A Comprehensive Study of a New 1.75 Hydrate of Ciprofloxacin Salicylate: SCXRD Structure Determination, Solid Characterization, Water Stability, Solubility, and Dissolution Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Methods
2.3.1. Multicomponent Salt Formation
Ciprofloxacin Base Preparation
Binary Phase Diagram Construction
Ciprofloxacin Salicylate 1.75 Hydrate Salt Formation
2.3.2. Characterization
Fourier-Transform Infrared Spectroscopy (FTIR)
Raman Spectroscopy
Powder X-ray Diffractometry (PXRD)
Single Crystal X-ray Diffractometry (SCXRD)
Thermal Analysis
2.3.3. Performance Evaluation
Solubility Study
Dissolution Study
2.3.4. Dehydration
3. Results
3.1. Multicomponent Salt Formation
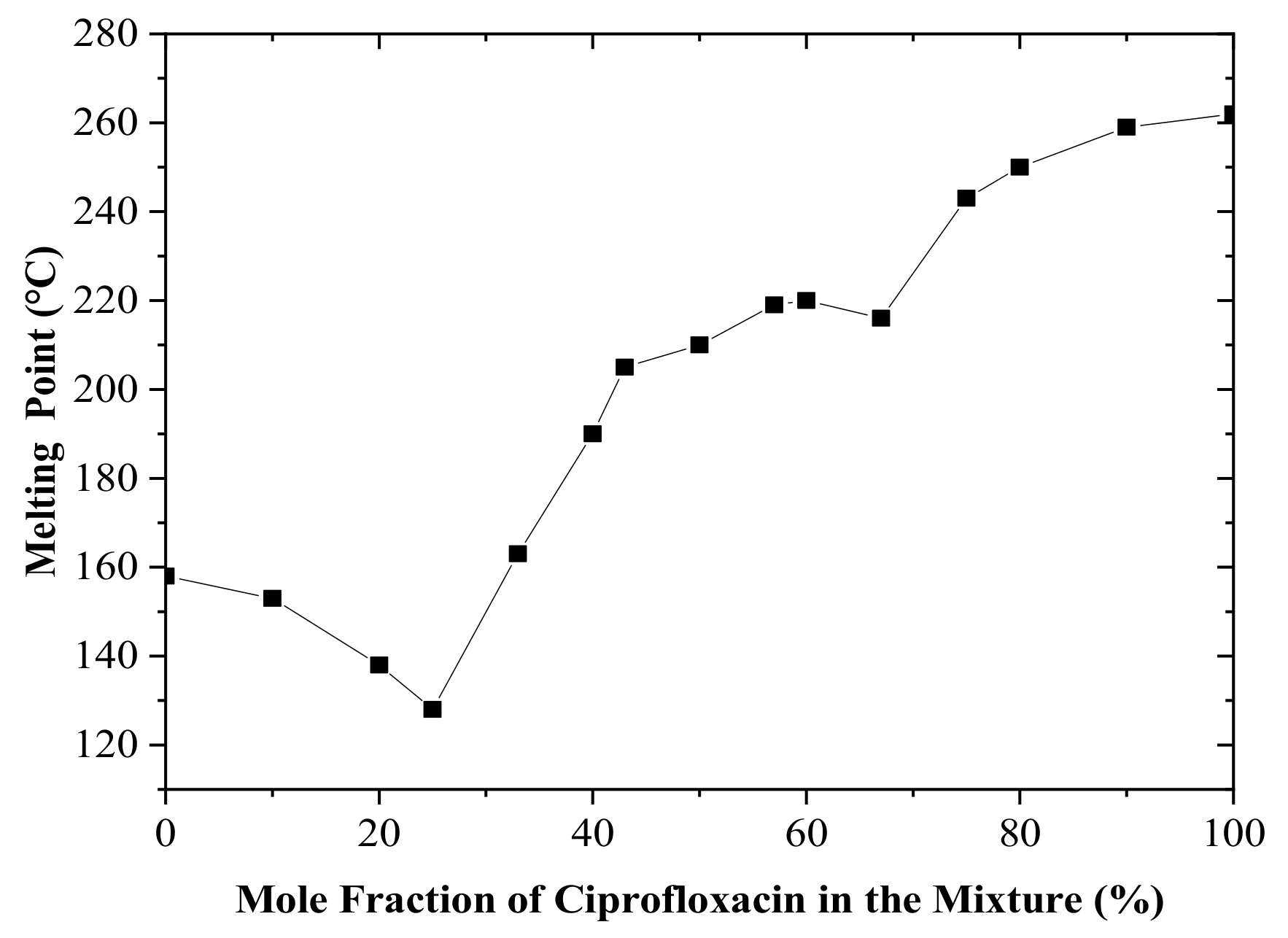
3.2. Binary Phase Diagram Construction
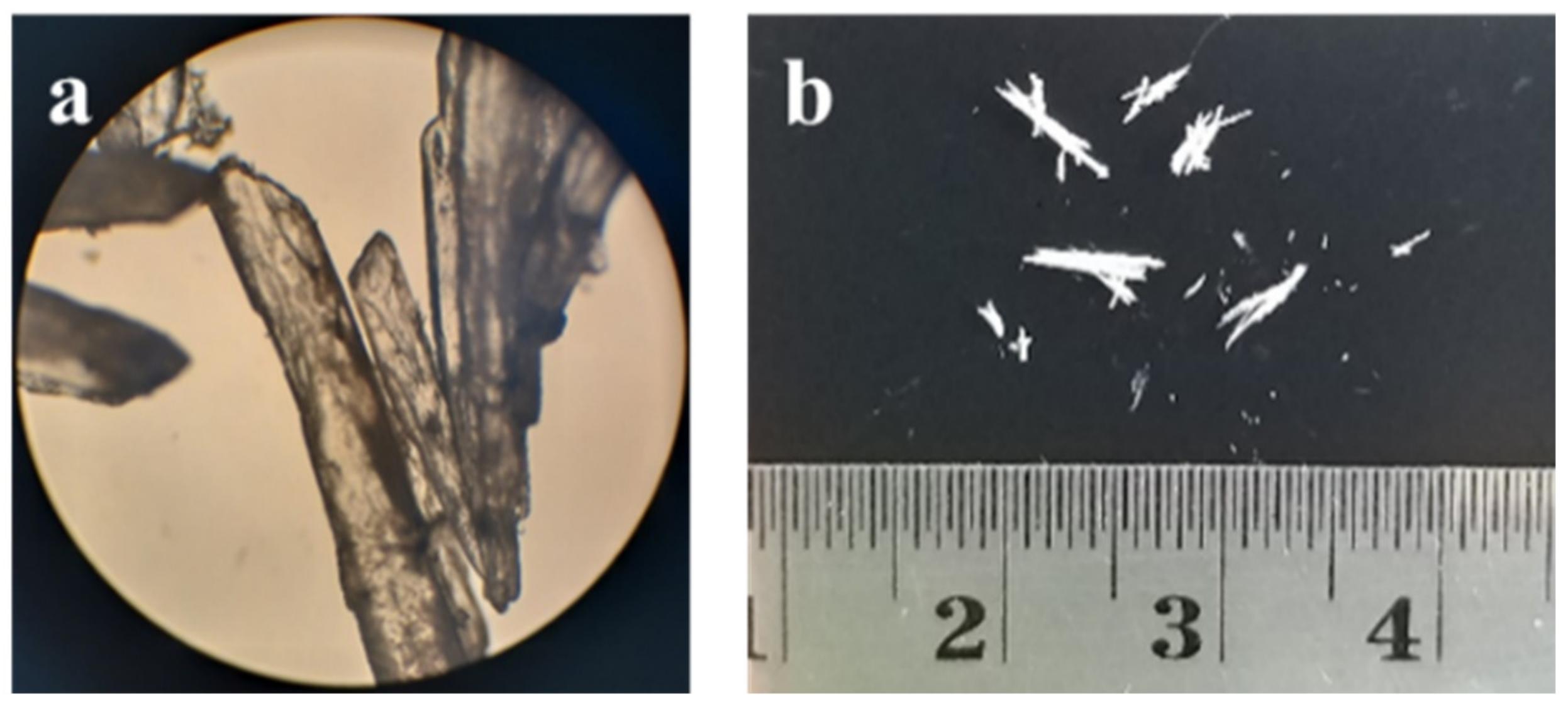
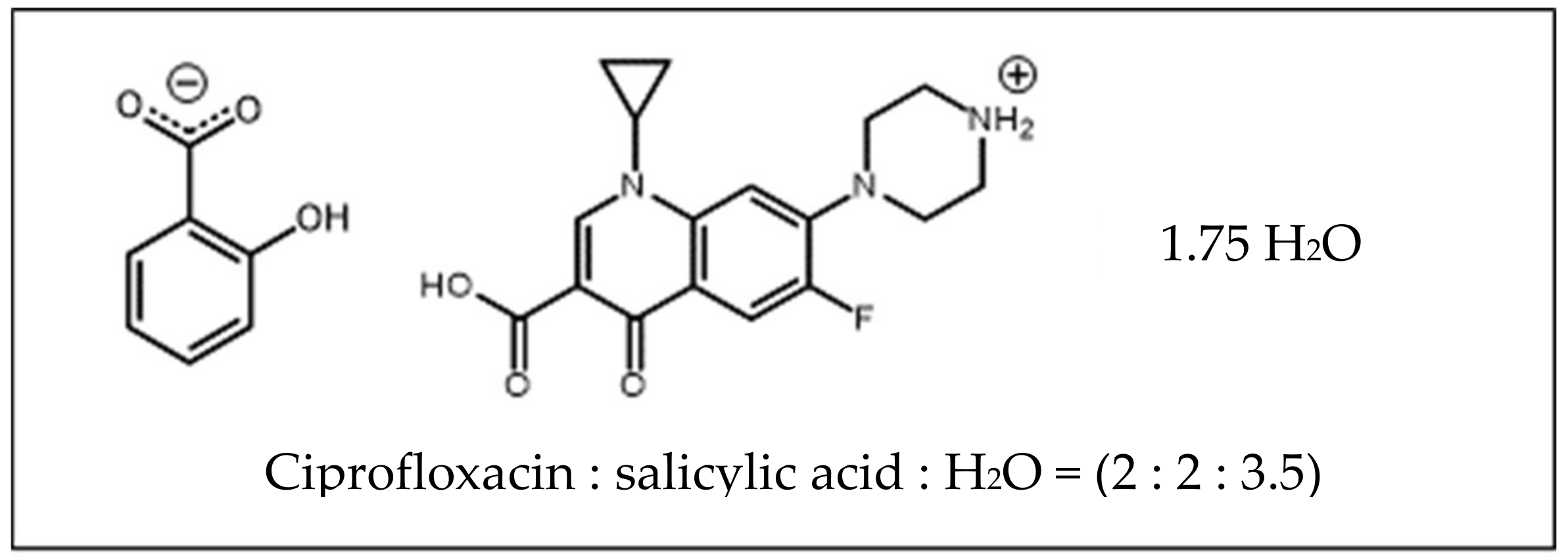
3.3. Ciprofloxacin Salicylate 1.75 Hydrate Crystal Preparation
3.4. Characterization
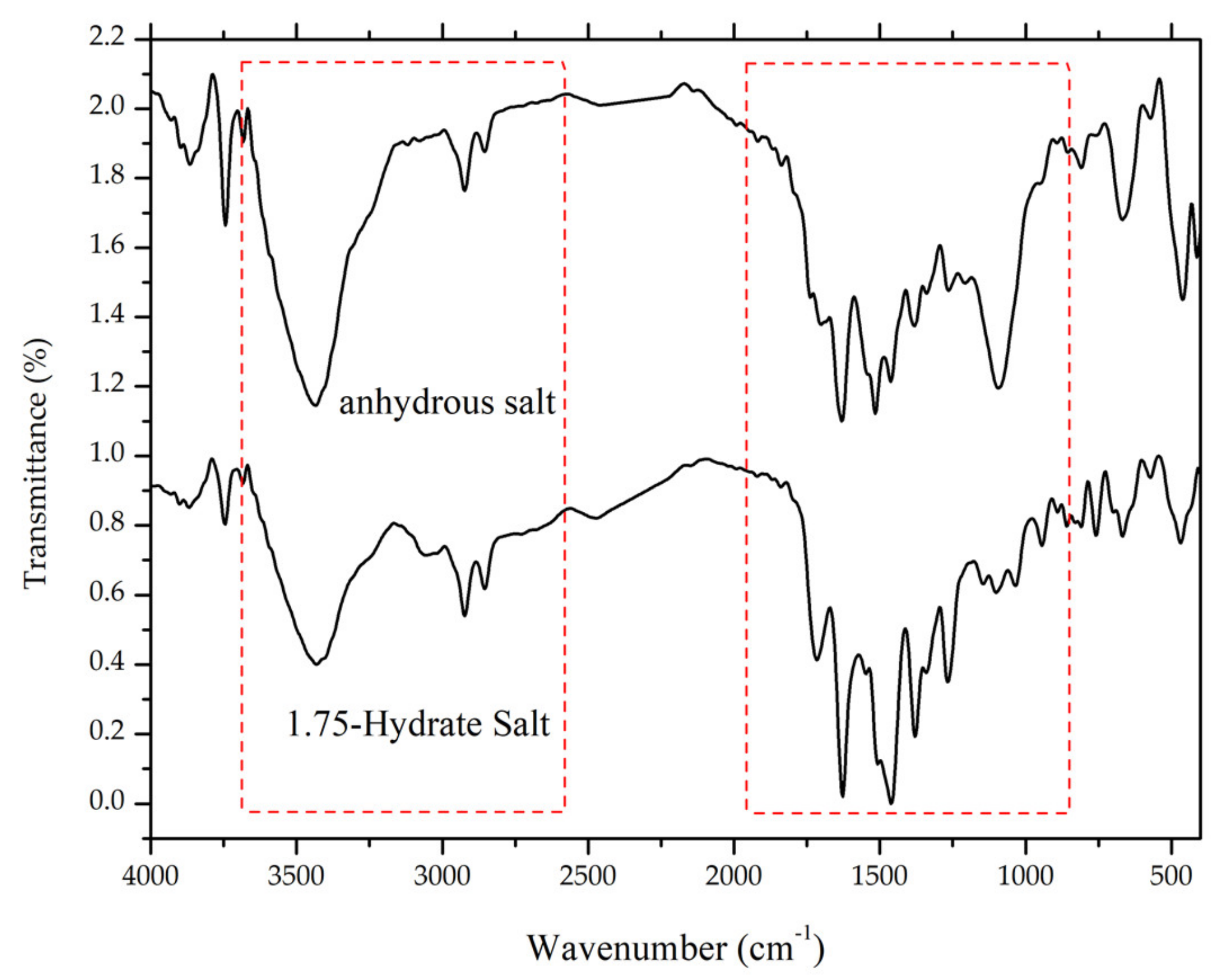
3.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
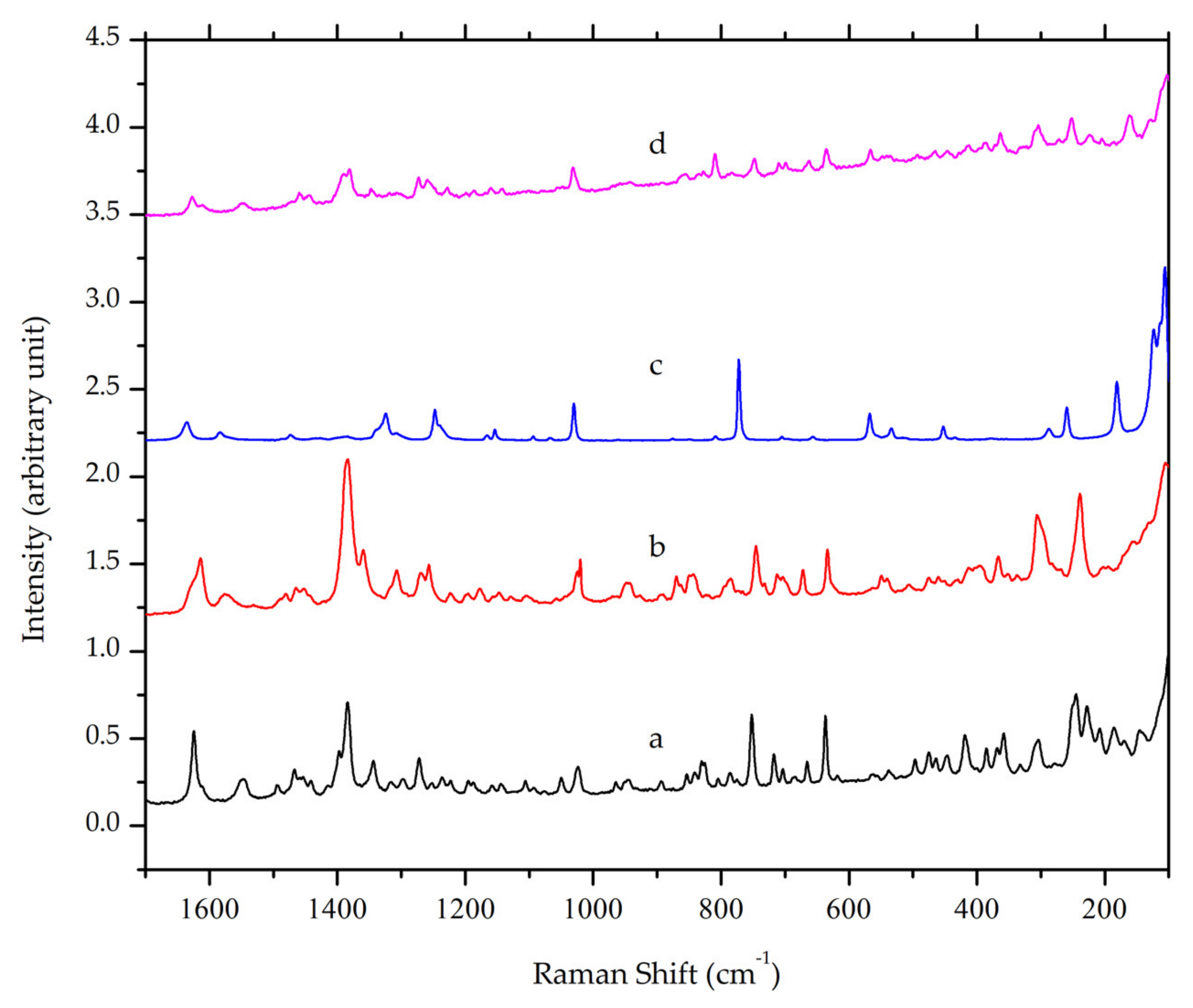
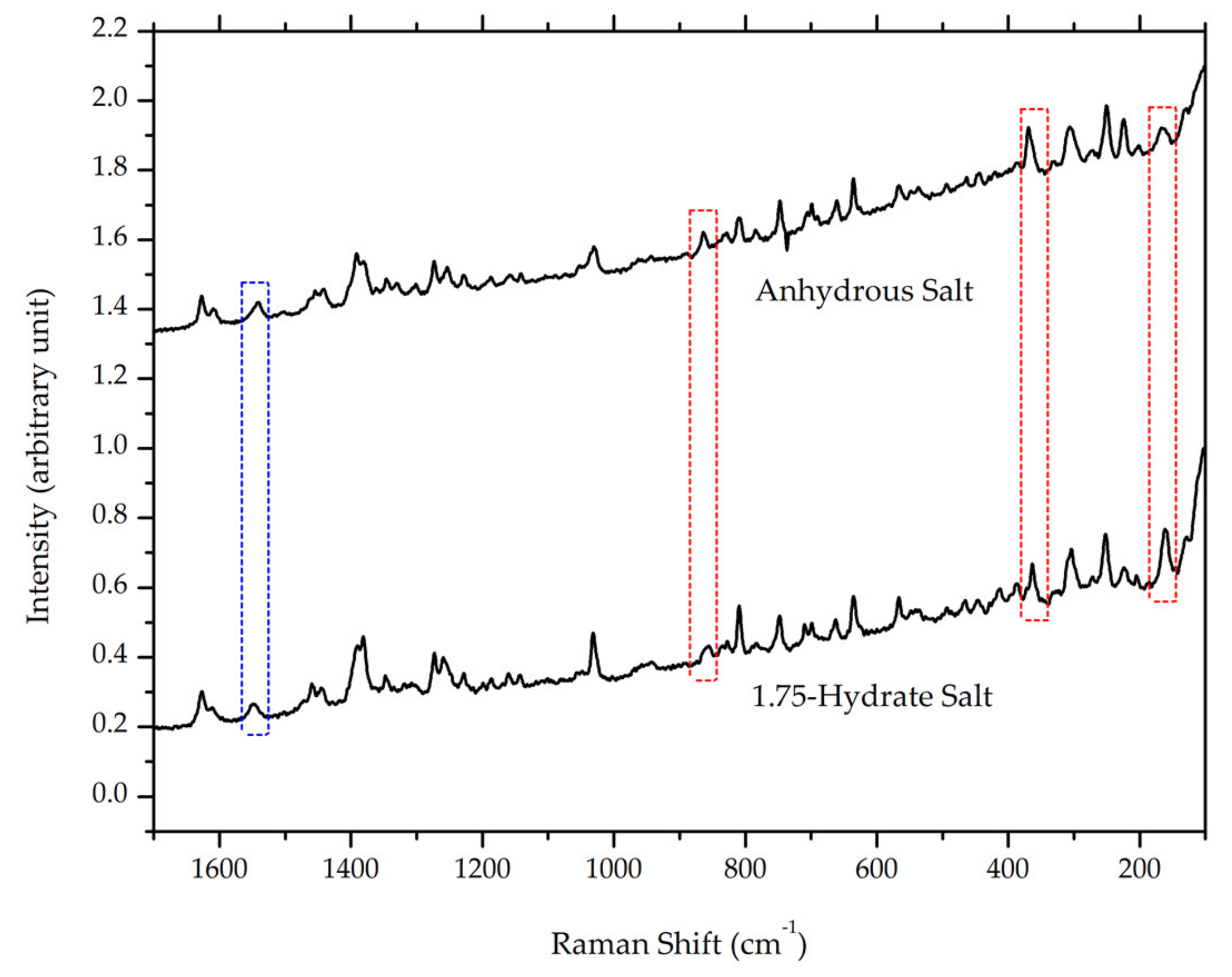
3.4.2. Raman Spectroscopy
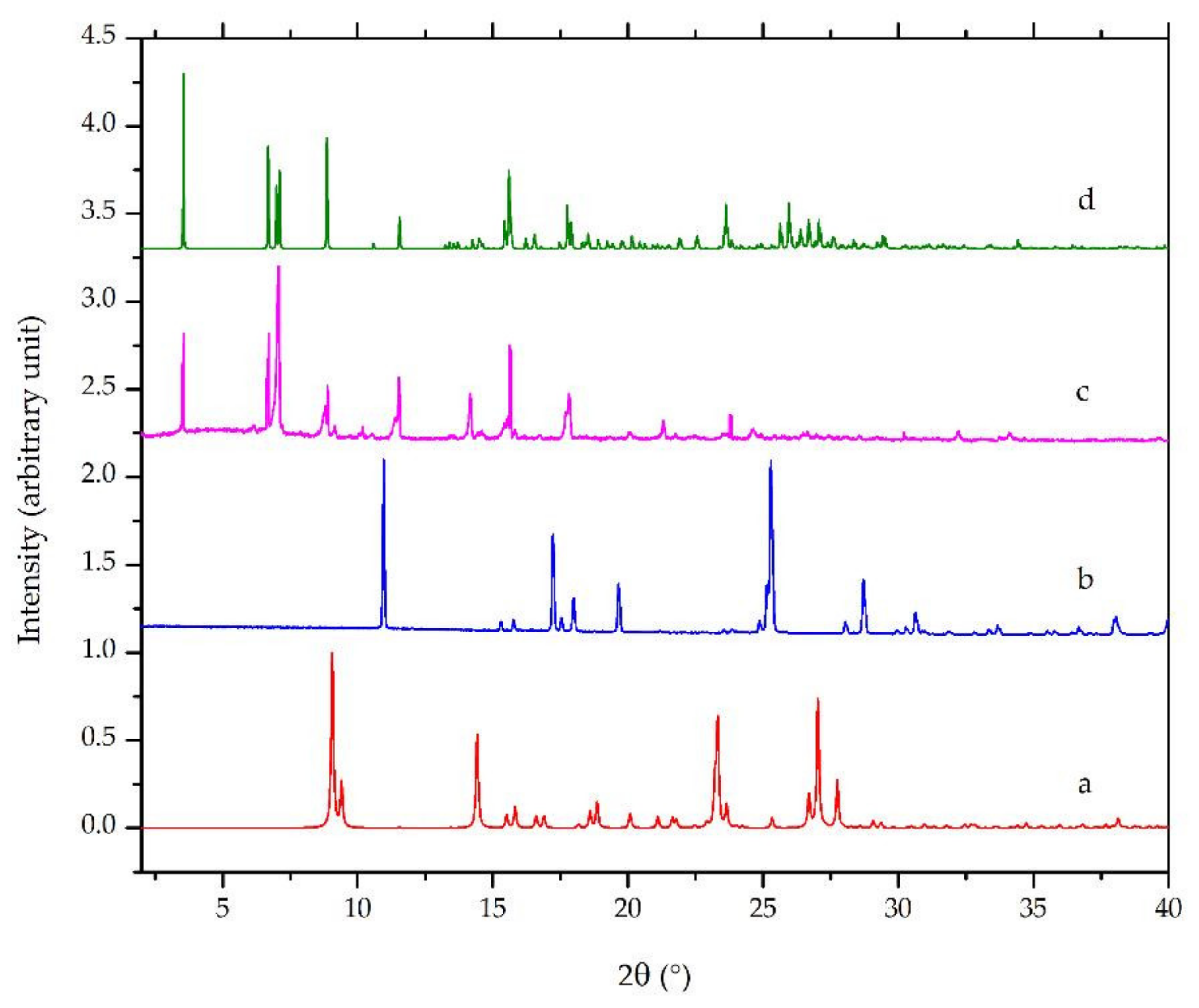
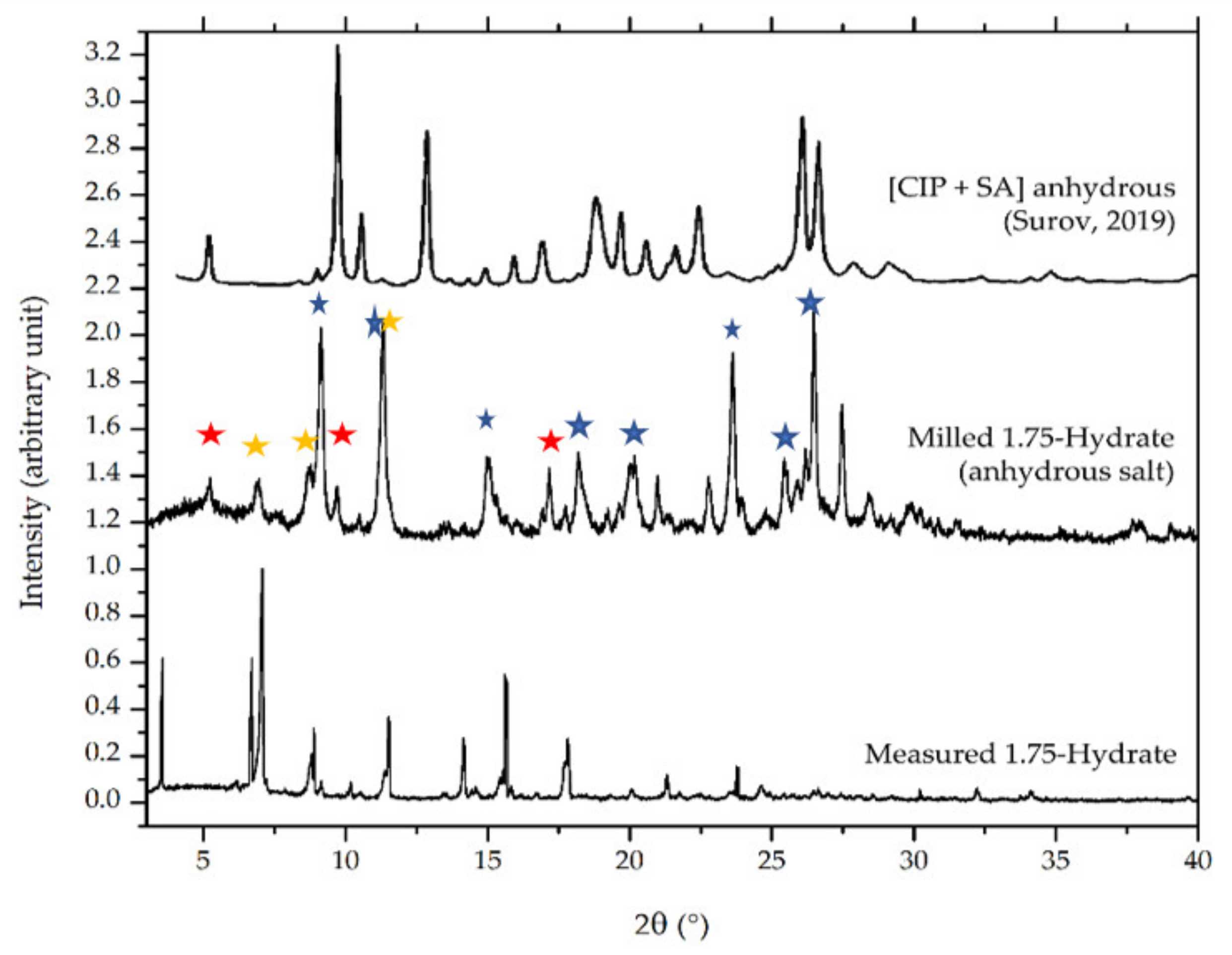
3.4.3. Powder X-ray Diffractometry (PXRD)
3.4.4. Single Crystal X-ray Diffractometry (SCXRD)
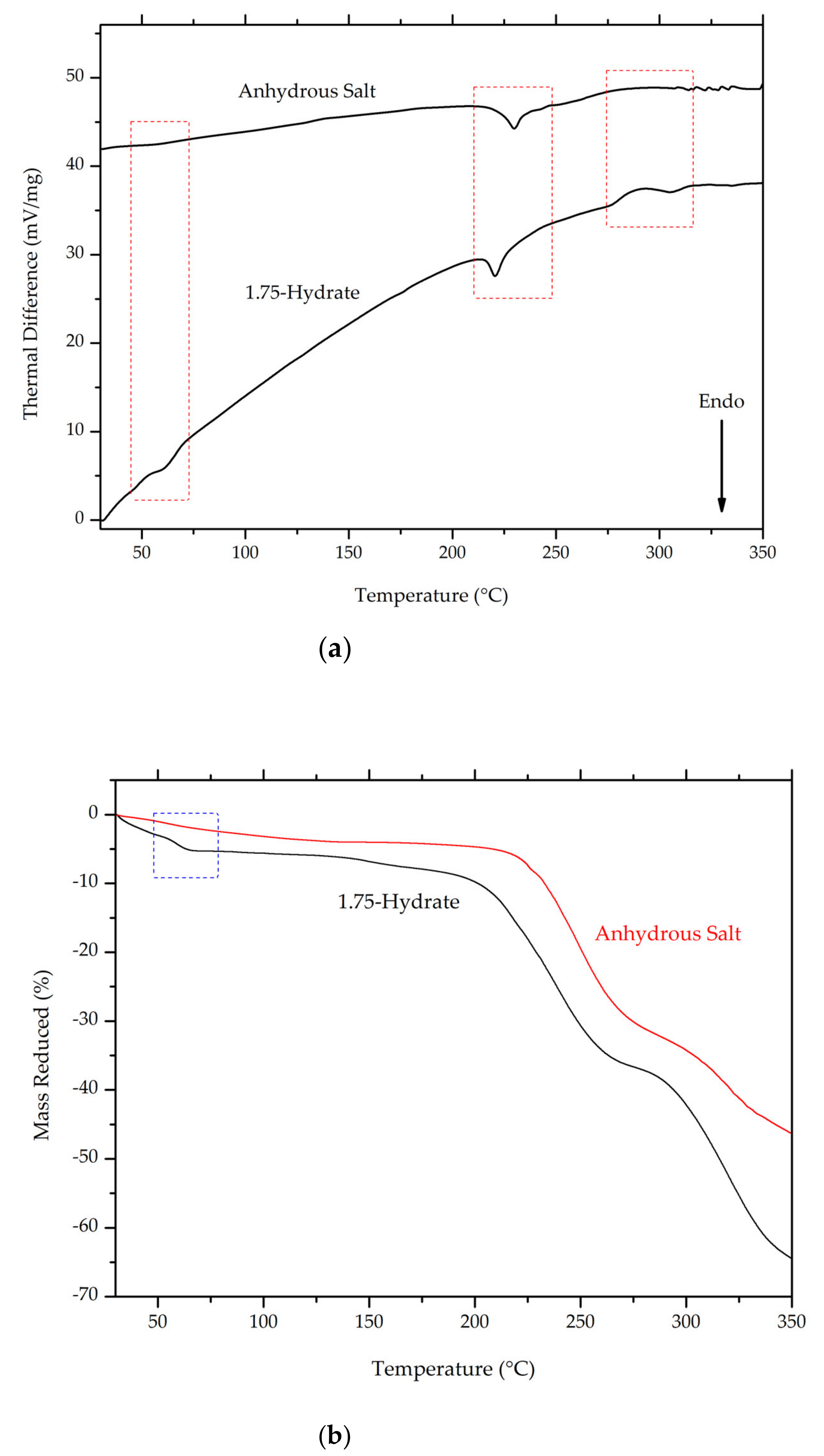
3.4.5. Thermal Analysis
3.5. Performance Evaluation
3.5.1. Solubility Study
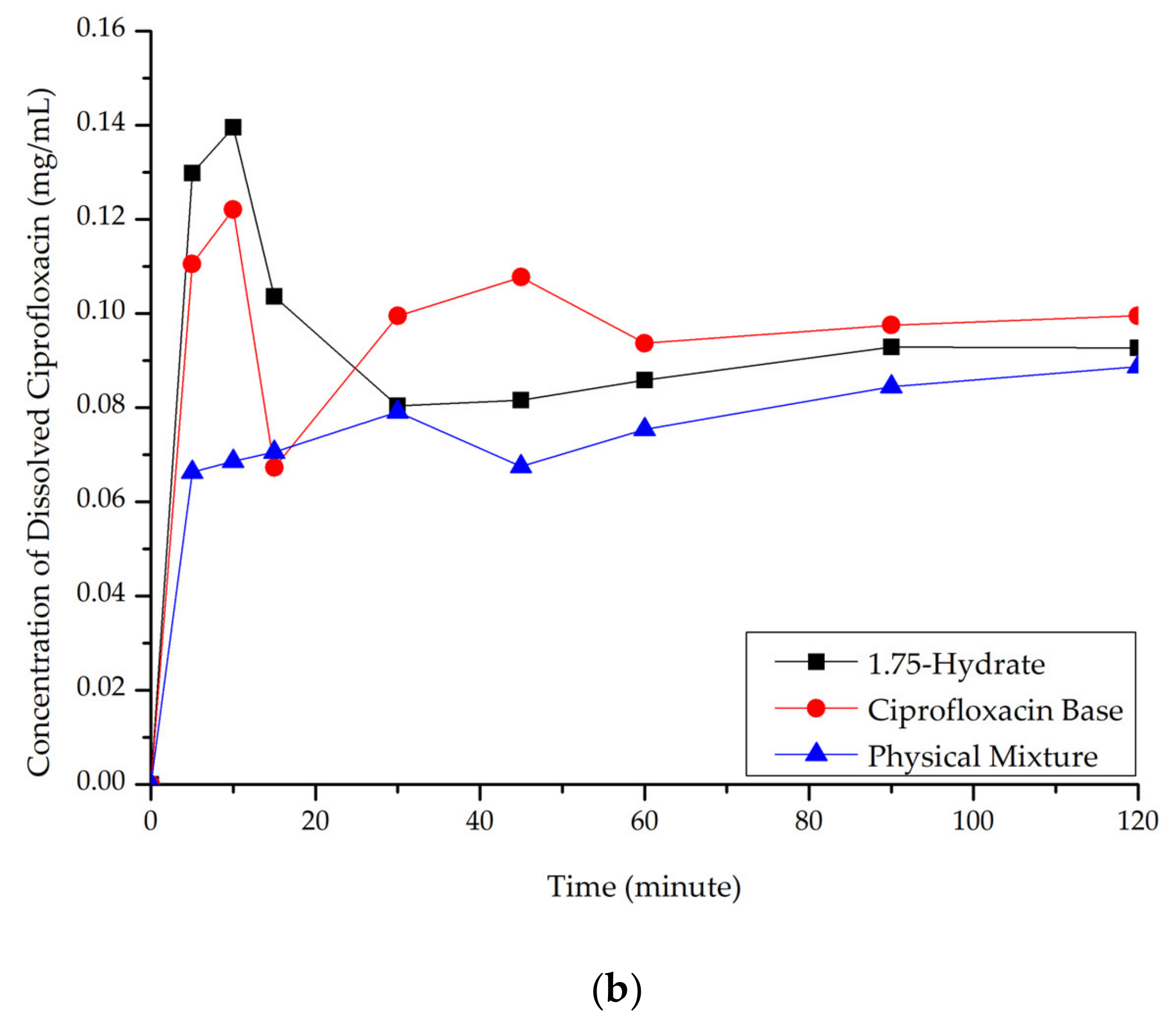
3.5.2. Dissolution Study
3.6. Dehydration by Milling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Troughton, J.A.; Millar, G.; Smyth, E.T.M.; Doherty, L.; McMullan, R. Ciprofloxacin use and susceptibility of Gram-negative organisms to quinolone and non-quinolone antibiotics. J. Antimicrob. Chemother. 2011, 66, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.X.; Murray, J. Biochemistry, Dissolution and Solubility. StatPearls Publishing: Treasure Island, Florida, 2019. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28613752 (accessed on 20 November 2019).
- Khadka, P.; Roa, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2019, 9, 304–316. [Google Scholar] [CrossRef]
- Olivera, M.E.; Manzo, R.H.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Ciprofloxacin hydrochloride. J. Pharm. Sci. 2011, 100, 22–33. [Google Scholar] [CrossRef]
- Cavanagh, K.L.; Maheshwari, C.; Hornedo, N.R. Understanding the Differences between Cocrystal and Salt Aqueous Solubilities. J. Pharm. Sci. 2018, 107, 113–120. [Google Scholar] [CrossRef]
- Bannigan, P.; Verma, V.; Hudsan, S.P. Overcoming the Common Ion Effect for Weakly Basic Drugs: Inhibiting the Crystallization of Clofazimine Hydrochloride in Simulated Gastrointestinal Media. Cryst. Growth Des. 2019, 19, 1599–1609. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeey, M.S.; Lesnikov, M.K.; Atuchin, V.V. Two salts and the salt cocrystal of ciprofloxacin with thiobarbituric and barbituric acids: The structure and properties. J. Phys. Org. Chem. 2017, 31, 1–10. [Google Scholar] [CrossRef]
- Vioglio, P.C.; Chieroitti, M.R.; Gobetto, R. Pharmaceutical aspects of salt and cocrystal forms of APIS and characterization challenges. Adv. Drug Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Nagalapalli, R.; Bheem, S.Y. Synthesis, Crystal Structure, and Hirshfeld Surface Analysis of Ciprofloxacin-Salicylic Acid Molecular Salt. J. Crystallogr. 2014, 2014, 936174. [Google Scholar] [CrossRef]
- Surov, A.O.; Vasilev, N.A.; Churakov, A.V.; Stroh, J.; Emmerling, F.; Perlovich, G.L. Solid Forms of Ciprofloxacin Salicylate: Polymorphism, Formation Pathways, and Thermodynamic Stability. Cryst. Growth Des. 2019, 19, 2979–2990. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53 Pt 1, 226–235. [Google Scholar] [CrossRef]
- Dalhoff, A.; Schubert, S.; Vente, A. Pharmacodynamics of Finafloxacin, Ciprofloxacin, and Levofloxacin in Serum and Urine against TEM- and SHV-Type Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae Isolates from Patients with Urinary Tract Infections. Antimicrob. Agents Chemother. 2017, 61, e02446-16. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Hirakura, Y.; Yuda, M.; Teramura, T.; Terada, K. Detection of Cocrystal Formation Based on Binary Phase Diagrams Using Thermal Analysis. Pharm. Res. 2013, 30, 70–80. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Row, T.N.G. Comprehending the Formation of Eutectics and Cocrystals in Terms of Design and Their Structural Interrelationships. Cryst. Growth. Des. 2014, 14, 4187–4198. [Google Scholar] [CrossRef]
- Stoler, E.; Warner, J.C. Non-Covalent Derivatives: Cocrystals and Eutectics. Molecules 2015, 20, 14833–14848. [Google Scholar] [CrossRef]
- Chadha, R.; Gupta, S.; Shukla, G. Crystal habit, characterization and pharmacological activity of various crystal forms of arteether. Acta Pharm. B 2011, 1, 129–135. [Google Scholar] [CrossRef][Green Version]
- Wermuth, C.; Stahl, P. Handbook of Pharmaceutical Salts, 2nd ed.; Verlag Helvetica Chimica Acta: Zurich, Switzerland, 2011. [Google Scholar]
- Rychkov, D.A.; Arkhipov, S.G.; Boldyreva, E.V. Simple and efficient modifications of well-known techniques for reliable growth of high-quality crystals of small bioorganic molecules. J. Appl. Crystallogr. 2014, 47, 1435–1442. [Google Scholar] [CrossRef]
- Rager, T.; Hilfiker, R. Cocrystal Formation from Solvent Mixtures. Cryst. Growth. Des. 2012, 10, 3237–3241. [Google Scholar] [CrossRef]
- Pubchem: Ciprofloxacin. Available online: Pubchem.ncbi.nlm.nih.gov/compound/Ciprofloxacin (accessed on 20 November 2019).
- Pubchem: Salicylic Acid. Available online: Pubchem.ncbi.nlm.nih.gov/compound/Salicylic-acid (accessed on 20 November 2019).
- Han, L.; Zhang, K.; Ishida, H.; Froimowicz, P. Study of the Effects of Intramolecular and Intermolecular Hydrogen-Bonding Systems on the Polymerization of Amide-Containing Benzoxazines. Macromol. Chem. Phys. 2017, 218, 1600562. [Google Scholar] [CrossRef]
- Schkesinger, C.; Bolte, M.; Schmidt, M.U. Challenging structure determination from powder diffraction data: Two pharmaceutical salts and one cocrystal with Z′ = 2. Z. Krist-Cryst. Mater. 2018, 234, 257–268. [Google Scholar] [CrossRef]
- Zheng, H.; Xiong, J.; Zhao, Z.; Qiao, J.; Xu, D.; Miao, M.; He, L.; Wu, X. Preparation of Progesterone Co-Crystals Based on Crystal Engineering Strategies. Molecules 2019, 24, 3936. [Google Scholar] [CrossRef] [PubMed]
- Basavoju, S.; Boström, D.; Velaga, S. Pharmaceutical cocrystal and salts of norfloxacin. Cryst. Growth Des. 2006, 6, 2699–2708. [Google Scholar] [CrossRef]
- Martinez-Alejo, J.M.; Dominguez-Chavez, J.G.; Rivera-Islas, J.; Herrera-Ruiz, D.; Hoepfl, H.; Morales-Rojas, H.; Senosiain, J.P. A Twist in Cocrystals of Salts: Changes in Packing and Chloride Coordination Lead to Opposite Trends in the Biopharmaceutical Performance of Fluoroquinolone Hydrochloride Cocrystals. Cryst. Growth Des. 2014, 14, 3078–3095. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Shettigar, H.; Bairwa, K.; Jana, S. Fourier transform infrared and ultraviolet-visible spectroscopic characterization of biofield treated salicylic acid and sparfloxacin. Nat. Prod. Chem. Res. 2015, 3, 1000186. [Google Scholar] [CrossRef]
- Neugebauer, U.; Szeghalmi, A.; Schmitt, M.; Kiefer, W.; Popp, J.; Holzgrabe, U. Vibrational spectroscopic characterization of fluoroquinolones. Spectrochim. Acta A 2005, 61, 1505–1517. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Zhang, S.; Zhou, X. Synthesis, Crystal Structure, and Solubility Analysis of a Famotidine Cocrystal. Crystals 2019, 9, 360. [Google Scholar] [CrossRef]
- An, H.; Choi, I.; Kim, I.W. Melting diagrams of adefovir dipivoxil and dicarboxylic acids: An approach to assess cocrystal compositions. Crystals 2019, 9, 70. [Google Scholar] [CrossRef]
- Poppler, A.C.; Corlett, E.K.; Pearce, H.; Seymour, M.P.; Reid, M.; Montgomery, M.G.; Brown, S.P. Single-crystal X-ray diffraction and NMR crystallography of a 1:1 cocrystal of dithianon and pyrimethanil. Acta Cryst. 2017, 73, 149–156. [Google Scholar]
- Aitipamula, S.; Vangala, V.R. X-Ray crystallography and its role in understanding the physicochemical properties of pharmaceutical cocrystals. J. Indian Inst. Sci. 2017, 97, 227–243. [Google Scholar] [CrossRef]
- Mahapatra, S.; Venugopala, K.N.; Row, T.N.G. A Device to Crystallize Organic Solids: Structure of ciprofloxacin, midazolam, and ofloxacin as targets. Crys. Growth Des. 2010, 10, 1866–1870. [Google Scholar] [CrossRef]
- Woinska, M.; Grabowsky, S.; Dominiak, P.M.; Wozniak, K.; Jayatilaka, D. Hydrogen atoms can be located accurately and precisely by x-ray crystallography. Sci. Adv. 2016, 2, e1600192. [Google Scholar] [CrossRef] [PubMed]
- Saganowska, P.; Wesolowski, M. DSC as a screening tool for rapid co-crystal detection in binary mixtures of benzodiazepines with co-formers. J. Therm. Anal. Calorim. 2018, 133, 785–795. [Google Scholar] [CrossRef]
- Khan, S.B.; Alamry, K.A.; Alyahyawi, N.A.; Asiri, A.M. Nanohybrid based on antibiotic encapsulated layered double hydroxide as a drug delivery system. Appl. Biochem. Biotechnol. 2014, 175, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.T.; Wang, C.J.; Li, Z. Intercalation of ciprofloxacin accompanied by dehydration in rectorite. Appl. Clay Sci. 2013, 74, 74–80. [Google Scholar] [CrossRef]
- Badea, M.; Olar, R.; Marinescu, D.; Uivarosi, V.; Iacob, D. Thermal decomposition of some biologically active complexes of ruthenium (III) with quinolone derivatives. J. Therm. Anal. Calorim. 2009, 97, 735. [Google Scholar] [CrossRef]
- Satisharan, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, E108. [Google Scholar] [CrossRef]
- Emami, S.; Shadbad, M.S.; Adibkia, K.; Jalali, M.B. Recent advances in improving oral drug bioavailability by cocrystals. Bioimpacts 2018, 8, 305–320. [Google Scholar] [CrossRef]
- Scherer, R.; Pereira, J.; Firme, J.; Lemos, M.; Lemos, M. Determination of Ciprofloxacin in Pharmaceutical Formulations Using HPLC Method with UV Detection. Indian J. Pharm. Sci. 2014, 76, 541. [Google Scholar]
- Emami, J.; Rezazadeh, M. A simple and sensitive high-performance liquid chromatography method for determination of ciprofloxacin in bioavailability studies of conventional and gastroretentive prolonged-release formulations. Adv. Biomed. Res. 2016, 5, 163. [Google Scholar] [PubMed]
- Naveed, S.; Waheed, N.; Simple, U.V. Spectrophotometruc Assay of Ciprofloxacin. Mintage J. Pharm. Med. Sci. 2014, 5, 10–13. [Google Scholar]
- Badel, D.A.P.; Rivas, B.L.; Urbano, B. Ultrafiltration membranes with three water-soluble polyelectrolyte copolymers to remove ciprofloxacin from aqueous systems. Chem. Eng. J. 2018, 351, 85–93. [Google Scholar]
- Perez, H.A.; Butos, A.; Taranto, M.P. Effects of Lysozyme on the Activity of Ionic of Fluoroquinolone Species. Molecules 2018, 23, 741. [Google Scholar] [CrossRef]
- Guo, H.B.; He, F.; Gu, B.; Liang, L. Time-Dependent Density Functional Theory Assessment of UV Absorption of Benzoic Acid Derivatives. J. Phys. Chem. A 2012, 116, 11870–11879. [Google Scholar] [CrossRef]
- Ahmad, I.; Sheraz, M.A.; Ahmed, S.; Anwar, Z. Multicomponent spectrometric analysis of drugs and their preparations. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–413. [Google Scholar]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Roussel, P.; Perlovich, G.L. Diversity of crystal structures and physicochemical properties of ciprofloxacin and norfloxacin salts with fumaric acid. CrystEngComm 2018, 6, 755–767. [Google Scholar] [CrossRef]
- Bavishi, D.D.; Borkhataria, C.H. Spring and parachute: How cocrystals enhance solubility. Prog. Cryst. Growth Charact. 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef]
- Bezerra, D.M.; Zapelini, I.W.; Franke, K.M.; Ribeiro, M.E.; Cardoso, D. Investigation of the structural order and stability of mesoporous silicas under a humid atmosphere. Mater. Charact. 2019, 154, 103–115. [Google Scholar] [CrossRef]
- Nugrahani, I.; Ibrahim, S.; Mauludin, R.; Almira, M. Hydrate transformation study of fluoroquinolone antibiotics using Fourier transform infrared spectroscopy (FTIR). Int. J. Pharm. Pharm. Sci. 2015, 7, 246–252. [Google Scholar]
- Nugrahani, I.; Min, S.S. Hydrate transformation of sodium sulfacetamide and neomycin sulphate. Int. J. Pharm. Pharm. Sci. 2015, 7, 409–415. [Google Scholar]
- Scaramuzza, D.; Rauber, G.S.; Voinovich, D.; Hasa, D. Dehydration without heating: Use of polymer-assisted grinding for understanding the stability of hydrates in the presence of polymeric excipients. Cryst. Growth Des. 2018, 18, 5245–5253. [Google Scholar] [CrossRef]
- Ranu, B.; Stolle, A. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; RSC: Cambridge, UK, 2014; pp. 167–175. [Google Scholar]

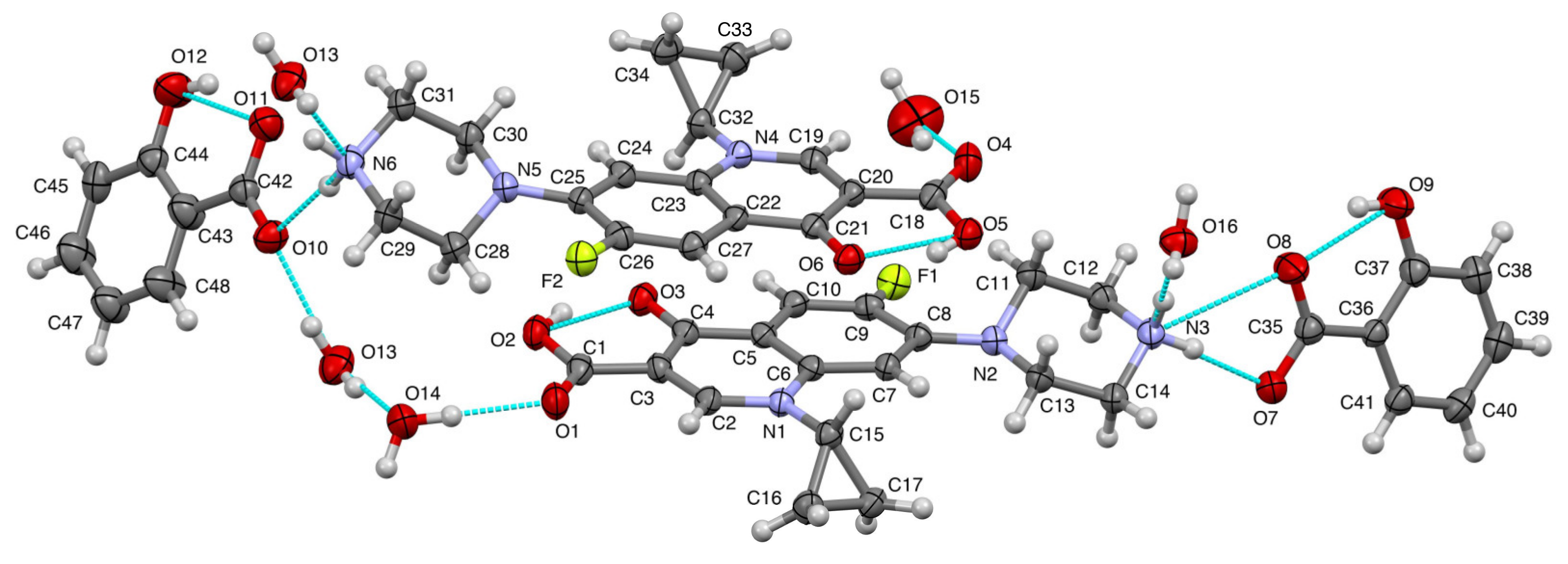
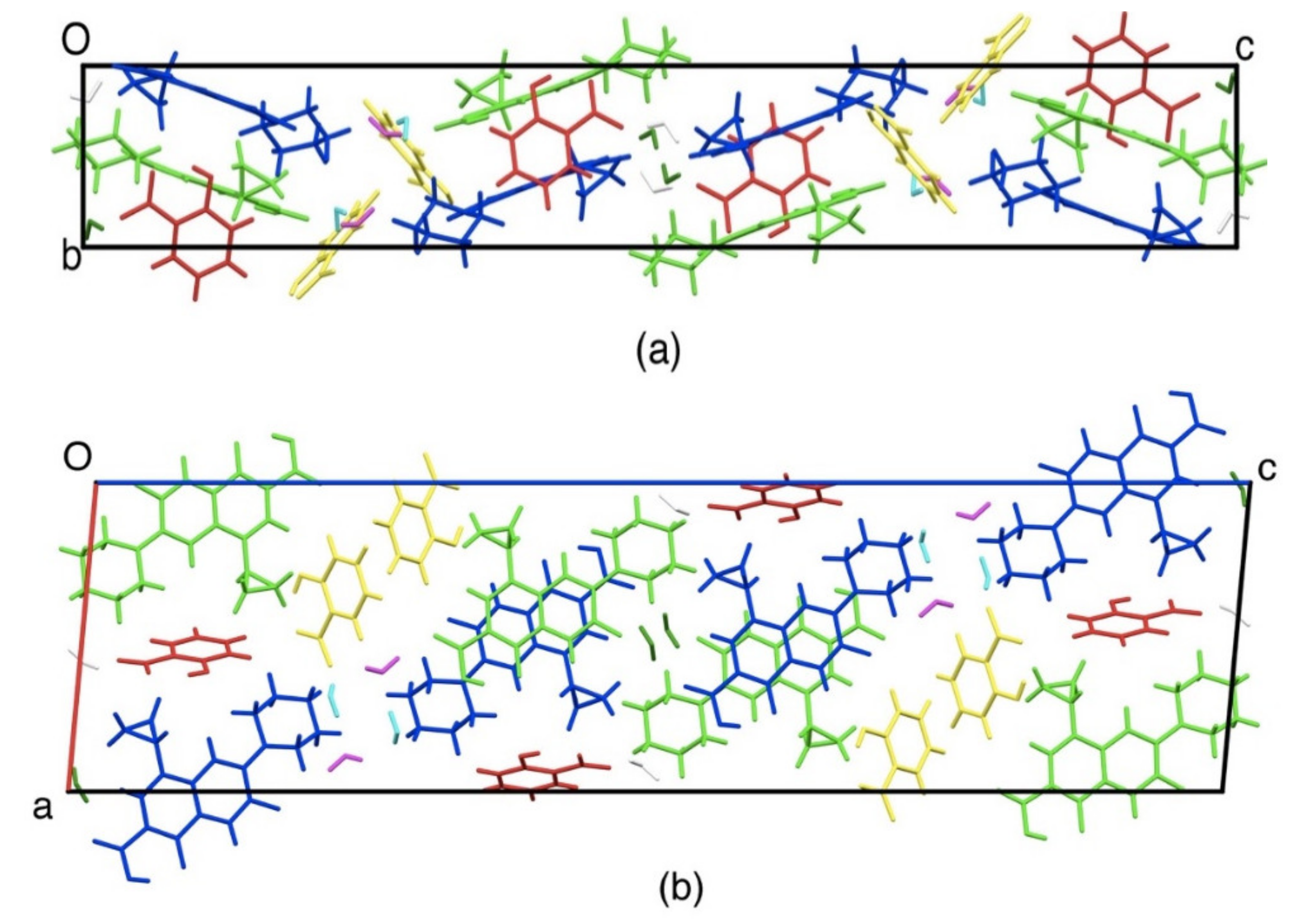
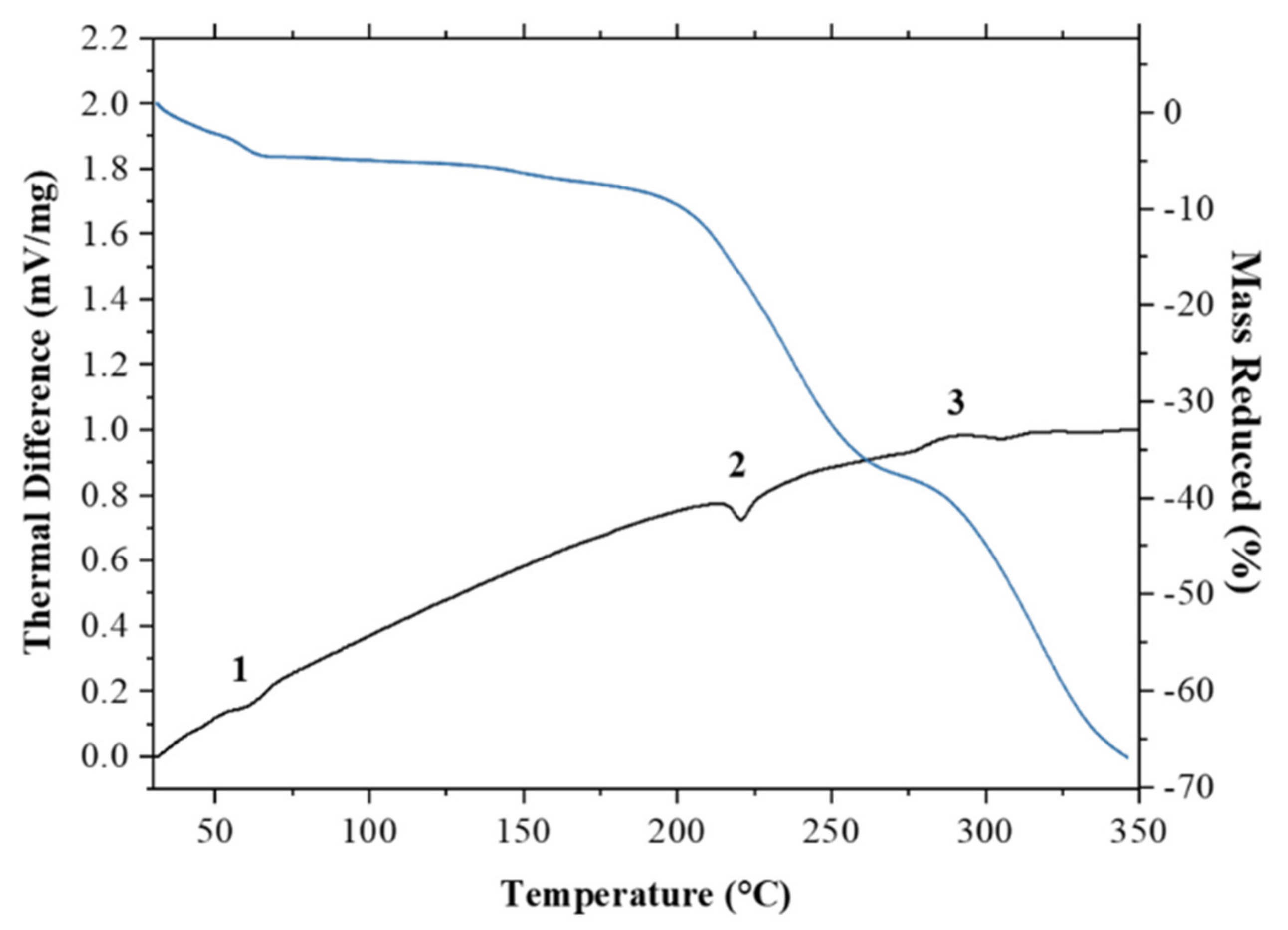
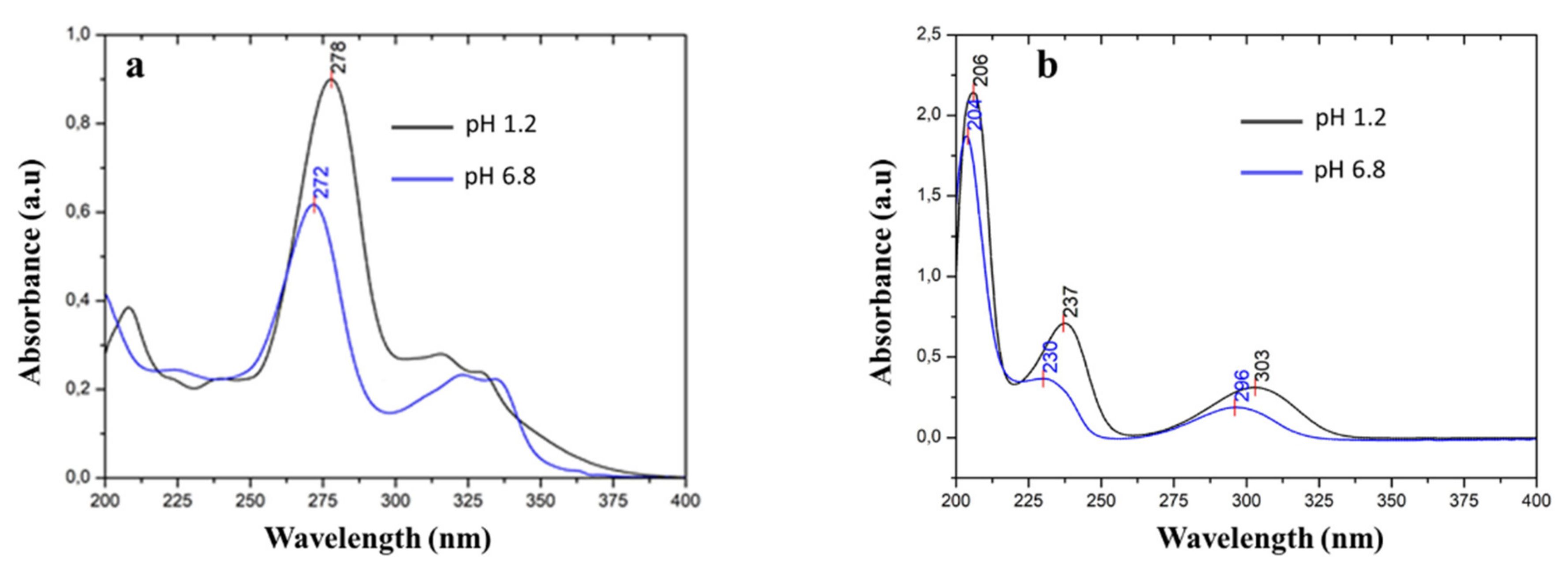

| Formula | (C17H19FN3O3)2(C7H5O3)2(H2O)3.5 |
|---|---|
| Crystal System | Monoclinic |
| Space group | P 21/n |
| a/Å | 13.4283(3) |
| b/Å | 7.01490(10) |
| c/Å | 49.9625(9) |
| β/° | 95.1830(10) |
| V/Å3 | 4687.13(15) |
| Z | 4 |
| R1/% | 8.49 |
| HCl Buffer pH 1.2 | Phosphate Buffer pH 6.8 | ||
|---|---|---|---|
| Ciprofloxacin (ppm) | Salicylic Acid (ppm) | Ciprofloxacin (ppm) | Salicylic Acid (ppm) |
| 8 | 0.5 | 0.5 | 8 |
| 6 | 4 | 1 | 6 |
| 5 | 1.5 | 2 | 4 |
| 4 | 2 | 4 | 2 |
| 3 | 3 | 6 | 1 |
| 2 | 1 | 8 | 0.5 |
| Medium | Equation | R2 |
|---|---|---|
| pH 1.2 | A 278 = −0.0772 + 0.0997 C ciprofloxacin + 0.0115 C salicylic | R2 = 0.999 |
| pH 1.2 | (dA/dλ) 287 = −0.0003 - 0.0056 C ciprofloxacin + 0.0016 C salicylic | R2 = 0.999 |
| pH 6.8 | A 272 = −0.0459 + 0.0891 C ciprofloxacin + 0.0093 C salicylic | R2 = 0.999 |
| pH 6.8 | (dA/dλ) 281 = 0.0013 − 0.0053 C ciprofloxacin + 0.0008 C salicylic | R2 = 0.999 |
| Medium | Sample | Solubility (mg/mL) | Factor of Multiplication |
|---|---|---|---|
| pH 1.2 | ciprofloxacin salicylate • 1.75 H2O | 10.7 ± 0.8 | 0.96 |
| ciprofloxacin salicylate • H2O polymorph I [13] | 8.8 ± 0.6 | 1.18 | |
| ciprofloxacin salicylate • H2O polymorph II [13] | 7.09 ± 0.02 | 1.46 | |
| pH 6.8 | ciprofloxacin salicylate • 1.75 H2O | 1.30 ± 0.18 | 0.13 |
| ciprofloxacin salicylate • H2O polymorph I [13] | 1.10 ± 0.13 | 0.16 | |
| ciprofloxacin salicylate • H2O polymorph II [13] | 0.67 ± 0.04 | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugrahani, I.; Tjengal, B.; Gusdinar, T.; Horikawa, A.; Uekusa, H. A Comprehensive Study of a New 1.75 Hydrate of Ciprofloxacin Salicylate: SCXRD Structure Determination, Solid Characterization, Water Stability, Solubility, and Dissolution Study. Crystals 2020, 10, 349. https://doi.org/10.3390/cryst10050349
Nugrahani I, Tjengal B, Gusdinar T, Horikawa A, Uekusa H. A Comprehensive Study of a New 1.75 Hydrate of Ciprofloxacin Salicylate: SCXRD Structure Determination, Solid Characterization, Water Stability, Solubility, and Dissolution Study. Crystals. 2020; 10(5):349. https://doi.org/10.3390/cryst10050349
Chicago/Turabian StyleNugrahani, Ilma, Billgerd Tjengal, Tutus Gusdinar, Ayano Horikawa, and Hidehiro Uekusa. 2020. "A Comprehensive Study of a New 1.75 Hydrate of Ciprofloxacin Salicylate: SCXRD Structure Determination, Solid Characterization, Water Stability, Solubility, and Dissolution Study" Crystals 10, no. 5: 349. https://doi.org/10.3390/cryst10050349
APA StyleNugrahani, I., Tjengal, B., Gusdinar, T., Horikawa, A., & Uekusa, H. (2020). A Comprehensive Study of a New 1.75 Hydrate of Ciprofloxacin Salicylate: SCXRD Structure Determination, Solid Characterization, Water Stability, Solubility, and Dissolution Study. Crystals, 10(5), 349. https://doi.org/10.3390/cryst10050349








