Initial Growth and Crystallization Onset of Plasma Enhanced-Atomic Layer Deposited ZnO
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
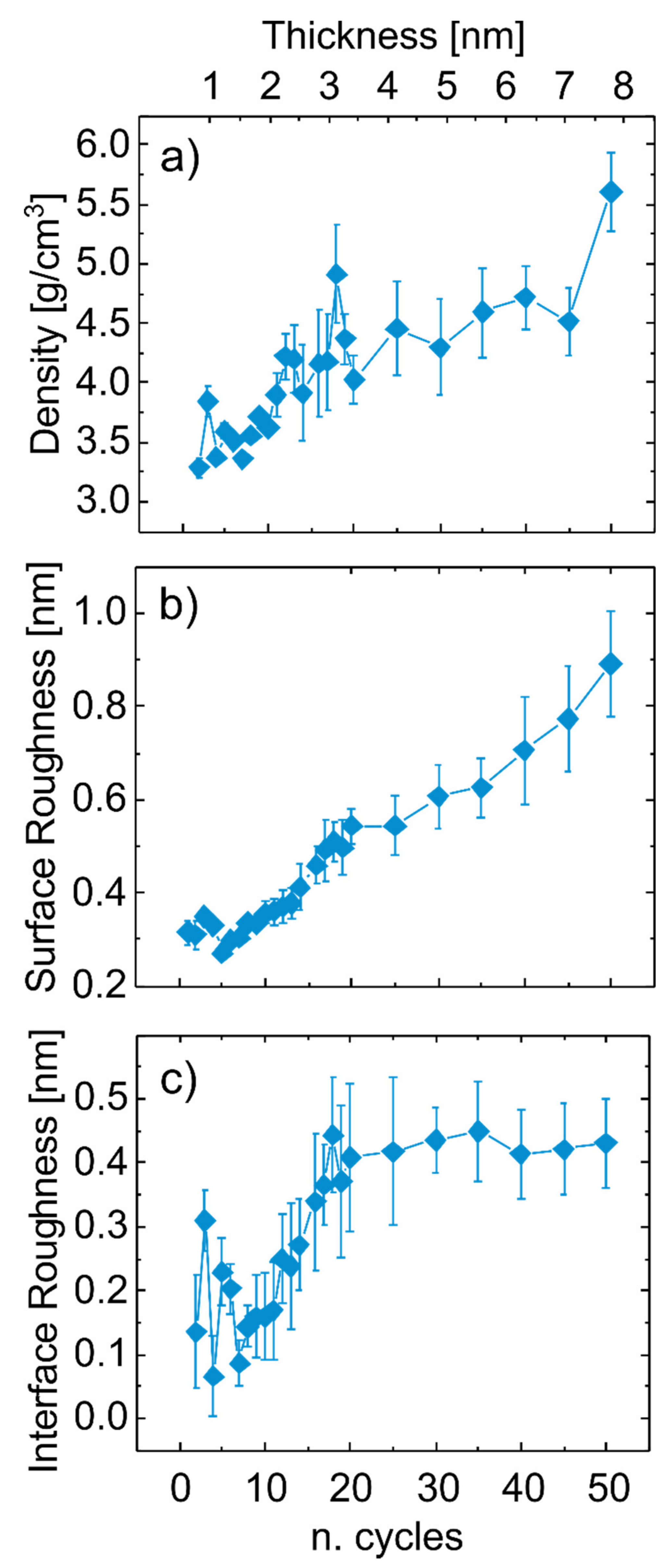
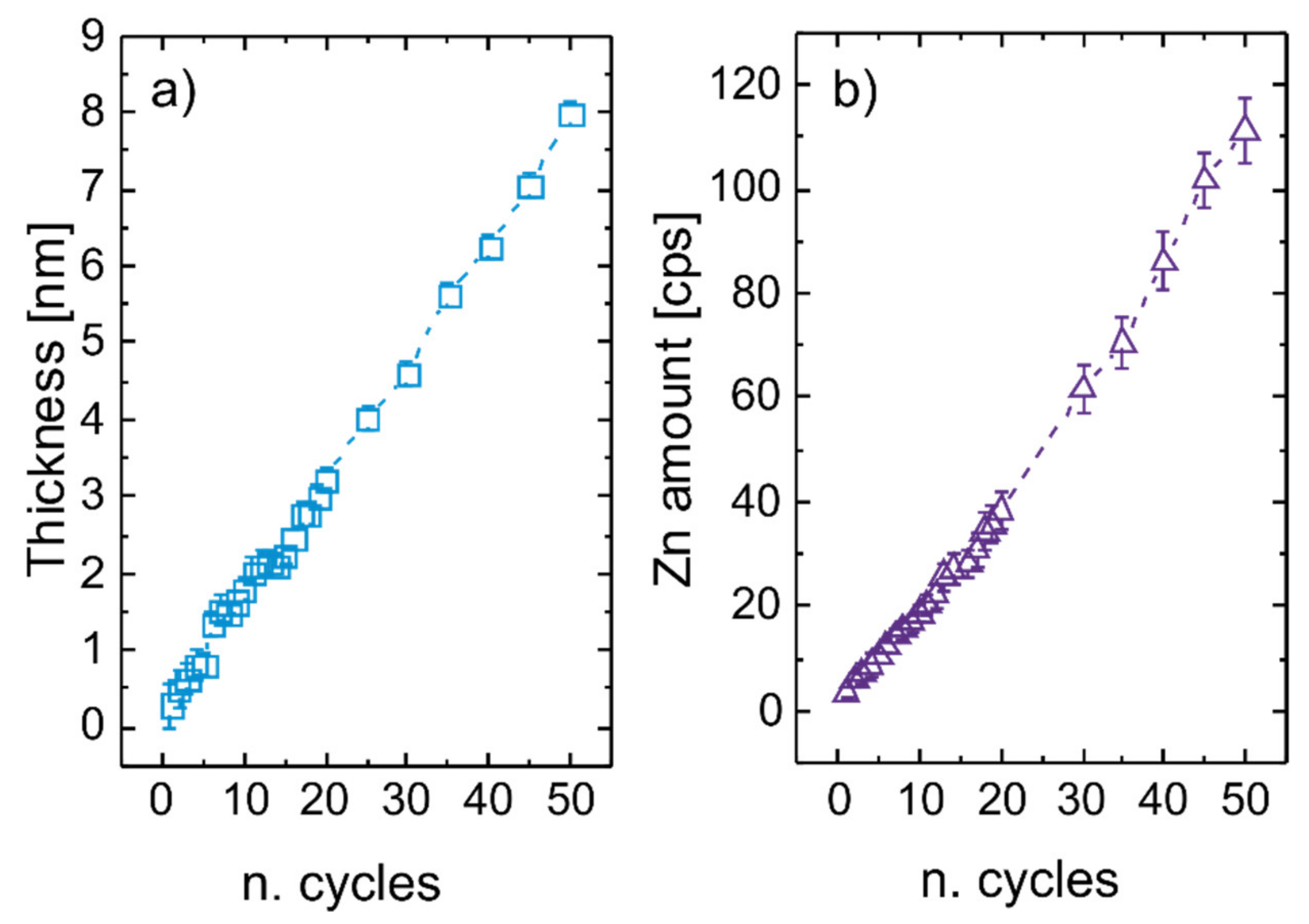
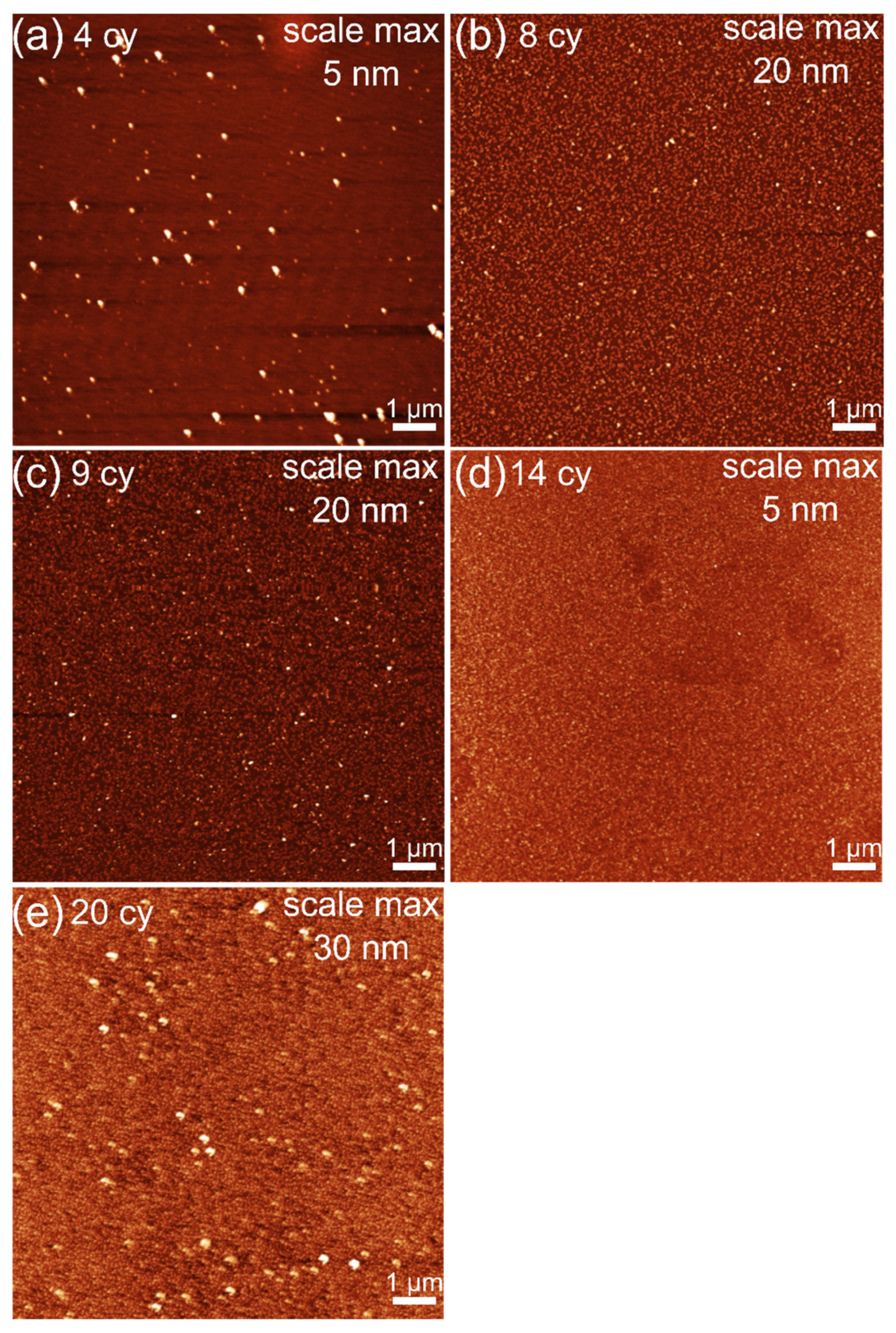
3.1. Thickness and Morphology
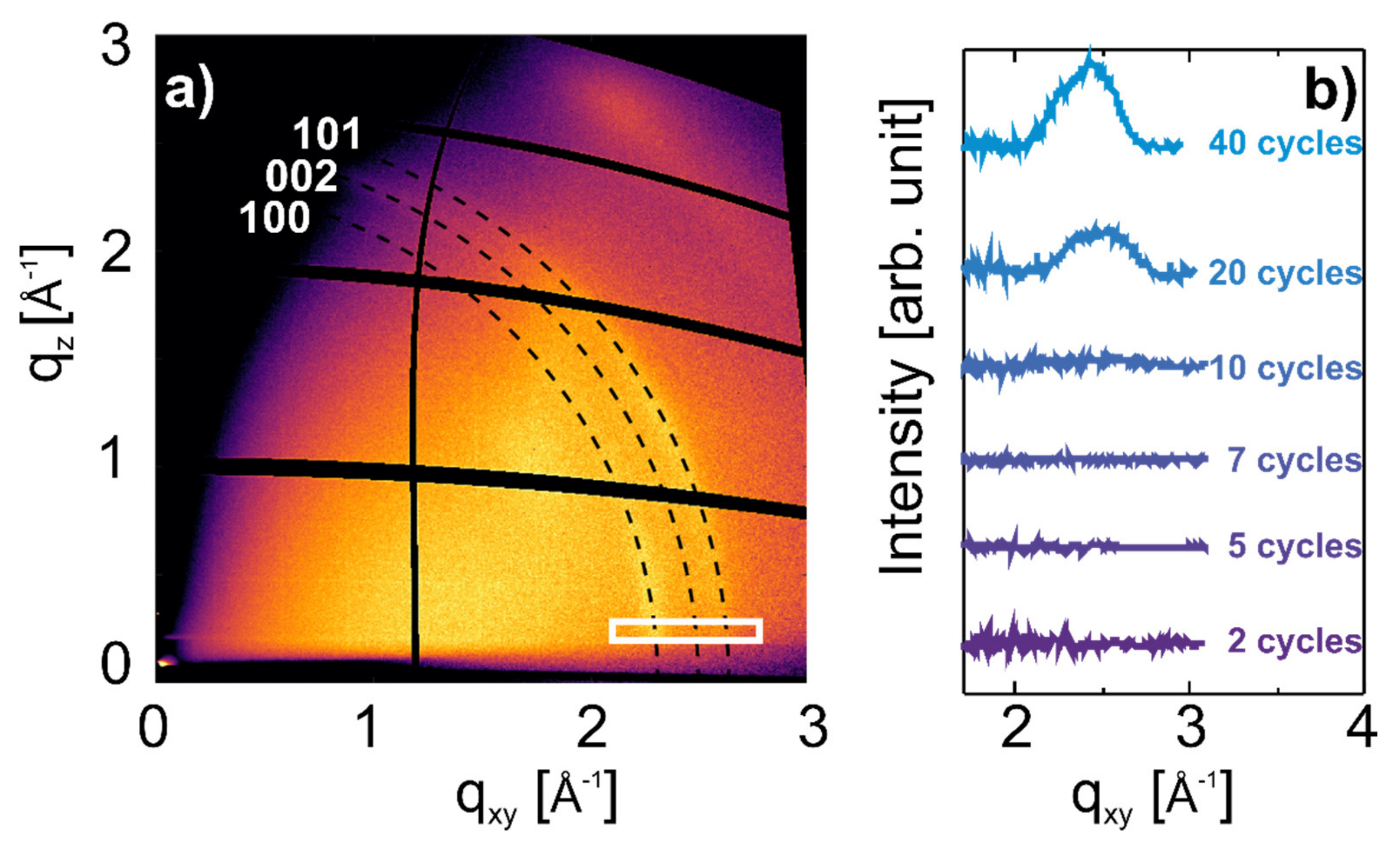
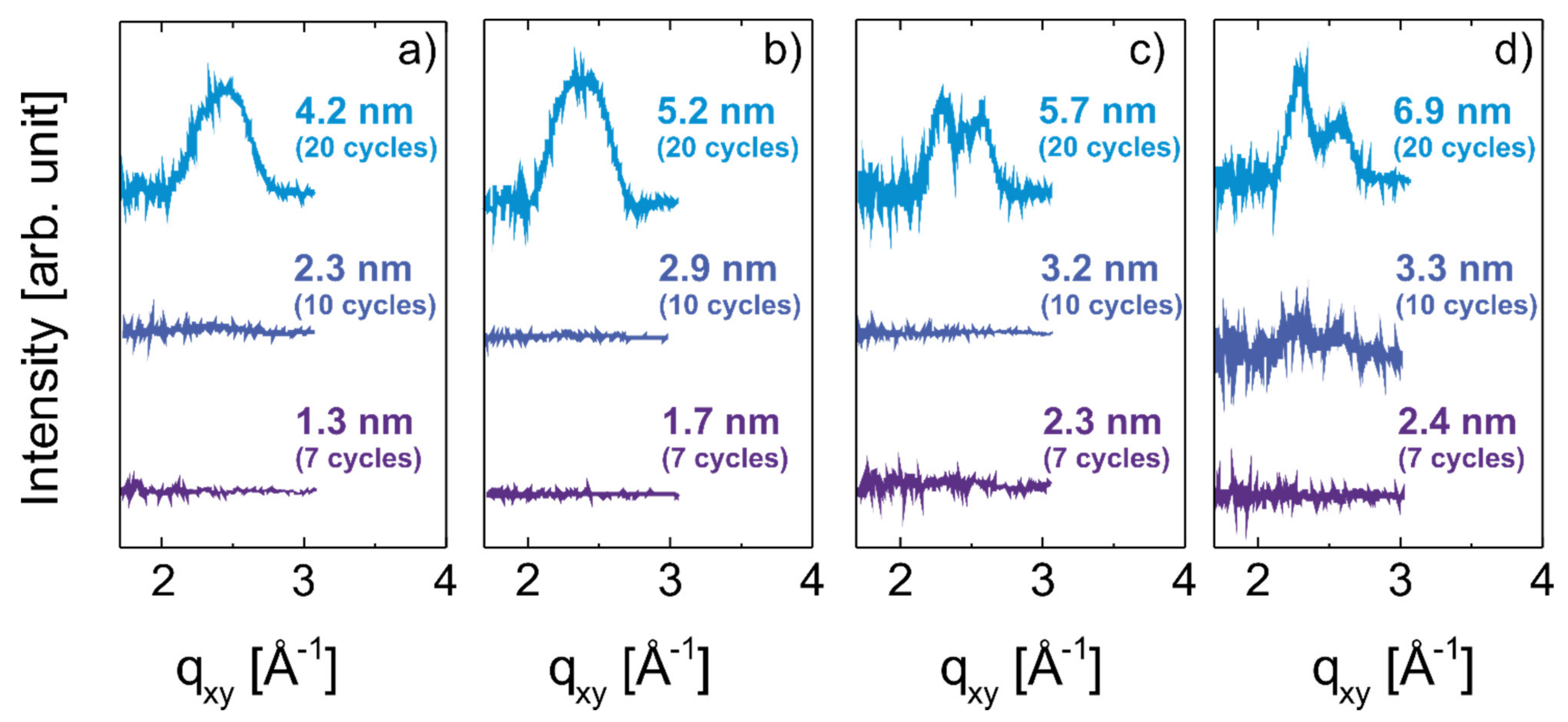
3.2. Development of Crystallites
3.3. Crystallization at High Temperature
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Klingshirn, C.; Fallert, J.; Zhou, H.; Sartor, J.; Thiele, C.; Maier-Flaig, F.; Schneider, D.; Kalt, H. 65 years of ZnO research–old and very recent results. Phys. Status Solidi 2010, 247, 1424–1447. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond. Sci. Technol. 2014, 29, 043001. [Google Scholar] [CrossRef]
- Cachoncinlle, C.; Hebert, C.; Perrière, J.; Nistor, M.; Petit, A.; Millon, E. Random lasing of ZnO thin films grown by pulsed-laser deposition. Appl. Surf. Sci. 2015, 336, 103–107. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Wu, Y.; Hermkens, P.M.; Van de Loo, B.W.H.; Knoops, H.C.M.; Potts, S.E.; Verheijen, M.A.; Roozeboom, F.; Kessels, W.M.M. Electrical transport and Al doping efficiency in nanoscale ZnO films prepared by atomic layer deposition. J. Appl. Phys. 2013, 114, 024308. [Google Scholar] [CrossRef]
- Lee, D.-J.; Kim, H.-M.; Kwon, J.-Y.; Choi, H.; Kim, S.-H.; Kim, K.-B. Structural and Electrical Properties of Atomic Layer Deposited Al-Doped ZnO Films. Adv. Funct. Mater. 2011, 21, 448–455. [Google Scholar] [CrossRef]
- Horwat, D.; Mickan, M.; Chamorro, W. New strategies for the synthesis of ZnO and Al-doped ZnO films by reactive magnetron sputtering at room temperature. Phys. Status Solidi 2016, 13, 951–957. [Google Scholar] [CrossRef]
- Wisz, G.; Virt, I.; Sagan, P.; Potera, P.; Yavorskyi, R. Structural, Optical and Electrical Properties of Zinc Oxide Layers Produced by Pulsed Laser Deposition Method. Nanoscale Res. Lett. 2017, 12, 253. [Google Scholar] [CrossRef]
- Kim, D.; Kang, H.; Kim, J.M.; Kim, H. The properties of plasma-enhanced atomic layer deposition (ALD) ZnO thin films and comparison with thermal ALD. Appl. Surf. Sci. 2011, 257, 3776–3779. [Google Scholar] [CrossRef]
- Baitimirova, M.; Viter, R.; Andzane, J.; Van der Lee, A.; Voiry, D.; Iatsunskyi, I.; Coy, E.; Mikoliunaite, L.; Tumenas, S.; Załęski, K.; et al. Tuning of Structural and Optical Properties of Graphene/ZnO Nanolaminates. J. Phys. Chem. C 2016, 120, 23716–23725. [Google Scholar] [CrossRef]
- Graniel, O.; Weber, M.; Balme, S.; Miele, P.; Bechelany, M. Atomic layer deposition for biosensing applications. Biosens. Bioelectron. 2018, 122, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Abou Chaaya, A.; Viter, R.; Bechelany, M.; Alute, Z.; Erts, D.; Zalesskaya, A.; Kovalevskis, K.; Rouessac, V.; Smyntyna, V.; Miele, P. Evolution of microstructure and related optical properties of ZnO grown by atomic layer deposition. Beilstein J. Nanotechnol. 2013, 4, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Oruc, F.B.; Aygun, L.E.; Donmez, I.; Biyikli, N.; Okyay, A.K.; Yu, H.Y. Low temperature atomic layer deposited ZnO photo thin film transistors. J. Vac. Sci. Technol. A Vacuum Surf. Film 2015, 33, 01A105. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Rowlette, P.C.; Allen, C.G.; Bromley, O.B.; Dubetz, A.E.; Wolden, C.A. Plasma-enhanced atomic layer deposition of semiconductor grade ZnO using dimethyl zinc. Chem. Vap. Depos. 2009, 15, 15–20. [Google Scholar] [CrossRef]
- Pilz, J.; Perrotta, A.; Christian, P.; Tazreiter, M.; Resel, R.; Leising, G.; Griesser, T.; Coclite, A.M. Tuning of material properties of ZnO thin films grown by plasma-enhanced atomic layer deposition at room temperature. J. Vac. Sci. Technol. A Vacuum Surf. Film 2018, 36, 01A109. [Google Scholar] [CrossRef]
- Thomas, M.A.; Cui, J.B. Highly tunable electrical properties in undoped ZnO grown by plasma enhanced thermal-atomic layer deposition. ACS Appl. Mater. Interfaces 2012, 4, 3122–3128. [Google Scholar] [CrossRef]
- Thomas, M.A.; Cui, J. The Effects of an O2 Plasma on the Optical Properties of Atomic Layer Deposited ZnO. ECS Trans. 2012, 45, 87–95. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; Van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A Vacuum Surf. Film 2011, 29, 050801. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Fong, D.D.; Eastman, J.A.; Kim, S.K.; Fister, T.T.; Highland, M.J.; Baldo, P.M.; Fuoss, P.H. In situ synchrotron x-ray characterization of ZnO atomic layer deposition. Appl. Phys. Lett. 2010, 97, 191904. [Google Scholar] [CrossRef]
- Baji, Z.; Lábadi, Z.; Horváth, Z.E.; Molnár, G.; Volk, J.; Bársony, I.; Barna, P. Nucleation and Growth Modes of ALD ZnO. Cryst. Growth Des. 2012, 12, 5615–5620. [Google Scholar] [CrossRef]
- Klug, J.A.; Weimer, M.S.; Emery, J.D.; Yanguas-Gil, A.; Seifert, S.; Schlepütz, C.M.; Martinson, A.B.F.; Elam, J.W.; Hock, A.S.; Proslier, T. A modular reactor design for in situ synchrotron x-ray investigation of atomic layer deposition processes. Rev. Sci. Instrum. 2015, 86, 113901. [Google Scholar] [CrossRef] [PubMed]
- Boichot, R.; Tian, L.; Richard, M.-I.; Crisci, A.; Chaker, A.; Cantelli, V.; Coindeau, S.; Lay, S.; Ouled, T.; Guichet, C.; et al. Evolution of Crystal Structure During the Initial Stages of ZnO Atomic Layer Deposition. Chem. Mater. 2016, 28, 592–600. [Google Scholar] [CrossRef]
- Chu, M.H.; Tian, L.; Chaker, A.; Cantelli, V.; Ouled, T.; Boichot, R.; Crisci, A.; Lay, S.; Richard, M.-I.; Thomas, O.; et al. An Atomistic View of the Incipient Growth of Zinc Oxide Nanolayers. Cryst. Growth Des. 2016, 16, 5339–5348. [Google Scholar] [CrossRef]
- Chu, M.-H.; Tian, L.; Chaker, A.; Skopin, E.; Cantelli, V.; Ouled, T.; Boichot, R.L.; Crisci, A.; Lay, S.; Richard, M.-I.; et al. Evaluation of Alternative Atomistic Models for the Incipient Growth of ZnO by Atomic Layer Deposition. J. Electron. Mater. 2017, 46, 3512–3517. [Google Scholar] [CrossRef]
- Skopin, E.V.; Rapenne, L.; Roussel, H.; Deschanvres, J.-L.; Blanquet, E.; Ciatto, G.; Fong, D.D.; Richard, M.-I.; Renevier, H. The initial stages of ZnO atomic layer deposition on atomically flat In0.53Ga0.47As substrates. Nanoscale 2018, 10, 11585–11596. [Google Scholar] [CrossRef]
- Skopin, E.V.; Deschanvres, J.-L.; Renevier, H. In Situ Ellipsometry Study of the Early Stage of ZnO Atomic Layer Deposition on In0.53Ga0.47As. Phys. Status Solidi 2020, 86, 1900831. [Google Scholar] [CrossRef]
- Liao, M.-H.; Chang, L.C. Experimental demonstration for the implant-free In0.53Ga0.47As quantum-well metal-insulator-semiconductor field-effect transistors with ultra-low source/drain resistance. Appl. Phys. Lett. 2013, 103, 072102. [Google Scholar] [CrossRef]
- Hyun Kim, S.; Yeong Joo, S.; Soo Jin, H.; Kim, W.-B.; Joo Park, T. Ultrathin ZnS and ZnO Interfacial Passivation Layers for Atomic-Layer-Deposited HfO2 Films on InP Substrates. ACS Appl. Mater. Interfaces 2016, 8, 20880–20884. [Google Scholar]
- Puurunen, R.L.; Vandervorst, W. Island growth as a growth mode in atomic layer deposition: A phenomenological model. J. Appl. Phys. 2004, 96, 7686–7695. [Google Scholar] [CrossRef]
- Langereis, E.; Heil, S.B.S.; Van de Sanden, M.C.M.; Kessels, W.M.M. In situ spectroscopic ellipsometry study on the growth of ultrathin TiN films by plasma-assisted atomic layer deposition. J. Appl. Phys. 2006, 100, 023534. [Google Scholar] [CrossRef]
- Langereis, E.; Heil, S.B.S.; Knoops, H.C.M.; Keuning, W.; Van de Sanden, M.C.M.; Kessels, W.M.M. In situ spectroscopic ellipsometry as a versatile tool for studying atomic layer deposition. J. Phys. D Appl. Phys. 2009, 42, 073001. [Google Scholar] [CrossRef]
- Dendooven, J.; Solano, E.; Minjauw, M.M.; Van de Kerckhove, K.; Coati, A.; Fonda, E.; Portale, G.; Garreau, Y.; Detavernier, C. Mobile setup for synchrotron based in situ characterization during thermal and plasma-enhanced atomic layer deposition. Rev. Sci. Instrum. 2016, 87, 113905. [Google Scholar] [CrossRef]
- Perrotta, A.; Pilz, J.; Pachmajer, S.; Milella, A.; Coclite, A.M. On the transformation of “zincone”-like into porous ZnO thin films from sub-saturated plasma enhanced atomic layer deposition. Beilstein J. Nanotechnol. 2019, 10, 746–759. [Google Scholar] [CrossRef]
- Perrotta, A.; Pilz, J.; Milella, A.; Coclite, A.M. Opto-chemical control through thermal treatment of plasma enhanced atomic layer deposited ZnO: An in situ study. Appl. Surf. Sci. 2019, 483, 10–18. [Google Scholar] [CrossRef]
- Pilz, J.; Perrotta, A.; Leising, G.; Coclite, A.M. ZnO Thin Films Grown by Plasma-Enhanced Atomic Layer Deposition: Material Properties within and Outside the “ALD Window.”. Phys. Status Solidi 2019, 1900256. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Van de Loo, B.W.H.; Smit, S.; Ponomarev, M.V.; Weber, J.-W.; Sharma, K.; Kessels, W.M.M.; Creatore, M. Optical modeling of plasma-deposited ZnO films: Electron scattering at different length scales. J. Vac. Sci. Technol. A Vacuum Surf. Film 2015, 33, 021509. [Google Scholar] [CrossRef]
- Danauskas, S.M.; Li, D.; Meron, M.; Lin, B.; Lee, K.Y.C. Stochastic fitting of specular X-ray reflectivity data using StochFit. J. Appl. Crystallogr. 2008, 41, 1187–1193. [Google Scholar] [CrossRef]
- Lausi, A.; Polentarutti, M.; Onesti, S.; Plaisier, J.R.; Busetto, E.; Bais, G.; Barba, L.; Cassetta, A.; Campi, G.; Lamba, D.; et al. Status of the crystallography beamlines at Elettra. Eur. Phys. J. Plus 2015, 130, 43. [Google Scholar] [CrossRef]
- Schrode, B.; Pachmajer, S.; Dohr, M.; Röthel, C.; Domke, J.; Fritz, T.; Resel, R.; Werzer, O. IUCr GIDVis: A comprehensive software tool for geometry-independent grazing-incidence X-ray diffraction data analysis and pole-figure calculations. J. Appl. Crystallogr. 2019, 52, 683–689. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Kim, S.W.; Peng, L.; Miller, A.; Beyer, G.; Beyne, E.; Lee, C.S. Permanent wafer bonding in the low temperature by using various plasma enhanced chemical vapour deposition dielectrics. In Proceedings of the 2015 International 3D Systems Integration Conference, 3DIC 2015, Sendai, Japan, 31 August–2 September 2015; Institute of Electrical and Electronics Engineers Inc.: Tokyo, Japan, 2015; pp. TS7.2.1–TS7.2.4. [Google Scholar]
- Van Bui, H.; Wiggers, F.B.; Gupta, A.; Nguyen, M.D.; Aarnink, A.A.I.; De Jong, M.P.; Kovalgin, A.Y. Initial growth, refractive index, and crystallinity of thermal and plasma-enhanced atomic layer deposition AlN films. J. Vac. Sci. Technol. A Vacuum Surf. Film 2015, 33, 01A111. [Google Scholar] [CrossRef]
- Napari, M.; Lahtinen, M.; Veselov, A.; Julin, J.; Østreng, E.; Sajavaara, T. Room-temperature plasma-enhanced atomic layer deposition of ZnO: Film growth dependence on the PEALD reactor configuration. Surf. Coat. Technol. 2017, 326, 281–290. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of the structure of hexagonal ZnO. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 1233–1236. [Google Scholar] [CrossRef]
- Koch, M.H.; Hartmann, A.J.; Lamb, R.N.; Neuber, M.; Grunze, M. Self-Texture in the Initial Stages of ZnO Film Growth. J. Phys. Chem. B 1997, 101, 8231–8236. [Google Scholar] [CrossRef]
- Kajikawa, Y. Texture development of non-epitaxial polycrystalline ZnO films. J. Cryst. Growth 2006, 289, 387–394. [Google Scholar] [CrossRef]
- Park, S.-H.K.; Hwang, C.-S.; Kwack, H.-S.; Lee, J.-H.; Chu, H.Y. Characteristics of ZnO Thin Films by Means of Plasma-Enhanced Atomic Layer Deposition. Electrochem. Solid-State Lett. 2006, 9, G299–G301. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Zhang, Q.; Dong, S.; Luo, J.K. Structural, optical, electrical and resistive switching properties of ZnO thin films deposited by thermal and plasma-enhanced atomic layer deposition. Appl. Surf. Sci. 2013, 282, 390–395. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrotta, A.; Pilz, J.; Resel, R.; Werzer, O.; Coclite, A.M. Initial Growth and Crystallization Onset of Plasma Enhanced-Atomic Layer Deposited ZnO. Crystals 2020, 10, 291. https://doi.org/10.3390/cryst10040291
Perrotta A, Pilz J, Resel R, Werzer O, Coclite AM. Initial Growth and Crystallization Onset of Plasma Enhanced-Atomic Layer Deposited ZnO. Crystals. 2020; 10(4):291. https://doi.org/10.3390/cryst10040291
Chicago/Turabian StylePerrotta, Alberto, Julian Pilz, Roland Resel, Oliver Werzer, and Anna Maria Coclite. 2020. "Initial Growth and Crystallization Onset of Plasma Enhanced-Atomic Layer Deposited ZnO" Crystals 10, no. 4: 291. https://doi.org/10.3390/cryst10040291
APA StylePerrotta, A., Pilz, J., Resel, R., Werzer, O., & Coclite, A. M. (2020). Initial Growth and Crystallization Onset of Plasma Enhanced-Atomic Layer Deposited ZnO. Crystals, 10(4), 291. https://doi.org/10.3390/cryst10040291




