About the Role of Fluorine-Bearing Apatite in the Formation of Oxalate Kidney Stones
Abstract
1. Introduction
2. Materials and Methods
3. Results
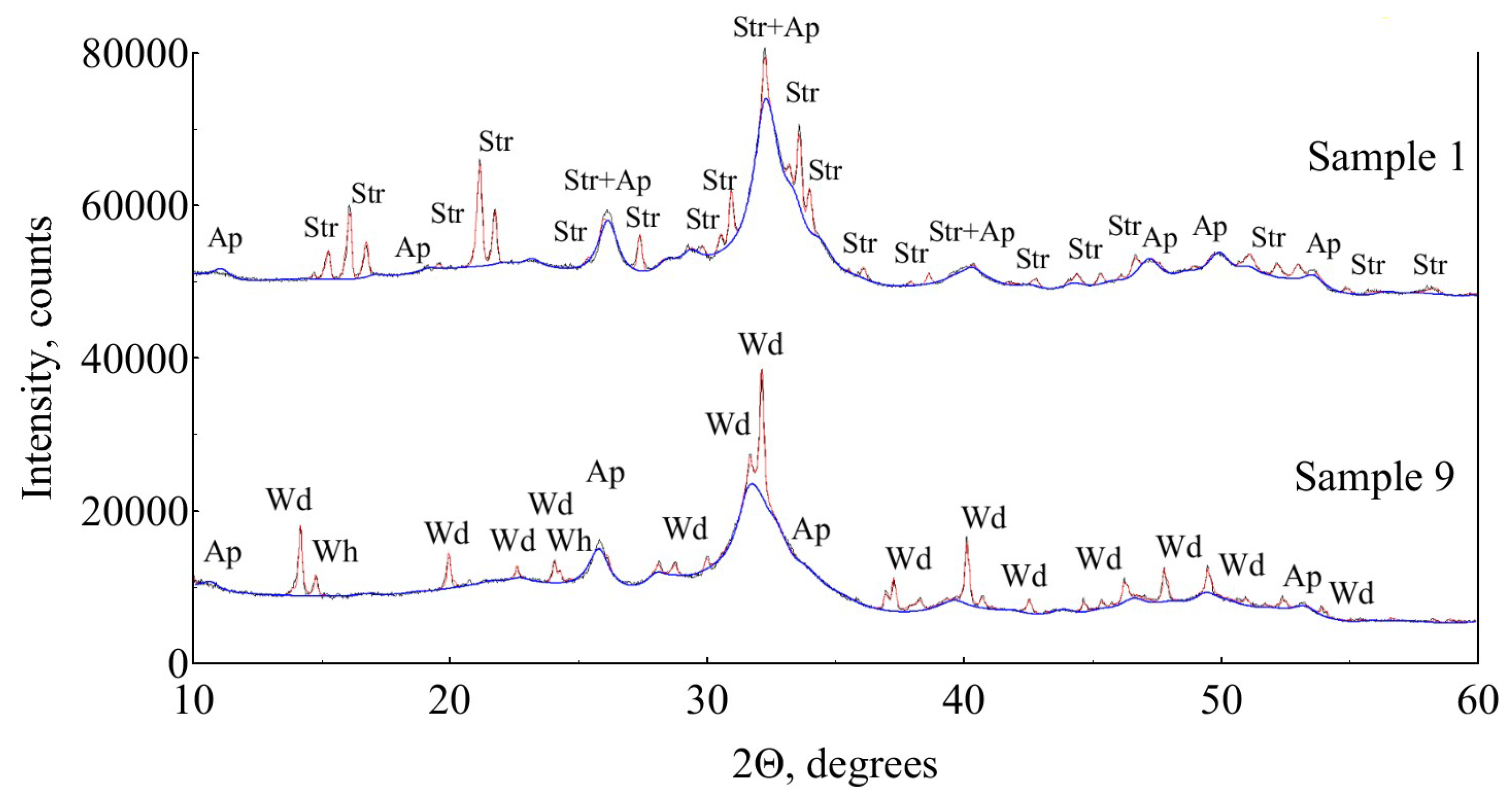
3.1. X-ray Diffraction
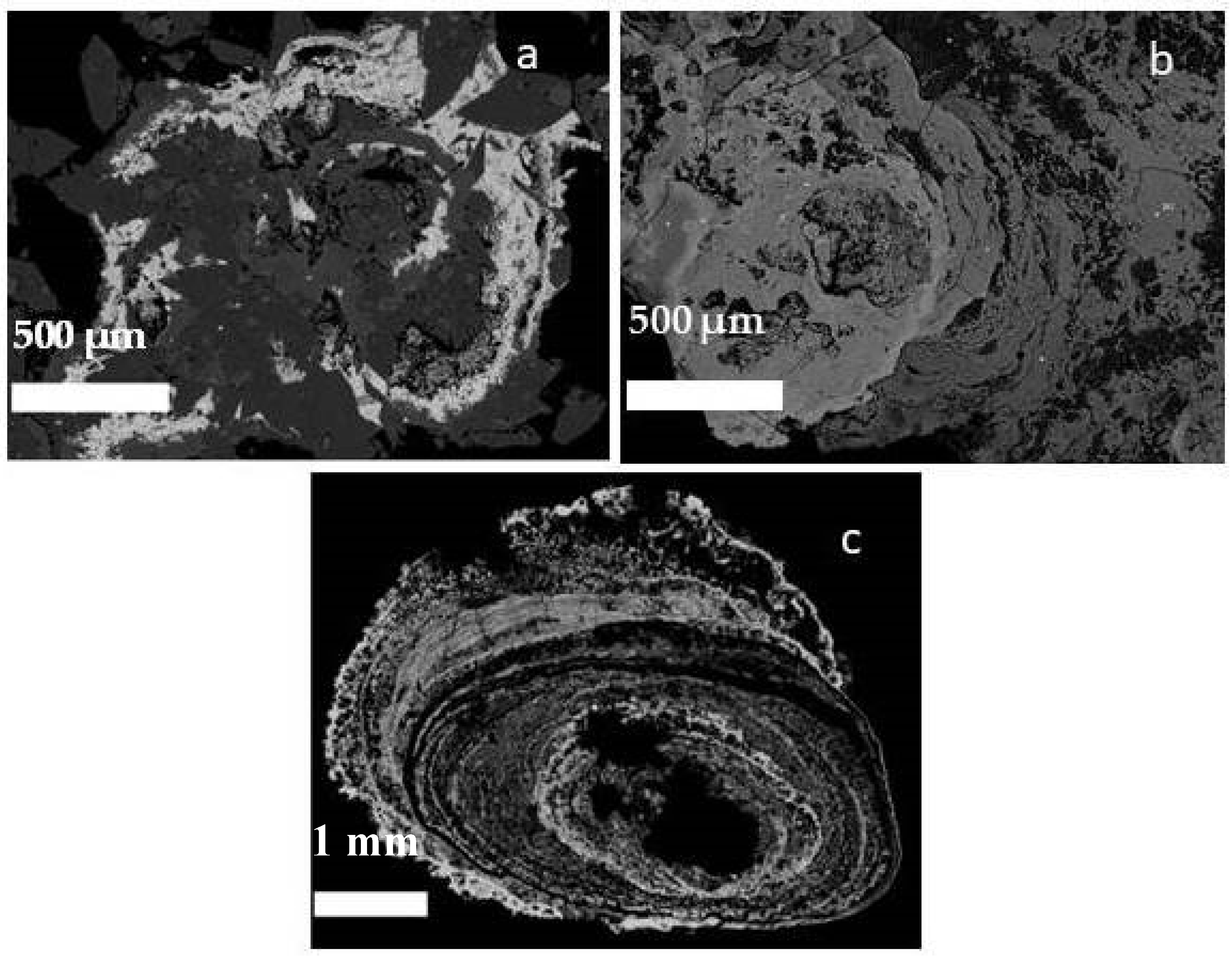
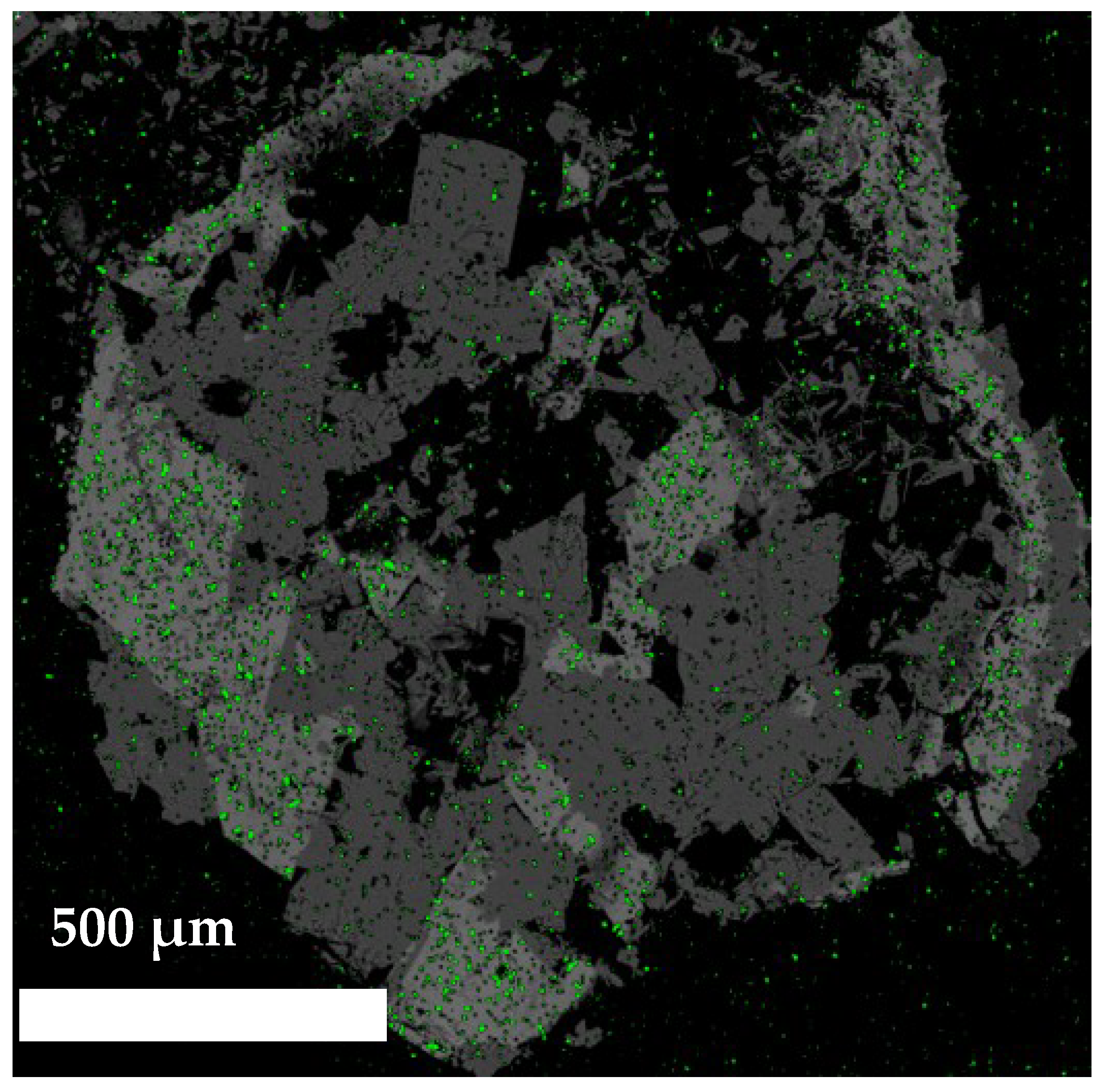
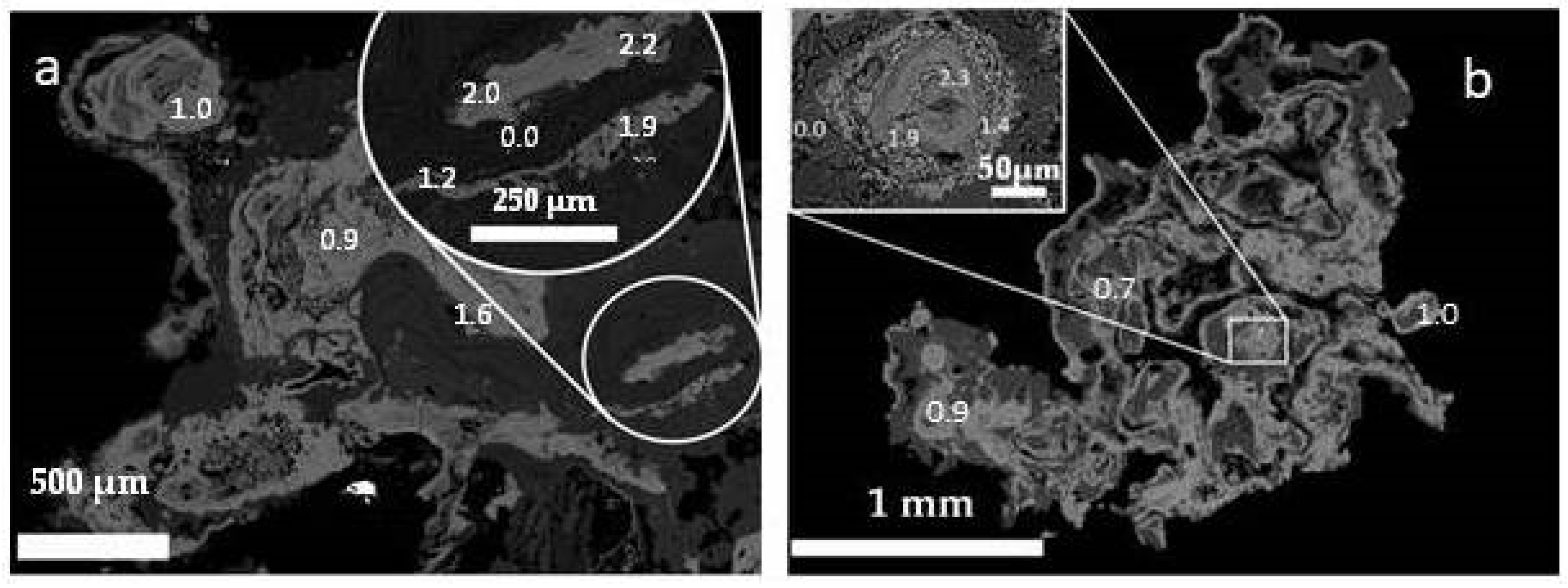
3.2. SEM and EDX
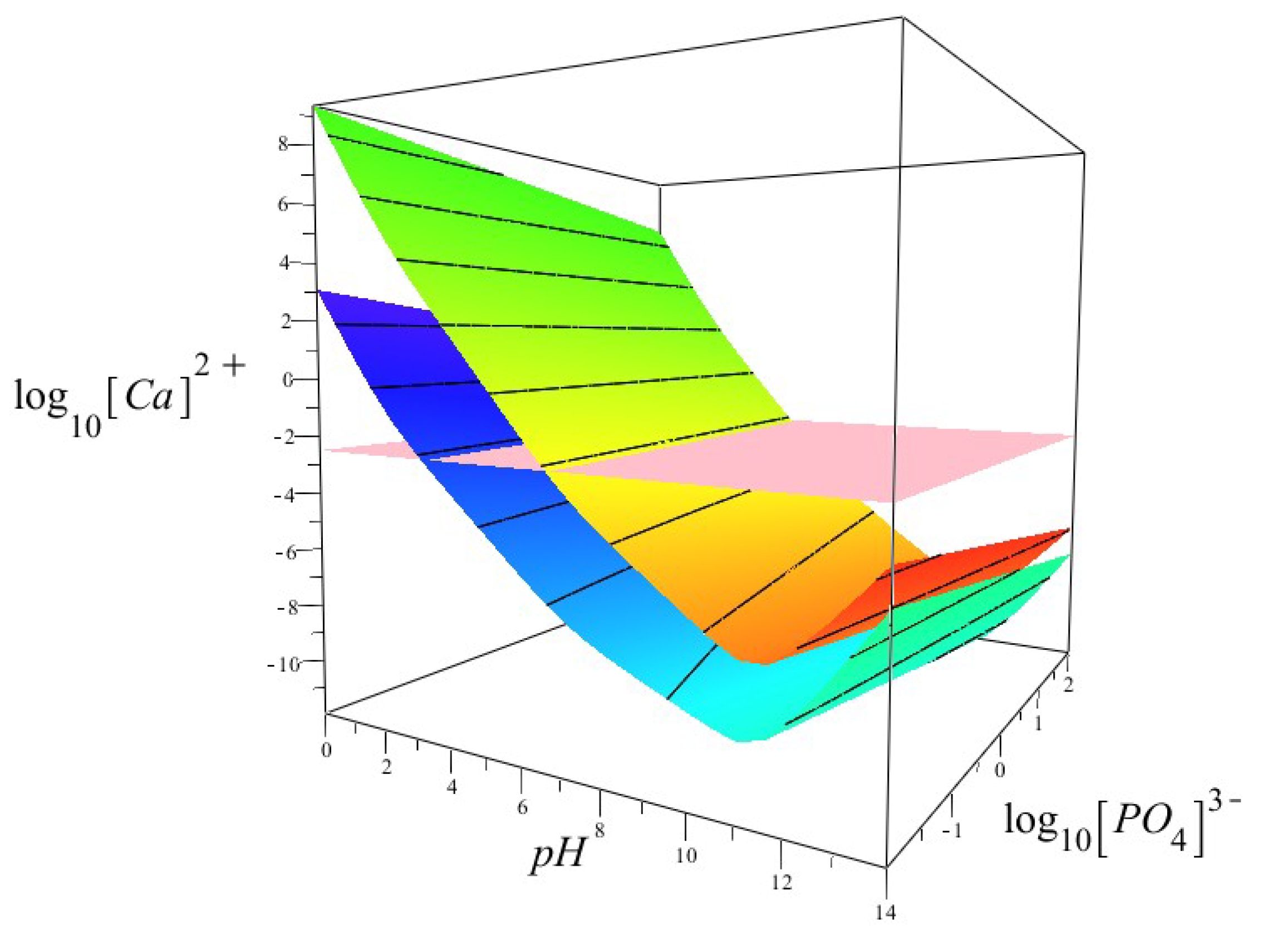
3.3. Thermodynamic Modelling
4. Discussion
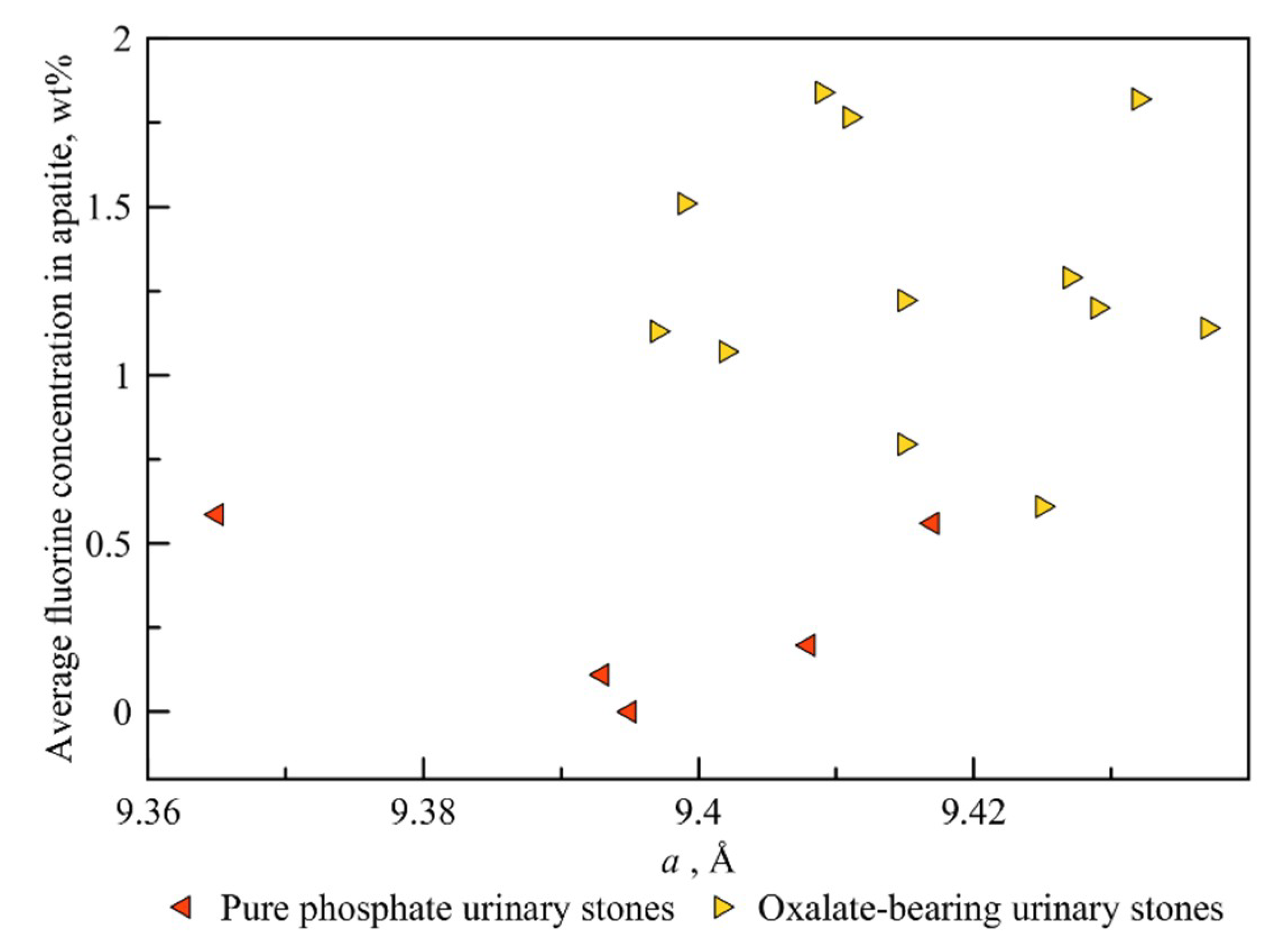
4.1. Variation of Apatite Unit Cell Parameters
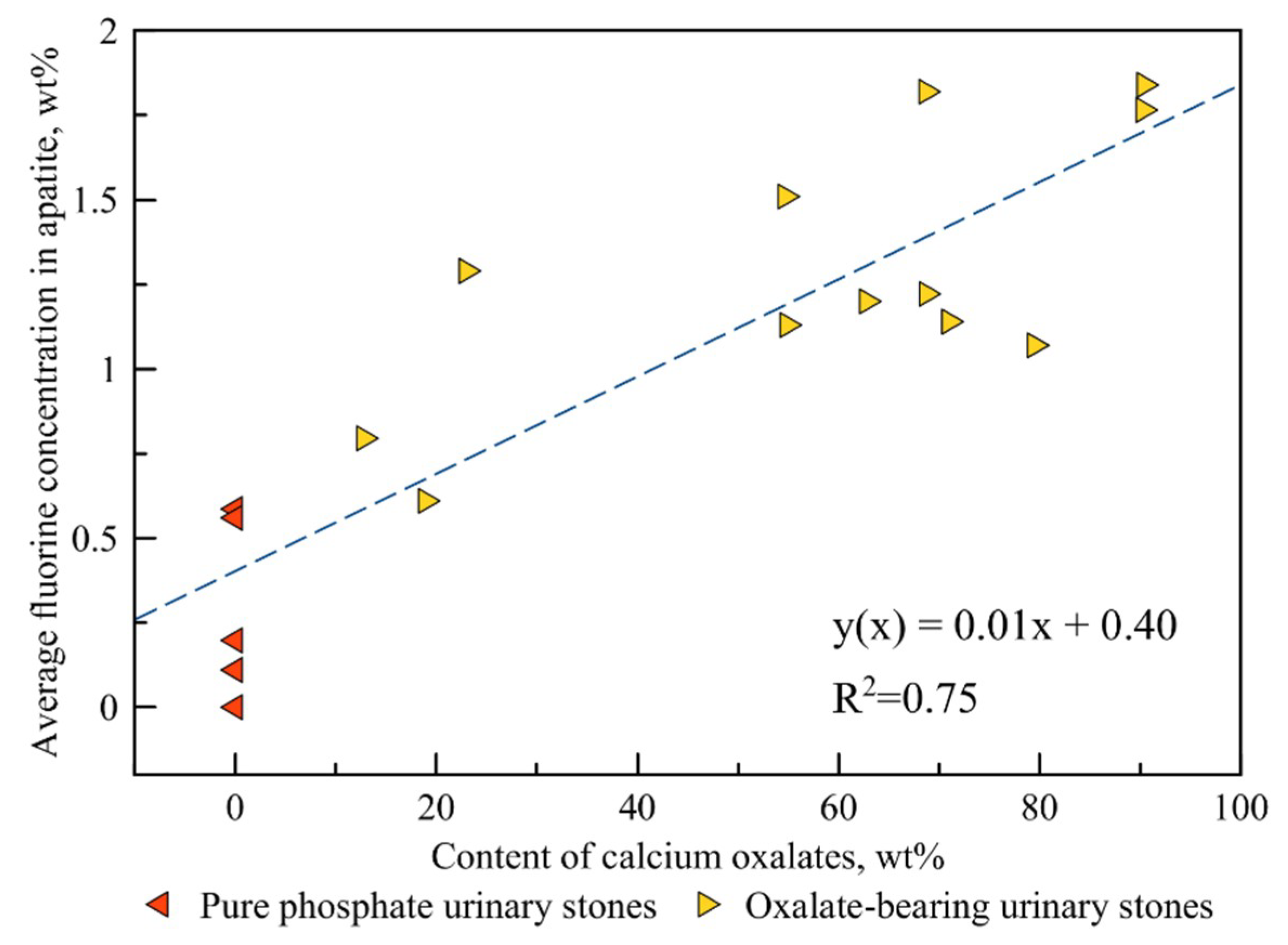
4.2. Correlation between the Fluorine Content and the Amount of Oxalate Mineral Phases in a Kidney Stone
4.3. The Effect of pH on the Thermodynamic Stability of Fluorapatite
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shattock, S.G. A prehistoric or predynastic Egyptian calculus. Trans. Pathol. Soc. Lond. 1905, 56, 275–290. [Google Scholar]
- Chatterjee, P.; Chakraborty, A.; Mukherjee, A.K. Phase composition and morphological characterization of human kidney stones using IR spectroscopy, scanning electron microscopy and X-ray Rietveld analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-H.; Li, C.-C.; Hsu, H.; Chang, W.-C.; Liu, C.-C.; Li, W.-M.; Ke, H.-L.; Lee, M.-H.; Liu, M.-E.; Pan, S.-C.; et al. Renal function in patients with urinary stones of varying compositions. Kaohsiung J. Med Sci. 2011, 27, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Golovanova, O.A.; Punin Yu, O.; Izatulina, A.R.; Yelnikov, V.Y.; Plotkina Yu, V. Structural-tex-tural features and ontogenetic regularities of renal stone formation. Vestn. St. Peterbg. Univ. Seriya Geol. I Geogr. 2009, 1, 26–34. (In Russian) [Google Scholar]
- Otnes, B. Correlation Between Causes and Composition of Urinary Stones. Scand. J. Urol. Nephrol. 1983, 17, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Evan, A.P. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr. Nephrol. 2010, 25, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Tiselius, H.; Lindbäck, B.; Fornander, A.; Nilsson, M. Studies on the role of calcium phosphate in the process of calcium oxalate crystal formation. Urol. Res. 2009, 37, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Randall, A. The etiology of primary renal calculus. Int. Abstr. Surg. 1940, 71, 209–240. [Google Scholar]
- Evan, A.P.; Lingeman, J.E.; Coe, F.L.; Parks, J.H.; Bledsoe, S.B.; Shao, Y.; Sommer, A.J.; Paterson, R.F.; Kuo, R.L.; Grynpas, M. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J. Clin. Investig. 2003, 111, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Sethmann, I.; Wendt-Nordahl, G.; Knoll, T.; Enzmann, F.; Simon, L.; Kleebe, H.-J. Microstructures of Randall’s plaques and their interfaces with calcium oxalate monohydrate kidney stones reflect underlying mineral precipitation mechanisms. Urolithiasis 2017, 45, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Tiselius, H. Hypothesis of calcium stone formation: An interpretation of stone research during the past decades. Urol. Res. 2011, 39, 231–243. [Google Scholar] [CrossRef] [PubMed]
- El’nikov, V.Y.; Rosseeva, E.V.; Golovanova, O.A.; Frank-Kamenetskaya, O.V. Thermodynamic and Experimental Modeling of the Formation of Major Mineral Phases of Uroliths. Russ. J. Inorg. Chem. 2007, 52, 150–157. [Google Scholar]
- Izatulina, A.R.; Yelnikov, V. Structure, chemistry and crystallization conditions of calcium oxalates—The main components of kidney stones. In Minerals as Advanced Materials I; Krivovichev, S., Ed.; Springer: Berlin, Germany, 2008; pp. 231–241. [Google Scholar]
- Xie, B.; Halter, T.J.; Borah, B.M.; Nancollas, G.H. Aggregation of calcium phosphate and oxalate phases in the formation of renal stones. Cryst. Growth Des. 2014, 15, 3038–3045. [Google Scholar] [CrossRef] [PubMed]
- Frank-Kamenetskaya, O.V.; Izatulina, A.R.; Kuz’mina, M.A. Ion substitutions, non-stoichiometry, and formation conditions of oxalate and phosphate minerals of the human. In Biogenic-Abiogenic Interactions in Natural and Anthropogenic Systems; Frank-Kamenetskaya, O.V., Panova, E.G., Vlasov, D.Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 425–442. [Google Scholar]
- Frank-Kamenetskaya, O.V. Crystal chemistry and synthesis of carbonate apatites—Main minerals in living organisms. In Proceedings of the 9th International Congress for Applied Mineralogy, Brisbane, Australia, 8–10 September 2008; Australasian Institute of Mining and Metallurgy: Carlton, Australia, 2008. [Google Scholar]
- Topas V4.2: General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2009.
- Robie, R.A.; Hemingway, B.S.; Fisher, J.R. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 bar (105 Pascals) Pressure and at Higher Temperatures, 2nd ed.; United States Government Printing Office: Washington, DC, USA, 1979; 464p.
- Naumov, G.B.; Ryzhenko, B.N.; Khodakovsky, I.L. Handbook of Thermodynamic Quantities (for Geologists), 1st ed.; Atomizdat: Moscow, Russia, 1971; 240p. (In Russian) [Google Scholar]
- Tušl, J. Direct determination of fluoride in human urine using the fluoride electrode. Clin. Chim. Acta 1970, 27, 216–218. [Google Scholar] [CrossRef]
- Borodin, E.A. Biochemical Diagnosis; V. 1,2; Amuruprpoligraphizdat: Blagoveschensk, Russia, 1989; p. 77. (In Russian) [Google Scholar]
- Moskalev, Y.I. Mineral Exchange; Medicine: Moscow, Russia, 1985; p. 288. (In Russian) [Google Scholar]
- Izatulina, A.R.; Gurzhiy, V.V.; Krzhizhanovskaya, M.G.; Chukanov, N.V.; Panikorovskii, T.L. Thermal Behavior and Phase Transition of Uric Acid and Its Dihydrate Form, the Common Biominerals Uricite and Tinnunculite. Minerals 2019, 9, 373. [Google Scholar] [CrossRef]
- Izatulina, A.R.; Punin, Y.O.; Golovanova, O.A. To the formation of aggregate structures of kidney stones. J. Struct. Chem. 2014, 55, 1225–1231. [Google Scholar] [CrossRef]
- Brown, P.W.; Constantz, B. Hydroxyapatite and Related Materials; CRC: Boca Raton, FL, USA, 1994; 368p. [Google Scholar]
- Hovis, G.L.; Scott, B.T.; Altomare, C.M.; Leaman, A.R.; Morris, M.D.; Tomaino, G.P.; McCubbin, F.M. Thermal expansion of fluorapatite-hydroxylapatite crystalline solutions. Am. Mineral. 2014, 99, 2171–2175. [Google Scholar] [CrossRef][Green Version]
- Driessens, F.C.M. Relation between apatite solubility and anti-cariogenic effect of fluoride. Nature 1973, 243, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Kresak, M.; Zahradnik, R.T. Fluoridated hydroxyapatite solubility and caries formation. Nature 1974, 247, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Kuz’mina, M.A.; Nikolaev, A.M.; Frank-Kamenetskaya, O.V. The formation of calcium and magnesium phosphates of the renal stones depending on the composition of the crystallization medium. In Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature; Frank-Kamenetskaya, O.V., Vlasov, D.Y., Panova, E.G., Lessovaia, S.N., Eds.; Springer: Cham, Switzerland, 2019; pp. 107–118. [Google Scholar]
- Bretherton, T.; Rodgers, A. Crystallization of calcium oxalate in minimally diluted urine. J. Cryst. Growth 1998, 192, 448–455. [Google Scholar] [CrossRef]


| Composition/Ion | ΔfG°298, kJ/mol | Reference |
|---|---|---|
| Fluorapatite Ca5(PO4)3F | −6455.778 | [18] |
| hydroxylapatite Ca5(PO4)3OH | −6286.093 | [18] |
| Ca2+ | −552.706 | [19] |
| Ca(OH)+ | −716.970 | |
| Ca(OH)2 | −897.008 | |
| PO43− | −1018.804 | |
| HPO42− | −1089.263 | |
| H2PO4− | −1130.391 | |
| H3PO4 | −1142.650 | |
| OH− | −157.293 | |
| F− | −279.993 | |
| HF | −293.825 | |
| H2O | −237.178 |
| Sample | Whewellite, % | Weddellite, % | Struvite, % | Apatite, % |
|---|---|---|---|---|
| 1 | 0.0 | 0.0 | 23.8 | 76.2 |
| 2 | 7.0 | 64.0 | 0.0 | 29.0 |
| 3 | 46.8 | 7.9 | 0.0 | 45.3 |
| 4 | 80.8 | 9.6 | 0.0 | 9.6 |
| 5 | 23.2 | 45.5 | 0.0 | 31.3 |
| 6 | 11.8 | 1.0 | 5.4 | 81.8 |
| 7 | 76.6 | 2.9 | 0.0 | 20.5 |
| 8 | 5.3 | 57.5 | 0.0 | 37.2 |
| 9 | 2.3 | 16.6 | 0.0 | 81.1 |
| 10 | 63.4 | 5.3 | 0.0 | 31.3 |
| 11 | 49.2 | 5.7 | 0.0 | 45.1 |
| 12 | 0.0 | 0.0 | 54.7 | 45.3 |
| 13 | 48.4 | 41.9 | 0.0 | 9.7 |
| 14 * | 0.0 | 6.0 | 0.0 | 77.0 |
| 15 | 0.0 | 0.0 | 50.0 | 50.0 |
| 16 | 0.0 | 0.0 | 5.4 | 94.6 |
| 17 | 0.0 | 0.0 | 55.0 | 45.0 |
| Sample | a, Å | c, Å | Crystallite Size, nm |
|---|---|---|---|
| 2 | 9.437 | 6.872 | 10(1) |
| 3 | 9.399 | 6.854 | 20(3) |
| 4 | 9.409 | 6.882 | 19(4) |
| 5 | 9.432 | 6.871 | 11(1) |
| 6 | 9.415 | 6.878 | 8(1) |
| 7 | 9.402 | 6.865 | 50(30) |
| 8 | 9.429 | 6.874 | 10(1) |
| 9 | 9.425 | 6.854 | 8(1) |
| 10 | 9.415 | 6.874 | 21(5) |
| 11 | 9.397 | 6.861 | 34(11) |
| 13 | 9.411 | 6.880 | 13(5) |
| 14 | 9.427 | 6.875 | 8(1) |
| 1 | 9.393 | 6.878 | 7(1) |
| 12 | 9.408 | 6.870 | 8(1) |
| 15 | 9.365 | 6.878 | 7(1) |
| 16 | 9.417 | 8.867 | 3(1) |
| 17 | 9.395 | 6.869 | 9(1) |
| Sample | F, wt% |
|---|---|
| Stones with calcium oxalates (oxalate-bearing) | |
| 2 | 1.14 |
| 3 | 1.51 |
| 4 | 1.84 |
| 5 | 1.82 |
| 6 | 0.79 |
| 7 | 1.07 |
| 8 | 1.20 |
| 9 | 0.61 |
| 10 | 1.22 |
| 11 | 1.13 |
| 13 | 1.77 |
| 14 | 1.29 |
| Stones with no calcium oxalate (pure phosphate) | |
| 1 | 0.12 |
| 12 | 0.37 |
| 15 | 0.59 |
| 16 | 0.56 |
| 17 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korneev, A.V.; Frank-Kamenetskaya, O.V.; Izatulina, A.R. About the Role of Fluorine-Bearing Apatite in the Formation of Oxalate Kidney Stones. Crystals 2020, 10, 486. https://doi.org/10.3390/cryst10060486
Korneev AV, Frank-Kamenetskaya OV, Izatulina AR. About the Role of Fluorine-Bearing Apatite in the Formation of Oxalate Kidney Stones. Crystals. 2020; 10(6):486. https://doi.org/10.3390/cryst10060486
Chicago/Turabian StyleKorneev, Anatolii V., Olga V. Frank-Kamenetskaya, and Alina R. Izatulina. 2020. "About the Role of Fluorine-Bearing Apatite in the Formation of Oxalate Kidney Stones" Crystals 10, no. 6: 486. https://doi.org/10.3390/cryst10060486
APA StyleKorneev, A. V., Frank-Kamenetskaya, O. V., & Izatulina, A. R. (2020). About the Role of Fluorine-Bearing Apatite in the Formation of Oxalate Kidney Stones. Crystals, 10(6), 486. https://doi.org/10.3390/cryst10060486







