Study on Visible Light Catalysis of Graphite Carbon Nitride-Silica Composite Material and Its Surface Treatment of Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphite Carbon Nitride-Silica Composite Materials
2.3. Characterization of g-C3N4-SiO2
2.4. Surface Treatment of Cement with g-C3N4-SiO2
2.5. Evaluation of Visible Light Catalytic Performance of Photocatalytic Cement
2.6. Exploration of the Binding Mechanism of Graphite Carbon Nitride-Silicon Dioxide Composite Materials and Cement
3. Results and Discussion
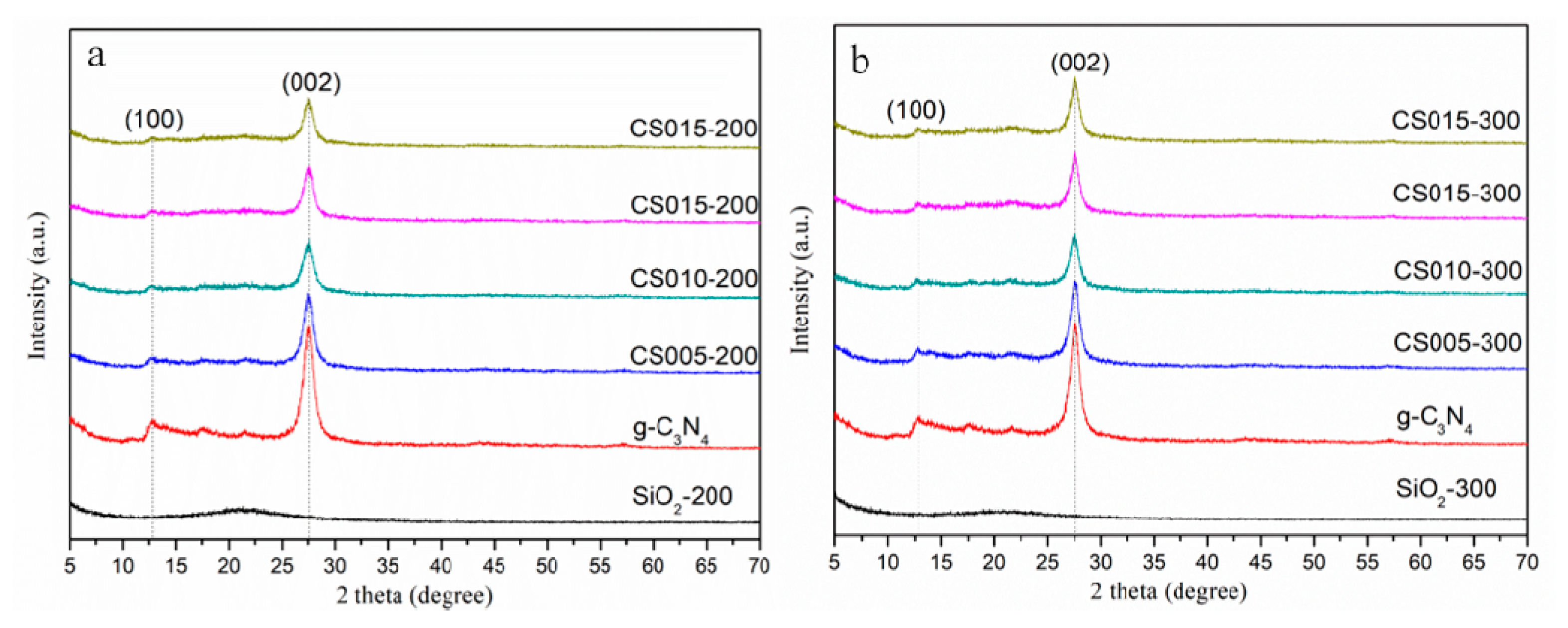
3.1. Crystal Structure
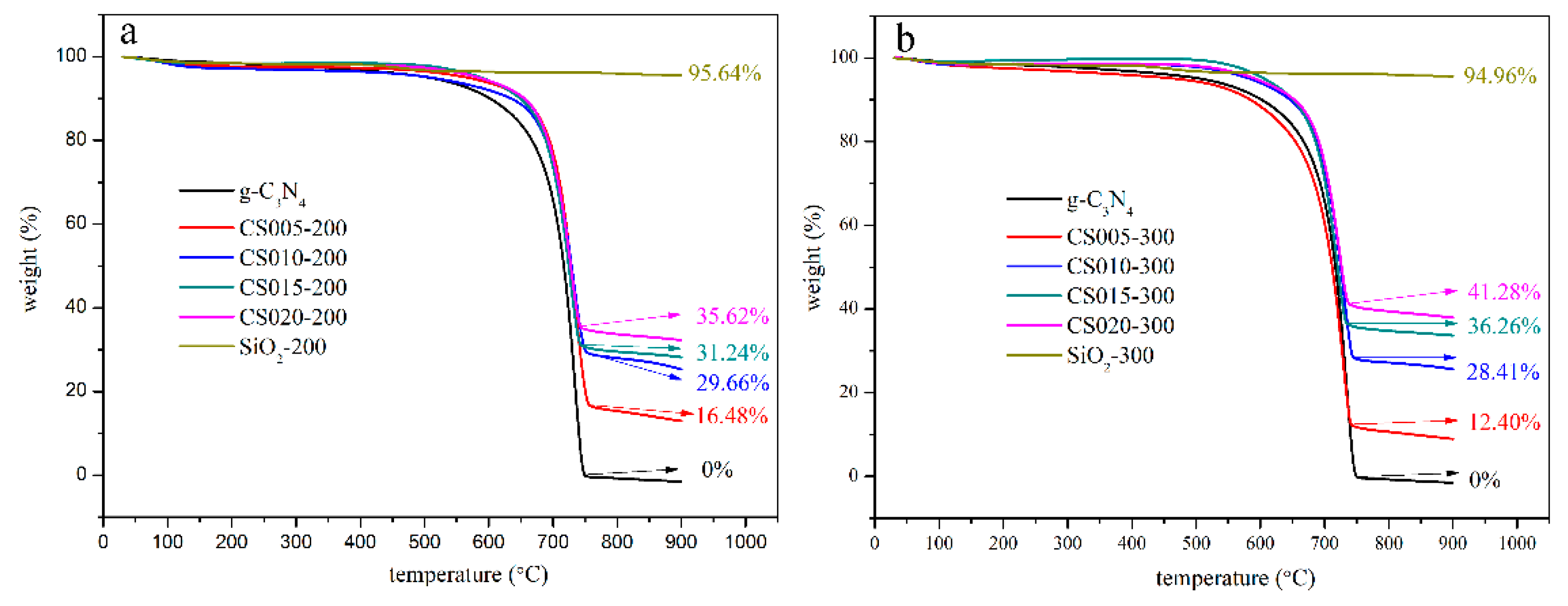
3.2. Composition
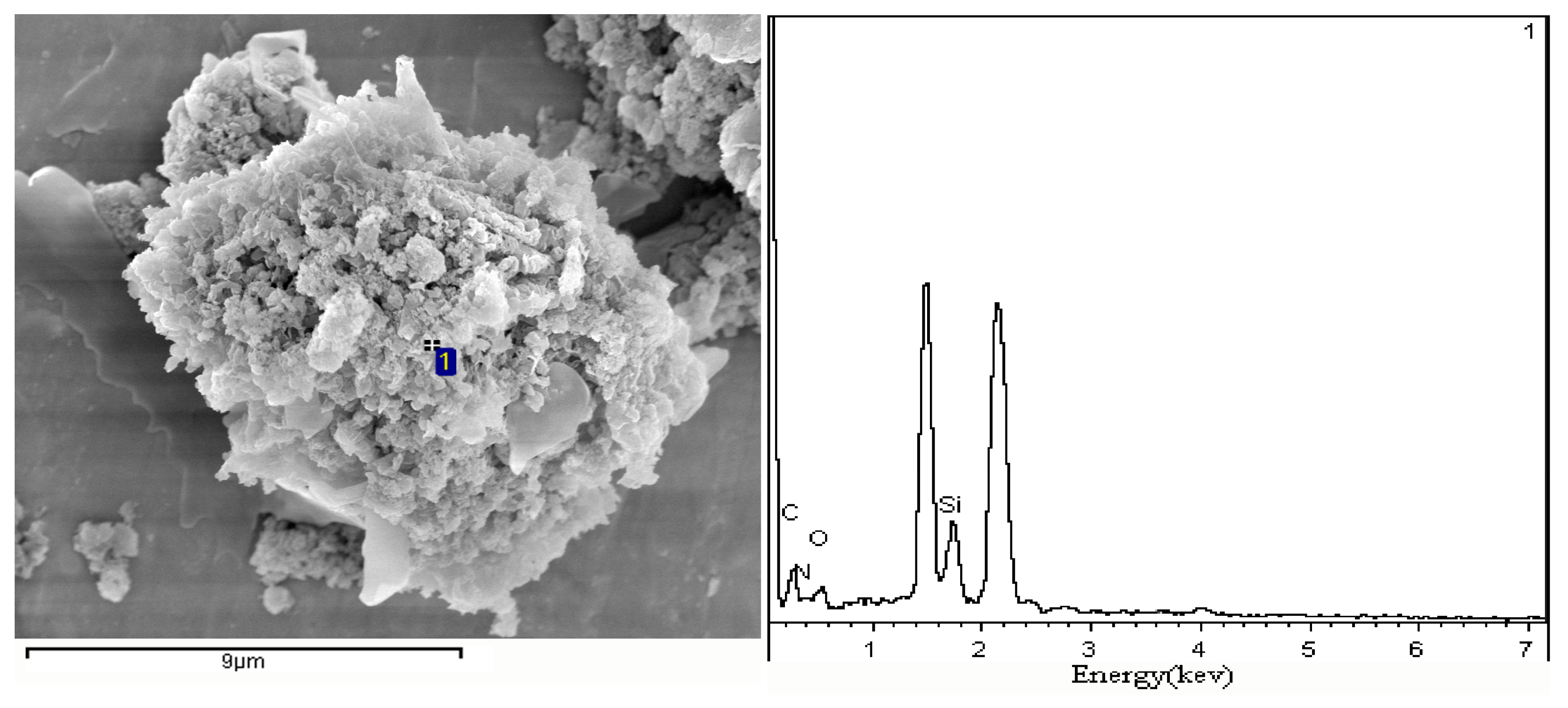
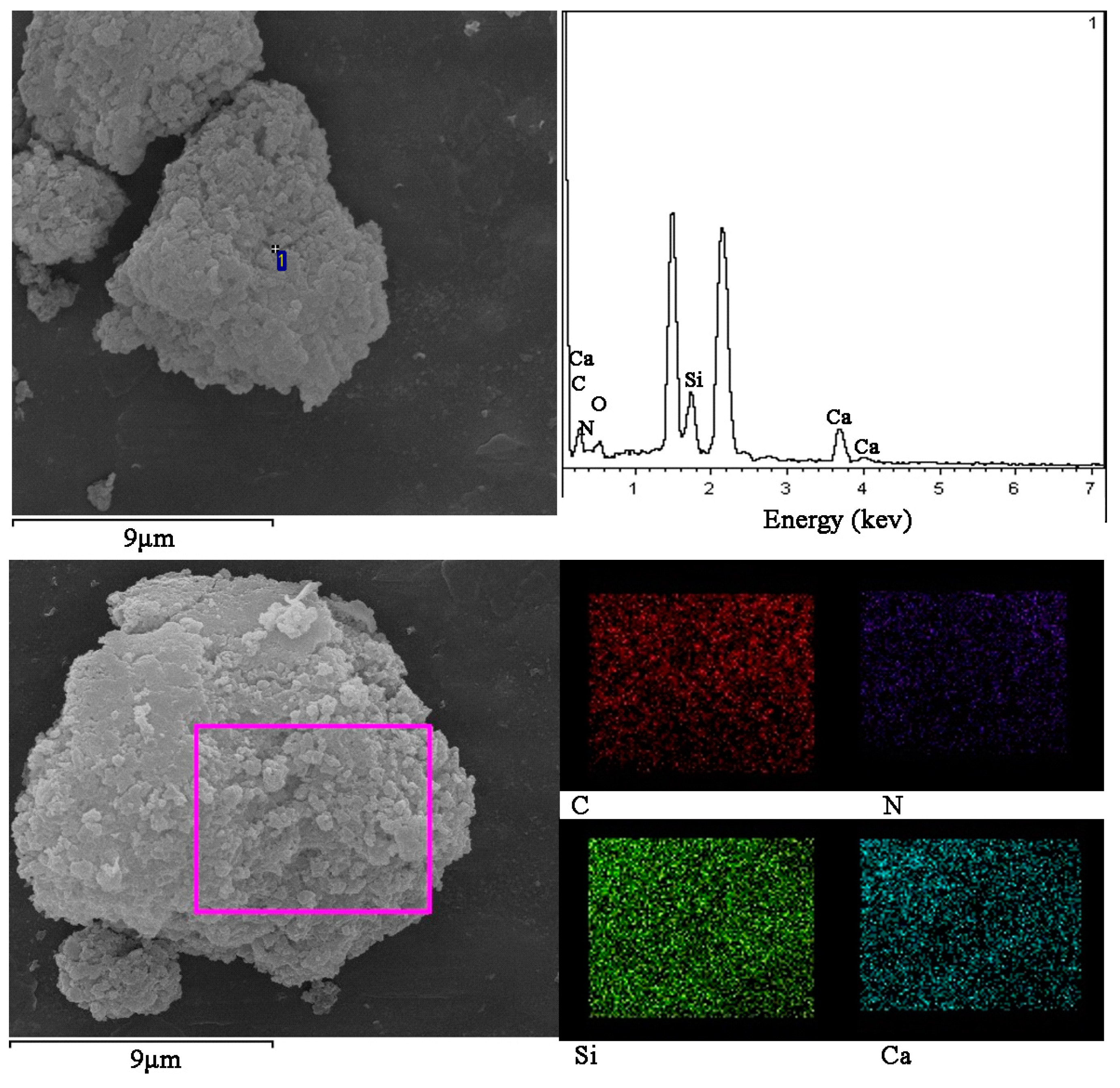
3.3. Surface Morphology and Structure
3.4. Specific Surface Area and Porosity
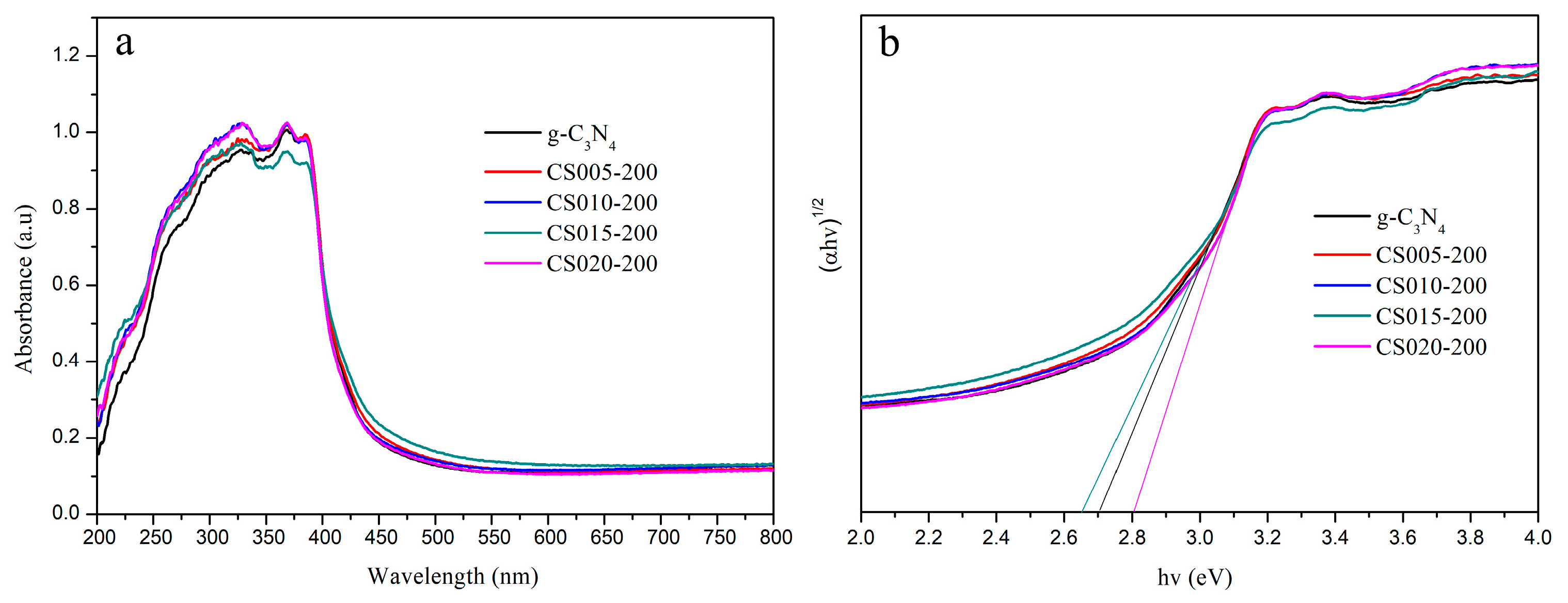
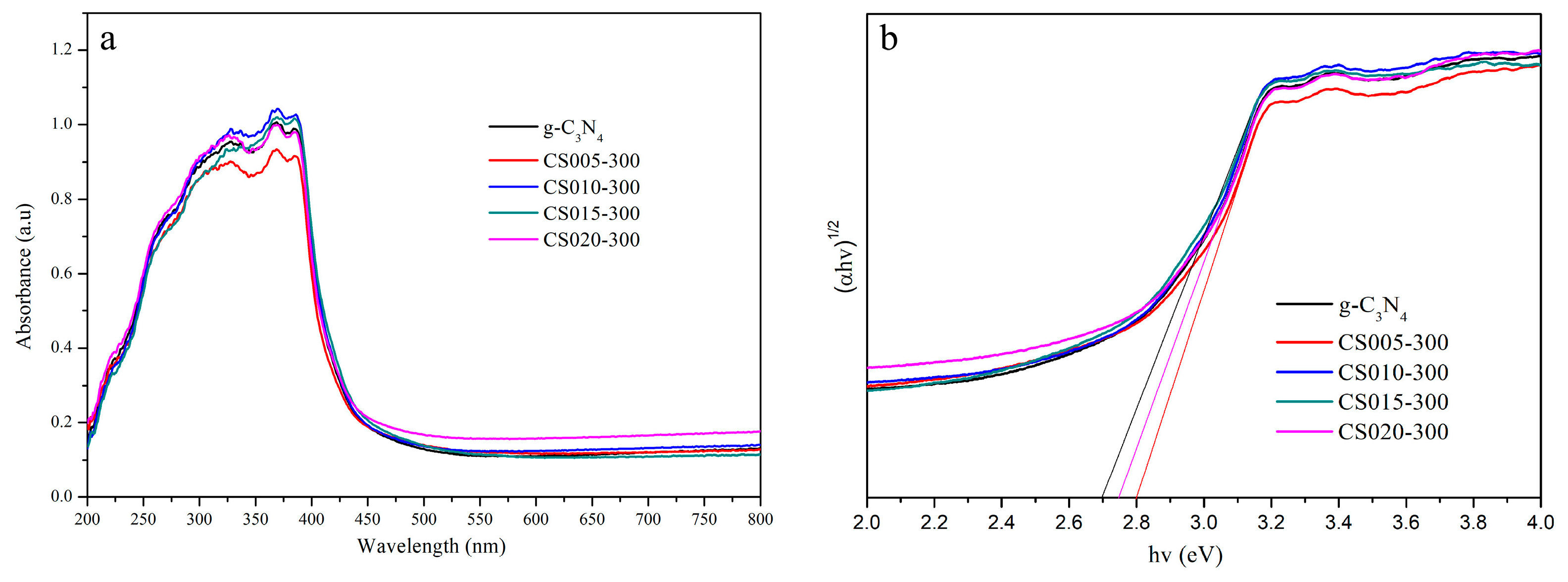
3.5. Band Gap and Absorption Edge
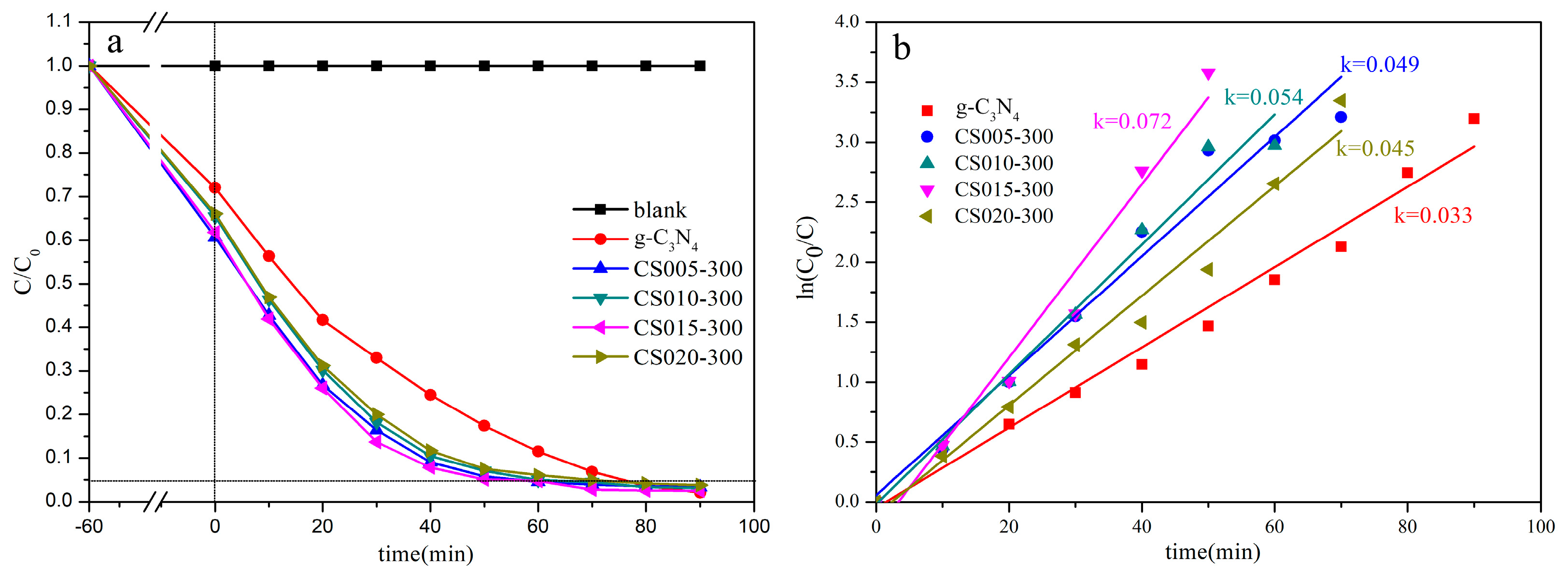
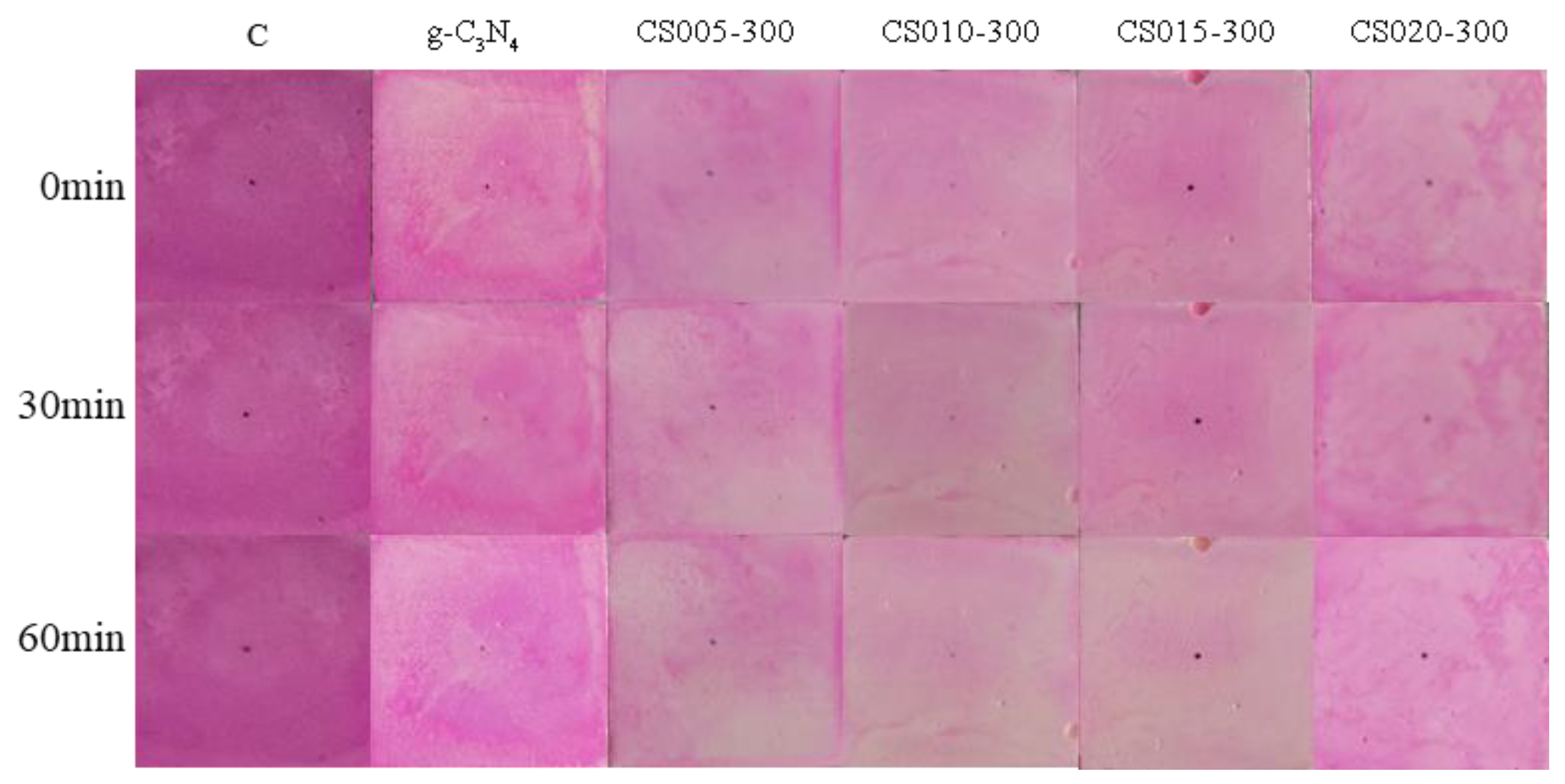
3.6. Photocatalytic Evaluation
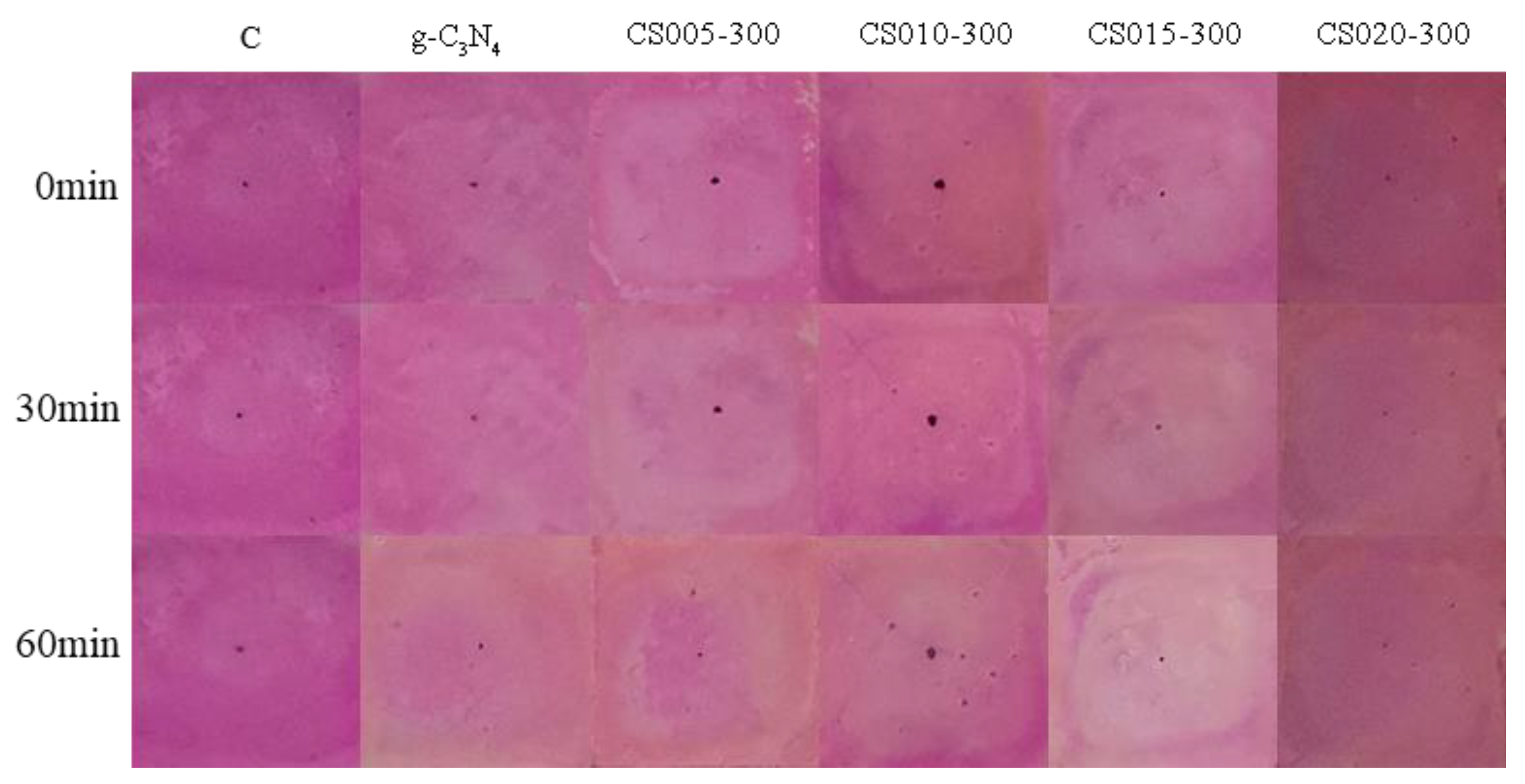
3.7. Surface Treatment Evaluation
3.8. Performance Evaluation of Photocatalytic Cement
3.9. Exploration of Binding Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jafari, H.; Afshar, S. Improved photodegradation of organic contaminants using nano-TiO2 and TiO2-SiO2 deposited on Portland cement concrete blocks. Photochem. Photobiol. 2016, 92, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Papoulis, D.; Kordouli, E.; Lampropoulou, P.; Rapsomanikis, A.; Kordulis, C.; Panagiotaras, D.; Theophylaktou, K.; Stathatos, E.; Komarneni, S. Characterization and photocatalytic activities of fly ash-TiO2 nanocomposites for the mineralization of azo dyes in water. J. Surf. Interfaces Mater. 2014, 2, 261–266. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.H.; Du, A.J.; Fu, W.; Sun, D.D.; Leckie, J.O. Combination of one-dimensional TiO2 nanowire photocatalytic oxidation with microfiltration for water treatment. Water Res. 2009, 43, 1186. [Google Scholar] [CrossRef] [PubMed]
- Canterino, M.; Somma, I.D.; Marotta, R.; Andreozzi, R.; Caprio, V. Energy recovery in wastewater decontamination: Simultaneous photocatalytic oxidation of an organic substrate and electricity generation. Water Res. 2009, 43, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Huesken, G.; Hunger, M.; Brouwers, H.J.H. Experimental study of photocatalytic concrete products for air purification. Build Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- Lee, B.Y.; Jayapalan, A.R.; Bergin, M.H.; Kurtis, K.E. Photocatalytic cement exposed to nitrogen oxides: Effect of oxidation and binding. Cem Concr. Res. 2014, 60, 30–36. [Google Scholar] [CrossRef]
- Maggos, T.; Plassais, A.; Bartzis, J.G.; Vasilakos, C.; Moussiopoulos, N.; Bonafous, L. Photocatalytic degradation of NOx in a pilot street canyon configuration using TiO2-mortar panels. Environ. Monit. Assess. 2008, 136, 35–44. [Google Scholar] [CrossRef]
- Chen, M.; Chu, J. NOx photocatalytic degradation on active concrete road surface-from experiment to real -scale application. J. Clean. Prod. 2011, 19, 1266–1272. [Google Scholar] [CrossRef]
- Lin, H.J.; Yang, T.S.; Hsi, C.S.; Wang, M.C.; Lee, K.C. Optical and photocatalytic properties of Fe3+-doped TiO2 thin films prepared by a sol-gel spin coating. Ceram. Int. 2014, 40, 10633–10640. [Google Scholar] [CrossRef]
- Cheng, X.; Gotoh, K.; Nakagawa, Y.; Usami, N. Effect of substrate type on the electrical and structural properties of TiO2 thin films deposited by reactive DC sputtering. J. Cryst. Growth 2018, 491, 120–125. [Google Scholar] [CrossRef]
- Chen, X.B. Titanium dioxide nanomaterials and their energy applications. Chin. J. Catal. 2009, 30, 830–851. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Sikora, P.; Horszczaruk, E.; Rucinska, T. The effect of nanosilica and titanium dioxide on the mechanical and self-cleaning properties of water-glass cement mortar. Procedia Eng. 2015, 108, 146–153. [Google Scholar] [CrossRef]
- Mendoza, C.; Valle, A.; Castellote, M.; Bahamonde, A.; Faraldos, M. TiO2 and TiO2-SiO2 coated cement: Comparison of mechanic and photocatalytic properties. Appl. Catal. B 2014, 178, 155–164. [Google Scholar] [CrossRef]
- Umar, I.G.; Abdul, H.A. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar]
- Chen, J.; Kou, S.C.; Poon, C. Photocatalytic cement-based materials: Comparison of nitrogen oxides and toluene removal potentials and evaluation of self-cleaning performance. Build Environ. 2011, 46, 1827–1833. [Google Scholar] [CrossRef]
- Ye, Q.; Mo, R.H.; Yu, Y.C.; Li, G.H.; Huang, Z.Z. Application of polymer cement mortar modified with nitrogen-doped nano-TiO2 photocatalytic material. New Build. Mater. 2009, 36, 15–17. [Google Scholar]
- Gao, J.W.; Yang, H.; Shen, Q.H. Research progress of TiO2 in green building materials. J. Ceram. 2007, 28, 237–239. [Google Scholar]
- Wang, C.M.; Shi, H.S.; Li, Y. Research progress of nano-TiO2 photocatalytic functional building materials. New Chem. Mater. 2011, 39, 10–12. [Google Scholar]
- Dong, R.; Shen, W.G.; Zhong, J.B.; Liao, G.; Chen, H.; Tan, Y. Research progress of photocatalytic self-cleaning concrete. Concrete 2011, 8, 62–65. [Google Scholar]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of nano-TiO2 on the mechanical properties of cement mortar. Constr. Build Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Peng, F.P.; Ni, Y.R.; Zhou, Q.; Kou, J.H.; Lu, C.H.; Xue, Z.Z. New g-C3N4 based photocatalytic cement with enhanced visible-light photocatalytic activity by constructing muscovite sheet/SnO2 structures. Constr. Build Mater. 2018, 179, 315–325. [Google Scholar] [CrossRef]
- Bossa, N.; Chaurand, P.; Levard, C.; Borschneck, D.; Miche, H.; Vicente, J.; Geantet, C.; Aguerre-Chariol, O.; Michel, F.M.; Rose, J.; et al. Environmental exposure to TiO2 nanomaterials incorporated in building material. Environ. Pollut. 2016, 220 Pt B, 1160–1170. [Google Scholar] [CrossRef]
- Xu, J.; Teng, Y.; Teng, F. Effect of surface defect states on valence band and charge separation and transfer efficiency. Sci. Rep. 2016, 6, 32457. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Liu, Z.; Zhang, A.; Li, M. Photocatalytic performances of Ag3PO4 polypods for degradation of dye pollutant under natural indoor weak light irradiation. Environ. Sci. Technol. 2015, 49, 9489–9494. [Google Scholar] [CrossRef]
- Teng, F.; Chen, M.; Li, N.; Hua, X.; Wang, K.; Xu, T. Effect of TiO2 surface structure on the hydrogen production activity of the Pt@CuO/TiO2 photocatalysts for water splitting. Chem. Cat. Chem. 2014, 6, 842–847. [Google Scholar]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Yan, H.J.; Yang, H.X. TiO2-g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. J. Alloys Compd. 2011, 509, 126–129. [Google Scholar] [CrossRef]
- Kang, H.W.; Lim, S.N.; Song, D.; Park, S.B. Organic-inorganic composite of g-C3N4-SrTiO3: RH photocatalyst for improved H2 evolution under visible light irradiation. Int. J. Hydrogen Energy 2012, 37, 11602–11610. [Google Scholar] [CrossRef]
- Fu, Q.; Jiu, J.T.; Cai, K.; Wang, H.; Cao, C.B.; Zhu, H.S. Attempt to deposit carbon nitride films by electrodeposition from an organic liquid. Phys. Rev. B 1999, 59, 1693–1696. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhao, H.; Wang, H. Atomic single layer graphitic-C3N4: Fabrication and its high photocatalytic performance under visible light irradiation. Rsc. Adv. 2014, 4, 624–628. [Google Scholar] [CrossRef]
- Sun, X.D.; Li, Y.Y.; Zhou, J.; Ma, C.H.; Wang, Y.; Zhua, J.H. Facile synthesis of high photocatalytic active porous g-C3N4 with ZnCl2 template. J. Colloid Interface Sci. 2015, 451, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, W.; Dong, X.A.; Wang, H.; Dong, F. In situ FT-IR investigation on the reaction mechanism of visible light photocatalytic NO oxidation with defective g-C3N4. Sci. Bull. 2018, 63, 117–125. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Cen, W.; Sun, Y.; Lee, S.C.; Dong, F. Steering the interlayer energy barrier and charge flflow via bioriented transportation channels in g-C3N4: Enhanced photocatalysis and reaction mechanism. J. Catal. 2017, 352, 351–360. [Google Scholar] [CrossRef]
- Cui, W.; Li, J.; Dong, F.; Sun, Y.; Jiang, G.; Cen, W.; Lee, S.C.; Wu, Z. Highly effificient performance and conversion pathway of photocatalytic NO oxidation on SrO@clusters amorphous carbon nitride. Environ. Sci. Technol. 2017, 51, 10682–10690. [Google Scholar] [CrossRef]
- Shi, L. Preparation and Properties of Carbon Nitride-Based Photocatalytic Materials. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2017. [Google Scholar]
- Liao, G.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Graphene oxide modifified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. J. Mater. Chem. 2012, 22, 2721–2726. [Google Scholar] [CrossRef]
- Hao, Q.; Niu, X.; Nie, C.; Hao, S.; Zou, W.; Ge, J.; Chen, D.; Yao, W. A highly efficient g-C3N4/SiO2 heterojunction: The role of SiO2 in the enhancement of visible light photocatalytic activity. Phys. Chem. Chem. Phys. 2016, 18, 31410–31418. [Google Scholar] [CrossRef]
- Wang, D.; Yang, P.; Hou, P.K.; Zhang, L.N.; Zhang, X.Z.; Zhou, Z.H.; Xie, N.; Huang, S.F.; Cheng, X. Cement-based composites endowed with novel functions through controlling interface microstructure from Fe3O4@SiO2 nanoparticles. Cem. Con. Com. 2017, 80, 268–276. [Google Scholar] [CrossRef]
- Lin, B.; Xue, C.; Yan, X.; Yang, G.; Yang, G.; Yang, B. Facile fabrication of novel SiO2/g-C3N4 core-shell nanosphere photocatalysts with enhanced visible light activity. Appl. Surf. Sci. 2015, 357, 346–355. [Google Scholar] [CrossRef]
- Lei, J.; Ying, C.; Wang, L.; Liu, Y.D.; Zhang, J.L. Highly condensed g-C3N4-modified TiO2 catalysts with enhanced photo degradation performance toward acid orange 7. J. Mater. Sci. 2015, 50, 3467–3476. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Chang, H.L.; Liu, X.; Ye, S.X.; Shu, X.; Ran, Q.P. The significance of dispersion of nano-SiO2 on early age hydration of cement paste. Mater. Des. 2020, 186, 108320. [Google Scholar] [CrossRef]
- Shen, W.Z.; Ren, L.W.; Zhou, H.; Zhang, S.; Fan, W. Facile one-pot synthesis of bimedal mesepormm carbon nitride and its function as a lipase immobilization support. J. Mater. Chem. 2011, 21, 3890–3894. [Google Scholar] [CrossRef]
- Wang, X.X.; Wang, S.S.; Hu, W.D.; Cai, J.; Zhang, L.; Dong, L. Synthesis and photocatalytic activity of SiO2/g-C3N4 composite photocatalyst. Mater. Lett. 2014, 115, 53–66. [Google Scholar] [CrossRef]
- Tolman, R.C. The effect of droplet size on surface tension. J. Chem. Phys. 2004, 17, 333–337. [Google Scholar] [CrossRef]
- Khandaker, M.; Anwar, H. Volcanic ash and pumice as cement additives: Pozzolanic, alkali-silica reaction and autoclave expansion characteristics. Cem. Concr. Res. 2005, 35, 1141–1144. [Google Scholar]
- Wang, D.; Yang, P.; Hou, P.K.; Cheng, X. BiOBr@SiO2 flower-like nanospheres chemically-bonded on cement-based materials for photocatalysis. App. Surf. Sci. 2018, 30, 539–548. [Google Scholar]

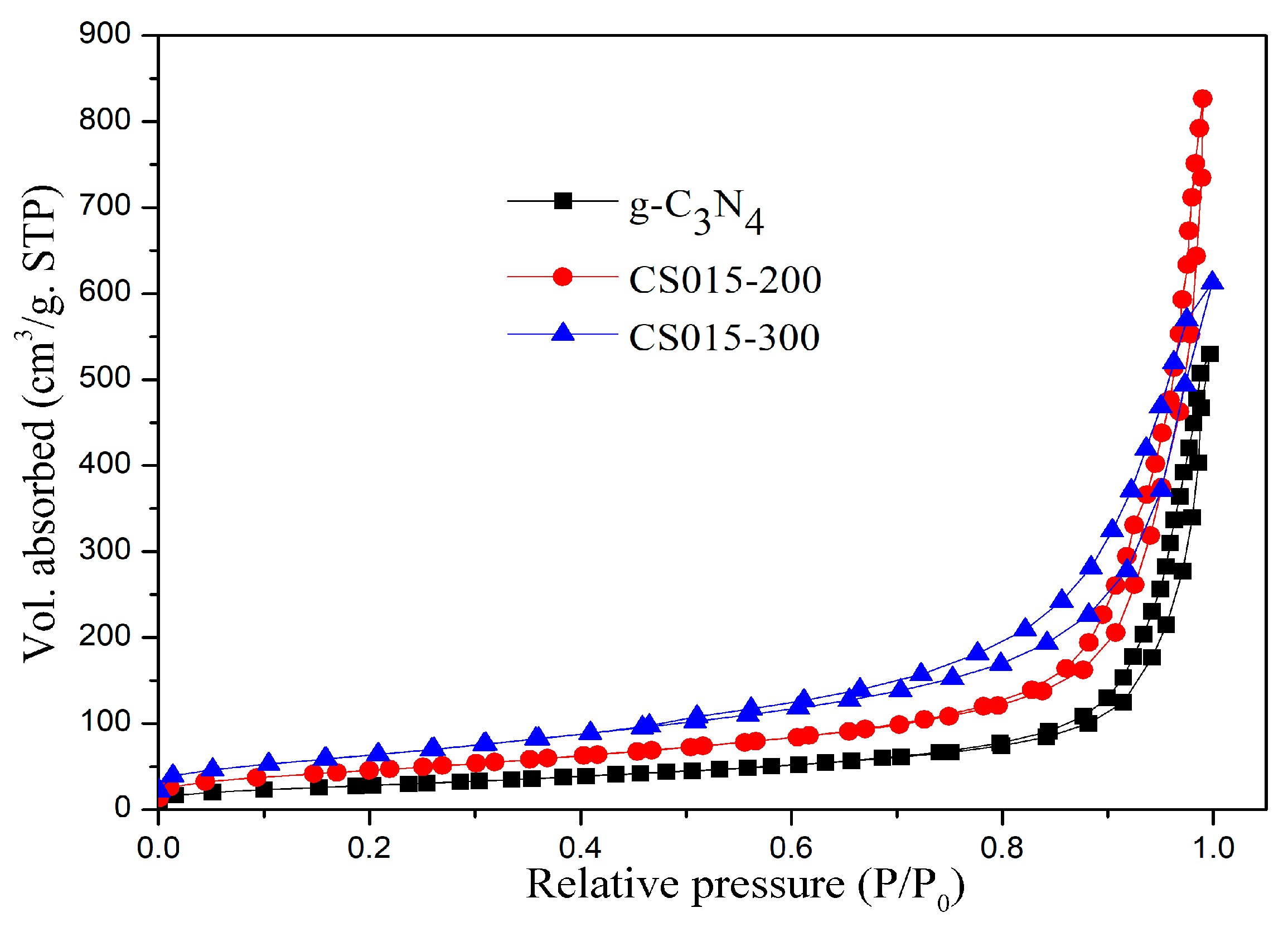




| Sample | Specific Surface Area | Total Pore Volume | Average Hole Radius |
|---|---|---|---|
| g-C3N4 | 105.45 m2/g | 0.81 cm3/g | 15.53 nm |
| CS015-200 | 170.49 m2/g | 1.27 cm3/g | 15.00 nm |
| CS015-300 | 238.68 m2/g | 0.94 cm3/g | 7.30 nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.; Wang, D.; Jiang, C.; Lu, X.; Zhang, L.; Cheng, X. Study on Visible Light Catalysis of Graphite Carbon Nitride-Silica Composite Material and Its Surface Treatment of Cement. Crystals 2020, 10, 490. https://doi.org/10.3390/cryst10060490
Zhong W, Wang D, Jiang C, Lu X, Zhang L, Cheng X. Study on Visible Light Catalysis of Graphite Carbon Nitride-Silica Composite Material and Its Surface Treatment of Cement. Crystals. 2020; 10(6):490. https://doi.org/10.3390/cryst10060490
Chicago/Turabian StyleZhong, Weiguang, Dan Wang, Congcong Jiang, Xiaolei Lu, Lina Zhang, and Xin Cheng. 2020. "Study on Visible Light Catalysis of Graphite Carbon Nitride-Silica Composite Material and Its Surface Treatment of Cement" Crystals 10, no. 6: 490. https://doi.org/10.3390/cryst10060490
APA StyleZhong, W., Wang, D., Jiang, C., Lu, X., Zhang, L., & Cheng, X. (2020). Study on Visible Light Catalysis of Graphite Carbon Nitride-Silica Composite Material and Its Surface Treatment of Cement. Crystals, 10(6), 490. https://doi.org/10.3390/cryst10060490





