Polymeric Planar Microcavities Doped with a Europium Complex
Abstract
1. Introduction
2. Materials and Methods
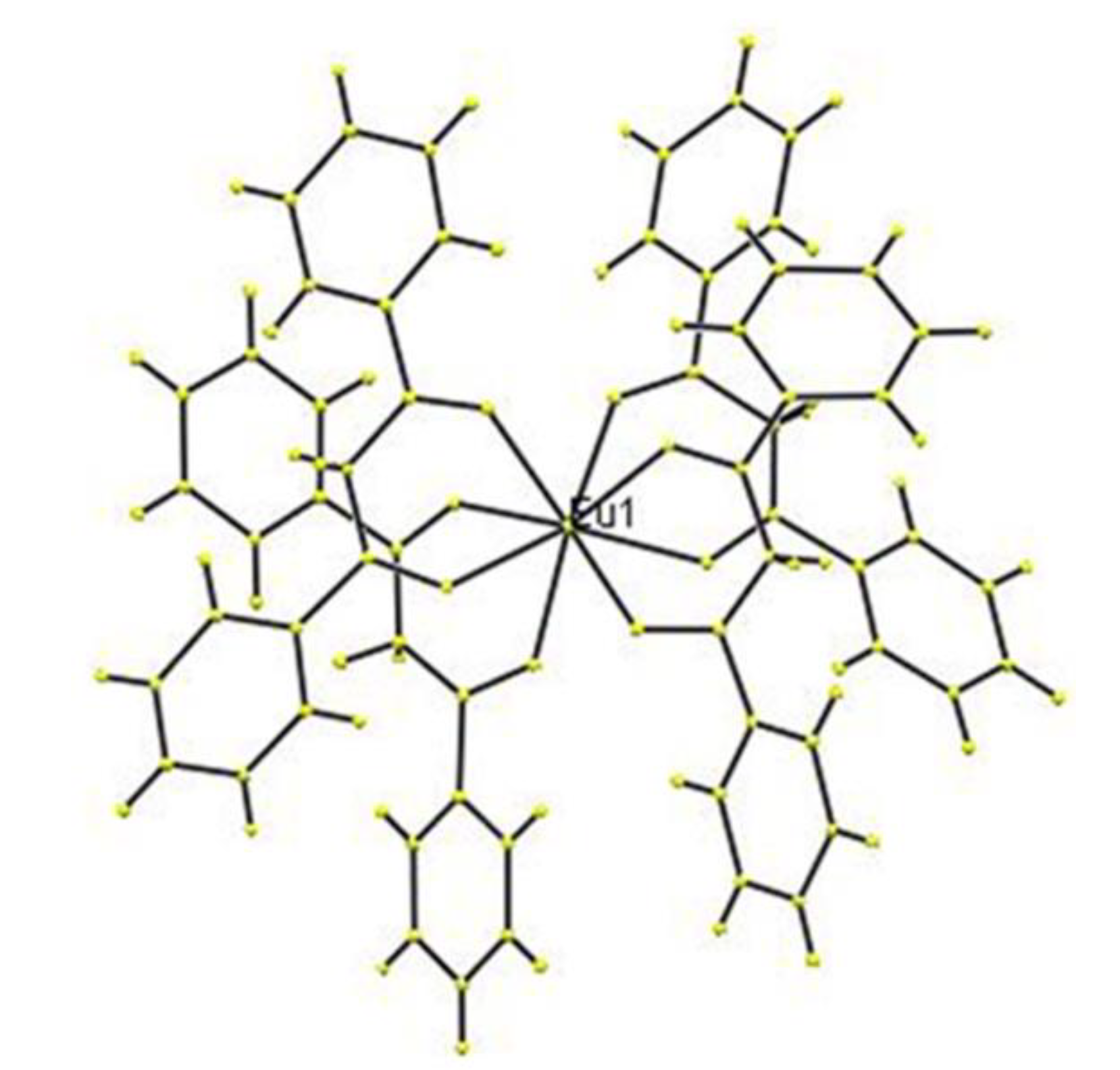
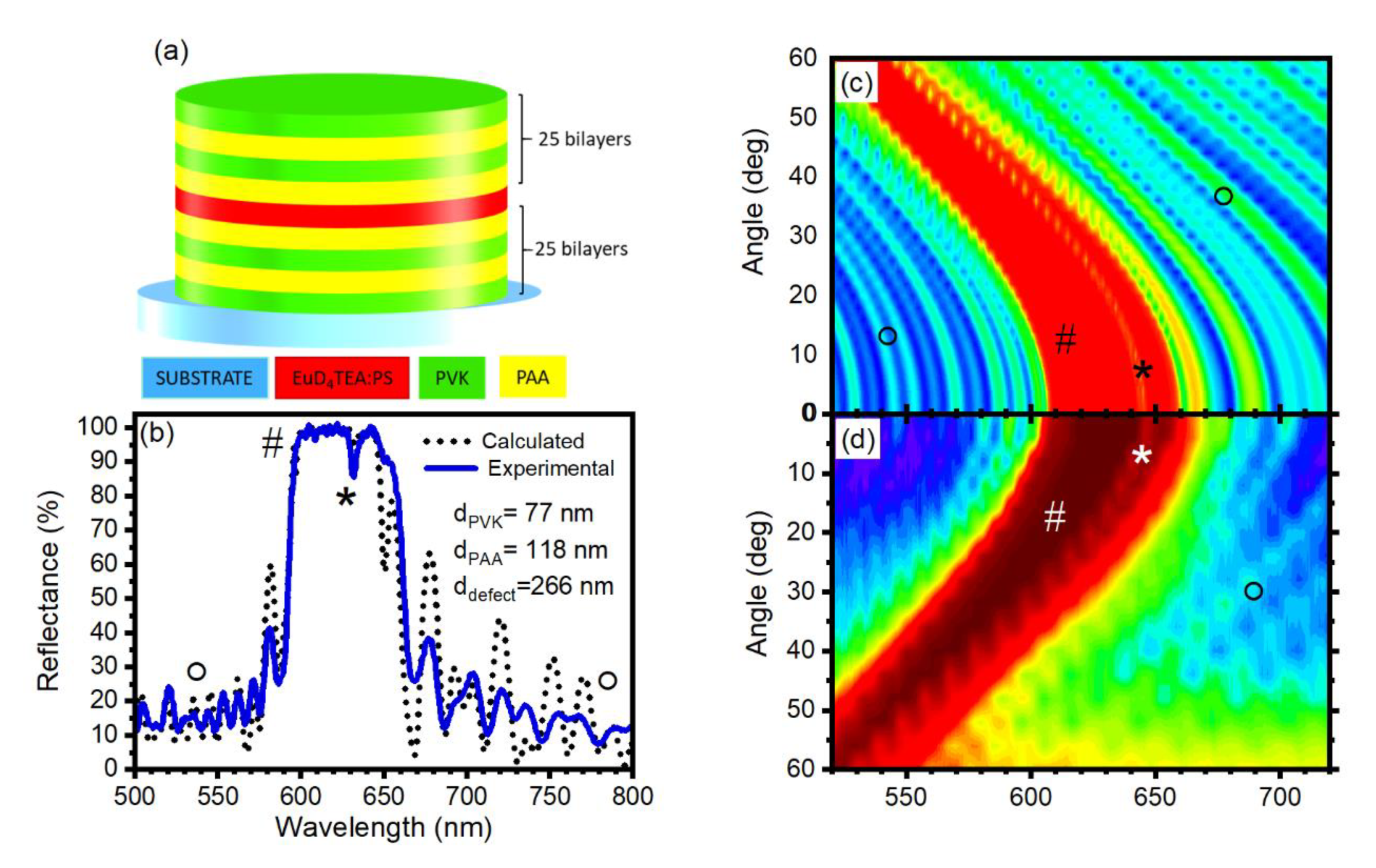
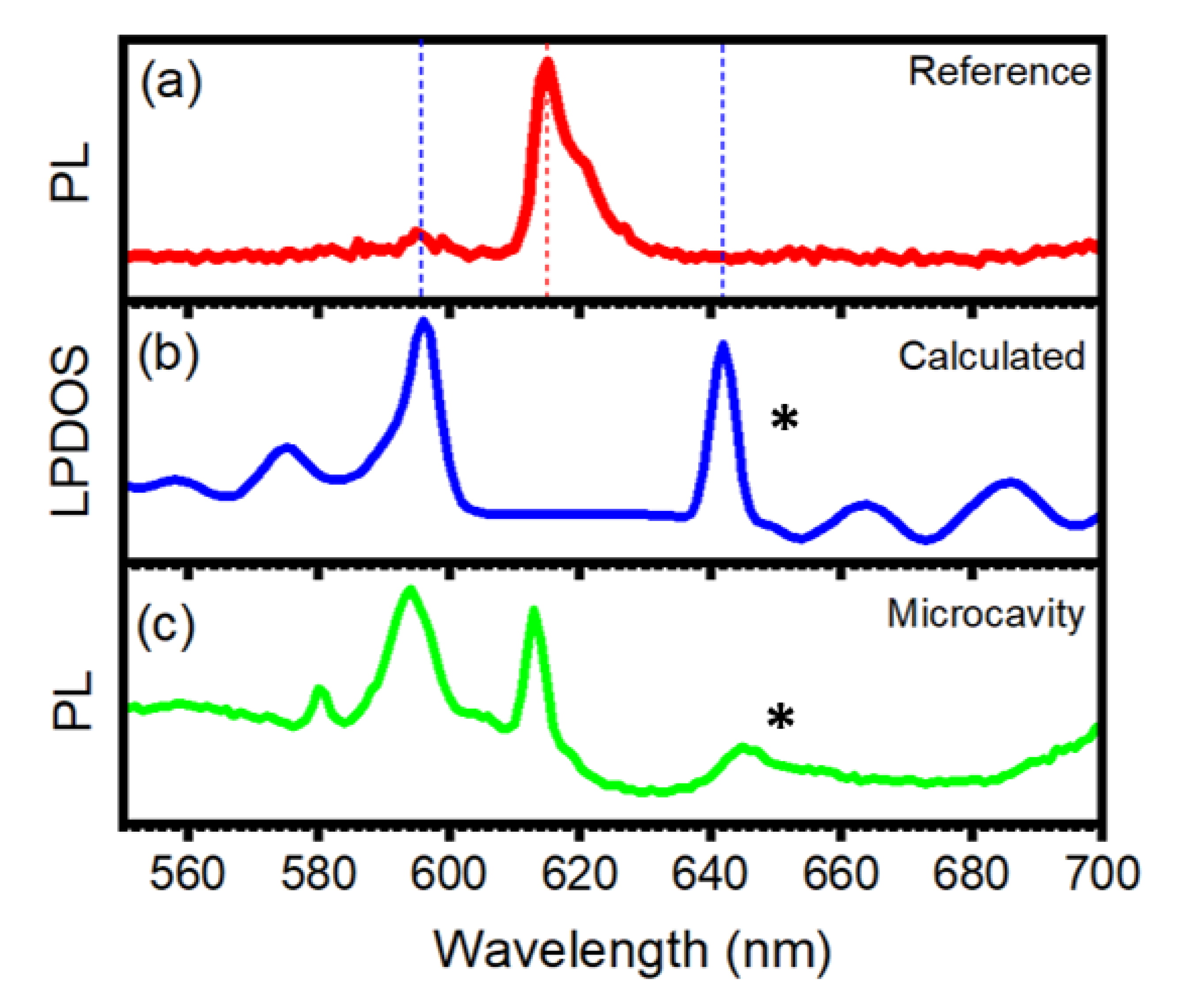
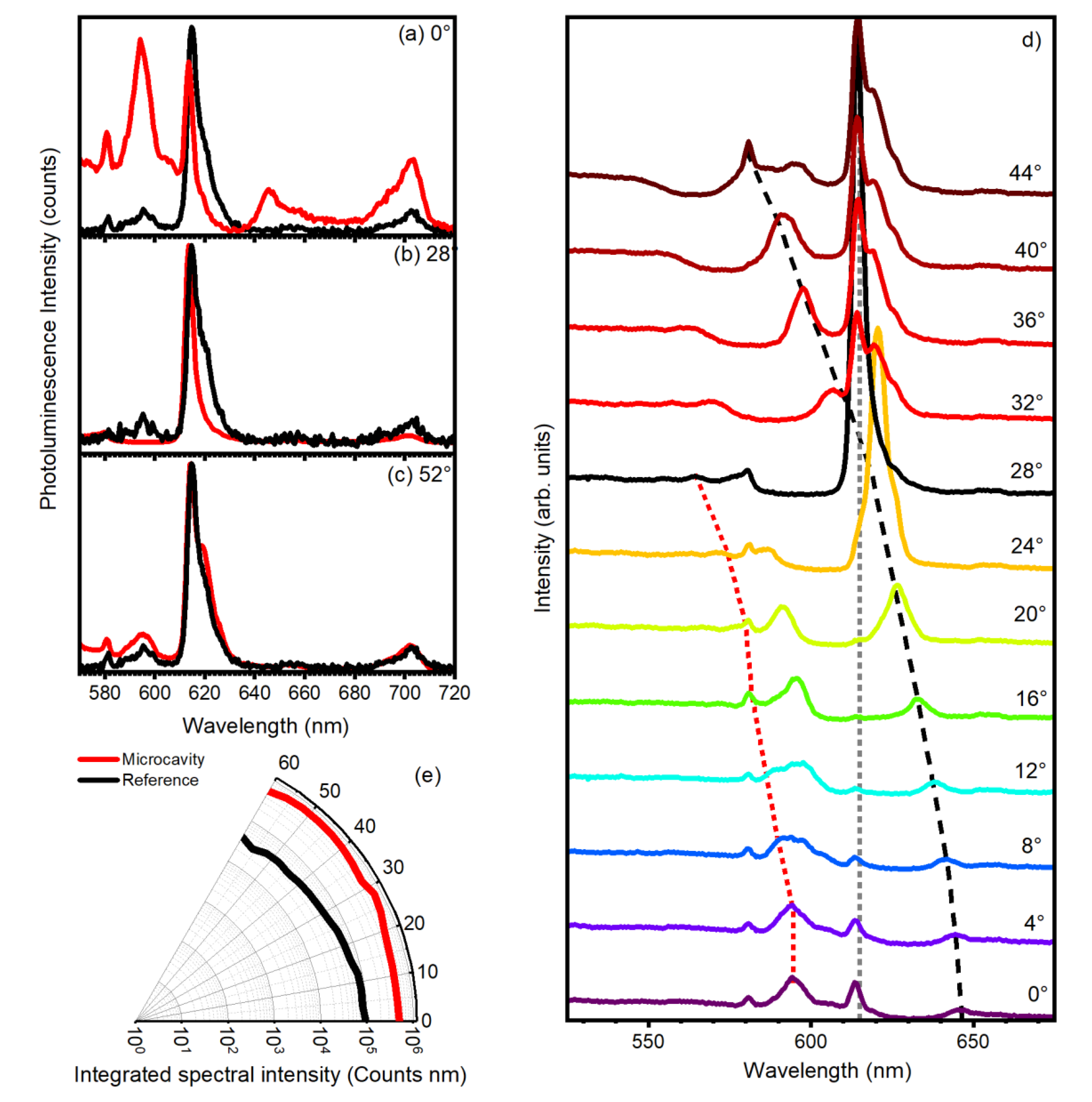
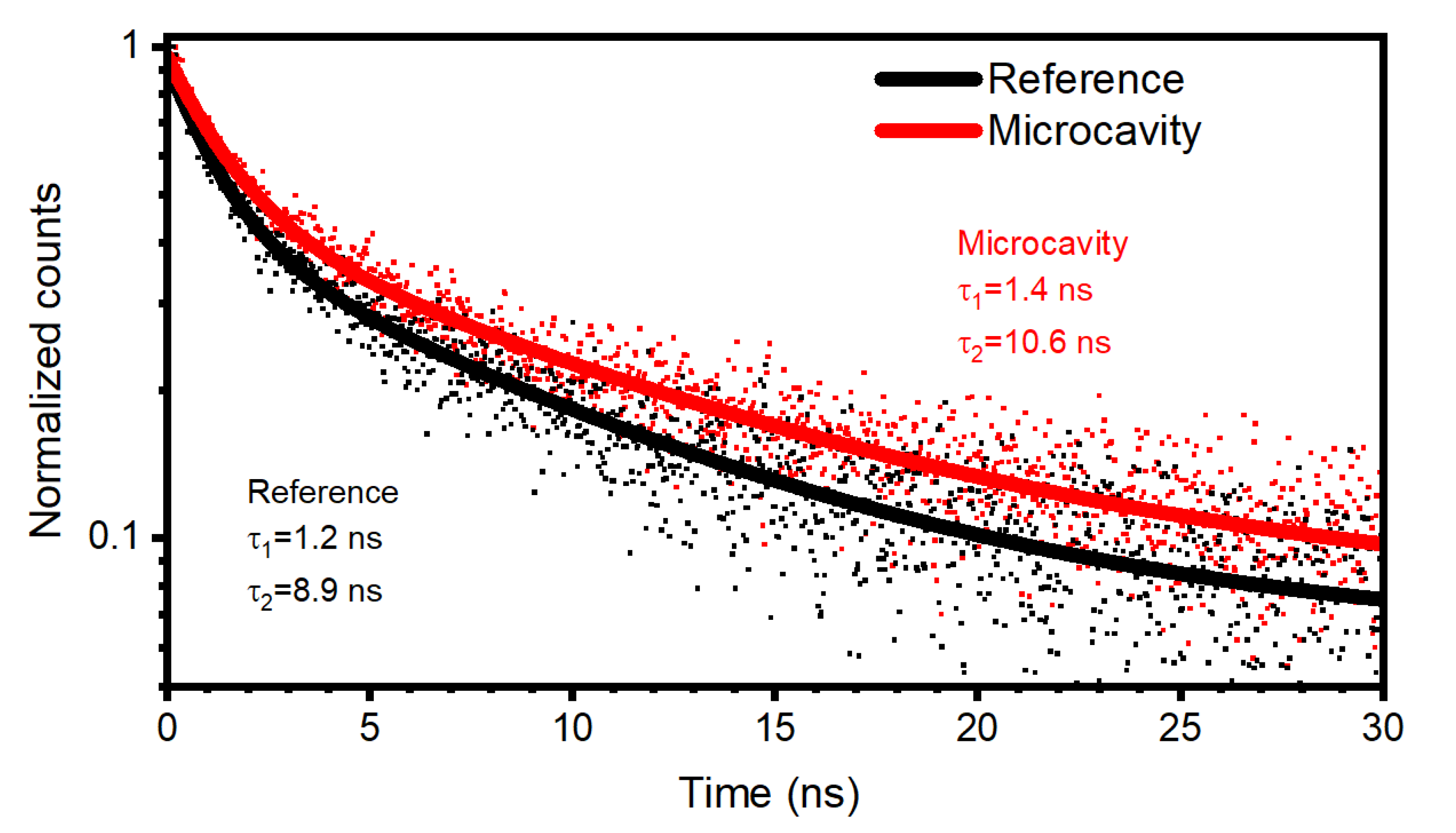
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beer, P.D.; Hayes, E.J. Transition Metal and Organometallic Anion Complexation Agents. Coord. Chem. Rev. 2003, 240, 167–189. [Google Scholar] [CrossRef]
- Vogler, A.; Kunkely, H. Charge Transfer Excitation of Organometallic Compounds: Spectroscopy and Photochemistry. Coord. Chem. Rev. 2004, 248, 273–278. [Google Scholar] [CrossRef]
- İncel, A.; Varlikli, C.; McMillen, C.D.; Demir, M.M. Triboluminescent Electrospun Mats with Blue-Green Emission under Mechanical Force. J. Phys. Chem. C 2017, 121, 11709–11716. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Bhat, K.N.; Hollerman, W.A.; Aggarwal, M.D.; Nguyen, K.M. Comparison of the Triboluminescent Yield and Decay Time for Europium Dibenzoylmethide Triethylammonium Synthesized Using Different Solvents. CrystEngComm 2012, 14, 1382. [Google Scholar] [CrossRef]
- Hurt, C.R.; McAvoy, N.; Bjorklund, S.; Filipescu, N. High Intensity Triboluminescence in Europium Tetrakis (Dibenzoylmethide)-triethylammonium. Nature 1966, 212, 179. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Wei, Y.; Xie, G.; Xue, Q.; Deng, Z.; Huang, W. A Unique White Electroluminescent One-Dimensional Europium(III) Coordination Polymer. J. Mater. Chem. C 2015, 3, 1893–1903. [Google Scholar] [CrossRef]
- Jin, X.; Deng, M.; Kaps, S.; Zhu, X.; Hölken, I.; Mess, K.; Adelung, R.; Mishra, Y.K. Study of Tetrapodal ZnO-PDMS Composites: A Comparison of Fillers Shapes in Stiffness and Hydrophobicity Improvements. PLoS ONE 2014, 9, e106991. [Google Scholar] [CrossRef]
- Fontenot, R.S.; Hollerman, W.A.; Bhat, K.N.; Aggarwal, M.D. Synthesis and Characterization of Highly Triboluminescent Doped Europium Tetrakis Compounds. J. Lumin. 2012, 132, 1812. [Google Scholar] [CrossRef]
- Sweeting, L.M.; Rheingold, A.L. Crystal Disorder and Triboluminescence—Triethylammonium Tetrakis(dibenzoylmethanato)europate. J. Am. Chem. Soc. 1987, 109, 2652. [Google Scholar] [CrossRef]
- İncel, A.; Emirdag-Eanes, M.; McMillen, C.D.; Demir, M.M. Integration of Triboluminescent EuD4TEA Crystals to Transparent Polymers: Impact Sensor Application. ACS Appl. Mater. Interfaces 2017, 9, 6488–6496. [Google Scholar] [CrossRef]
- İncel, A.; Reddy, S.M.; Demir, M.M. A New Method to Extend the Stress Response of Triboluminescent Crystals by Using Hydrogels. Mater. Lett. 2017, 186, 210. [Google Scholar] [CrossRef]
- Walton, A.J. Triboluminescence. Adv. Phys. 1977, 26, 887–948. [Google Scholar] [CrossRef]
- Hardy, G.E.; Kaska, W.C.; Chandra, B.P.; Zink, J.I. Triboluminescence-Structure Relationships in Polymorphs of Hexaphenylcarbodiphosphorane and Anthranilic Acid, Molecular Crystals, and Salts. JACS 1981, 103, 1074–1079. [Google Scholar] [CrossRef]
- Yablonovitch, E. Photonic Crystals: Semiconductors of Light. Sci. Am. 2001, 12, 47. [Google Scholar] [CrossRef]
- Sadrai, M.; Hadel, L.; Sauers, R.R.; Husain, S.; Krogh-Jespersen, K.; Westbrook, J.D.; Bird, G.R. Lasing Action in a Family of Perylene Derivatives: Singlet Absorption and Emission Spectra, Triplet Absorption and Oxygen Quenching Constants, and Molecular Mechanics and Semiempirical Molecular Orbital Calculations. J. Phys. Chem. 1992, 96, 7988–7996. [Google Scholar] [CrossRef]
- Paternò, G.M.; Iseppon, C.; D’Altri, A.; Fasanotti, C.; Merati, G.; Randi, M.; Desii, A.; Pogna, E.A.A.; Viola, D.; Cerullo, G.; et al. Solution Processable and Optically Switchable 1D Photonic Structures. Sci. Rep. 2018, 8, 3517. [Google Scholar] [CrossRef]
- Paternò, G.M.; Moscardi, L.; Donini, S.; Ariodanti, D.; Kriegel, I.; Zani, M.; Parisini, E.; Scotognella, F.; Lanzani, G. Hybrid One-Dimensional Plasmonic–Photonic Crystals for Optical Detection of Bacterial Contaminants. J. Phys. Chem. Lett. 2019, 10, 4980–4986. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic Crystals for Chemical Sensing and Biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef] [PubMed]
- von Freymann, G.; Kitaev, V.; Lotsch, B.V.; Ozin, G.A. Bottom-up Assembly of Photonic Crystals. Chem. Soc. Rev. 2013, 42, 2528–2554. [Google Scholar] [CrossRef]
- Ye, Z.; Park, J.-M.; Constant, K.; Kim, T.-G.; Ho, K.-M. Photonic Crystal: Energy-Related Applications. J. Photonics Energy 2012, 2, 021012–13. [Google Scholar] [CrossRef]
- David, A.; Benisty, H.; Weisbuch, C. Photonic Crystal Light-Emitting Sources. Rep. Prog. Phys. 2012, 75, 126501. [Google Scholar] [CrossRef] [PubMed]
- Ralf, B.W.; Johannes, Ü. 3D Photonic Crystals for Photon Management in Solar Cells. J. Opt. 2012, 14, 024003. [Google Scholar]
- Yin, J.; Migas, D.B.; Panahandeh-Fard, M.; Chen, S.; Wang, Z.; Lova, P.; Soci, C. Charge Redistribution at GaAs/P3HT Heterointerfaces with Different Surface Polarity. J. Phys. Chem. Lett. 2013, 4, 3303–3309. [Google Scholar] [CrossRef]
- Sprengard, R.; Bonrad, K.; Daeubler, T.K.; Frank, T.; Hagemann, V.; Köhler, I.; Pommerehne, J.; Ottermann, C.R.; Voges, F.; Vingerling, B. OLED Devices for Signage Applications: A Review of Recent Advances and Remaining Challenges. In Organic Light-Emitting Materials and Devices VIII; SPIE: Bellingham, WA, USA, 2004; Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/5519/0000/OLED-devices-for-signage-applications--a-review-of-recent/10.1117/12.567131.short?SSO=1 (accessed on 9 April 2020).
- Shah, A.; Torres, P.; Tscharner, R.; Wyrsch, N.; Keppner, H. Photovoltaic Technology: The Case for Thin-Film Solar Cells. Science 1999, 285, 692–698. [Google Scholar] [CrossRef]
- Appold, M.; Gallei, M. Bio-Inspired Structural Colors Based on Linear Ultrahigh Molecular Weight Block Copolymers. ACS Appl. Polym. Mater. 2019, 1, 239–250. [Google Scholar] [CrossRef]
- Kang, H.S.; Lee, J.; Cho, S.M.; Park, T.H.; Kim, M.J.; Park, C.; Lee, S.W.; Kim, K.L.; Ryu, D.Y.; Huh, J.; et al. Printable and Rewritable Full Block Copolymer Structural Color. Adv. Mater. 2017, 29, 1700084. [Google Scholar] [CrossRef]
- Nucara, L.; Piazza, V.; Greco, F.; Robbiano, V.; Cappello, V.; Gemmi, M.; Cacialli, F.; Mattoli, V. Ionic Strength Responsive Sulfonated Polystyrene Opals. ACS Appl. Mater. Interfaces 2017, 9, 4818–4827. [Google Scholar] [CrossRef]
- Mele, E.; Di Benedetto, F.; Persano, L.; Cingolani, R.; Pisignano, D. Multilevel, Room-Temperature Nanoimprint Lithography for Conjugated Polymer-Based Photonics. Nano Lett. 2005, 5, 1915–1919. [Google Scholar] [CrossRef]
- Giusto, P.; Lova, P.; Manfredi, G.; Gazzo, S.; Srinivasan, B.; Radice, S.V.; Comoretto, D. Colorimetric Detection of Perfluorinated Compounds by All-Polymer Photonic Transducers. ACS Omega 2018, 3, 7517–7522. [Google Scholar] [CrossRef]
- Lova, P. Selective Polymer Distributed Bragg Reflector Vapor Sensors. Polymers 2018, 10, 1161. [Google Scholar] [CrossRef]
- Lova, P.; Manfredi, G.; Bastianini, C.; Mennucci, C.; Buatier de Mongeot, F.; Servida, A.; Comoretto, D. Flory-Huggins Photonic Sensors for the Optical Assessment of Molecular Diffusion Coefficients in Polymers. ACS Appl. Mater. Interfaces 2019, 11, 16872–16880. [Google Scholar] [CrossRef] [PubMed]
- Lova, P.; Manfredi, G.; Comoretto, D. Advances in Functional Solution Processed Planar One-Dimensional Photonic Crystals. Adv. Opt. Mater. 2018, 6, 1800730. [Google Scholar] [CrossRef]
- Song, H.; Singer, K.; Wu, Y.; Zhou, J.; Lott, J.; Andrews, J.; Hiltner, A.; Baer, E.; Weder, C.; Bunch, R.; et al. Layered Polymeric Optical Systems Using Continuous Coextrusion. Proc. SPIE 2009, 7467, 74670A. [Google Scholar]
- Singer, K.D.; Kazmierczak, T.; Lott, J.; Song, H.; Wu, Y.; Andrews, J.; Baer, E.; Hiltner, A.; Weder, C. Melt-processed All-polymer Distributed Bragg Reflector Laser. Opt. Express 2008, 16, 10358–10363. [Google Scholar] [CrossRef] [PubMed]
- Palatnik, A.; Tischler, Y.R. Solid-state Rhodamine 6G Microcavity Laser. IEEE Photonics Technol. Lett. 2016, 28, 1823–1826. [Google Scholar] [CrossRef]
- Lee, T.-W.; Park, O.O.; Cho, H.N.; Kim, D.Y.; Kim, Y.C. Low-threshold Lasing in A Microcavity of Fluorene-Based Liquid-Crystalline Polymer Blends. J. Appl. Phys. 2003, 93, 1370. [Google Scholar] [CrossRef][Green Version]
- Tessler, N.; Denton, G.J.; Friend, R.H. Lasing from Conjugated-Polymer Microcavities. Nature 1996, 382, 695–697. [Google Scholar] [CrossRef]
- Manfredi, G.; Lova, P.; Di Stasio, F.; Rastogi, P.; Krahne, R.; Comoretto, D. Lasing From Dot-in-Rod Nanocrystals in Planar Polymer Microcavities. RSC Adv. 2018, 8, 13026–13033. [Google Scholar] [CrossRef]
- Galfsky, T.; Sun, Z.; Considine, C.R.; Chou, C.-T.; Ko, W.-C.; Lee, Y.-H.; Narimanov, E.E.; Menon, V.M. Broadband Enhancement of Spontaneous Emission in Two-Dimensional Semiconductors Using Photonic Hypercrystals. Nano Lett. 2016, 16, 4940–4945. [Google Scholar] [CrossRef]
- Valappil, N.V.; Luberto, M.; Menon, V.M.; Zeylikovich, I.; Gayen, T.K.; Franco, J.; Das, B.B.; Alfano, R.R. Solution Processed Microcavity Structures with Embedded Quantum Dots. Photonics Nanostruct. Fundam. Appl. 2007, 5, 184–188. [Google Scholar] [CrossRef]
- Menon, V.M.; Luberto, M.; Valappil, N.V.; Chatterjee, S. Lasing from InGaP Quantum Dots in a Spin-Coated Flexible Microcavity. Opt. Express 2008, 16, 19535–19540. [Google Scholar] [CrossRef] [PubMed]
- Lova, P.; Grande, V.; Manfredi, G.; Patrin, M.; Herbst, S.; Würthner, F.; Comoretto, D. All-Polymer Photonic Microcavities Doped with Perylene Bisimide J-Aggregates. Adv. Opt. Mater. 2017, 5, 1700523. [Google Scholar] [CrossRef]
- Lova, P.; Giusto, P.; Stasio, F.D.; Manfredi, G.; Paternò, G.M.; Cortecchia, D.; Soci, C.; Comoretto, D. All-Polymer Methylammonium Lead Iodide Perovskite Microcavity. Nanoscale 2019, 11, 8978–8983. [Google Scholar] [CrossRef] [PubMed]
- Lova, P.; Cortecchia, D.; Soci, C.; Comoretto, D. Solution Processed Polymer-ABX4 Perovskite-Like Microcavities. Appl. Sci. 2019, 9, 5203. [Google Scholar] [CrossRef]
- Comoretto, D.; Cuniberti, C.; Musso, G.F.; Dellepiane, G.; Speroni, F.; Botta, C.; Luzzati, S. Optical Properties and Long-Lived Charged Photoexcitations in Polydiacetylenes. Phys. Rev. B 1994, 49, 8059–8066. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Salvi, P.R.; Gellini, C.; Dellepiane, G.; Comoretto, D.; Cuniberti, C. Two-photon Spectroscopy of Pi-Conjugated Polymers: The Case of Poly 1,6-Bis(3,6-Dihexadecyl-N-Carbazolyl)-2,4-Hexadiyne (Polydchd-Hs). J. Phys. Chem A 2001, 105, 7759–7764. [Google Scholar] [CrossRef]
- Frezza, L.; Patrini, M.; Liscidini, M.; Comoretto, D. Directional Enhancement of Spontaneous Emission in Polymer Flexible Microcavities. J. Phys. Chem. C 2011, 115, 19939–19946. [Google Scholar] [CrossRef]
- Rybin, M.V.; Zherzdev, A.V.; Feoktistov, N.A.; Pevtsov, A.B. Effect of photonic crystal stop-band on photoluminescence of a − Si1−xCx: H. Phys. Rev. B 2017, 95, 165118. [Google Scholar] [CrossRef]
- Berti, L.; Cucini, M.; Di Stasio, F.; Comoretto, D.; Galli, M.; Marabelli, F.; Manfredi, N.; Marinzi, C.; Abbotto, A. Spectroscopic Investigation of Artificial Opals Infiltrated with a Heteroaromatic Quadrupolar Dye. J. Phys. Chem. C 2010, 114, 2403–2413. [Google Scholar] [CrossRef]
- Morandi, V.; Marabelli, F.; Amendola, V.; Meneghetti, M.; Comoretto, D. Light Localization Effect on the Optical Properties of Opals Doped with Gold Nanoparticles. J. Phys. Chem. C 2008, 112, 6293–6298. [Google Scholar] [CrossRef]
- Barth, M.; Gruber, A.; Cichos, F. Spectral and Angular Redistribution of Photoluminescence near a Photonic Stop Band. Phys. Rev. B 2005, 72, 085129. [Google Scholar] [CrossRef]
- Hagler, T.W.; Pakbaz, K.; Voss, K.F.; Heeger, A.J. Enhanced Order and Electronic Delocalization in Conjugated Polymers Oriented by Gel Processing in Polyethylene. Phys. Rev. B 1991, 44, 8652–8666. [Google Scholar] [CrossRef] [PubMed]
- Björk, G.; Machida, S.; Yamamoto, Y.; Igeta, K. Modification of Spontaneous Emission Rate in Planar Dielectric Microcavity Structures. Phys. Rev. A 1991, 44, 669–681. [Google Scholar] [CrossRef]
- Björk, G. On the Spontaneous Lifetime Change in an Ideal Planar Microcavity—Transition from a Mode Continuuum to Quantized Modes. IEEE J. Quantum Electron. 1994, 30, 2314–2318. [Google Scholar] [CrossRef]
- Vos, W.L.; Sprik, R.; van Blaaderen, A.; Imhof, A.; Lagendijk, A.; Wegdam, G.H. Strong Effects of Photonic Band Structures on the Diffraction of Colloidal Crystals. Phys. Rev. B 1996, 53, 16231. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Sano, D. Low-Threshold Lasing and Purcell Effect in Microdisk Lasers at Room Temperature. IEEE J. Sel. Top. Quantum Electron. 2003, 9, 1340–1346. [Google Scholar] [CrossRef]
- Gerard, J.M.; Gayral, B. Strong Purcell Effect for Inas Quantum Boxes in Three-Dimensional Solid-State Microcavities. J. Light. Technol. 1999, 17, 2089–2095. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lova, P.; Olivieri, M.; Surace, A.; Topcu, G.; Emirdag-Eanes, M.; Demir, M.M.; Comoretto, D. Polymeric Planar Microcavities Doped with a Europium Complex. Crystals 2020, 10, 287. https://doi.org/10.3390/cryst10040287
Lova P, Olivieri M, Surace A, Topcu G, Emirdag-Eanes M, Demir MM, Comoretto D. Polymeric Planar Microcavities Doped with a Europium Complex. Crystals. 2020; 10(4):287. https://doi.org/10.3390/cryst10040287
Chicago/Turabian StyleLova, Paola, Marco Olivieri, Alba Surace, Gokhan Topcu, Mehtap Emirdag-Eanes, Mustafa M. Demir, and Davide Comoretto. 2020. "Polymeric Planar Microcavities Doped with a Europium Complex" Crystals 10, no. 4: 287. https://doi.org/10.3390/cryst10040287
APA StyleLova, P., Olivieri, M., Surace, A., Topcu, G., Emirdag-Eanes, M., Demir, M. M., & Comoretto, D. (2020). Polymeric Planar Microcavities Doped with a Europium Complex. Crystals, 10(4), 287. https://doi.org/10.3390/cryst10040287




