Silver(I) and Copper(I) Complexation with Decachloro-Closo-Decaborate Anion
Abstract
:1. Introduction
2. Experimental
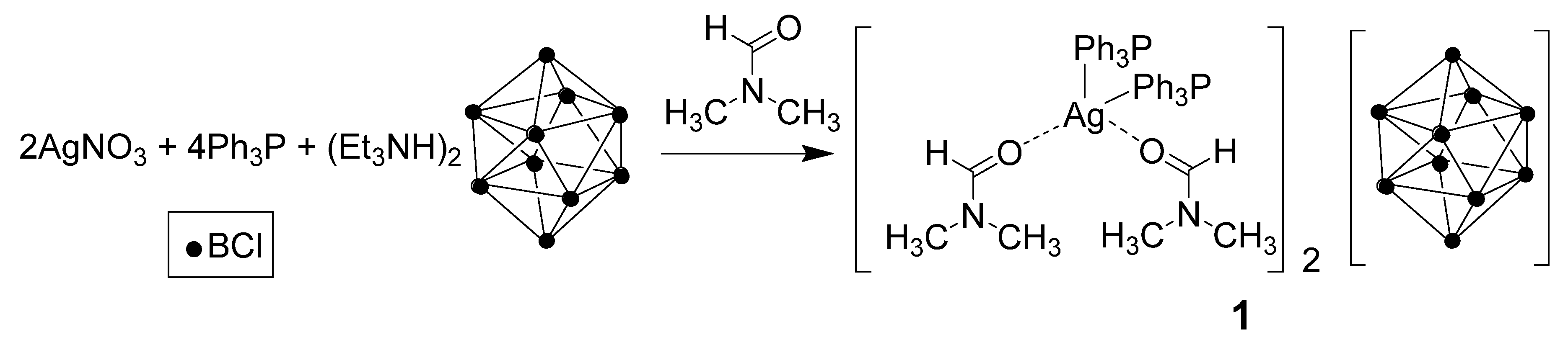
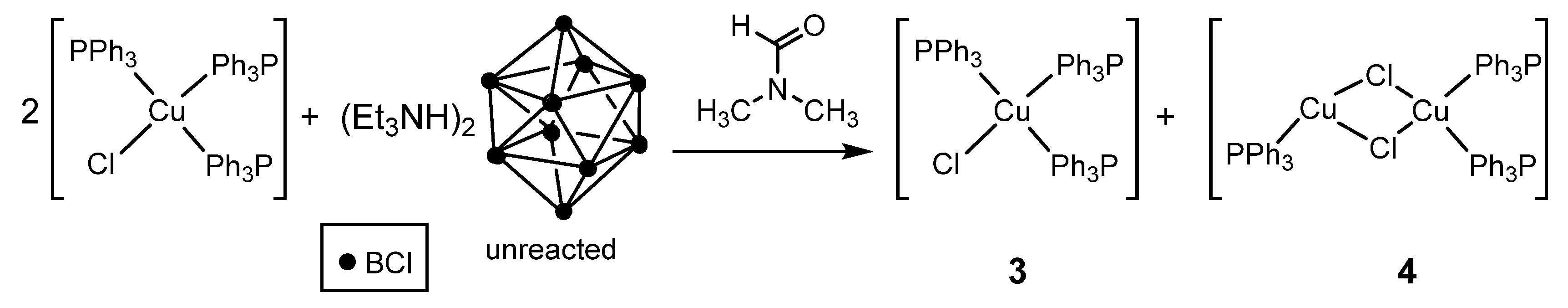
2.1. Synthesis
2.2. Methods
2.3. X-ray Diffraction
3. Results
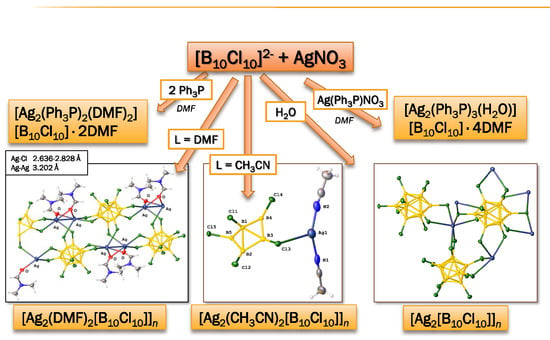
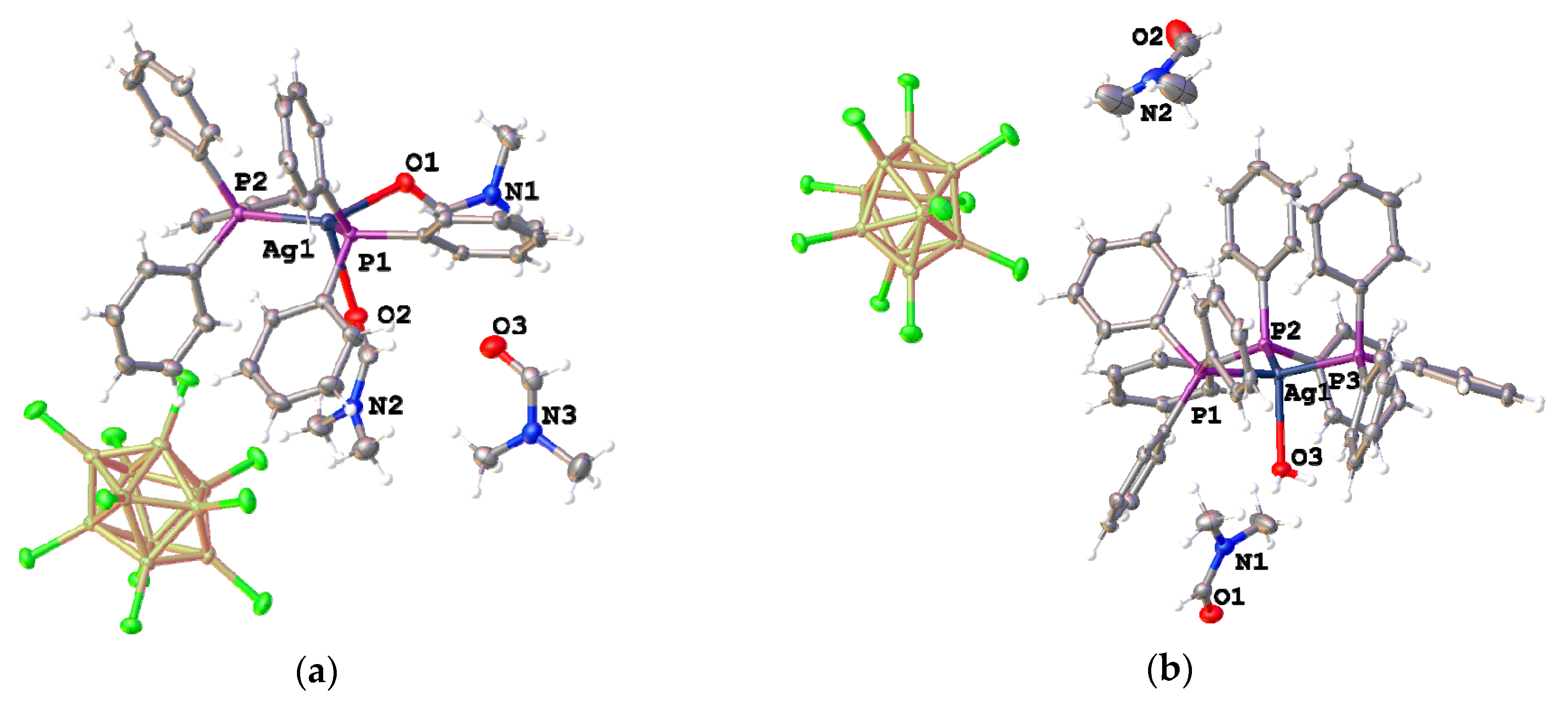
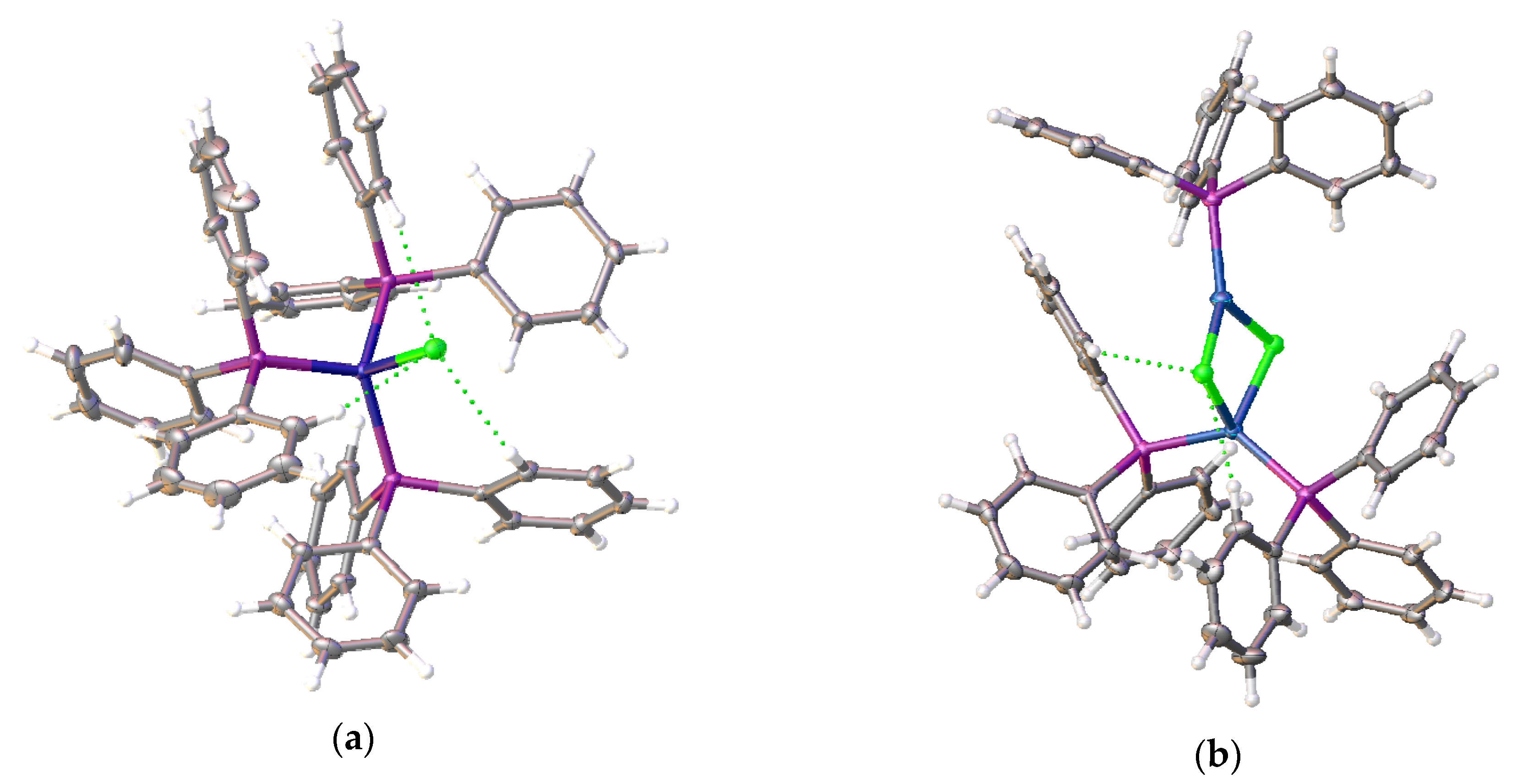
3.1. Complexes with Non-Coordinated [B10Cl10]2− Anion
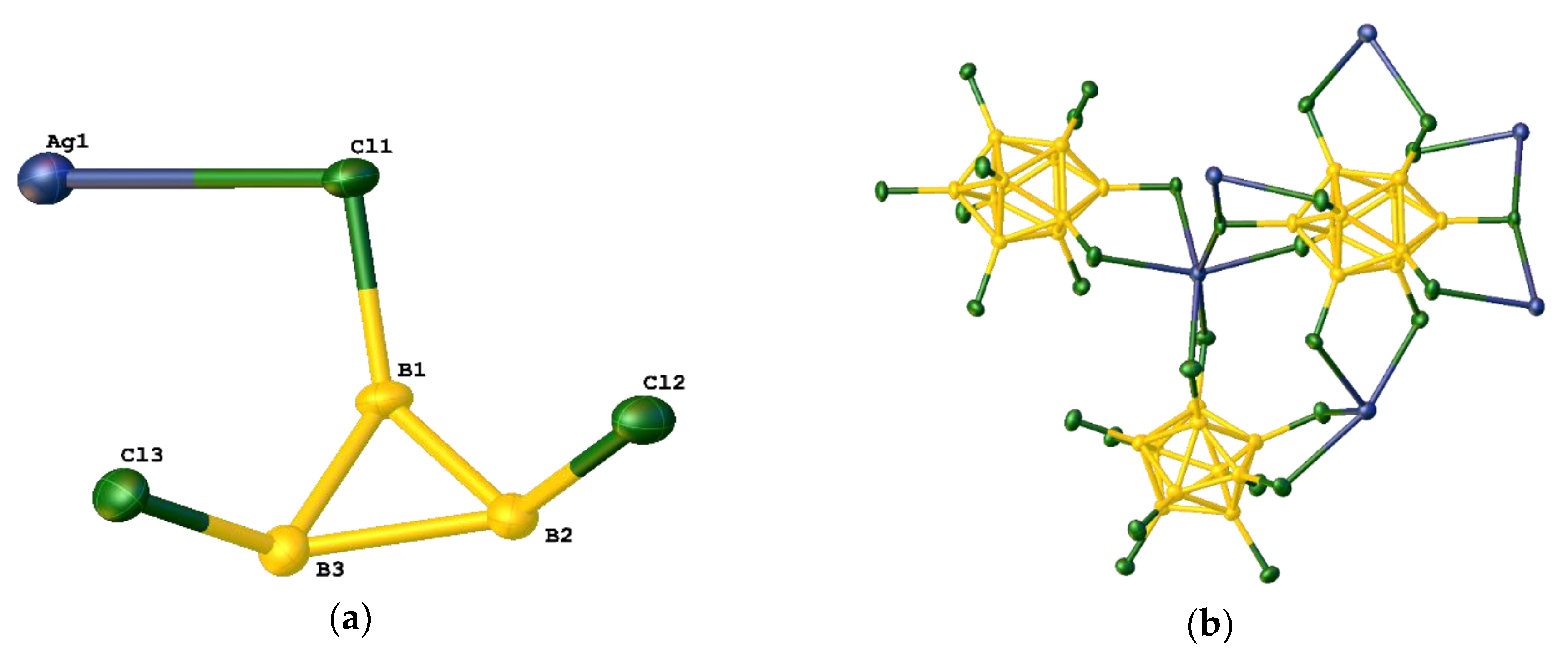
3.2. Silver Complexes with Coordinated [B10Cl10]2− Anion
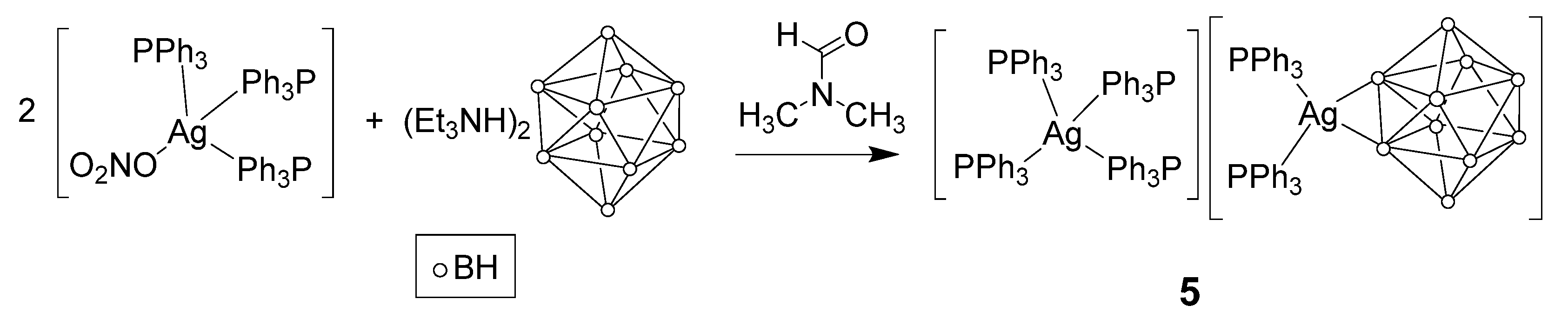
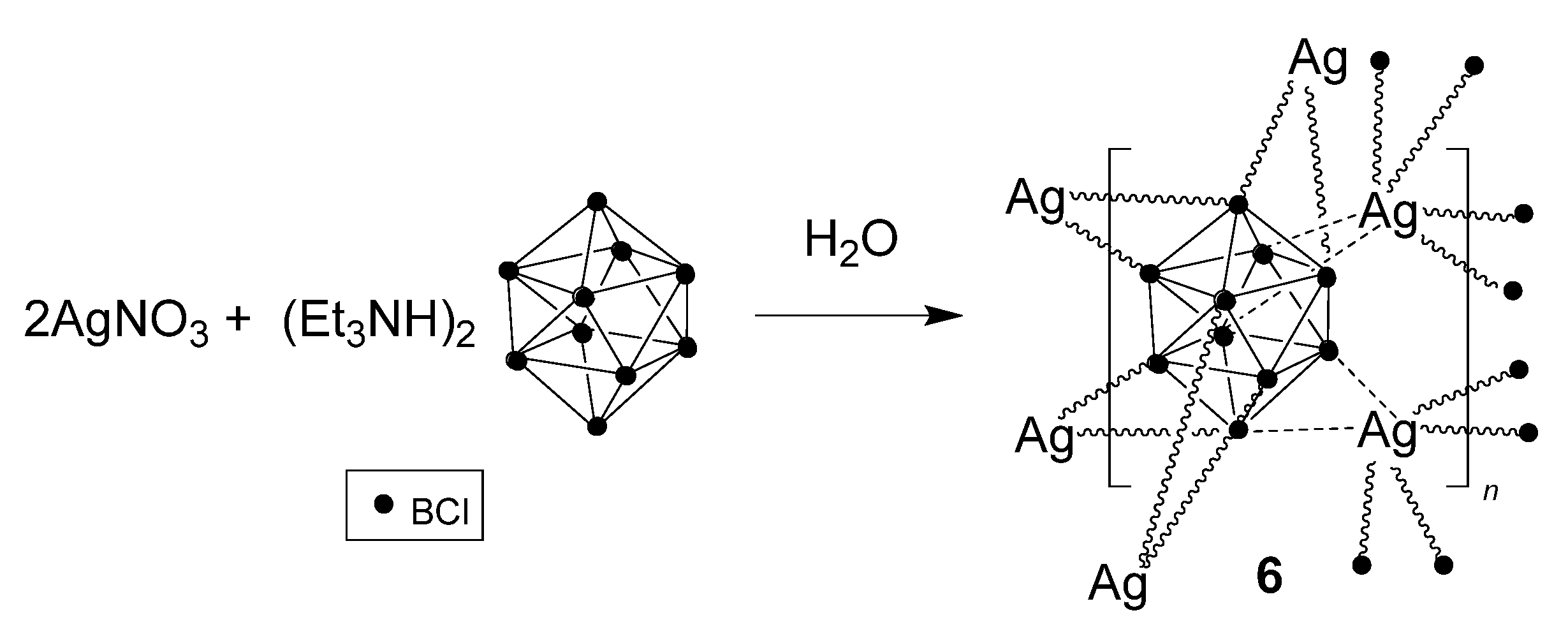
3.2.1. Synthesis in Water
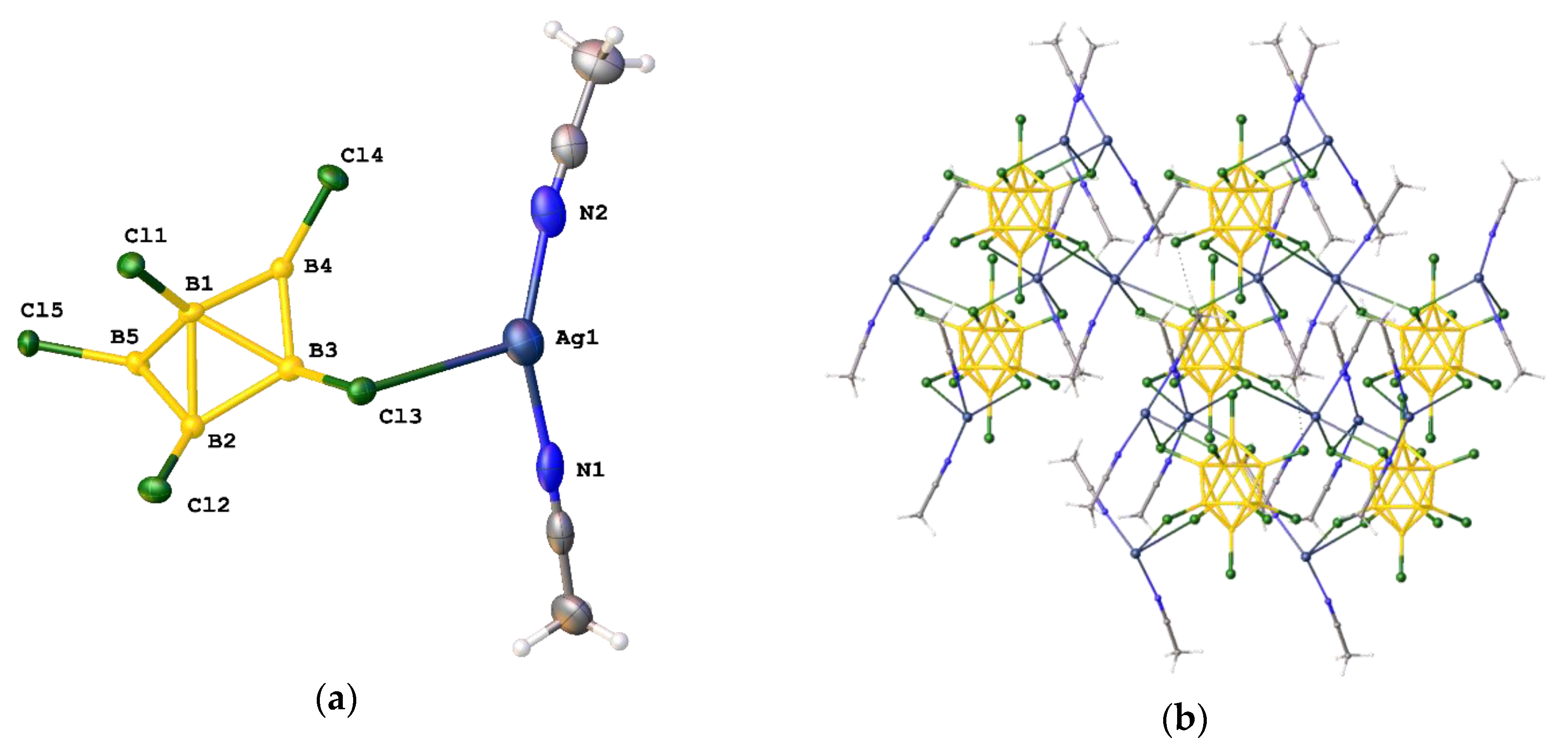
3.2.2. Synthesis in DMF
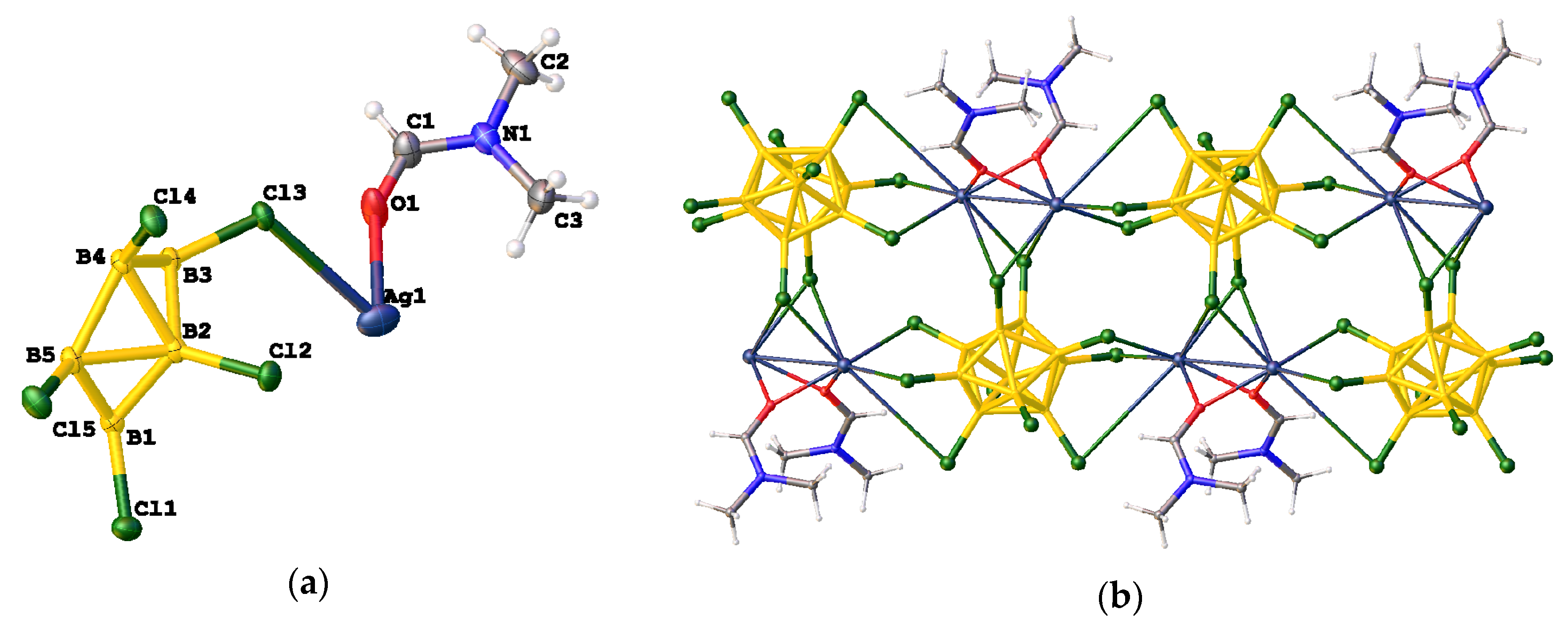
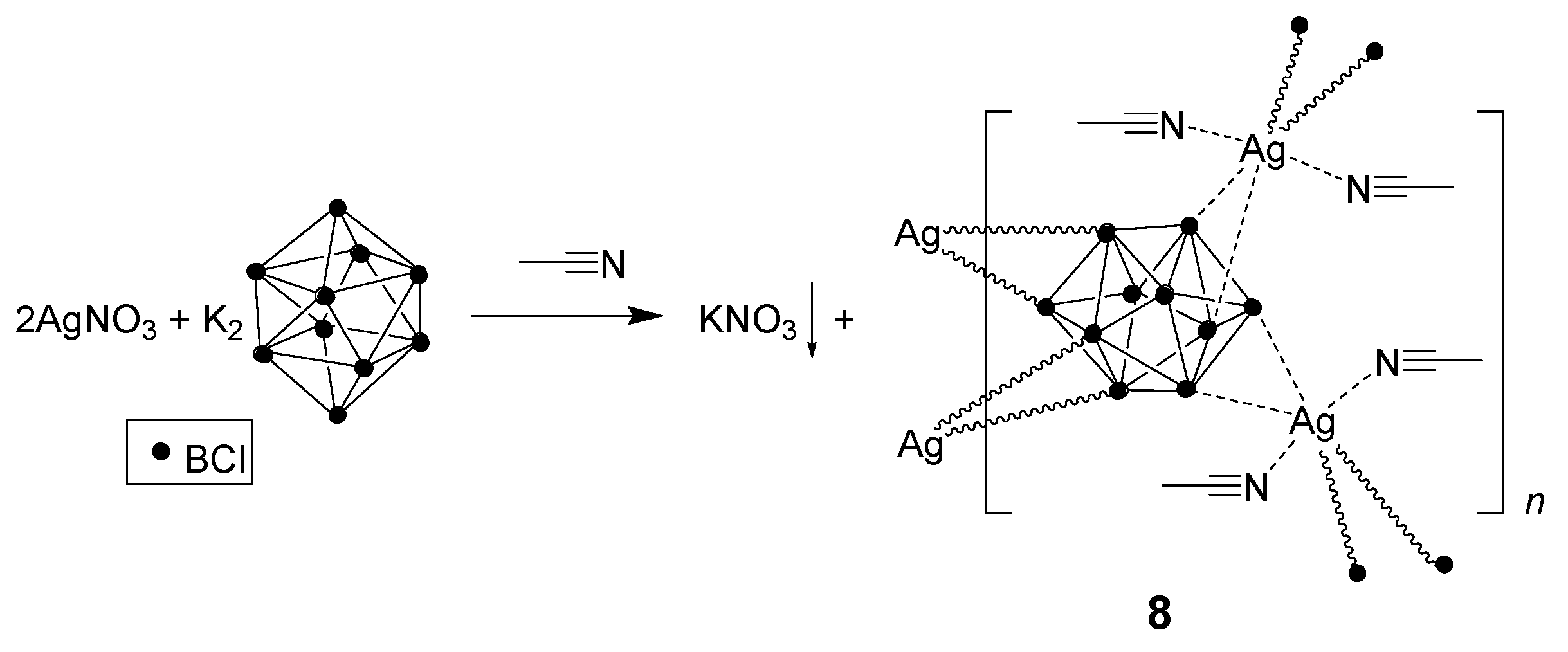
3.2.3. Synthesis in Acetonitrile
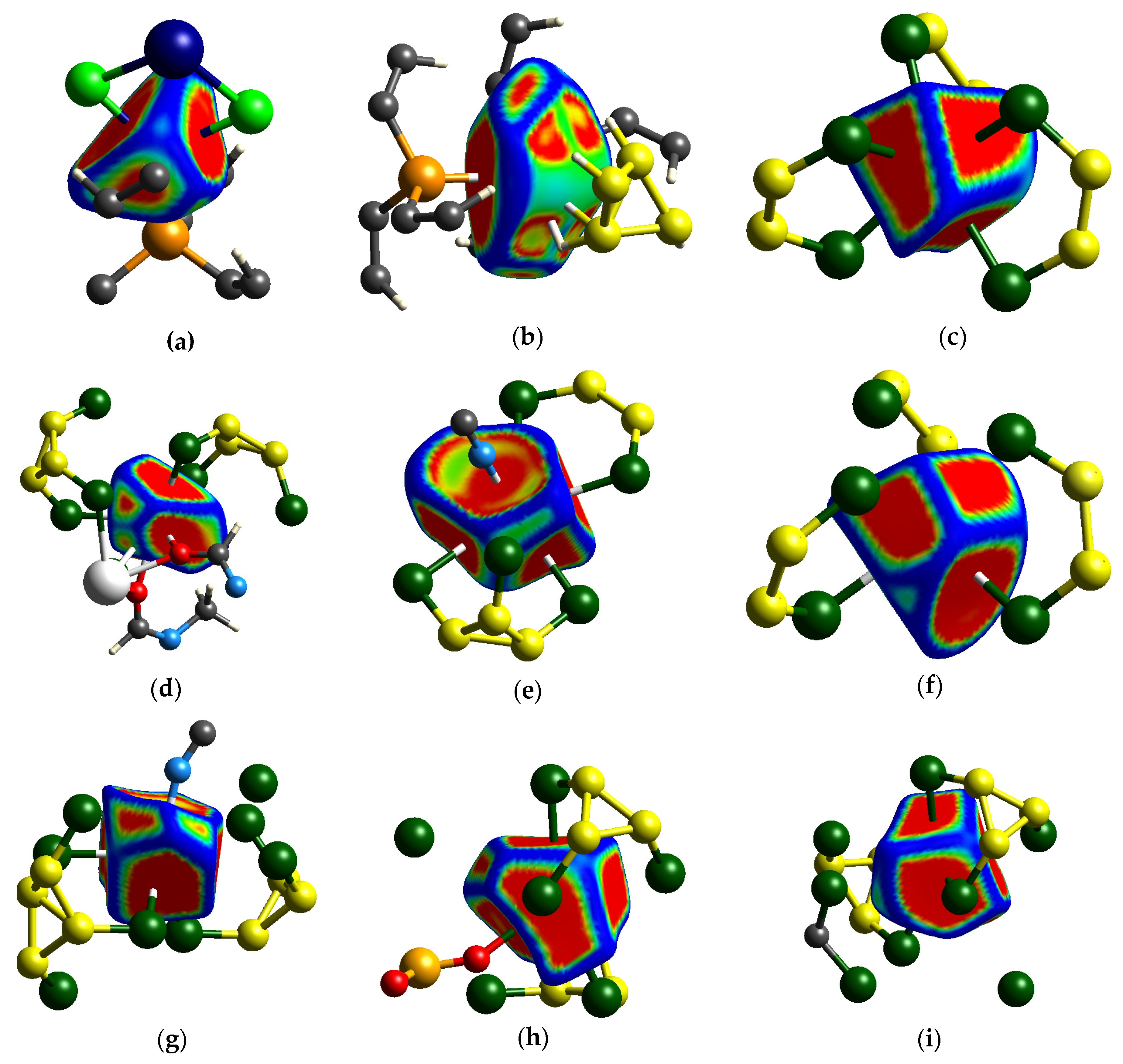
3.3. The Hirshfeld Analysis of Ag…Cl–B Bonding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishnan, K.M. Fundamentals and Applications of Magnetic Materials; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Muetterties, E.L.; Knoth, W.H. Polyhedral Boranes; Dekker: New York, NY, USA, 1968. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Boron Science: New Technologies and Applications; Hosmane, N.S. (Ed.) CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Zhizhin, K.Y.; Zhdanov, A.P.; Kuznetsov, N.T. Derivatives of closo-decaborate anion [B10H10]2− with exo-polyhedral substituents. Russ. J. Inorg. Chem. 2010, 55, 2089–2127. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Naoufal, D. Fifty years of the closo-decaborate anion chemistry. Coll. Czech. Chem. Commun. 2010, 75, 1149–1199. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of closo-Dodecaborate Anion [B12H12]2−: A Review. Collect. Czech. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Silver and copper complexes with closo-polyhedral borane, carborane and metallacarborane anions: Synthesis and X-ray structure. Crystals 2016, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Malinina, E.A.; Avdeeva, V.V.; Goeva, L.V.; Kuznetsov, N.T. Coordination compounds of electron-deficient boron cluster anions BnHn2− (n = 6, 10, 12). Russ. J. Inorg. Chem. 2010, 55, 2148–2202. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Coordination chemistry of iron triad metals with organic N-donor ligands and boron cluster anions [B10H10]2−, [B12H12]2−, and [B10Cl10]2−: Complexation and accompanying processes. Rus. J. Inorg. Chem. 2017, 62, 1673–1702. [Google Scholar] [CrossRef]
- Goswami, L.N.; Ma, L.; Chakravarty, S.; Cai, Q.; Jalisatgi, S.S.; Hawthorne, M.F. Discrete Nanomolecular Polyhedral Borane Scaffold Supporting Multiple Gadolinium(III) Complexes as a High Performance MRI Contrast Agent. Inorg. Chem. 2013, 52, 1694–1700. [Google Scholar] [CrossRef]
- Plesek, J. Potential applications of the boron cluster compounds. Chem. Rev. 1992, 92, 269–278. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Kuznetsov, N.T. Derivatives of the closo-dodecaborate anion and their application in medicine. Russ. Chem. Bull. 2002, 51, 1362–1374. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 11, 1433–1450. [Google Scholar] [CrossRef]
- Teixidor, F.; Vinas, C.; Demonceau, A.; Nunez, R. Boron clusters: Do they receive the deserved interest? Pure Appl. Chem. 2003, 75, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Isomerism in complexes with the decahydro-closo-decaborate anion. Polyhedron 2016, 105, 205–221. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Polyakova, I.N.; Vologzhanina, A.V.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Positional isomers of mononuclear silver(I) anionic complex [Ag(Ph3P)2(B10H10-xClx)]− (x = 0 or 1) with apically and equatorially coordinated decahydro-closo-decaborate and 2-chlorononahydro-closo-decaborate ligands. Polyhedron 2017, 123, 396–403. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Buzin, M.I.; Dmitrienko, A.O.; Dorovatovskii, P.V.; Malinina, E.A.; Kuznetsov, N.T.; Voronova, E.D.; Zubavichus, Y.V.; Vologzhanina, A.V. Solid-State Reactions of Eicosaborate [B20H18]2− Salts and Complexes. Chem. Eur. J. 2017, 23, 16819–16828. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.A. Carboranes: A new class of weakly coordinating anions for strong electrophiles, oxidants, and superacids. Acc. Chem. Res. 1998, 31, 133–139. [Google Scholar] [CrossRef]
- Strauss, S.H. Search for larger and more weakly coordinating anions. Chem. Rev. 1993, 93, 927–942. [Google Scholar] [CrossRef]
- Knapp, C. Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 651–679. [Google Scholar]
- Engesser, T.A.; Lichtenthaler, M.R.; Schleep, M.; Krossing, I. Reactive p-Block cations stabilized by weakly coordinating anions. Chem. Soc. Rev. 2016, 45, 789–899. [Google Scholar] [CrossRef] [Green Version]
- Avdeeva, V.V.; Kravchenko, E.A.; Gippius, A.A.; Vologzhanina, A.V.; Malinina, E.A.; Zhurenko, S.V.; Buzanov, G.A.; Kuznetsov, N.T. Decachloro-closo-decaborate anion in copper(II) complexation reactions with N-donor ligands: 35Cl NQR and X-ray studies. Polyhedron 2017, 127, 238–247. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Vologzhanina, A.V.; Goeva, L.V.; Malinina, E.A.; Kuznetsov, N.T. Reactivity of boron cluster anions [B10H10]2−, [B10Cl10]2− and [B12H12]2− in cobalt(II)/cobalt(III) complexation with 1,10-phenanthroline. Inorg. Chim. Acta 2015, 428, 154–162. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Vologzhanina, A.V.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Boron Cluster Anions [B10X10]2− (X = H, Cl) in Manganese(II) Complexation with 2,2′-Bipyridyl. Russ. J. Coord. Chem. 2019, 45, 301–306. [Google Scholar] [CrossRef]
- Kravchenko, E.A.; Gippius, A.A.; Korlyukov, A.A.; Vologzhanina, A.V.; Avdeeva, V.V.; Malinina, E.A.; Ulitin, E.O.; Kuznetsov, N.T. Secondary interactions in decachloro-closo-decaborates R2[B10Cl10] (R = [Ag(NH3)2]+, Ph4P+, Et3NH+): 35Cl NQR, PW-DFT, and X-ray studies. Inorg. Chim. Acta 2016, 447, 22–31. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Powell, D.R.; Wehmschulte, R.J. Chlorination of 1-Carba-closo-dodecaborate and 1-Ammonio-closo-dodecaborate Anions. Inorg. Chem. 2016, 55, 10617–10627. [Google Scholar] [CrossRef] [PubMed]
- Hague, C.; Patmore, N.J.; Frost, C.G.; Mahon, M.F.; Weller, A.S. [(PPh3)Ag(CB11H6Y6)] (Y = H, Br): Highly active, selective and recyclable Lewis acids for a hetero-Diels–Alder reaction. Chem. Commun. 2001, 21, 2286–2287. [Google Scholar] [CrossRef]
- Patmore, N.J.; Ingleson, M.J.; Mahon, M.F.; Weller, A.S. Investigation of the synthesis of {Mo(η5-C5H5)(CO)3}+ fragments partnered with the monoanionic carboranes [closo-CB11H11Br]−, [closo-CB11H6Br6]− and [closo-HCB11Me11]− by silver salt metathesis and hydride abstraction. Dalton Trans. 2003, 14, 2894–2904. [Google Scholar] [CrossRef]
- Cunha-Silva, L.; Carr, M.J.; Kennedy, J.D.; Hardie, M.J. Silver-Dabco Coordination Networks with Distinct Carbaborane Anions: Investigating Ag···H–B and Ag···I–B Interactions. Cryst. Growth Des. 2013, 13, 3162–3170. [Google Scholar] [CrossRef]
- Jenne, C.; Wegener, B. Silver Salts of the Weakly Coordinating Anion [Me3NB12Cl11]−. Z. Anorg. Allg. Chem. 2018, 644, 1123–1132. [Google Scholar] [CrossRef]
- Tsang, C.-W.; Yang, Q.; Sze, E.T.-P.; Mak, T.C.W.; Chan, D.T.W.; Xie, Z. Weakly Coordinating Nature of a Carborane Cage Bearing Different Halogen Atoms. Synthesis and Structural Characterization of Icosahedral Mixed Halocarborane Anions, 1-H-CB11Y5X6− (X, Y = Cl, Br, I). Inorg. Chem. 2000, 39, 5851–5858. [Google Scholar] [CrossRef]
- Tiritiris, I.; Schleid, T. The Crystal Structure of Solvent-Free Silver Dodecachloro-closo-dodecaborate Ag2[B12Cl12] from Aqueous Solution. Z. Anorg. Allg. Chem. 2003, 629, 581–583. [Google Scholar] [CrossRef]
- Volkov, O.; Hu, C.; Paetzold, P. Silver-Hydrogen Interactions in Crystalline Silver Dodecahydrododecaborates. Z. Anorg. Allg. Chem. 2005, 631, 1107–1112. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pitochelli, A.R. The reaction of bis-acetonitrile decaborane with amines. J. Am. Chem. Soc. 1959, 81, 5519. [Google Scholar] [CrossRef]
- Knoth, W.H.; Miller, H.C.; Sauer, J.C.; Balthis, J.H.; Chia, Y.T.; Muetterties, E.L. Chemistry of Boranes. IV. Halogenation of B10H102− and B12H122−. Inorg. Chem. 1964, 3, 159–167. [Google Scholar] [CrossRef]
- Barron, P.F.; Dyason, J.C.; Healy, P.C.; Engelhardt, L.M.; Skelton, B.W.; White, A.H. Lewis base adducts of Group 11 metal compounds. Part 24. Co-ordination of triphenylphosphine with silver nitrate. A solid-state cross-polarization magic angle spinning 31P nuclear magnetic resonance, crystal structure, and infrared spectroscopic study of Ag(PPh3)3NO3 (n = 1–4). J. Chem. Soc. Dalton Trans. 1986, 1965–1970. [Google Scholar] [CrossRef] [Green Version]
- Barron, P.F.; Dyason, J.C.; Healy, P.C.; Engelhardt, L.M.; Pakawatchai, C.; Patrick, V.A.; White, A.H. Lewis-base adducts of Group 11 metal(I) compounds. Part 28. Solid-state phosphorus-31 cross-polarization magic-angle spinning nuclear magnetic resonance and structural studies on the mononuclear 3:1 adducts of triphenylphosphine with copper(I) halides. J. Chem. Soc. Dalton Trans. 1987, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–7. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Schaper, T.; Preetz, W. Hexahydro-closo-hexaborate as a ligand in coordination compounds: Syntheses and crystal structure of [M2(μ-bis-η3-B6H6)(PPh3)2] (M = Cu, Au). Chem. Ber. 1997, 130, 405–408. [Google Scholar] [CrossRef]
- Kabbani, R.F.; Rheingold, A.L.; Lam, K.-C.; Margulis, Y.; Vovchenko, M. Synthesis and Crystal Structure of a New Hexahydro-closo-hexaborate Copper(I) Complex Cu2[(C6H5)3P]4B6H6. Inorg. Chem. 1999, 38, 3748–3750. [Google Scholar] [CrossRef]
- Gill, J.T.; Lippard, S.J. Transition metal hydroborate complexes. VIII. Structure of [(PPh3)2Cu]2B10H10⋅CHCl3. Inorg. Chem. 1975, 14, 751–761. [Google Scholar] [CrossRef]
- Drozdova, V.V.; Malinina, E.A.; Polyakova, I.N.; Kuznetsov, N.T. Coordination Isomerism in Complexes of IB Group Metals with the closo-Decaborate Anion [B10H10]2− and Triphenylphosphine. Dokl. Chem. 2008, 418, 30–33. [Google Scholar] [CrossRef]
- Chaozhou, N.; Yilin, M.; Cuifang, S. Crystal and molecular structure of [(C6H5)3P]2Cu}2B12H12. Acta Chim. Sin. 1985, 43, 411. [Google Scholar]
- Lazarou, K.; Bednarz, B.; Kubicki, M.; Verginadis, I.I.; Charalabopoulos, K.; Kourkoumelis, N.; Hadjikakou, S.K. Structural, photolysis and biological studies of the bis(μ2-chloro)-tris(triphenylphosphine)-di-copper(I) and chloro-tris(triphenylphosphine)-copper(I) complexes. Study of copper(I)–copper(I) interactions. Inorg. Chim. Acta 2010, 363, 763–772. [Google Scholar] [CrossRef]
- Folting, K.; Huffman, J.; Mahoney, W.; Stryker, J.M.; Caulton, K.G. Structure of chlorotris(triphenylphosphine)copper(I)-tetrahydrofuran (1/3). Acta Crystallogr. Sect. C 1987, 43, 1490–1492. [Google Scholar] [CrossRef]
- Krauter, T.; Neumuller, B. Triphenylphosphane complexes of copper(I): Structural and 31P NMR investigations. Polyhedron 1996, 15, 2851–2857. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Balthis, J.H.; Chia, Y.T.; Knoth, W.H.; Miller, H.C. Chemistry of Boranes. VIII. Salts and Acids of B10H10−2 and B12H12−2. Inorg. Chem. 1964, 3, 444–451. [Google Scholar] [CrossRef]
- Malinina, E.A.; Zhizhin, K.Y.; Polyakova, I.N.; Lisovskiy, M.V.; Mustyatsa, V.N.; Kuznetsov, N.T. Silver(I) and copper(I) complexes with the closo-decoborate anion B10H102− as a ligand. Russ. J. Inorg. Chem. 2002, 47, 1158–1167. [Google Scholar]
- Paskevicius, M.; Hansen, B.R.S.; Jørgensen, M.; Richter, B.; Jensen, T.R. Multifunctionality of silver closo-boranes. Nat. Commun. 2017, 8, 15136. [Google Scholar] [CrossRef] [Green Version]
- Polyakova, I.N.; Malinina, E.A.; Drozdova, V.V.; Kuznetsov, N.T. Crystal structure of (μ5-decahydro-closo-decaborato) (μ2-O-dimethylformamide)disilver(I) [Ag2(B10H10)(DMF)]. Crystallogr. Rep. 2008, 53, 253–256. [Google Scholar] [CrossRef]
- Kravchenko, E.A.; Gippius, A.A.; Vologzhanina, A.V.; Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Secondary interactions in decachloro-closo-decaborates of alkali metals M2[B10Cl10] (M = K+ and Cs+): 35Cl NQR and X-ray studies. Polyhedron 2016, 117, 561–568. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Merrifield, R.E.; Miller, H.C.; Knoth, W.H., Jr.; Downin, J.R. Chemistry of Boranes. III. The Infrared and Raman Spectra of B12H12− and Related Anions. J. Am. Chem. Soc. 1962, 84, 2506–2508. [Google Scholar] [CrossRef]
- Martina, I.; Wiesinger, R.; Jembrih-Simbürger, D.; Schreiner, M. Micro-raman characterisation of silver corrosion products: Instrumental set up and reference Database. E-Preserv. Sci. 2012, 9, 1–8. [Google Scholar]
- Kravchenko, E.A.; Gippius, A.A.; Vologzhanina, A.V.; Avdeeva, V.V.; Malinina, E.A.; Demikhov, E.I.; Kuznetsov, N.T. Secondary interactions as defined by 35Cl NQR spectra in cesium decachloro-closo-decaborates prepared in non-aqueous solutions. Polyhedron 2017, 138, 140–144. [Google Scholar] [CrossRef]
- Vologzhanina, A.V.; Korlyukov, A.A.; Avdeeva, V.V.; Polyakova, I.N.; Malinina, E.A.; Kuznetsov, N.T. Theoretical QTAIM, ELI-D, and Hirshfeld Surface Analysis of the Cu–(H)B Interaction in [Cu2(bipy)2B10H10]. J. Phys. Chem. A 2013, 117, 13138–13150. [Google Scholar] [CrossRef] [PubMed]
- Smol’yakov, A.F.; Korlyukov, A.A.; Dolgushin, F.M.; Balagurova, E.V.; Chizhevsky, I.T.; Vologzhanina, A.V. Studies of Multicenter and Intermolecular Dihydrogen B–H···H–C Bonding in [4,8,8′-exo-{PPh3Cu}-4,8,8′-(μ-H)3-commo-3,3′-Co(1,2-C2B9H9)(1′,2′-C2B9H10)]. Eur. J. Inorg. Chem. 2015, 36, 5847–5855. [Google Scholar] [CrossRef]
- Savchenkov, A.V.; Klepov, V.V.; Vologzhanina, A.V.; Serezhkina, L.B.; Pushkin, D.V.; Serezhkin, V.N. Trinuclear {Sr[UO2L3]2(H2O)4} and pentanuclear {Sr[UO2L3]4}2− uranyl monocarboxylate complexes (L-acetate or n-butyrate ion). CrystEngComm 2015, 17, 740–746. [Google Scholar] [CrossRef]
- Kalaj, M.; Karter, K.P.; Savchenkov, A.V.; Pyrch, M.M.; Cahill, C.C. Syntheses, Structures, and Comparisons of Heterometallic Uranyl Iodobenzoates with Monovalent Cations. Inorg. Chem. 2017, 56, 9156–9168. [Google Scholar] [CrossRef]
- Skovsen, I.; Christensen, M.; Clausen, H.F.; Overgaard, J.; Stiewe, C.; Desgupta, T.; Mueller, E.; Spackman, M.A.; Iversen, B.B. Synthesis, Crystal Structure, Atomic Hirshfeld Surfaces, and Physical Properties of Hexagonal CeMnNi4. Inorg. Chem. 2010, 49, 9343–9349. [Google Scholar] [CrossRef]
- Jørgensen, M.R.V.; Skovsen, I.; Clausen, H.F.; Mi, J.-L.; Christensen, M.; Nishibori, E.; Spackman, M.A.; Iversen, B.B. Application of Atomic Hirshfeld Surface Analysis to Intermetallic Systems: Is Mn in Cubic CeMnNi4 a Thermoelectric Rattler atom? Inorg. Chem. 2012, 51, 1916–1924. [Google Scholar] [CrossRef]
- Kastbjerg, S.; Uvarov, C.A.; Kauzlarich, S.M.; Chen, Y.-S.; Nishibori, E.; Spackman, M.A.; Iversen, B.B. Crystal structure and chemical bonding of the intermetallic Zintl phase Yb11AlSb9. Dalton Trans. 2012, 41, 10347–10353. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17 (2017). University of Western Australia. Available online: http://hirshfeldsurface.net (accessed on 20 April 2020).
- Roy, D.; Furtak, T.E. Evidence for Ag cluster vibrations in enhanced Raman scattering from the Ag/Electrolyte Interface. Chem. Phys. Lett. 1986, 124, 299–303. [Google Scholar] [CrossRef]




| Parameter | 1∙ 2DMF | 2∙ 4DMF | 3∙ 2DMF | 4 | 5∙ 0.1H2O | 6 | 7 | 8 |
| Formula | C90H102Ag2B10Cl10N6O6P4 | C120H122Ag2B10Cl10N4O6P6 | C60H59ClCuN2O2P3 | C54H45Cl2Cu2P3 | C108H100.2Ag2B10O0.1P6 | Ag2B10Cl10 | C6H14Ag2B10Cl10N2O2 | C8H12Ag2B10Cl10N4 |
| Fw | 2165.99 | 2580.37 | 1031.99 | 984.76 | 1909.34 | 678.34 | 824.52 | 842.56 |
| Crystal system, space group | Monoclinic, C2/c | Monoclinic, C2/c | Triclinic, | Monoclinic, P21/c | Triclinic, | Hexagonal, P6222 | Monoclinic, C2/c | Tetragonal, P41212 |
| T (K) | 120.0(2) | 120.0(2) | 120.0(2) | 120.0(2) | 120.0(2) | 120.0(2) | 120.0(2) | 120.0(2) |
| a (Å) | 36.319(2) | 37.202(3) | 10.6772(4) | 18.942(2) | 13.5059(6) | 9.1869(7) | 16.195(4) | 11.2878(3) |
| b (Å) | 12.2841(7) | 13.1519(11) | 13.0436(5) | 9.7711(13) | 14.8623(7) | 9.1869(7) | 12.987(3) | 11.2878(3) |
| c (Å) | 23.3211(14) | 27.6119(19) | 20.3604(7) | 37.198(4) | 24.1499(11) | 19.476(3) | 12.289(3) | 22.2808(12) |
| α (°) | 90 | 90 | 108.352(1) | 90 | 91.598(1) | 90 | 90 | 90 |
| β (°) | 107.761(1) | 114.188(2) | 103.547(1) | 138.248(3) | 102.758(1) | 90 | 97.082(5) | 90 |
| γ (°) | 90 | 90 | 90.535(1) | 90 | 102.758(1) | 120 | 90 | 90 |
| V (Å3) | 9908.8(10) | 12,323.7(18) | 2605.85(17) | 4584.7(10) | 4719.1(4) | 1423.5(3) | 2564.9(11) | 2838.9(2) |
| Z | 4 | 4 | 2 | 4 | 2 | 3 | 2 | 4 |
| μ (cm−1) | 0.784 | 0.667 | 0.608 | 1.186 | 0.566 | 3.449 | 2.580 | 2.331 |
| No. of meas., indep. and obs. refl. | 72,769, 16,960, 12,277 | 57,468, 14,880, 11,554 | 43,302, 20,507, 14,307 | 48,895, 12,935, 6575 | 41,719, 18,266, 13,442 | 20,906, 1595, 1387 | 18,259, 4748, 3764 | 28,810, 4927, 3373 |
| Rint | 0.0712 | 0.0595 | 0.0431 | 0.1904 | 0.0434 | 0.1126 | 0.0594 | 0.0702 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.0422, 0.0944, 1.004 | 0.0425, 0.1151, 0.999 | 0.0481, 0.1203, 1.006 | 0.0655, 0.0921, 1.007 | 0.0411, 0.0944, 0.987 | 0.0343, 0.0694, 1.058 | 0.0395, 0.1018, 0.996 | 0.0403, 0.0855, 0.998 |
| Δρmax, Δρmin (e Å−3) | 0.91, −0.33 | 2.45, −1.09 | 1.01, −0.52 | 0.98, −0.88 | 1.28, −0.57 | 0.79, −0.80 | 2.15, −1.51 | 0.75, −0.49 |
| Bond | 1 · 2DMF | 2 · 4DMF | 3 · 2DMF | 4 | 5 · 0.1H2O |
|---|---|---|---|---|---|
| M | AgI | AgI | CuI | CuI | AgI |
| M–P | 2.4174(5)–2.4262(5) | 2.4873(7)–2.4967(7) | 2.3125(5)–2.3197(4) | 2.177(1)–2.236(1) | 2.4921(8)–2.6516(8) |
| M–O | 2.374(2)–2.536(2) | 2.476(2) | |||
| M…B | 2.646(6)–2.758(6) | ||||
| M–Cl | 2.3358(4) | 2.245(1)–2.452(1) |
| Complex | M–Cl | M–N | M–O | Reference |
|---|---|---|---|---|
| 6 | 2.706(1)–2.893(1) | This work | ||
| 7 | 2.6364(9)–3.4908(8) | 2.391(2) | This work | |
| 8 | 3.000(1)–3.619(1) | 2.158(6)–2.159(6) | This work | |
| [Ag2[B12Cl12]]n | 2.825(2)–2.851(2) | [31] | ||
| [Ag(CH3CN)[(Me3N)B12Cl11]]n | 2.652(1)–3.016(1) | 2.188(3)–2.227(3) | [28] | |
| {[Ag2(SO2)[(Me3N)B12Cl11]2] · SO2}n | 2.652(1)–2.899(1) | 2.523(4) | [32] | |
| {[Ag[(Me3N)B12Cl11]] · 0.5CH2Cl2}n | 2.668(2)–2.957(1) | [32] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdeeva, V.V.; Buzanov, G.A.; Malinina, E.A.; Kuznetsov, N.T.; Vologzhanina, A.V. Silver(I) and Copper(I) Complexation with Decachloro-Closo-Decaborate Anion. Crystals 2020, 10, 389. https://doi.org/10.3390/cryst10050389
Avdeeva VV, Buzanov GA, Malinina EA, Kuznetsov NT, Vologzhanina AV. Silver(I) and Copper(I) Complexation with Decachloro-Closo-Decaborate Anion. Crystals. 2020; 10(5):389. https://doi.org/10.3390/cryst10050389
Chicago/Turabian StyleAvdeeva, Varvara V., Grigoriy A. Buzanov, Elena A. Malinina, Nikolay T. Kuznetsov, and Anna V. Vologzhanina. 2020. "Silver(I) and Copper(I) Complexation with Decachloro-Closo-Decaborate Anion" Crystals 10, no. 5: 389. https://doi.org/10.3390/cryst10050389
APA StyleAvdeeva, V. V., Buzanov, G. A., Malinina, E. A., Kuznetsov, N. T., & Vologzhanina, A. V. (2020). Silver(I) and Copper(I) Complexation with Decachloro-Closo-Decaborate Anion. Crystals, 10(5), 389. https://doi.org/10.3390/cryst10050389