CrossRef]
- Munitz, A.; Samuha, S.; Brosh, E.; Salhov, S.; Derimow, N.; Abbaschian, R. Liquid phase separation phenomena in Al2.2CrCuFeNi2 HEA. Intermet 2018, 97, 77–84. [Google Scholar] [CrossRef]
- Munitz, A.; Kaufman, M.J.; Chandler, J.P.; Kalaantari, H.; Abbaschian, R. Melt separation phenomena in CoNiCuAlCr high entropy alloy containing silver. Mater. Sci. Eng. A 2013, 560, 633–642. [Google Scholar] [CrossRef]
- Wu, P.H.; Liu, N.; Yang, W.; Zhu, Z.X.; Lu, Y.P.; Wang, X.J. Microstructure and solidification behavior of multicomponent CoCrCuxFeMoNi high-entropy alloys. Mater. Sci. Eng. A 2015, 642, 142–149. [Google Scholar] [CrossRef]
- Liu, N.; Wu, P.H.; Zhou, P.J.; Peng, Z.; Wang, X.J.; Lu, Y.P. Rapid solidification and liquid-phase separation of undercooled CoCrCuFexNi high-entropy alloys. Intermet 2016, 72, 44–52. [Google Scholar] [CrossRef]
- Wu, P.H.; Liu, N.; Zhou, P.J.; Peng, Z.; Du, W.D.; Wang, X.J.; Pan, Y. Microstructures and liquid phase separation in multicomponent CoCrCuFeNi high entropy alloys. Mater. Sci. Technol. 2016, 32, 576–580. [Google Scholar] [CrossRef]
- Wang, W.L.; Hu, L.; Luo, S.B.; Meng, L.J.; Geng, D.L.; Wei, B. Liquid phase separation and rapid dendritic growth of high-entropy CoCrCuFeNi alloy. Intermet 2016, 77, 41–45. [Google Scholar] [CrossRef]
- Guo, T.; Li, J.; Wang, J.; Wang, Y.; Kou, H.; Niu, S. Liquid-phase separation in undercooled CoCrCuFeNi high entropy alloy. Intermet 2017, 86, 110–115. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, N.; Zhang, S.Y.; Wu, P.H.; Wang, X.J. Liquid-phase separation of immiscible CrCuxFeMoyNi high-entropy alloys. Mater. Sci. Technol. 2017, 33, 1352–1359. [Google Scholar] [CrossRef]
- Munitz, A.; Kaufman, M.J.; Abbaschian, R. Liquid phase separation in transition element high entropy alloys. Intermet 2017, 86, 59–72. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; Feng, L.C.; Liu, Y.Y.; Zhang, P.; Hea, Y.Z.; Menga, Q.Q.; Zhanga, J.Y. Nano-phase formation accompanying phase separation in undercooled CoCrCuFeNi-3 at.% Sn high entropy alloy. Mater. Charact. 2018, 144, 516–521. [Google Scholar] [CrossRef]
- Munitz, A.; Dry, I.E.; Brosh, E.; Derimow, N.; MacDonald, B.E.; Lavernia, E.J.; Abbaschian, R. Liquid phase separation in AlCrFeNiMo0.3 high-entropy alloy. Intermet 2019, 112, 106517. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Nakano, T. Development of Ti-Zr-Hf-Y-La high-entropy alloys with dual hexagonal-close-packed structure. Scr. Mater. 2020, 186, 242–246. [Google Scholar] [CrossRef]
- Derimow, N.; Abbaschian, R. Liquid phase separation in high-entropy alloys—A review. Entropy 2018, 20, 890. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhy, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Ag-Co (Silver-Cobalt) system. J. Phase Equilib. 1986, 7, 259–263. [Google Scholar] [CrossRef]
- Venkatraman, M.; Neumann, J.P. The Ag-Cr (Silver-Chromium) system. J. Phase Equilib. 1990, 11, 263–265. [Google Scholar] [CrossRef]
- Swartzendruber, L.J. The Ag-Fe (Silver-Iron) system. J. Phase Equilib. 1984, 5, 560–564. [Google Scholar] [CrossRef]
- Karakaya, I.; Thompson, W.T. The Ag-Mn (silver-manganese) system. J. Phase Equilib. 1990, 11, 480–486. [Google Scholar] [CrossRef]
- Singleton, M.; Nash, P. The Ag-Ni (Silver-Nickel) system. J. Phase Equilib. 1987, 8, 119–121. [Google Scholar] [CrossRef]
- Nishizawa, T.; Ishida, K. The Co-Cu (Cobalt-Copper) system. J. Phase Equilib. 1984, 5, 161–165. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Z.P. The Fe-Cu system: A thermodynamic evaluation. Met. Trans. A 1995, 26, 417–426. [Google Scholar] [CrossRef]
- Wang, C.P.; Liu, X.J.; Ohnuma, I.; Kainuma, R.; Ishida, K. Thermodynamic database of the phase diagrams in Cu-Fe base ternary systems. J. Phase Equilib. Diffus. 2004, 25, 320–328. [Google Scholar] [CrossRef]
- Okamoto, H. Cr-Cu (chromium-copper). J. Phase Equilib. Diffus. 2012, 33, 342–343. [Google Scholar] [CrossRef]
- Nakagawa, Y. Liquid immiscibility in copper-iron and copper-cobalt systems in the supercooled state. Acta Met. 1958, 6, 704–711. [Google Scholar] [CrossRef]
- Sun, Z.B.; Wang, Y.H.; Guo, J. Liquid phase separation of Cu-Cr alloys during rapid cooling. Trans. Nonferrous Met. Soc. China 2006, 16, 998–1002. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; Yang, Z.; Sun, Z.; Yu, D.; Song, X.; Ding, B.; Yang, S. Liquid phase separation of Cu-Cr alloys during the vacuum breakdown. J. Alloy Compd. 2015, 509, 7116–7120. [Google Scholar] [CrossRef]
- Si, S.H.; Zhang, H.; He, Y.Z.; Li, M.X.; Guo, S. Liquid phase separation and the aging effect on mechanical and electrical properties of laser rapidly solidified Cu100-XCrx alloys. Metals 2015, 5, 2119–2127. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, G.; Tian, C.; He, X.; Li, S. Grain refinement of hypereutectic immiscible cu-50cr alloy during rapid melting and solidification induced by high power density laser beams. Met. 2019, 9, 585. [Google Scholar] [CrossRef]
- Yamauchi, I.; Ueno, N.; Shimaoka, M.; Ohnaka, I. Undercooling in Co-Cu alloys and its effect on solidification structure. J. Mater. Sci. 1998, 33, 371–378. [Google Scholar] [CrossRef]
- Nagase, T.; Yokoyama, A.; Umakoshi, Y. Formation of macroscopically phase separated cu-colored melt-spun ribbon in (Fe0.5Cu0.5)100-xBx (x = 0, 5, 10, and 20) alloys. J. Alloy. Compd. 2011, 509, 1178–1186. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Jain, A.; Hautier, G.; Moore, C.; Ong, S.P.; Fischer, C.; Mueller, T.; Persson, K.; Ceder, G. A high-throughput infrastructure for density functional theory calculations. Comp. Mater. Sci. 2011, 50, 2295–2310. [Google Scholar] [CrossRef]
- Chuang, Y.Y.; Schmid, R.; Chang, Y.A. Thermodynamic analysis of the iron-copper system I: The stable and metastable phase equilibria. Met. Trans. A 1984, 15, 1921–1930. [Google Scholar] [CrossRef]
- Wilde, G.; Willnecker, R.; Singh, R.N.; Sommer, F. The metastable miscibility gap in the system Fe-Cu. Z. Met. 1997, 88, 804–809. [Google Scholar]
- Turchanin, M.A.; Agraval, P.G.; Nikolaenko, I.V. Thermodynamics of alloys and phase equilibria in the copper-iron system. J. Phase Equilib. 2003, 24, 305–319. [Google Scholar] [CrossRef]
- Nagase, T.; Suzuki, M.; Tanaka, T. Formation of amorphous phase with crystalline globules in Fe-Cu-Nb-B immiscible alloys. J. Alloy. Compd. 2015, 619, 267–274. [Google Scholar] [CrossRef]
- Nagase, T.; Suzuki, M.; Tanaka, T. Formation of amorphous phase with crystalline globules in Fe-Cu-Si-B and Fe-Cu-Zr-B immiscible alloys. Intermet. 2015, 61, 56–65. [Google Scholar] [CrossRef]
- Nagase, T.; Suzuki, M.; Tanaka, T. Amorphous phase formation in Fe-Ag-based immiscible alloys. J. Alloy. Compd. 2015, 619, 311–318. [Google Scholar] [CrossRef]
- Nagase, T.; Umakoshi, Y. Amorphous phase formation in Co-Cu-Zr-B-based immiscible alloys. J. Alloy. Compd. 2015, 649, 1174–1181. [Google Scholar] [CrossRef]
- Nagase, T.; Takemura, M.; Matsumuro, M.; Matsumoto, M.; Fujii, Y. Design and microstructure analysis of globules in Al-Co-La-Pb immiscible alloys with an amorphous phase. Mater. Des. 2017, 117, 338–345. [Google Scholar] [CrossRef]
- Nagase, T.; Terai, T.; Kakeshita, T.; Matsumoto, M.; Fujii, Y. Microstructure and magnetic properties of Cu-Ag-La-Fe immiscible alloys with an amorphous phase. Mater. Trans. 2019, 60, 554–560. [Google Scholar] [CrossRef]
- Nagase, T.; Matsumoto, M.; Fujii, Y. Microstructure of Ti-Ag immiscible alloys with liquid phase separation. J. Alloy. Compd. 2018, 738, 440–447. [Google Scholar] [CrossRef]
- Matyja, H.; Giessen, B.C.; Grant, N.J. The effect of cooling rate on the dendrite spacing in splat cooled aluminium alloys. J. inst. Met. 1968, 96, 30–32. [Google Scholar]
- Naka, M.; Shibayanagi, T. Formation and application of rapidly quenched metals. J. High Temp. Soc. 1998, 24, 131–136. [Google Scholar]
- Nagase, T.; Takemura, M.; Matsumuro, M.; Maruyama, T. Solidification microstructure of AlCoCrFeNi2.1 eutectic high entropy alloy ingots. Mater. Trans. 2018, 59, 255–264. [Google Scholar] [CrossRef]
- Nagase, T.; Kakeshita, T.; Matsumura, K.; Nakazawa, K.; Furuya, S.; Ozoe, N.; Yoshino, K. Development of Fe-Co-Cr-Mn-Ni-C high entropy cast iron (HE cast iron) available for casting in air atmosphere. Mater. Des. 2019, 184, 108172. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Laurent-Brocq, M.; Akhatova, A.; Perriere, L.; Chebini, S.; Sauvage, X.; Leroya, E.; Champion, Y. Insights into the phase diagram of the CrMnFeCoNi high entropy alloy. Acta Mater. 2015, 88, 355–365. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, Y.L.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Tsau, C.H.; Chang, S.-Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Met. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
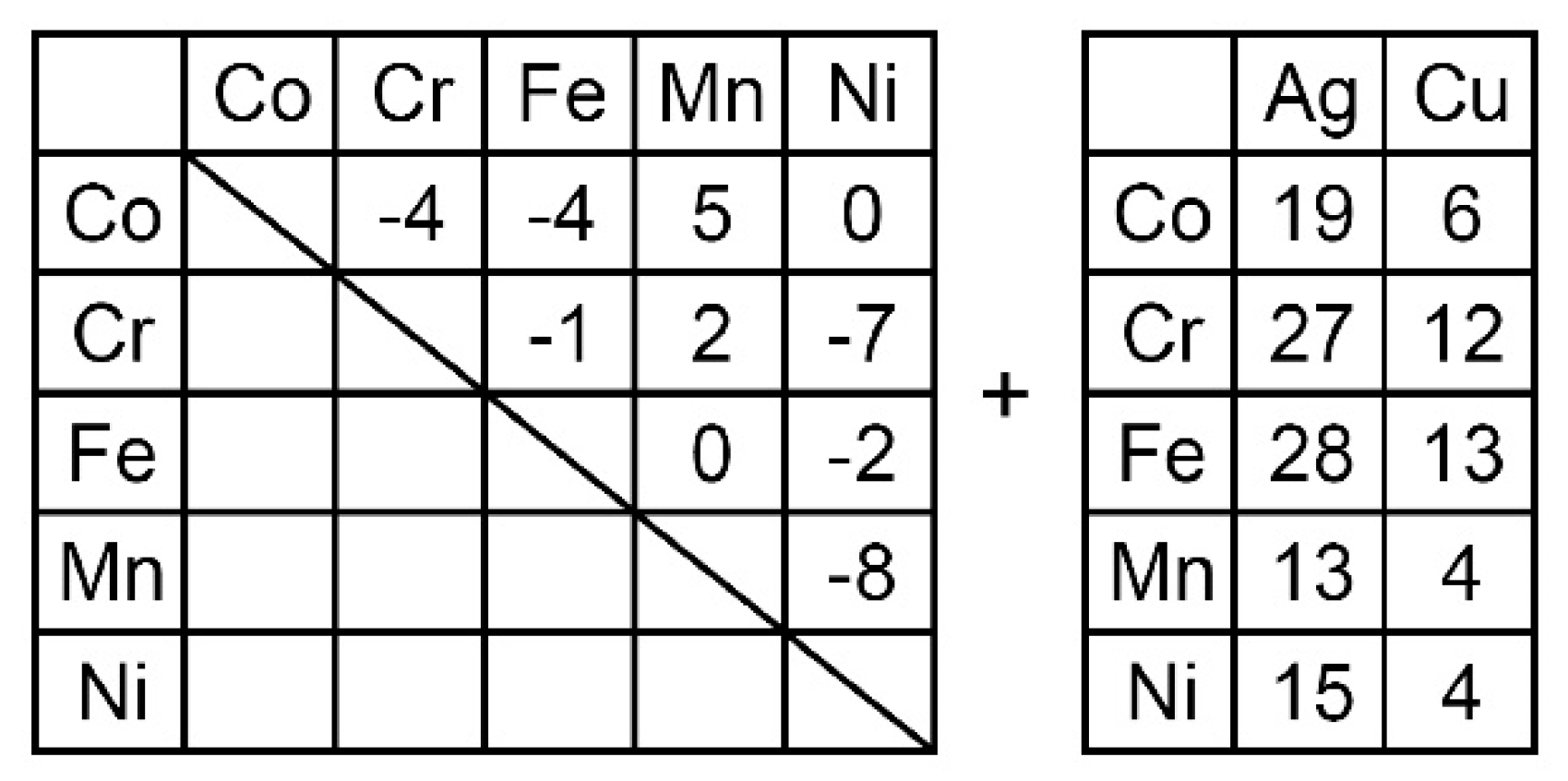
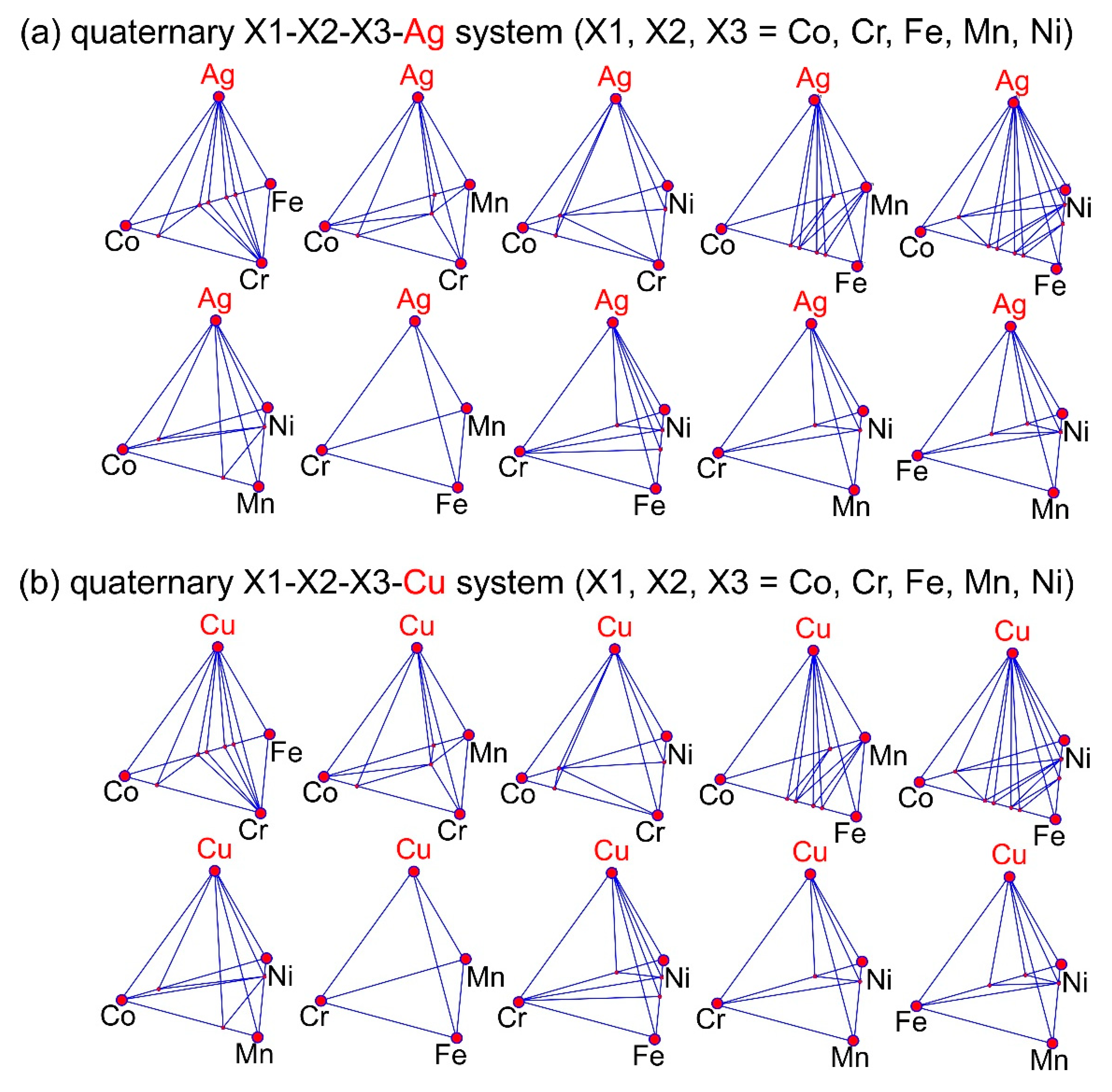
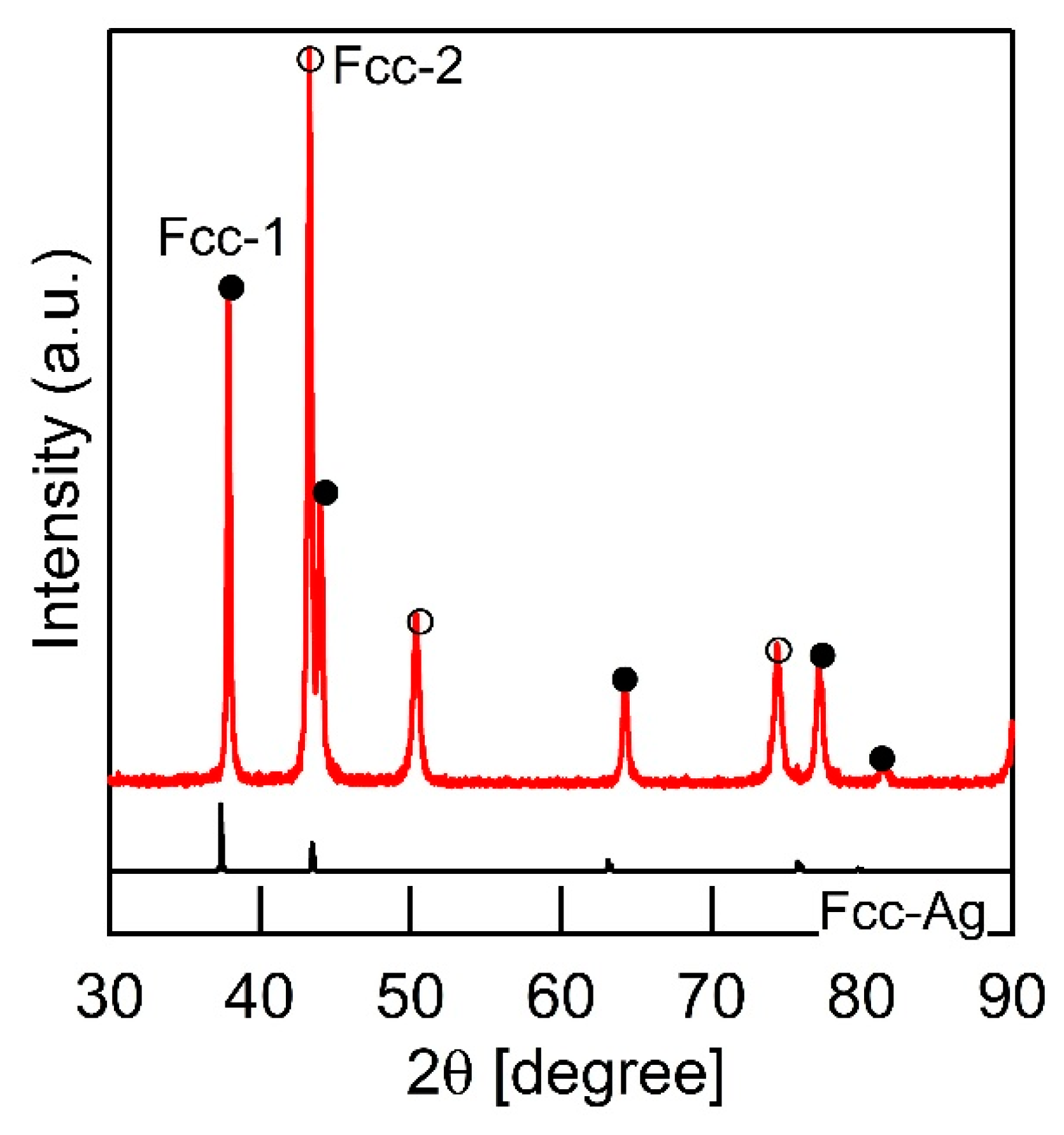
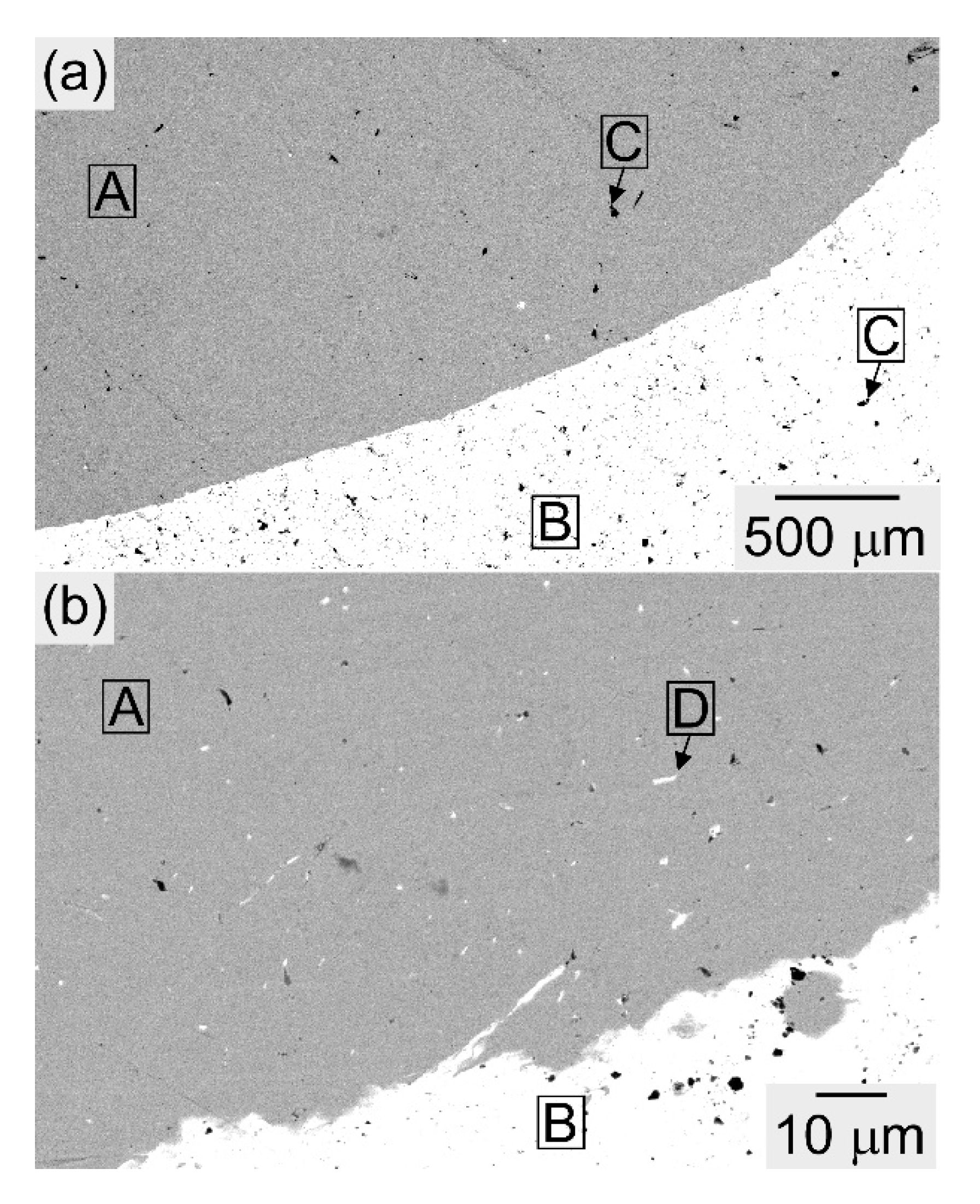
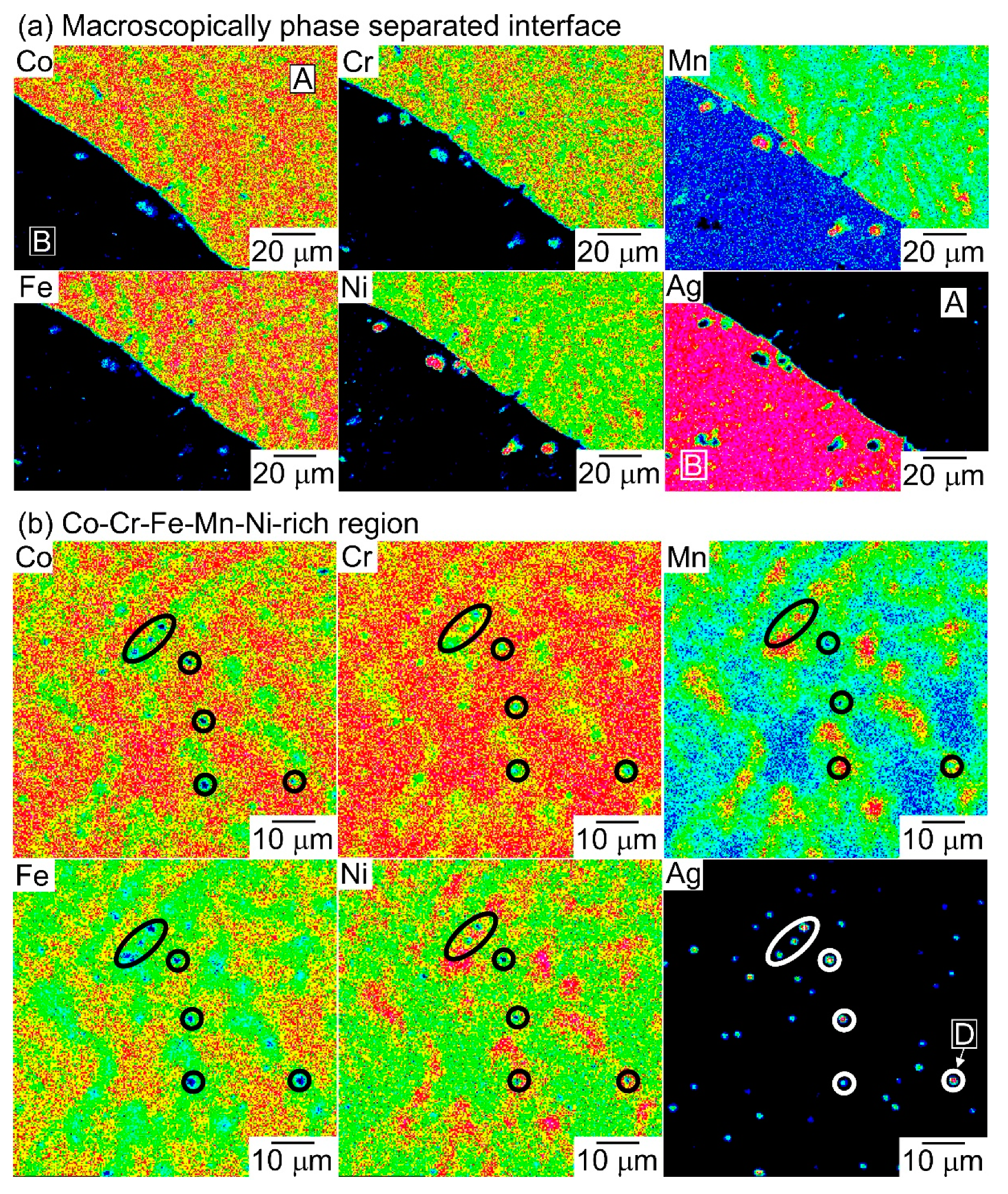
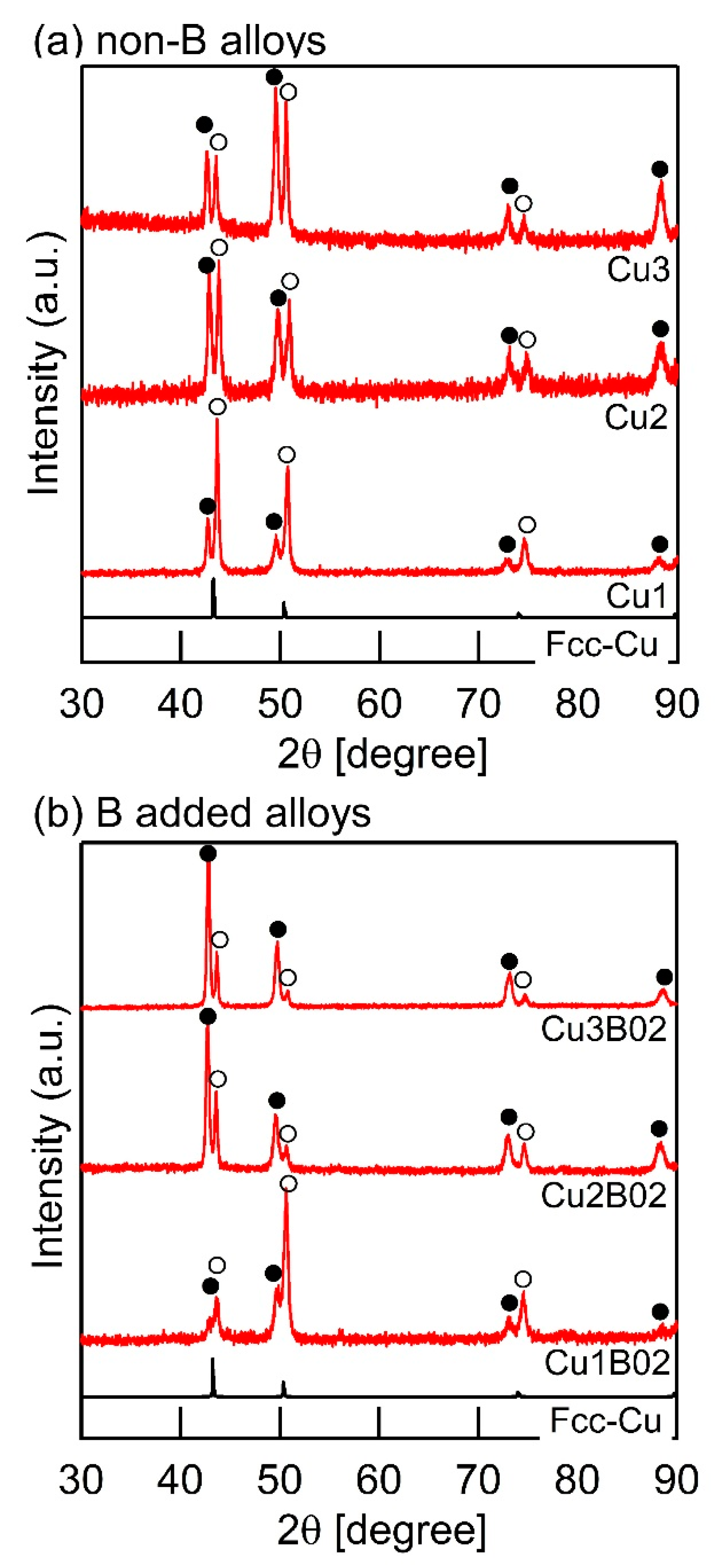
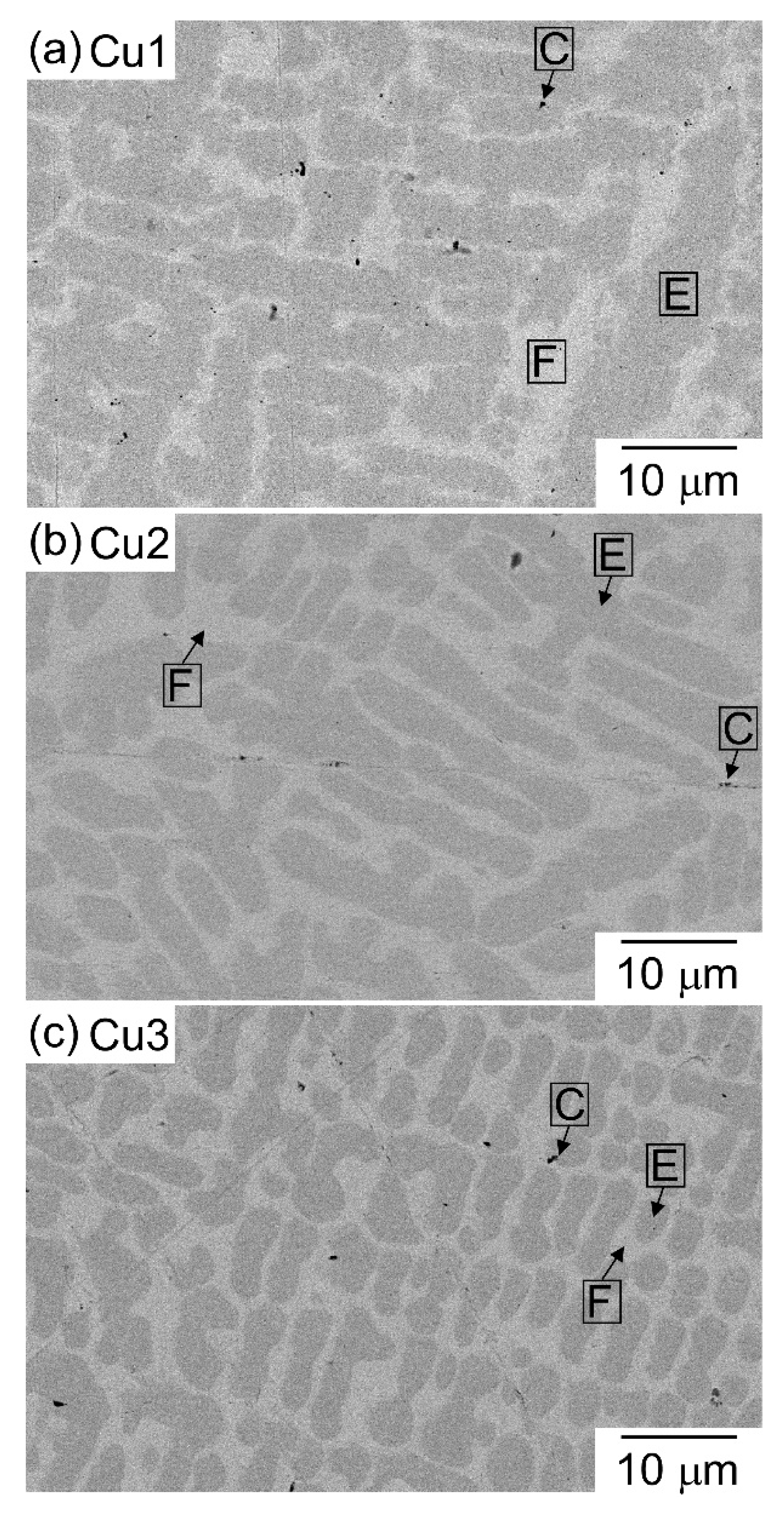
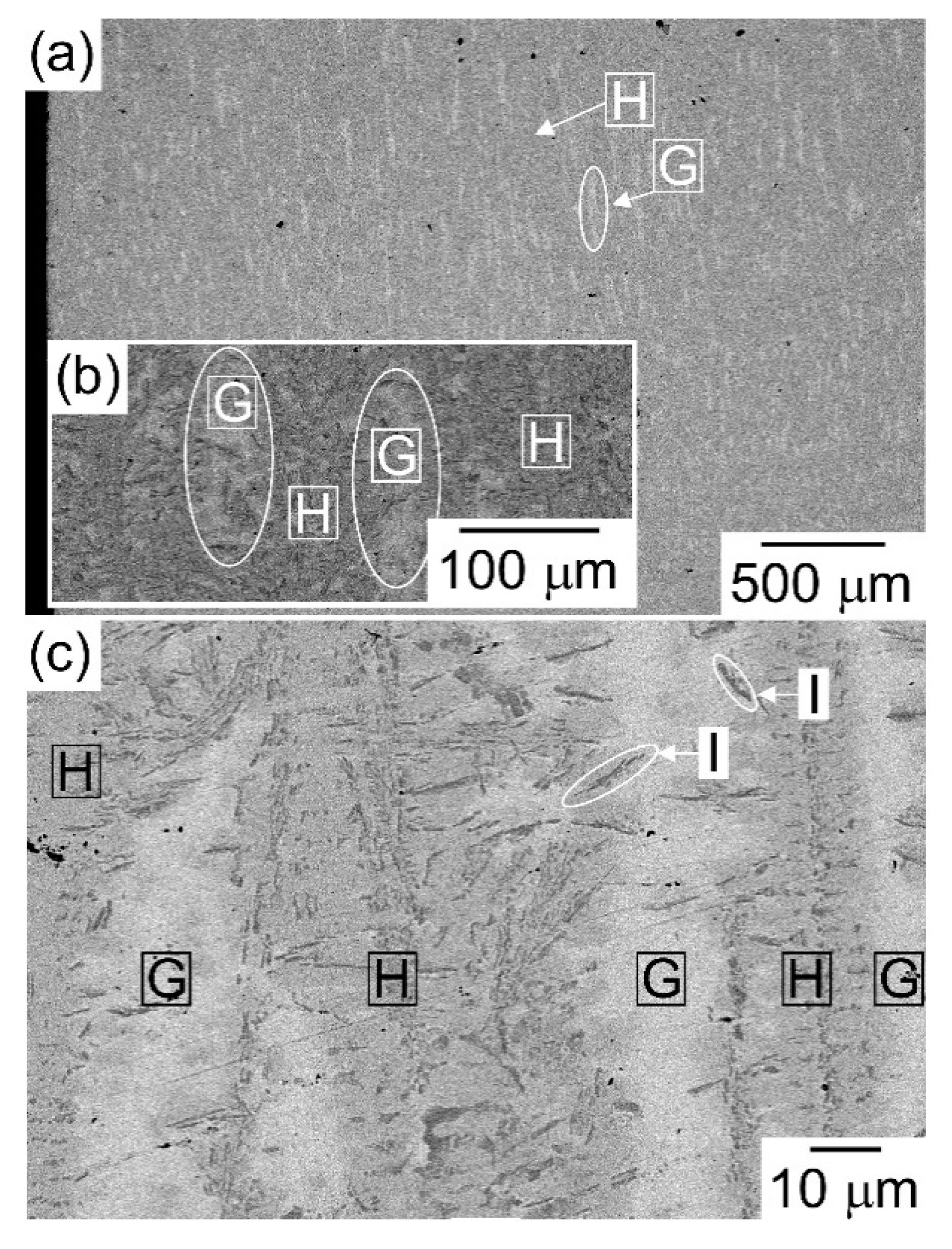
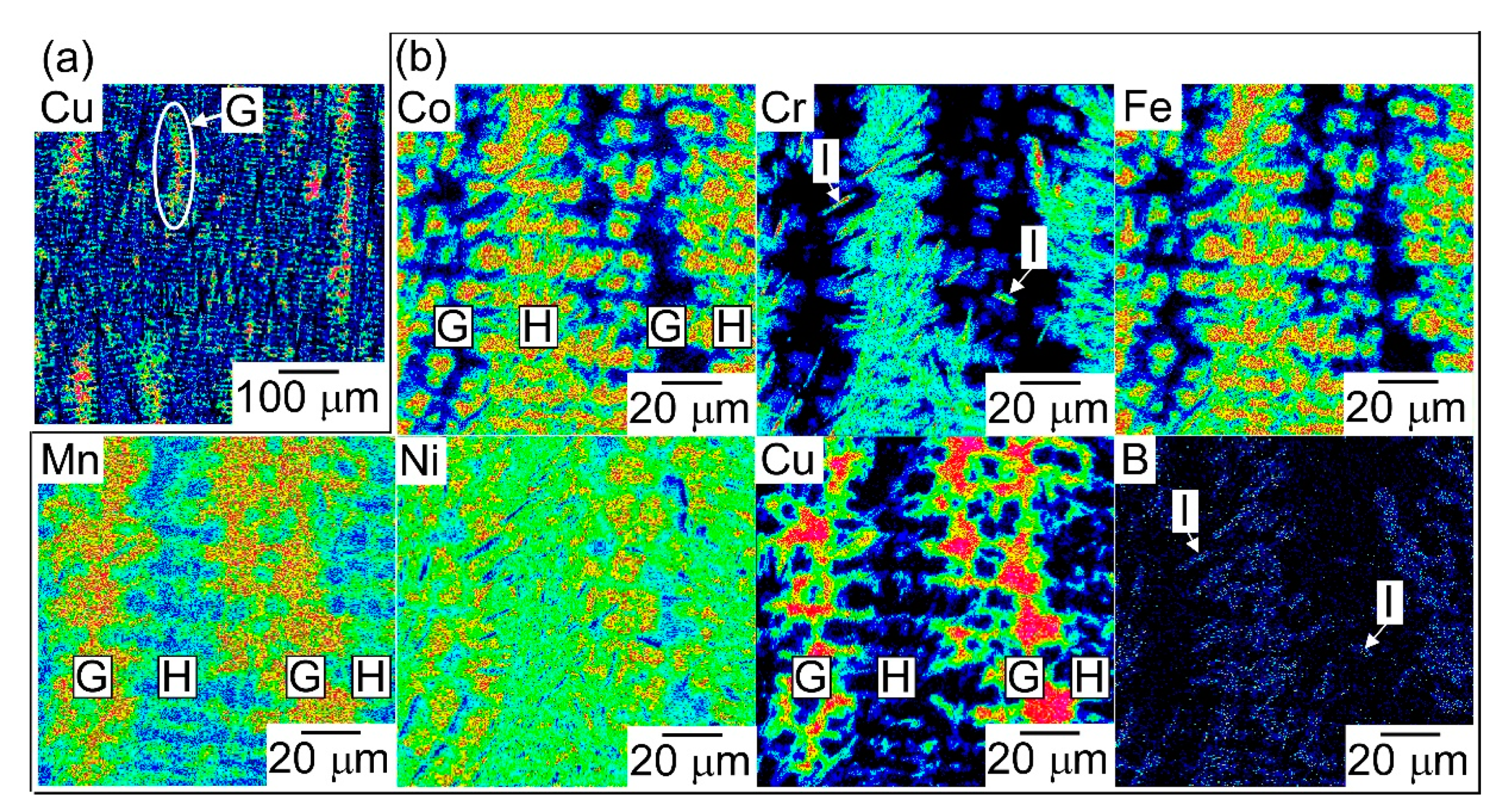
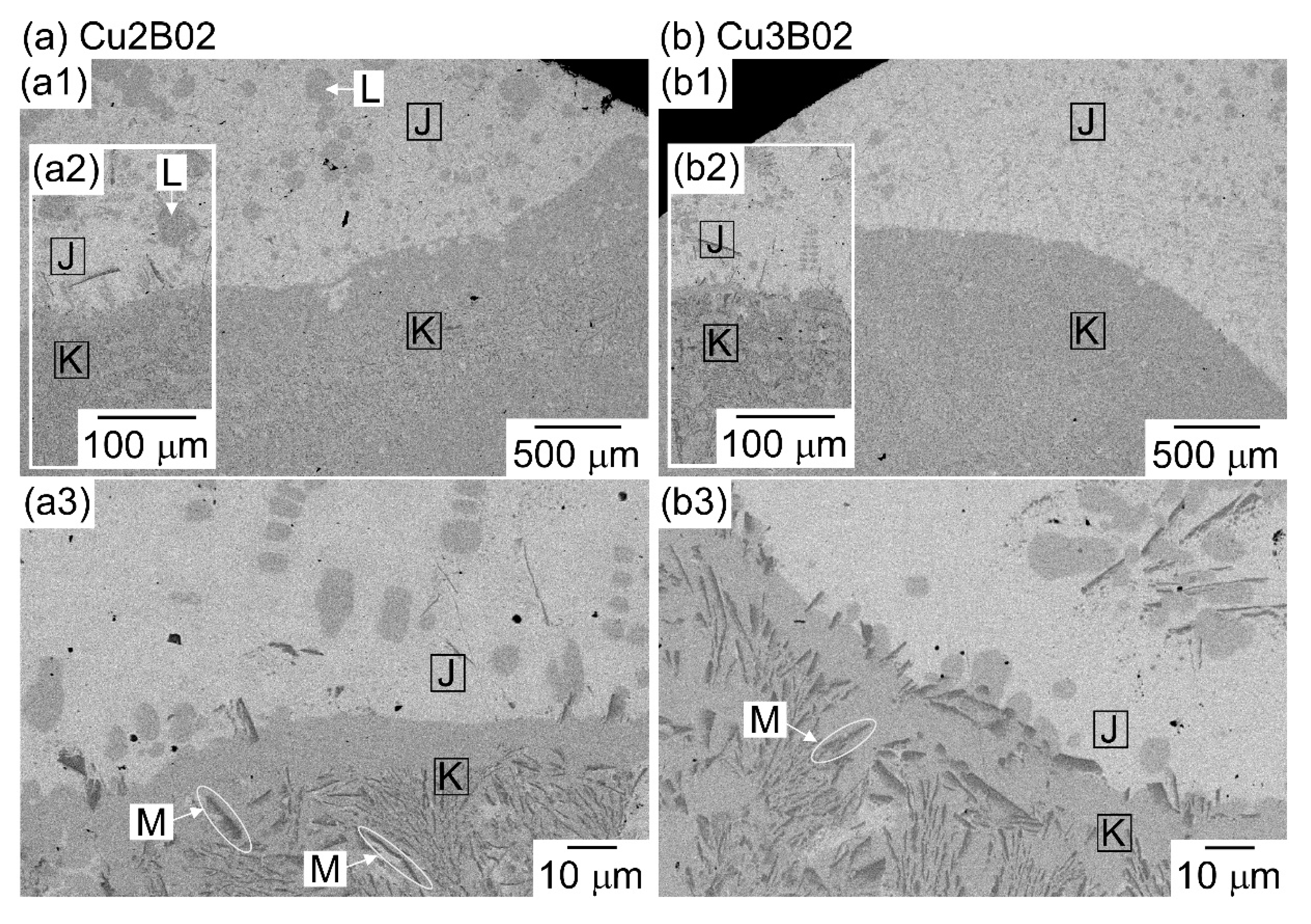
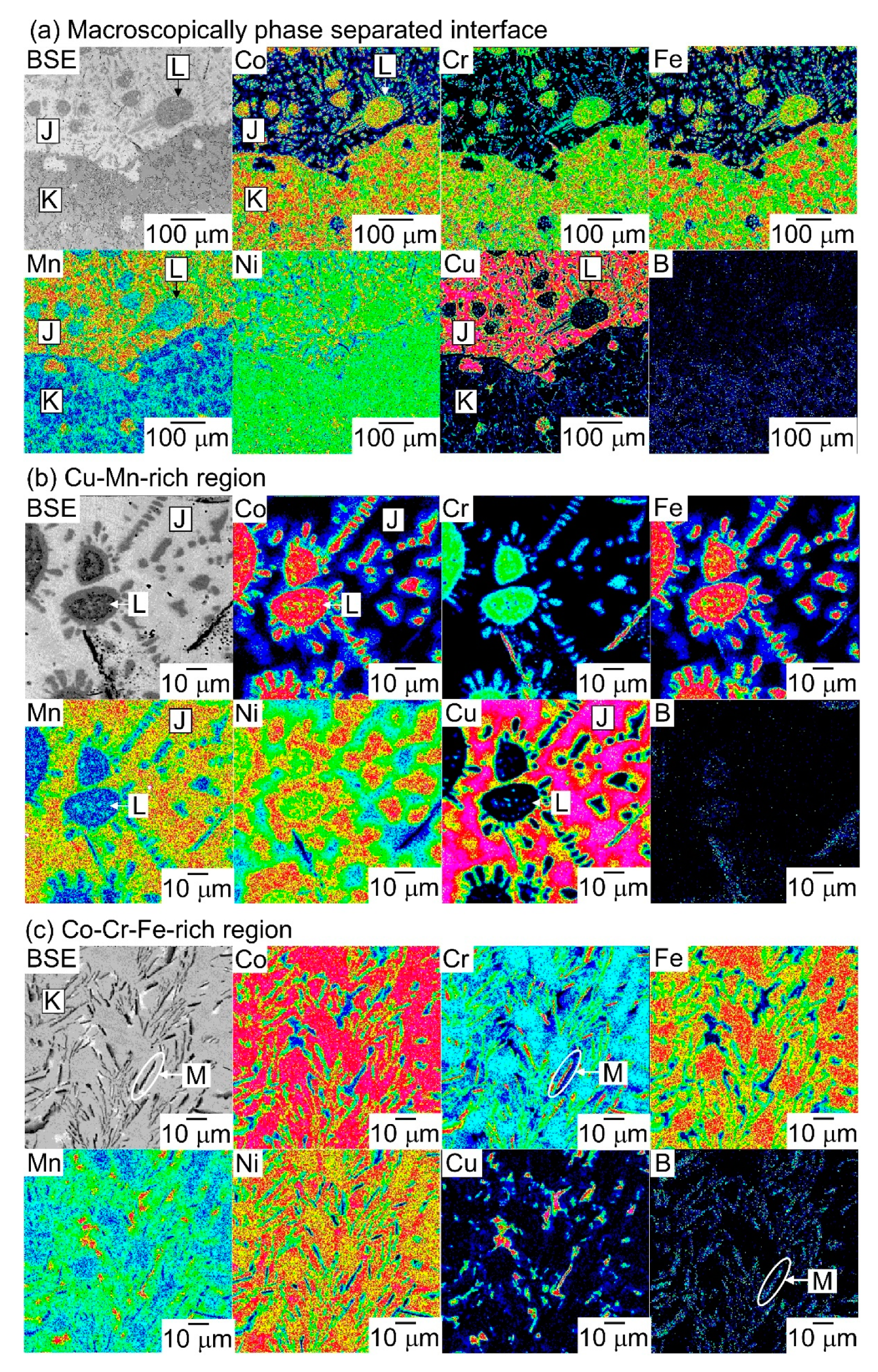


| (a) Atomic Ratio | ||||||||
| Alloy abbreviation | Ag | Co | Cr | Cu | Fe | Mn | Ni | B |
| Ag | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Cu1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Cu2 | 1 | 1 | 2 | 1 | 1 | 1 | ||
| Cu3 | 1 | 1 | 3 | 1 | 1 | 1 | ||
| Cu1B02 | 1 | 1 | 1 | 1 | 1 | 1 | 0.2 | |
| Cu2B02 | 1 | 1 | 2 | 1 | 1 | 1 | 0.2 | |
| Cu2B02 | 1 | 1 | 3 | 1 | 1 | 1 | 0.2 | |
| (b) Atomic Percent | ||||||||
| Alloy abbreviation | Ag | Co | Cr | Cu | Fe | Mn | Ni | B |
| Ag | 16.67 | 16.67 | 16.67 | 16.67 | 16.67 | 16.67 | ||
| Cu1 | 16.67 | 16.67 | 16.67 | 16.67 | 16.67 | 16.67 | ||
| Cu2 | 14.29 | 14.29 | 28.57 | 14.29 | 14.29 | 14.29 | ||
| Cu3 | 12.50 | 12.50 | 37.50 | 12.50 | 12.50 | 12.50 | ||
| Cu1B02 | 16.13 | 16.13 | 16.13 | 16.13 | 16.13 | 16.13 | 3.23 | |
| Cu2B02 | 13.89 | 13.89 | 27.78 | 13.89 | 13.89 | 13.89 | 2.78 | |
| Cu2B02 | 12.20 | 12.20 | 36.59 | 12.20 | 12.20 | 12.20 | 2.44 | |
| Position | Co | Cr | Cu | Fe | Mn | Ni |
|---|---|---|---|---|---|---|
| G | 2.9 | 1.8 | 57.3 | 2.2 | 23.2 | 12.7 |
| H | 21.1 | 20.7 | 9.1 | 22.1 | 10.5 | 16.4 |
| Position | Co | Cr | Cu | Fe | Mn | Ni |
|---|---|---|---|---|---|---|
| K | 22.9 | 21.5 | 6.6 | 24.2 | 9.5 | 15.2 |
| J | 2.1 | 1.2 | 64.9 | 1.6 | 20.1 | 10.0 |
| L | 21.2 | 24.3 | 7.9 | 21.5 | 10.7 | 14.5 |
| (a) Cu2B02, Cu-rich liquid | |||||||
| Temp. [K] | Co | Cr | Cu | Fe | Mn | Ni | B |
| 1500 | 4.5 | 3.3 | 67.8 | 3.6 | 13.1 | 7.2 | 0.4 |
| 1400 | 3.1 | 2.1 | 73.8 | 2.3 | 12.5 | 6.0 | 0.2 |
| (b) Cu2B02, Cu-poor liquid | |||||||
| Temp. [K] | Co | Cr | Cu | Fe | Mn | Ni | B |
| 1500 | 15.3 | 15.5 | 21.9 | 15.4 | 14.0 | 14.9 | 3.1 |
| 1400 | 16.7 | 16.9 | 15.9 | 16.9 | 14.3 | 15.9 | 3.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagase, T.; Todai, M.; Nakano, T. Liquid Phase Separation in Ag-Co-Cr-Fe-Mn-Ni, Co Cr-Cu-Fe-Mn-Ni and Co-Cr-Cu-Fe-Mn-Ni-B High Entropy Alloys for Biomedical Application. Crystals 2020, 10, 527. https://doi.org/10.3390/cryst10060527
Nagase T, Todai M, Nakano T. Liquid Phase Separation in Ag-Co-Cr-Fe-Mn-Ni, Co Cr-Cu-Fe-Mn-Ni and Co-Cr-Cu-Fe-Mn-Ni-B High Entropy Alloys for Biomedical Application. Crystals. 2020; 10(6):527. https://doi.org/10.3390/cryst10060527
Chicago/Turabian StyleNagase, Takeshi, Mitsuharu Todai, and Takayoshi Nakano. 2020. "Liquid Phase Separation in Ag-Co-Cr-Fe-Mn-Ni, Co Cr-Cu-Fe-Mn-Ni and Co-Cr-Cu-Fe-Mn-Ni-B High Entropy Alloys for Biomedical Application" Crystals 10, no. 6: 527. https://doi.org/10.3390/cryst10060527
APA StyleNagase, T., Todai, M., & Nakano, T. (2020). Liquid Phase Separation in Ag-Co-Cr-Fe-Mn-Ni, Co Cr-Cu-Fe-Mn-Ni and Co-Cr-Cu-Fe-Mn-Ni-B High Entropy Alloys for Biomedical Application. Crystals, 10(6), 527. https://doi.org/10.3390/cryst10060527