1. Introduction
In the service of man, wear and corrosion usually initiate from the surface of engineering materials. Modifying the surface of existing materials to enhance their surface properties while not affecting the properties in the sub-surface region is more economical than fabricating the bulk materials. Various surface treatment techniques, such as laser cladding, plasma spraying process, friction stir cladding, physical vapor deposition and chemical vapor deposition, have been developed over the years [
1,
2,
3,
4,
5]. Among these approaches, laser cladding has attracted a high level of interest of the scientific community due to its distinct advantages including faster processing rates, better control of coating thickness, minimal dilution, ease of equipment availability, maintainability and metallurgical substrate/coating bonding [
6]. It has been considered as an effective and sustainable remanufacturing technique to fabricate surface coatings which permits localized processing of the difficult-to-access regions and complex structures by inducing minimal thermal distortion [
7].
High entropy alloys (HEAs) are an emerging class of alloys which includes at least four or five principal elements in equimolar or close-to-equimolar ratios [
8]. Multi-principal elements give rise to high configurational entropy and thus the formation of randomized solid solution phases in HEAs instead of complex intermetallic compounds as expected in traditional alloys system [
9,
10]. Due to their simple solid solution structure, HEAs possess an outstanding combination of strength and ductility and excellent surface properties including hardness, wear resistance, oxidation and corrosion resistance, which make them suitable candidates for hard-facing applications [
11,
12]. Currently, encouraging researches on the manufacture of HEAs coating on the traditional materials have been reported [
13,
14,
15,
16,
17].
Rare earth elements (REEs) or their oxides have been successfully employed to tailor the quality and properties of various traditional bulk materials and coatings due to their excellent physicochemical properties such as unique shell structure, outstanding chemical activity and larger ionic radii. Various studies undertaken have concluded that the absorption of REEs in the cladding materials system can potentially reduce the dilution rate, improve coating toughness, reduce cracking tendency, enhance corrosion resistance and augment bioactivity of bioceramic reimplants [
18,
19,
20,
21,
22]. Inspired from the development of oxide dispersion strengthening (ODS) alloys, oxide particles, which act as effective pinning for grain boundaries and dislocations, were introduced into HEAs to improve their mechanical properties [
23,
24,
25,
26]. In addition, the reactive RE elements Y and Hf were doped into AlCoCrFeNi HEA by ingot metallurgy, which resulted in superior oxidation performance due to the beneficial REs-effects on the fast establishment of exclusive α-Al
2O
3 scale in early oxidation stage [
27]. However, investigations on the effect of REEs or their oxides in augmenting the HEA coatings by laser cladding is still very limited.
In this paper, laser cladding technique had been used to manufacture Al0.5CoCrCuFeNiSi HEA coatings without and with Y2O3 addition on H13 steel substrate. The microstructure and phase assemblage, microhardness, tribological and electrochemical properties of the HEA coatings without and with Y2O3 addition were investigated so as to provide an experimental basis to expand more promising application fields of both HEAs and REEs.
2. Materials and Methods
The H13 steel plate with dimensions of 40 × 40 × 10 mm3 was used as the substrate material. The HEA Al0.5CoCrCuFeNiSi coatings without and with 1 wt.% Y2O3 addition were fabricated on the substrate by laser cladding technique using elemental powders of Al, Co, Cr, Cu, Fe, Ni, Si and commercial Y2O3 powders with purity higher than 99.9%. Hereafter, for convenience, the HEA coatings without and with Y2O3 addition were denoted as HEAY0 and HEAY1, respectively. The size of the elemental powders is about 48 μm and that of the Y2O3 powder is in the range of 0.05–5 μm. Before laser cladding, the substrate was polished to obtain uniform roughness of ~0.2 μm and then ultrasonic-cleaned in acetone. The powders with the nominal chemical composition were mixed uniformly with α-cyanaloc acrylic resin adhesive and coated on the surface of the treated substrates to make a pre-coating about 1.5 mm in thickness. After that, the substrates with the pre-placed powders were dried in air for 2 days and then ground to a thickness of 1 mm by 1000 grade silicon carbide paper in dry condition. A JHM-1GY-800 pulsed Nd:YAG laser (CHUTIAN, Wuhan, China) with a maximum power of 800 W was used to re-melt the pre-placed layer by the relative movement between the laser beam and the substrate mounted on a four-axis numerical control working table. Argon gas blowing through a 4 mm diameter jet coaxial with the laser beam was used to shield the melt pool from oxidation and protect the laser optics from fumes and spattered particles. The experimental parameters including current, scanning speed, defocus and overlapping between tracks were optimized as 135 A, 200 mm/min, −45 mm and 45%, respectively.
An X Pertpro X-ray diffraction (XRD, PANalytical B. V., Almelo, the Netherlands) was used for the identification of the crystal structure of the laser cladding coatings without and with Y2O3 addition. The scanning was performed at a 2θ scan ranging from 20° to 100° at a speed of 2°/min and the typical radiation condition was 30 kV and 20 mA with a copper target. The microhardness from the laser cladding track regions to the substrate were measured using a 402MVD Vickers hardness tester (Yigong, Shanghai, China) under a load of 50 g with a dwell time of 10 s. The adhesive wear behavior of both the substrate and the laser cladding coatings were tested under dry sliding conditions at 500 °C using a MT-2000 pin-on-disk tribometer (Zhengli, Hebei, China) with a load of 100 N after 4000 rotations. The microstructure of the laser cladding coatings and the morphologies of the worn surfaces were examined using a SSX-550 scanning electron microscopy (SEM, Shimadzu, Kyoto, Japan) and the chemical compositions were determined by energy dispersive spectrometer (EDS) equipped with SEM. The microstructure observations and the EDS analysis were performed on the cross sections of the laser cladding coatings which were cut perpendicular to the laser tracks.
The electrochemical properties of specimen were measured by the CHI650D electrochemical analyzer (CH Instruments, Shanghai, China). The specimens after slightly polishing with silicon carbide paper (2500 grit) were decreased in benzene, cleaned ultrasonically and washed with distilled water prior to electrochemical tests. To study the corrosion behaviors of the substrate and the laser cladding coatings, a three-electrode cell arrangement was used for the electrochemical measurements with a saturated calomel electrode (SCE) as the reference electrode, a platinum plate as the auxiliary electrode and specimen as the working electrode with an exposed area of 1 cm2 in 3.5 vol.% NaCl solution at 23 ± 1 °C open to air and repeated several times to ensure the reproducibility of the data. Potentiodynamic polarization scan started from −0.65 V to 0.55 V with a sweep rate of 1 mVs−1 and the Tafel region was analyzed by using DC105 software (Shanghai, China).
3. Results
3.1. Microstructure and XRD Analysis
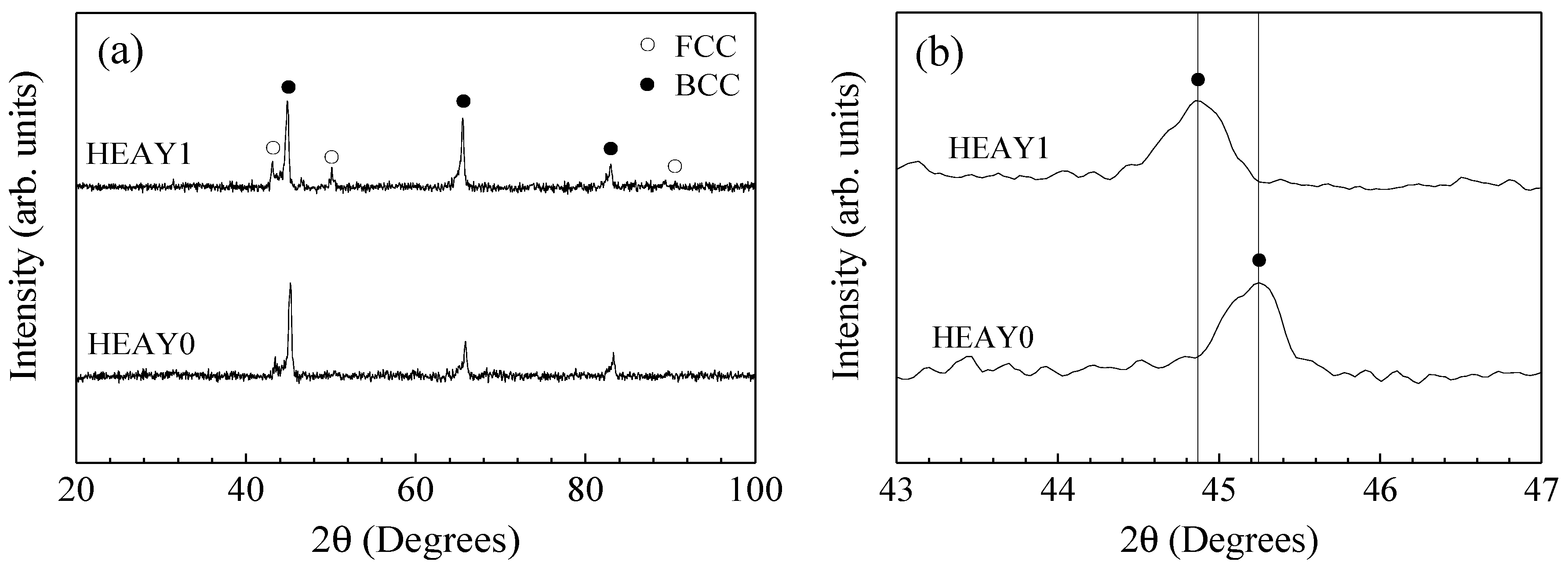
The XRD patterns of Al
0.5CoCrCuFeNiSi HEA coatings without and with Y
2O
3 addition are shown in
Figure 1. The Bragg peaks in the XRD pattern of the coating can be indexed to a mixture of body centered cubic (body centered cubic) and face centered cubic (FCC) simple solid solution structures in the laser cladding HEA coatings both without and with Y
2O
3 addition. The much stronger peak intensity of BCC phase suggests that the BCC structure is a dominative phase in the coatings. There are no peaks for Y
2O
3 or new generated phase detected in the XRD patterns of the laser cladding HEA coating with Y
2O
3 addition, which may be due to the small addition of Y
2O
3 which is beyond the detection limit of XRD. The average full width of half maximum (FWHM) of BCC diffraction peaks are 0.42 and 0.44 for the laser cladding coatings without and with Y
2O
3 addition, respectively. The average full width at half maximum (FWHM) of the BCC diffraction peaks increase with the addition of Y
2O
3 indicating that the BCC grains were refined after adding Y
2O
3.
Figure 1b shows an enlarged picture of the main peak of BCC phase in the laser cladding HEA coating without and with Y
2O
3 addition. It is also shown that the peaks of the BCC phases shifted leftwards with the addition of Y
2O
3, indicating that the lattice parameter of the BCC phases increases. By linearity extrapolation method, the lattice constants of the BCC phase were 2.834 Å and 2.856 Å for the laser cladding coatings without and with Y
2O
3 addition, respectively.
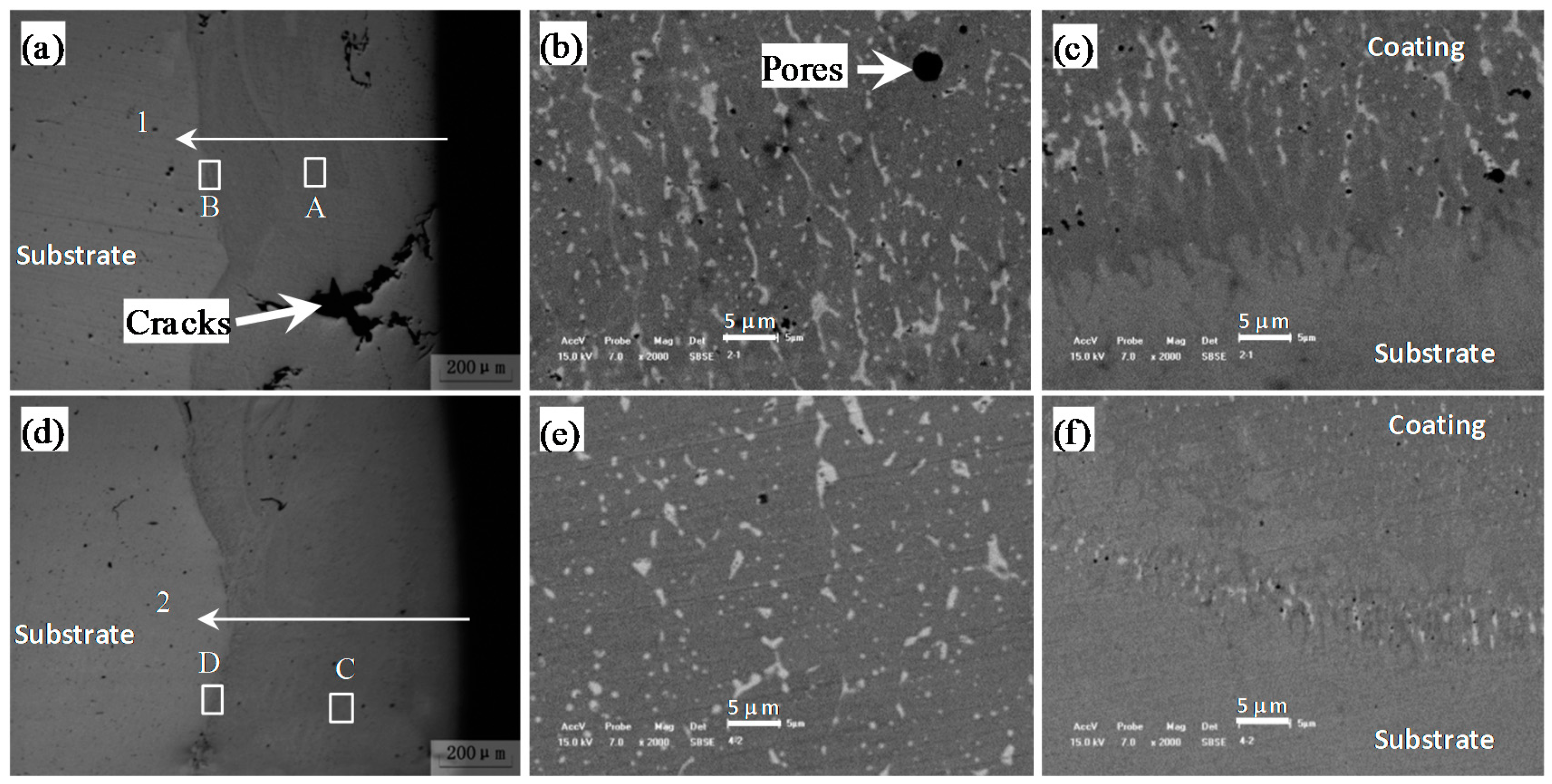
Figure 2 shows the cross-sectional microstructure of the laser cladding HEA coatings without (
Figure 2a–c) and with Y
2O
3 addition (
Figure 2d–f). For the laser cladding HEA coating without Y
2O
3, transverse cracks have been observed, which are perpendicular to the scanning direction (perpendicular to the paper) and stop at the coating/substrate interface. Furthermore, the cracks expand with radial shape. On the contrary, with the addition of 1 wt.% Y
2O
3, the cracks in the laser cladding HEA coating were inhibited, as shown in
Figure 2d.
Figure 2b,c represent the microstructure of the local amplification of area A and B as indicated by the squares in
Figure 2a.
Figure 2e,f represent of the local amplification of area C and D as indicated by the squares in
Figure 2d. The back scattered electron (BSE) images shows that the laser cladding coating both without and with Y
2O
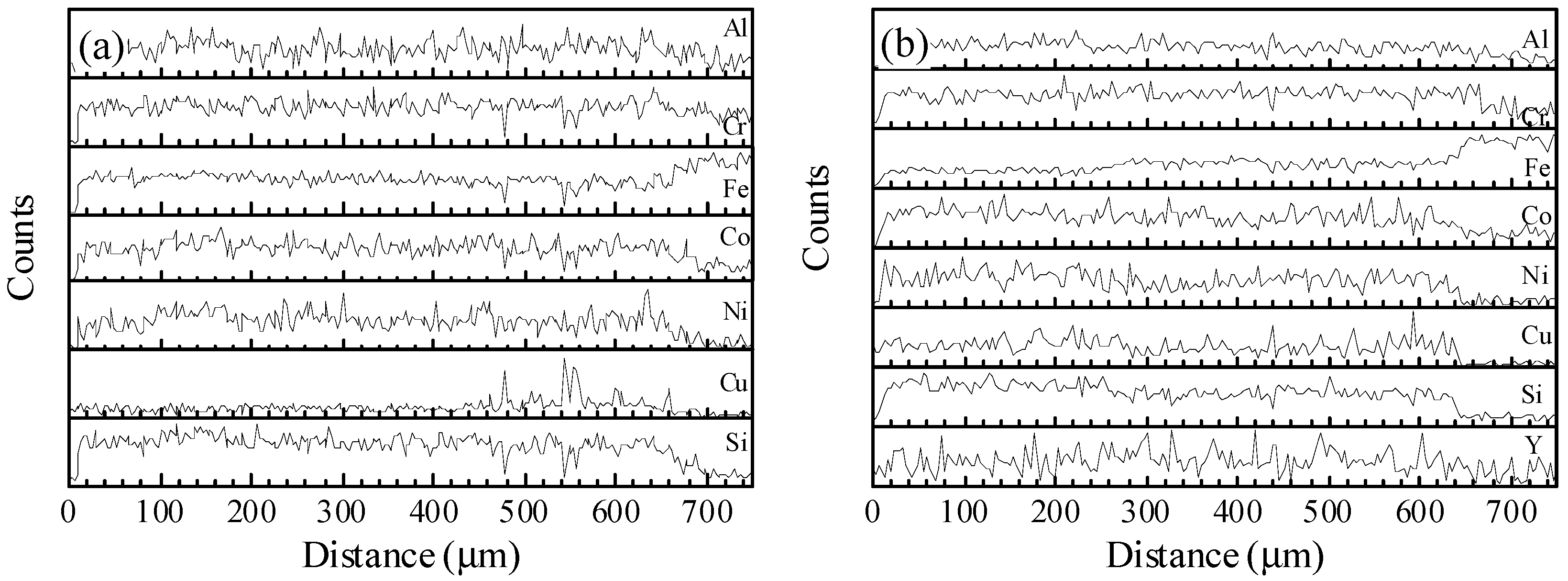
3 addition have a typical dendrite microstructure after solidification, i.e., a large amount of dendrite phases (dark contrast) with BCC structure and a small quantity of interdendrite phases (bright contrast) with FCC structure. The EDS analysis as shown in
Figure 3 indicates that there is Cu element segregation in the interdendrite region, and other elements are rich in the dendrite region, which is consistent with our previous report [
28]. For the laser cladding HEA coating without Y
2O
3 addition, significant porosities are observed and the dendrite near the coating/substrate interface is vertical, directional and epitaxial to the bonding interface. However, with the addition of Y
2O
3, both the number density and the size of the porosities in the coating were greatly reduced and the solidification microstructure near coating/substrate interface is no longer epitaxial and directional to the bonding interface.
3.2. Microhardness and Wear Resistance
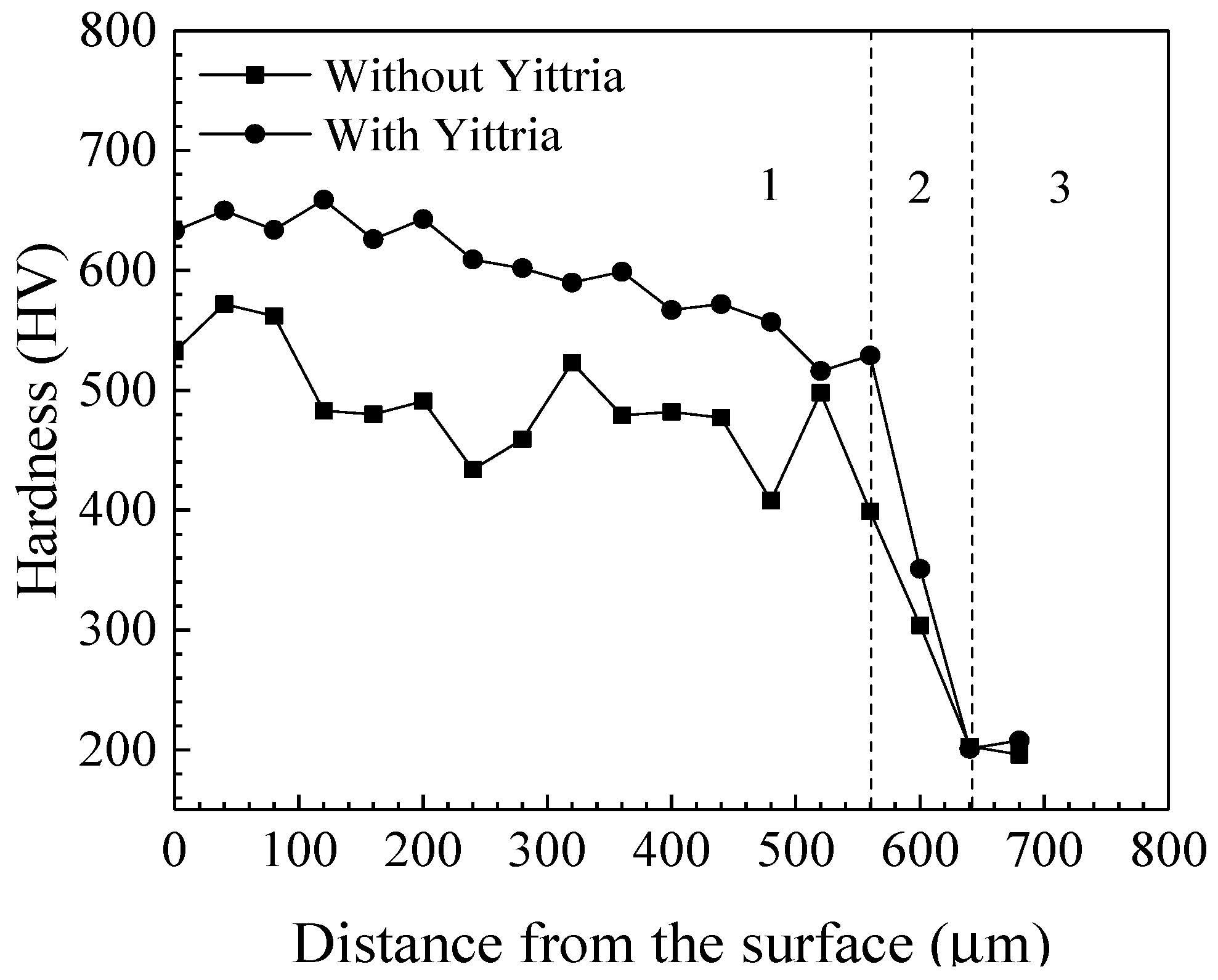
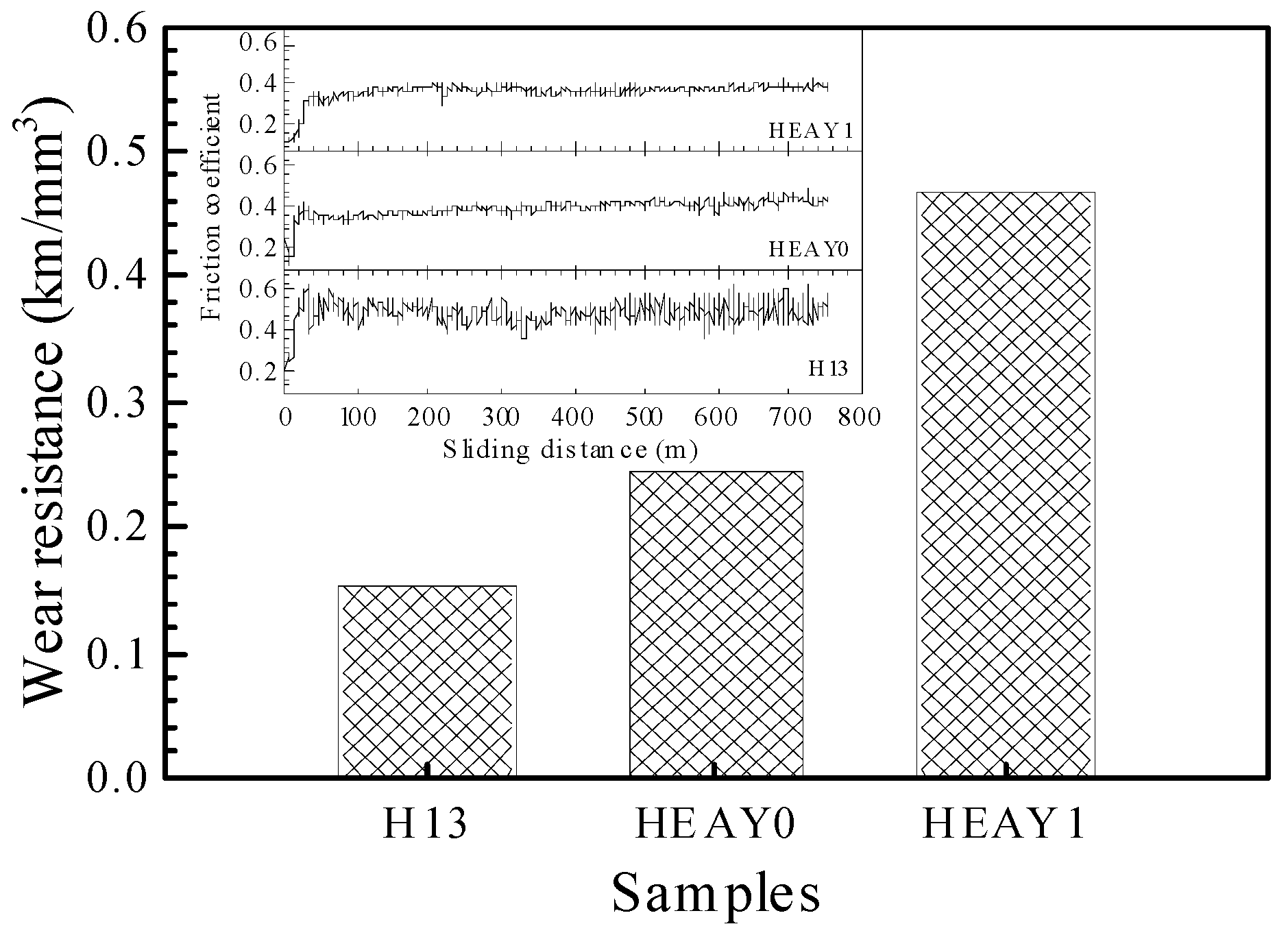
Figure 4 presents the microhardness variations ranging from the laser cladding coatings without and with Y
2O
3 addition to the substrate. It is apparent that both of the laser cladding coatings show higher hardness than the H13 steel substrate. The average hardness, 491 HV and 599 HV, of the laser cladding coatings without and with Y
2O
3 addition, respectively, are 2.43 and 2.97 times that, 202 HV, of the bare substrate. Furthermore, compared to the cladding coating without Y
2O
3 addition, the hardness of the cladding coating with Y
2O
3 addition increased and the hardness distribution throughout the coating in depth was much more uniform. The tribological performances of the laser cladding coatings were evaluated by subjecting them to adhesive wear at 500 °C and compared to that of the bare substrate tested at identical conditions. The wear resistance of the laser cladding coatings without and with Y
2O
3 addition, as well as that of the bare substrate, are shown in
Figure 5. The wear resistance, 0.24 km/mm
3 and 0.47 km/mm
3, for the laser cladding coating without and with Y
2O
3 addition, respectively, are 160% and 209% that, 0.15 km/mm
3, of the bare substrate. The insert in
Figure 5 represents the friction coefficients of the substrate and the laser cladding coatings. The friction coefficients of the laser cladding coatings are lower than that of the substrate, also corroborating the wear behavior improvement.
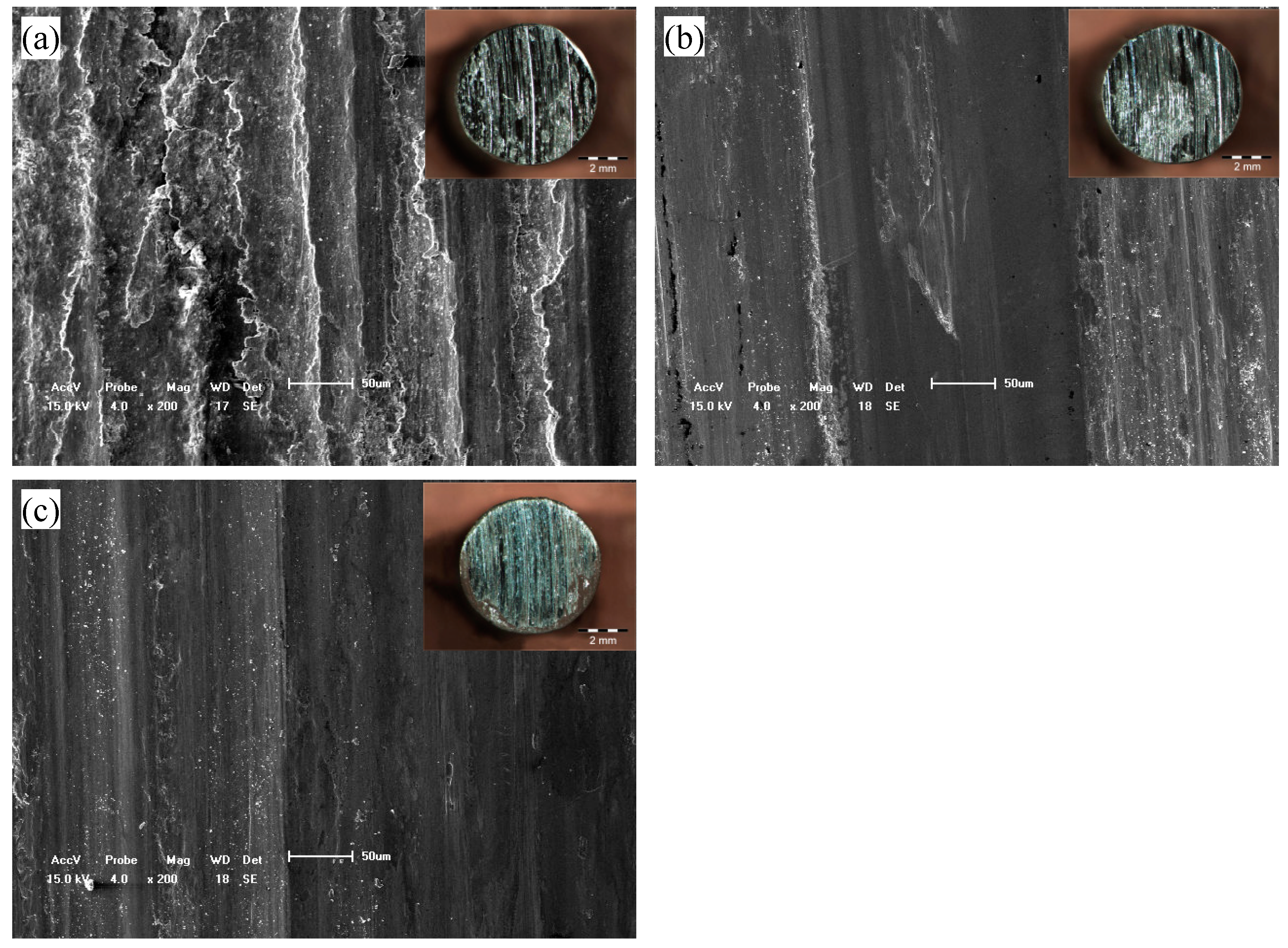
Figure 6 shows the morphologies of the worn surfaces of the bare substrate and the laser cladding coatings without and with Y
2O
3 addition. Distinct differences in the worn surface morphologies are seen. For the bare substrate (
Figure 6a), the worn surface was grooved and significant ductile deformation along the groove can be seen, and cracks are observed on the worn surface. It indicates that the adhesive wear of the bare substrate at 500 °C is predominantly of delamination wear by which the worn surface undergoes a periodic delamination fracture [
29]. However, for the laser cladding coating without Y
2O
3 addition, the plastic features and grooves were inhibited greatly and the worn surfaces are much smoother with fewer shallow grooves. The worn surface of the cladding layer with Y
2O
3 is even smoother than that of the cladding coating without Y
2O
3 addition. It is indicated that the laser cladding HEA coating with Y
2O
3 addition shows the best wear resistance, followed by the laser cladding HEA coating without Y
2O
3 addition, and the bare substrate the worst.
3.3. Corrosion Resistance
The polarization curves of laser cladding HEA coatings without and with Y
2O
3, as well as the bare substrate, tested in 3.5 vol.% NaCl solution under identical conditions are shown in
Figure 7. Tafel curve extrapolation method was carried out to determine the electrochemical parameters including the corrosion potential (E
corr) and the corrosion current density (I
corr) which were listed in
Table 1. The E
corr values of the laser cladding HEA coatings without and with Y
2O
3 addition are all more positive than that of the substrate, while the I
corr values of the laser cladding coatings are obviously lower than that of the substrate. Further, the laser cladding HEA coating with Y
2O
3 addition exhibits higher E
corr and lower I
corr compared to those of the HEA coating without Y
2O
3.
The results of the electrochemical impedance measurements obtained for the substrate and laser cladded specimens in 3.5% NaCl solution are displayed as Nyquist plots in
Figure 8. It can be seen that the Nyquist diagrams showed an unfinished semicircle arc for all the specimens. The semicircle arc represents film impedance at high frequencies and a low frequency electron transfer reaction response. The formation of such semicircle arc is attributed to the charge transfer process at the electrode/electrolyte interface, related to changes in the passive oxide property and their chemical composition. Differences were observed in all the impedance spectra between the laser cladded specimens and the substrate. For the substrates, the semicircle radius was smaller than the laser cladded coatings. The increase in semicircle radius for the laser cladded coating indicated an increase in the passive film stability and corrosion resistance. It is indicated that the HEA coating with Y
2O
3 addition provides the best corrosion resistance, followed by HEA coating without Y
2O
3 and the bare substrate the worst.
4. Discussion
For laser cladding of HEA coating, thermal entropy contribution adds to the configurational entropy making conditions suitable for solid solution phases formation at high temperature and rapid quenching ensures retention of the solid solution phase at room temperature. As indicated by the XRD and microstructural analysis, the Al
0.5CoCrCuFeNiSi HEA coatings consist of BCC structured dendrites and minor FCC structured interdendrites. In this HEA, the mixing enthalpy of Cu–Co, Cu–Cr, Cu–Fe atomic pairs have positive values. As for Si, though the mixing enthalpy between Si and Cu, −19 kJ/mol, is negative, it is still a relatively positive value among the mixing enthalpies between Si and the other components. Thus, the Cu atoms were rejected segregate to the interdendrites during solidification due to its relative positive mixing enthalpy values with the other components. Surface cracks with deep penetration into the cladding for the HEA coating without Y
2O
3 addition, as shown in
Figure 2a, is due to the strong convective flow and surface tension gradient in the melt pool and the rapid solidification leading to the existence of remnant stress in the coating after laser treatment [
20]. Porosities observed in the HEA cladding coating without Y
2O
3 addition could be explained by the gas trapping due to large fluid viscosity induced by material particles in the melt pool and the encapsulated bubbles generated during laser cladding process [
30].
Rare earth elements possess particular atomic structure and excellent chemical activity. With the addition of Y
2O
3 into cladding powders, the Y
2O
3 can be partially decomposed in the molten pool under the irradiation of high-energy laser beam and therefore the solidification pattern and surface tension gradients will be modified [
30]. The decomposed Y could act as surfactant which reduces the surface tension and interfacical energy and thus the work for forming nuclei with critical size improving the ratio of nucleation and refining the grains. Y
2O
3 could also be considered as nuclei in the melting pool and refine the microstructure due to its higher melting temperature. Due to its excellent chemical activity, the decomposed Y can react with elements such as oxygen, sulfur, nitrogen and so on, forming stable and high-melting-point compounds which will float on the melt and be cleaned off the clad layer as molten slag during laser cladding process. The changes of solidification pattern and surface tension gradients, the microstructural refinement and the purification of the coating due to the addition of Y
2O
3 will lower cracking tendency during solidification of the HEA coating.
Compared to the substrate, the improved mechanical properties of the laser cladding HEA coating without Y2O3 addition are attributed to the presence of BCC structured simple solid solution phase, as indicated by the XRD analysis, which are much harder and stronger due to its intrinsic structural characteristics. The increase in hardness and wear resistance of the laser cladding HEA coating with Y2O3 addition come from the refining effect of Y2O3 on the microstructure and solution hardening mechanism. The addition of Y2O3 into HEA coating leads to the refinement of the microstructure, which improves the hardness and strength of the cladding coating. For the solution hardening mechanism factor, the dissolution of Y atoms with larger radius (1.80 Å) into the BCC crystal lattice causes severe lattice distortion and thus increase the local elastic stress field which interacts with the stress field of dislocations and hinders the movements of dislocations causing strengthening. The uniform hardness distribution along depth in the laser cladding HEA coating with Y2O3 addition came from the elimination of porosities and cracks and the refinement of the microstructure. The enhancement of wear resistance for laser cladding coatings is due to the higher hardness which can resist the plastic deformation, particularly the laser cladding coating with Y2O3 addition.
In corrosion tests, the microstructures of coating played a dominate role. In the coating without Y
2O
3, cracks and porosities were observed, as indicated in
Figure 2a,b. The cracks could act as liquid channels and the corrosive medium might flow to the coating/substrate interface from the surface coating. Consequently, the substrate exposed to the electrolyte would be attacked by crevice corrosion. Due to the existence of porosities, the electrolyte could be trapped and stagnated in the pores causing the dissolution of the peripheral region, which results in the interconnection of the isolated pores forming a liquid channel. Either crevice corrosion, similar to that caused by cracks, or localized corrosion might occur depending on the width of the channels. With the addition of Y
2O
3 in the coating material, the cracks and porosities of coating were reduced greatly or even eliminated. Furthermore, due to the refining and purifying effect of Y
2O
3, the active center with different potential, which result in the formation of microcells and electrochemical corrosion, will be reduced or even eliminated and thus the surface potential will tend to be uniform.
5. Conclusions
The laser cladding HEA coating without and with Y2O3 addition consisted of a mixture of BCC structure dendrites and FCC interdendrites. The addition of Y2O3 has no effects on the crystal structure but the lattice constant of the BCC dendrites increased with the addition of Y2O3.
Cracks and porosities were observed in the laser cladding coating without Y2O3. With the addition of Y2O3, the cracks and porosities were inhibited and the microstructure was refined due to the improving of the ratio of nucleation.
The properties enhancement of the laser cladding coating without Y2O3 addition is due to the intrinsic characteristic of BCC structured solid solution phase. With the addition of Y2O3 in the coating material, the properties were further improved due to the inhibitation of cracks and porosities and the refining of the microstructure.
Author Contributions
Conceptualization, X.L.; data curation, J.L., Y.G. and X.L.; investigation, J.L., Y.G., X.X., P.P. and Q.D.; methodology, J.L., X.X., X.L. and Y.G.; supervision, X.L.; writing—Original draft, J.L. and X.X.; writing—Review and editing, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Training Program of Innovation and Entrepreneurship for Undergraduates (Grant number: 201910145257), the Fundamental Research Funds for the Central Universities (Project number: N182410001) and by the In-house funding from the Key Lab of EPM of NEU (Grant number: NEU-EPM-002).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.X.; Wu, F.Y.; Bai, B.; Wang, G.S.; Yang, L.; Zhou, S.F.; Lei, J.B. Effect of Cr additions on the microstructure and corrosion resistance of Diode laser clad CuAl10 coating. Surf. Coat. Technol. 2020, 381, 125215. [Google Scholar] [CrossRef]
- Bobzin, K.; Brogelmann, T.; Kalscheuer, C.; Welters, M. Structure, mechanical characteristics and thermal stability of high speed physical vapor deposition (Al,Cr)2O3 coatings. Thin Solid Films 2019, 690, 137529. [Google Scholar] [CrossRef]
- Kainz, C.; Schalk, N.; Tkadletz, M.; Saringer, C.; Winkler, M.; Stark, A.; Schell, N.; Julin, J.; Czettl, C. Thermo-physical properties of coatings in the Ti(B, N) system grown by chemical vapor deposition. Surf. Coat. Technol. 2020, 384, 125318. [Google Scholar] [CrossRef]
- Xiao, J.K.; Tan, H.; Wu, Y.Q.; Chen, J.; Zhang, C. Microstructure and wear behavior of FeCoNiCrMn high entropy alloy coating deposited by plasma spraying. Surf. Coat. Technol. 2020, 385, 125430. [Google Scholar] [CrossRef]
- Xiong, X.M.; Yang, Y.; Li, J.G.; Li, M.M.; Peng, J.; Wen, C.; Peng, X.D. Research on the microstructure and properties of a multi-pass friction stir processed 6061Al coating for AZ31 Mg alloy. J. Magnes. Alloys 2019, 7, 696–706. [Google Scholar] [CrossRef]
- Birger, E.M.; Moskvitin, G.V.; Polyakov, A.N.; Arkhipov, V.E. Industrial laser cladding: Current state and future. Welding Int. 2011, 25, 234–243. [Google Scholar] [CrossRef]
- Santo, L. Laser cladding of metals: A review. Int. J. Surf. Sci. Eng. 2008, 2, 327–335. [Google Scholar] [CrossRef]
- Zou, Y.; Maiti, S.; Steurer, W.; Spolenak, R. Size-dependent plasticity in an Nb25Mo25Ta25W25 refractory high entropy alloy. Acta Mater. 2014, 65, 85–97. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 23–218. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Cantor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.O.; Meng, G.H. Solidification behaviour in laser cladding of AlCoCrCuFeNi high-entropy alloy on magnesium substrates. J. Alloys Compd. 2014, 587, 588–593. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Li, J.; Juan, Y.F.; Lu, Z.J.; Jia, W.L. Evolution in microstructure and corrosion behavior of AlCoCrxFeNi high-entropy alloy coatings fabricated by laser cladding. J. Alloys Compd. 2017, 775, 1–14. [Google Scholar] [CrossRef]
- Cai, Z.B.; Wang, Y.D.; Cui, X.F.; Jin, G.; Li, Y.; Liu, Z.; Dong, M.L. Design and microstructure characterization of FeCoNiAlCu high-entropy alloy coating by plasma cladding: In comparison with thermodynamic calculation. Surf. Coat. Technol. 2017, 330, 163–169. [Google Scholar] [CrossRef]
- Zhang, G.J.; Tian, Q.W.; Yin, K.X.; Niu, S.Q.; Wu, M.H.; Wang, W.W.; Wang, Y.N.; Huang, J.C. Effect of Fe on microstructure and properties of AlCoCrFexNi (x = 1.5, 2.5) high entropy alloy coatings prepared by laser cladding. Intermetallics 2020, 119, 106722. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Qin, Z.; Dong, P.; Mi, Y.J.; Gong, D.Q.; Li, W.G. Microstructure and corrosion properties of FeCoNiCrMn high entropy alloy coatings prepared by high speed laser cladding and ultrasonic surface mechanical rolling treatment. Mater. Lett. 2020, 259, 126769. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, Q.B.; Sun, M.L.; Wei, X.G. Microstructural characteristics of laser clad coatings with rare earth metal elements. J. Mater. Process. Technol. 2003, 139, 448–452. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Li, G.J. Effect of Y2O3 on the sliding wear resistance of TiB/TiC-reinforced composite coatings fabricated by laser cladding. Wear 2014, 310, 72–82. [Google Scholar] [CrossRef]
- Wang, D.S.; Liang, E.J.; Chao, M.J.; Yuan, B. Investigation on the microstructure and cracking susceptibility of laser-clad V2O5/NiCrBSiC alloy coatings. Surf. Coat. Technol. 2008, 202, 1371–1378. [Google Scholar] [CrossRef]
- Zhu, R.; Li, Z.; Li, X.; Sun, Q. Microstructure and properties of the low-power-laser clad coatings on magnesium alloy with different amount of rare earth addition. Appl. Surf. Sci. 2015, 353, 405–413. [Google Scholar] [CrossRef]
- Li, H.C.; Wang, D.G.; Chen, C.Z.; Weng, F. Effect of CeO2 and Y2O3 on microstructure, bioactivity and degradability of laser cladding CaO-SiO2 coating on titanium alloy. Colloids Surf. B 2015, 127, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.; Singh, S.; Panigrahi, B.B. Mechanical activated synthesis of alumina dispersed FeNiCoCrAlMn high entropy alloy. J. Alloys Compd. 2017, 692, 720–726. [Google Scholar] [CrossRef]
- Praveen, S.; Anupam, A.; Sirasani, T.; Murty, B.S.; Kottada, R.S. Characterization of oxide dispersed AlCoCrFe high entropy alloy synthesised by mechanical alloying and spark plasma sintering. Trans. Indian Inst. Met. 2013, 66, 269–373. [Google Scholar] [CrossRef]
- Hadraba, H.; Chlup, Z.; Dlouhy, A.; Dobes, F.; Roupcova, P.; Vilemova, M.; Matejicek, J. Oxide dispersion strengthened CoCrFeNiMn high-entropy alloy. Mater. Sci. Eng. A 2017, 689, 252–256. [Google Scholar] [CrossRef]
- Gwalani, B.; Pohan, R.M.; Lee, J.; Lee, B.; Banerjee, R.; Ryu, H.J.; Hong, S.H. High-entropy alloy strengthened by in situ formation of entropy-stabilized nano-dispersoids. Sci. Rep. 2018, 8, 14085. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Ni, N.; Li, L.; He, L.M.; Mu, R.D.; Zhao, X.F.; Guo, F.W. Y/Hf-doped AlCoCrFeNi high-entropy alloy with ultra oxidation and spallation resistance. Corros. Sci. 2020, 166, 108426. [Google Scholar] [CrossRef]
- Liu, X.T.; Lei, W.B.; Ma, L.J.; Liu, J.; Liu, J.L.; Cui, J.Z. On the microstructures, phase assemblages and properties of Al0.5CoCrCuFeNiSix high-entropy alloys. J. Alloys Compd. 2015, 630, 151–157. [Google Scholar] [CrossRef]
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Li, M.Y.; Han, B.; Wang, Y.; Pu, K.J. Effects of La2O3 on the microstructure and property of laser cladding Ni-based ceramic coating. Optik 2017, 130, 1032–1037. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).