Extended π-Systems in Diimine Ligands in [Cu(P^P)(N^N)][PF6] Complexes: From 2,2′-Bipyridine to 2-(Pyridin-2-yl)Quinoline
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. 2-(6-Methylpyridin-2-yl)quinoline (2)
2.3. [Cu(POP)(1)][PF6]
2.4. [Cu(xantphos)(1)][PF6]
2.5. [Cu(POP)(2)][PF6]
2.6. [Cu(xantphos)(2)][PF6]
2.7. Crystallography
2.8. [Cu(xantphos)(1)][PF6]
2.9. [Cu(xantphos)(2)][PF6]
2.10. [Cu(POP)(2)][PF6]
3. Results and Discussion
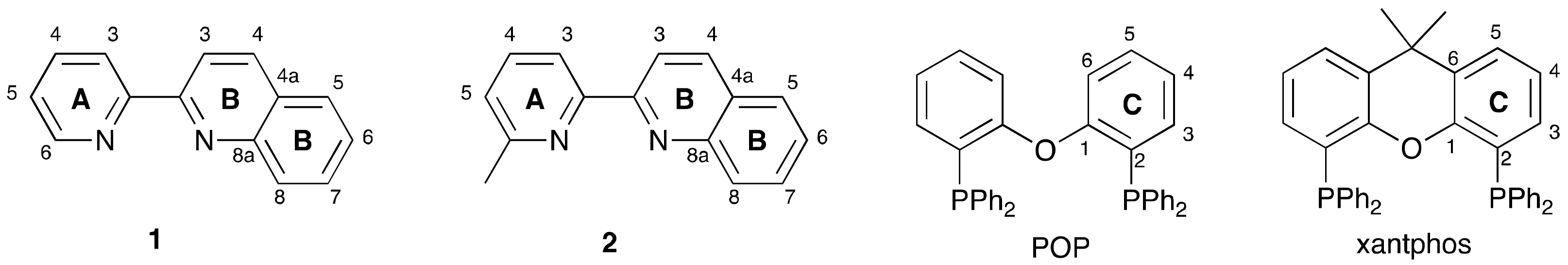
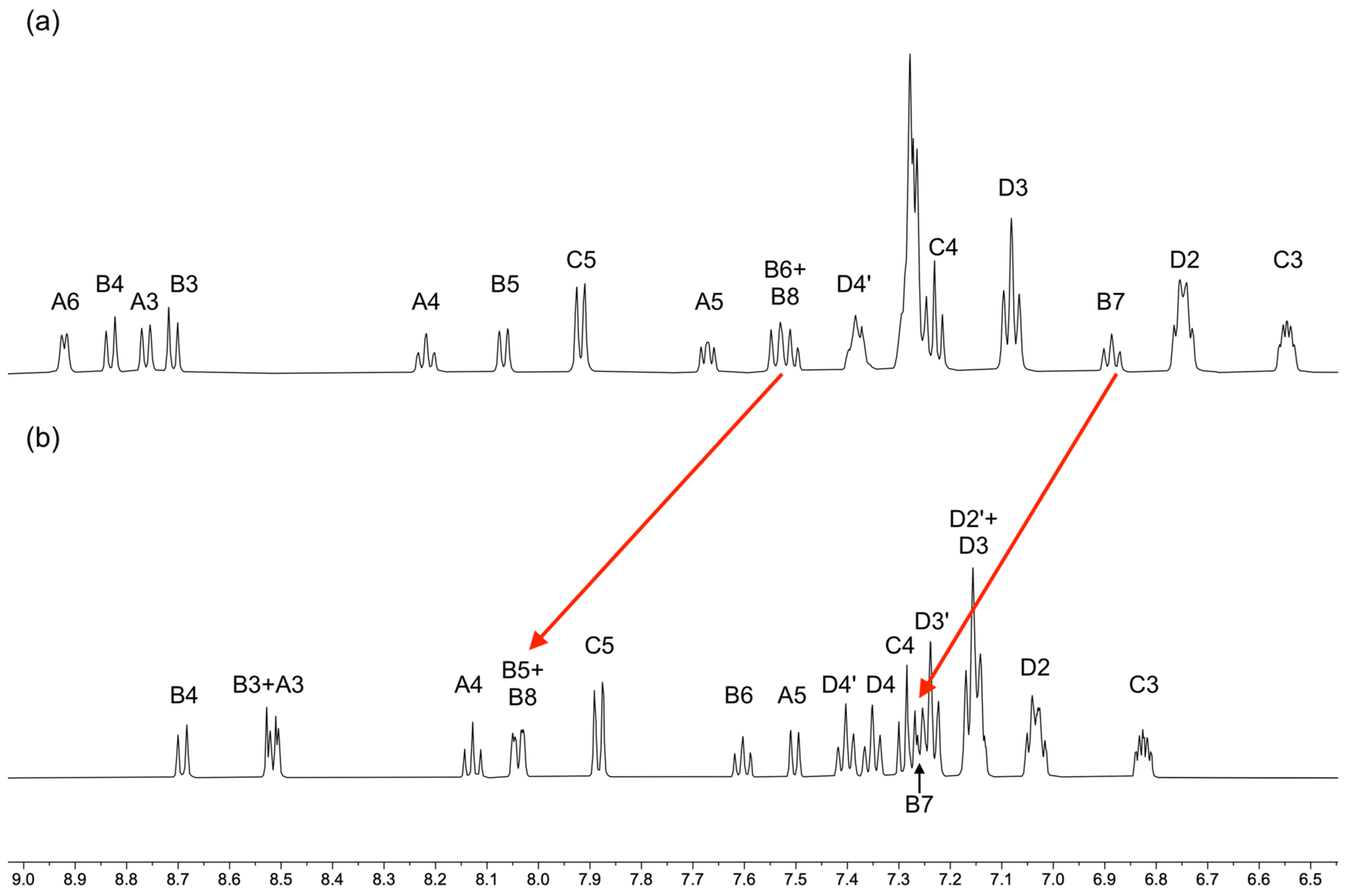
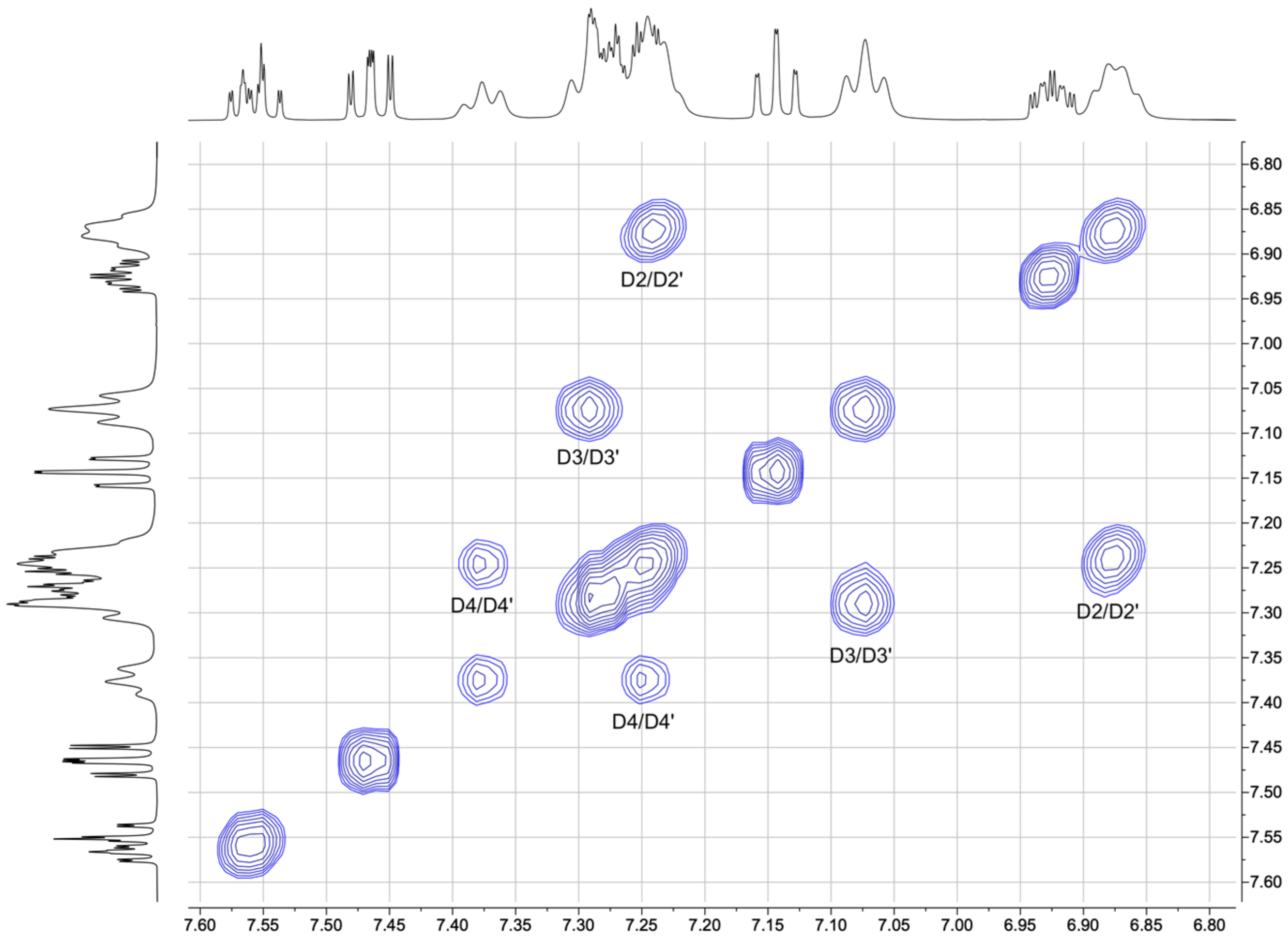
3.1. Characterization of Copper(I) Complexes
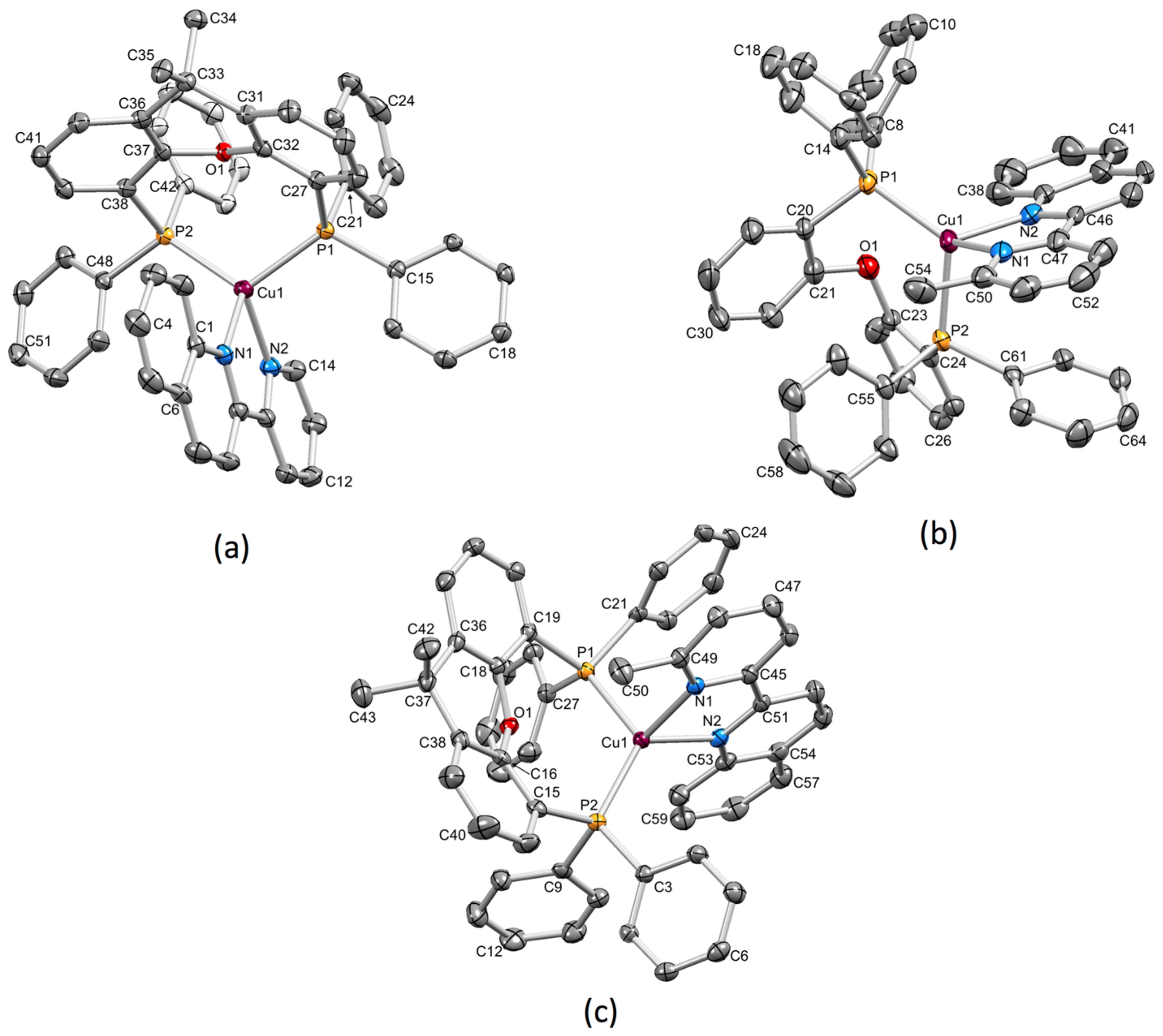
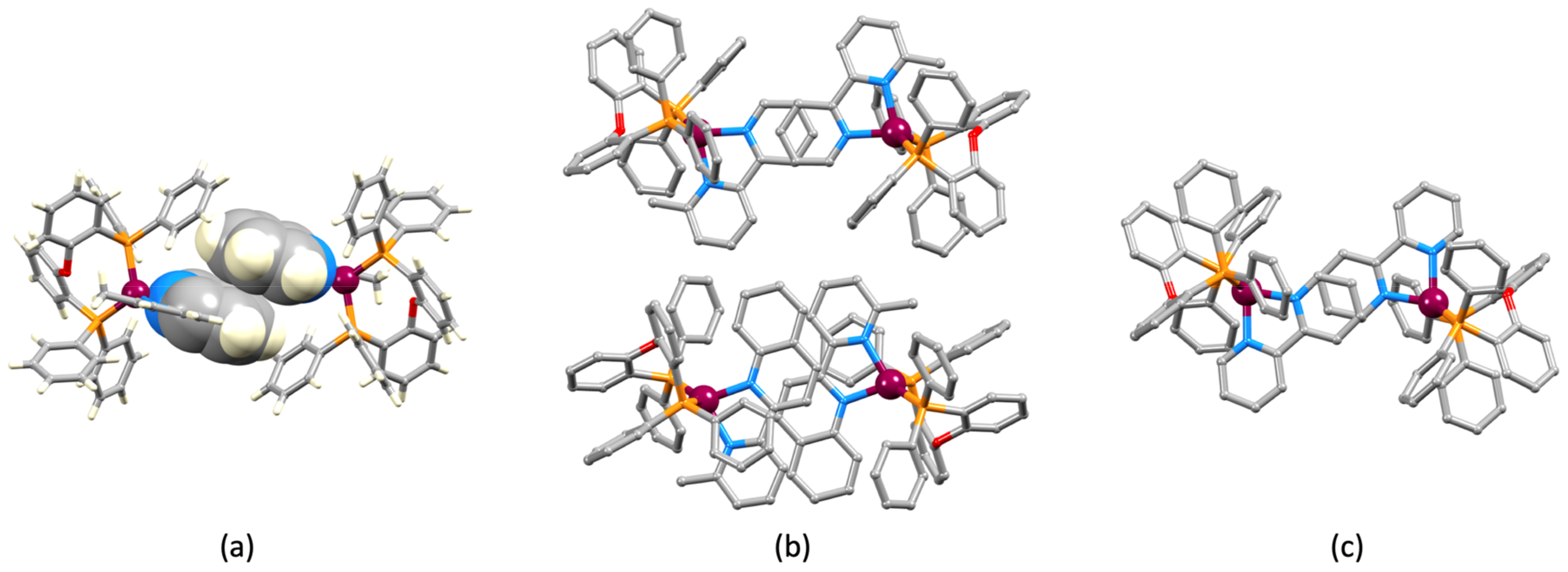
3.2. Crystal Structures of [Cu(xantphos)(1)][PF6], [Cu(xantphos)(2)][PF6] and [Cu(POP)(2)][PF6]
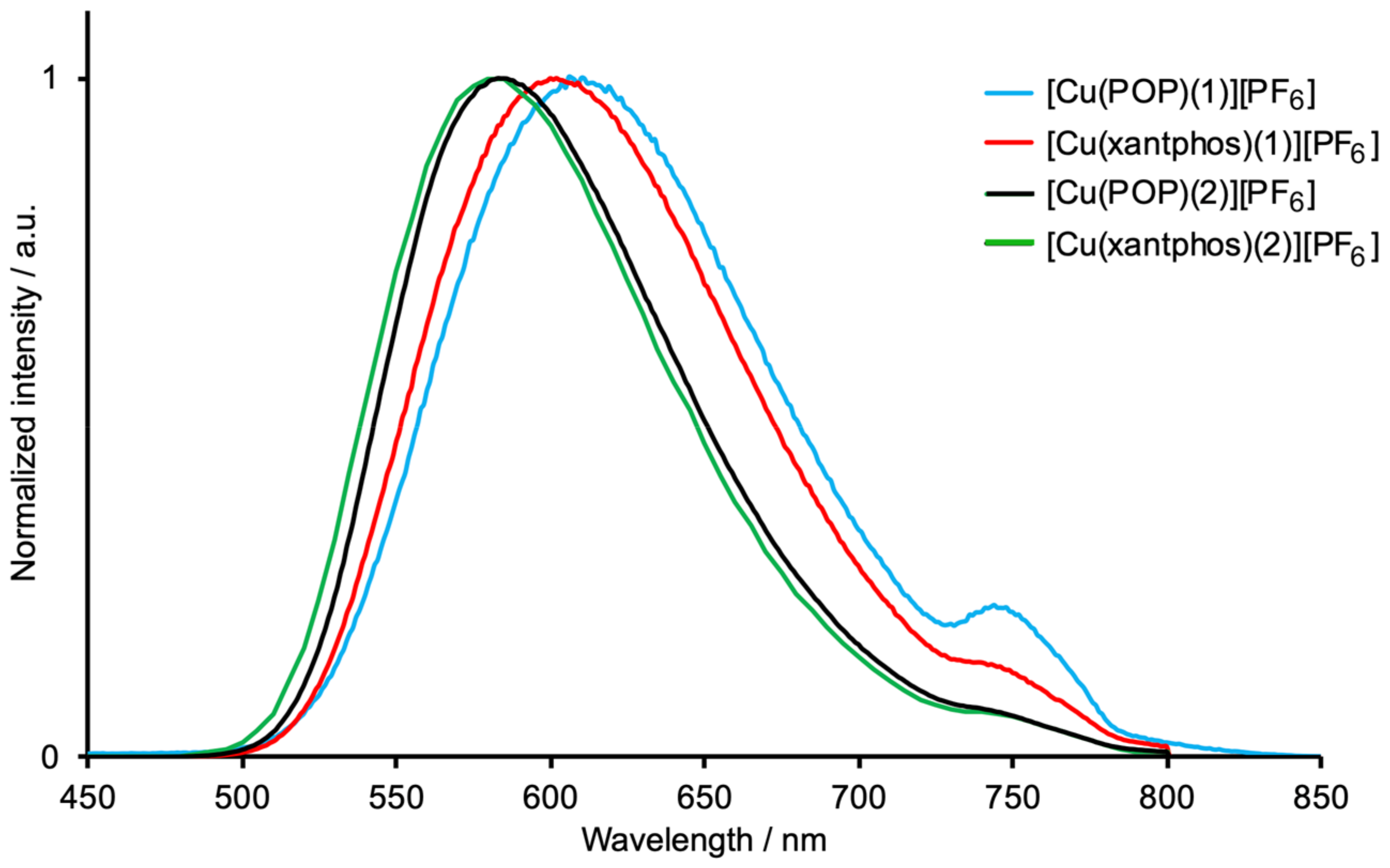
3.3. Photophysical Properties
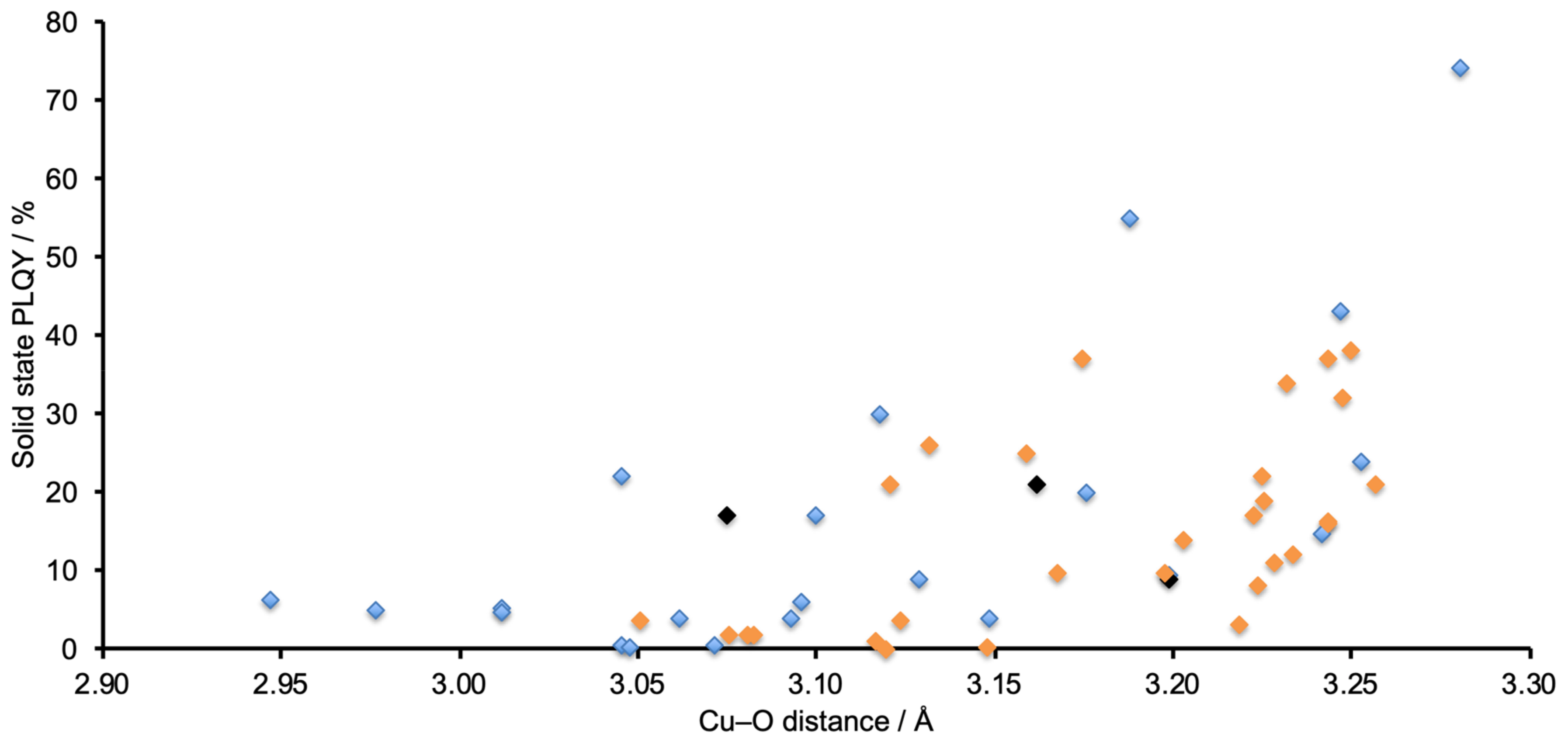
3.4. Comments on Structure–Property Relationships
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Wide Bite Angle Diphosphines: Xantphos Ligands in Transition Metal Complexes and Catalysis. Acc. Chem. Rev. 2001, 34, 895–904. [Google Scholar] [CrossRef]
- Costa, R.D. Light-Emitting Electrochemical Cells: Concepts, Advances and Challenges; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Fresta, E.; Costa, R.D. Beyond traditional light-emitting electrochemical cells – a review of new device designs and emitters. J. Mater. Chem. C 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Buckner, M.T.; McMillin, D.R. Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J. Chem. Soc. Chem. Commun. 1978, 759–761. [Google Scholar] [CrossRef]
- Rader, R.A.; McMillin, D.R.; Buckner, M.T.; Matthews, T.G.; Casadonte, D.J.; Lengel, R.K.; Whittaker, S.B.; Darmon, L.M.; Lytle, F.E. Photostudies of 2,2’-bipyridine bis(triphenylphosphine)copper(1+), 1,10-phenanthroline bis(triphenylphosphine)copper(1+), and 2,9-dimethyl-1,10-phenanthroline bis(triphenylphosphine)copper(1+) in solution and in rigid, low-temperature glasses. Simultaneous multiple emissions from intraligand and charge-transfer states. J. Am. Chem. Soc. 1981, 103, 5906–5912. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yerson, H. Cu(I) complexes—Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Bergmann, L.; Zink, D.M.; Bauman, T.; Volz, D.; Bräse, S. Metal-Organic and Organic TADF Materials: Status, Challenges and Characterization. Top. Curr. Chem. 2016, 374, 22. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Prescimone, A.; Longo, G.; Pertegás, A.; Sessolo, M.; Bolink, H.J. [Cu(bpy)(P^P)]+ containing light-emitting electrochemical cells: Improving performance through simple substitution. Dalton Trans. 2014, 43, 16593–16596. [Google Scholar] [CrossRef]
- Keller, S.; Pertegás, A.; Longo, G.; Martínez, L.; Cerdá, J.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Ortí, E.; et al. Shine bright or live long: Substituent effects in [Cu(N^N)(P^P)]+-based light-emitting electrochemical cells where N^N is a 6-substituted 2,2’-bipyridine. J. Mater. Chem. C 2016, 4, 3857–3871. [Google Scholar] [CrossRef]
- Alkan-Zambada, M.; Keller, S.; Martínez-Sarti, L.; Prescimone, A.; Junquera-Hernández, J.M.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. [Cu(P^P)(N^N)][PF6] compounds with bis(phosphane) and 6-alkoxy, 6-alkylthio, 6-phenyloxy and 6-phenylthio-substituted 2,2’-bipyridine ligands for light-emitting electrochemical cells. J. Mater. Chem. C 2018, 6, 8460–8471. [Google Scholar] [CrossRef]
- Fresta, E.; Volpi, G.; Milanesio, M.; Garino, C.; Barolo, C.; Costa, R.D. Novel Ligand and Device Designs for Stable Light-Emitting Electrochemical Cells Based on Heteroleptic Copper(I) Complexes. Inorg. Chem. 2018, 57, 10469–10479. [Google Scholar] [CrossRef]
- Leoni, E.; Mohanraj, J.; Holler, M.; Mohankumar, M.; Nierengarten, I.; Monti, F.; Sournia-Saquet, A.; Delavaux-Nicot, B.; Nierengarten, J.-F.; Armaroli, N. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands: Rationalization of the Photophysical and Electrochemical Properties. Inorg. Chem. 2018, 57, 15537–15549. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Vilar, J.M.; Fresta, E.; Armentano, D.; Costa, R.D.; Viciano-Chumillas, M.; Cano, J. Photoluminescent Cu(I) vs. Ag(I) complexes: Slowing down emission in Cu(I) complexes by pentacoordinate low-lying excited states. Dalton Trans. 2019, 48, 9765–9775. [Google Scholar] [CrossRef]
- Viciano-Chumillas, M.; Carbonell-Vilar, J.M.; Armentano, D.; Cano, J. Influence of Xantphos Derivative Ligands on the Coordination in Their Copper(I) and Silver(I) Complexes. Eur. J. Inorg. Chem. 2019, 2982–2989. [Google Scholar] [CrossRef]
- Costa, R.D.; Tordera, D.; Ortí, E.; Bolink, H.J.; Schönle, J.; Graber, S.; Housecroft, C.E.; Constable, E.C.; Zampese, J.A. Copper(I) Complexes for Sustainable Light-Emitting Electrochemical Cells. J. Mater. Chem. 2011, 21, 16108–16118. [Google Scholar] [CrossRef]
- Keller, S.; Brunner, F.; Junquera-Hernández, J.M.; Pertegás, A.; La-Placa, M.-G.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Ortí, E.; Housecroft, C.E. CF3 Substitution of [Cu(P^P)(bpy)][PF6] complexes: Effects on Photophysical Properties and Light-emitting Electrochemical Cell Performance. ChemPlusChem 2018, 83, 217–229. [Google Scholar] [CrossRef]
- Yang, L.; Feng, J.-K.; Ren, A.-M.; Zhang, M.; Ma, Y.-G.; Liu, X.-D. Structures, Electronic States and Electroluminescent Properties of a Series of CuI Complexes. Eur. J. Inorg. Chem. 2005, 2005, 1867–1879. [Google Scholar] [CrossRef]
- Kubas, G.J.; Monzyk, B.; Crumbliss, A.L. Tetrakis(acetonitrile)copper(I) hexafluorophosphate. Inorg. Synth. 1979, 19, 90–92. [Google Scholar] [CrossRef]
- Uenishi, J.; Tanaka, T.; Nishiwaki, K.; Wakabayashi, S.; Oae, S.; Tsukube, H. Synthesis of ω-(Bromomethyl)bipyridines and Related to ω-(Bromomethyl)pyridinoheteroaromatics: Useful Functional Tool for Ligands in Host Molecules. J. Org. Chem. 1993, 58, 4382–4388. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; He, Y.-M.; Li, C.; Fan, Q.-H. Enantioselective Synthesis of Tunable Chiral Pyridine–Aminophosphine Ligands and Their Application in Asymmetric Hydrogenation. Org. Biomol. Chem. 2019, 17, 5099–5105. [Google Scholar] [CrossRef]
- Software for the Integration of CCD Detector System Bruker Analytical X-ray Systems; Bruker Axs: Madison, WI, USA, 2013.
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip-A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A Computer Program for Topological Analysis of Discrete Electron Densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spartan ‘18 version 1.3; Wavefunction INC.: Irvine, CA, USA.
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Brunner, F.; Martínez-Sarti, L.; Keller, S.; Pertegás, A.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Housecroft, C.E. Peripheral halo-functionalization in [Cu(N^N)(P^P)]+ emitters: Influence on the performances of light-emitting electrochemical cells. Dalton Trans. 2016, 45, 15180–15192. [Google Scholar] [CrossRef]
- McCullough, B.J.; Neyhouse, B.J.; Schrage, B.R.; Reed, D.T.; Osinski, A.J.; Ziegler, C.J.; White, T.A. Visible-Light-Driven Photosystems Using Heteroleptic Cu(I) Photosensitizers and Rh(III) Catalysts To Produce H2. Inorg. Chem. 2018, 57, 2865–2875. [Google Scholar] [CrossRef]
- Kubiček, K.; Veedu, S.T.; Storozhuk, D.; Kia, R.; Techert, S. Geometric and electronic properties in a series of phosphorescent heteroleptic Cu(I) complexes: Crystallographic and computational studies. Polyhedron 2017, 124, 166–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, J.; Cheng, Y.; Wang, L.; Xie, Z.; Jing, X.; Wang, F. Novel Heteroleptic CuI Complexes with Tunable Emission Color for Efficient Phosphorescent Light-Emitting Diodes. Adv. Funct. Mater. 2007, 17, 2983–2990. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Cryst. 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Kuang, S.-M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and Structural Characterization of Cu(I) and Ni(II) Complexes that Contain the Bis[2-(diphenylphosphino)phenyl]ether Ligand: Novel Emission Properties for the Cu(I) Species. Inorg. Chem. 2002, 41, 3313–3323. [Google Scholar] [CrossRef]
- Linfoot, C.L.; Leitl, M.J.; Richardson, P.; Rausch, A.F.; Chepelin, O.; White, F.J.; Yersin, H.; Robertson, N. Thermally Activated Delayed Fluorescence (TADF) and Enhancing Photoluminescence Quantum Yields of [CuI(diimine)(diphosphine)]+ Complexes—Photophysical, Structural, and Computational Studies. Inorg. Chem. 2014, 53, 10854–10861. [Google Scholar] [CrossRef]


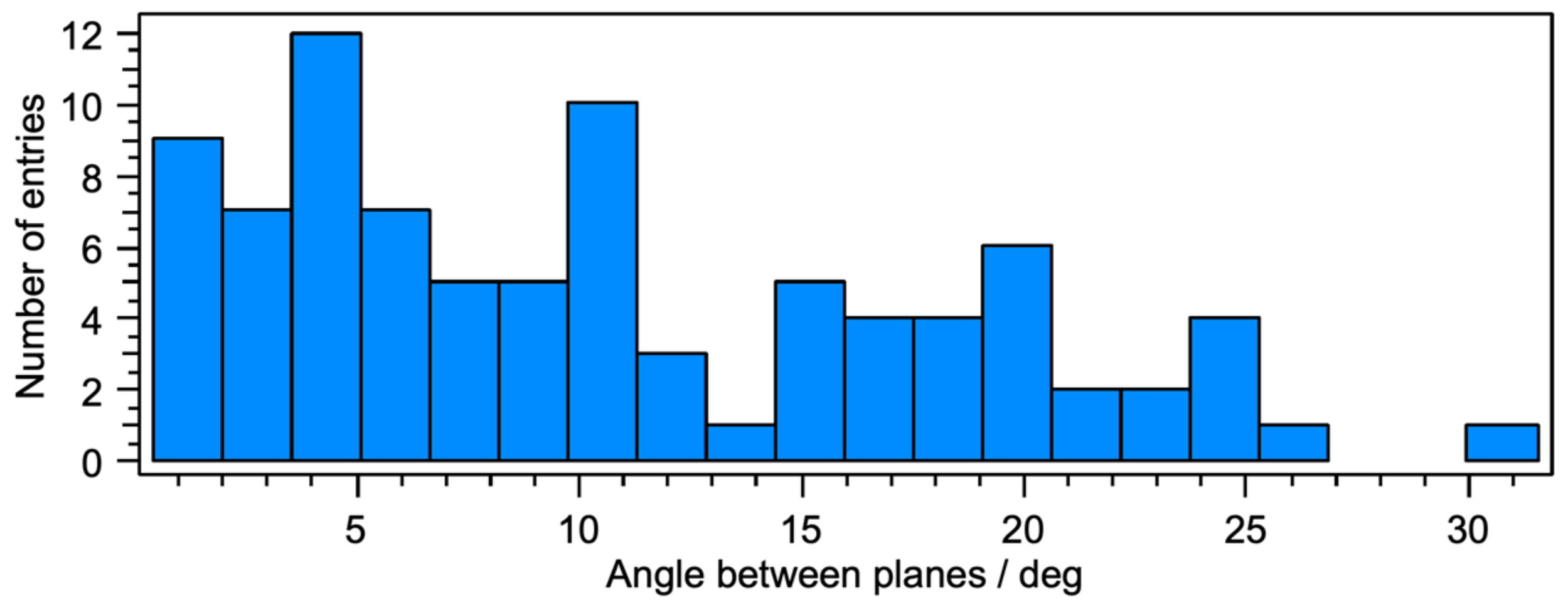
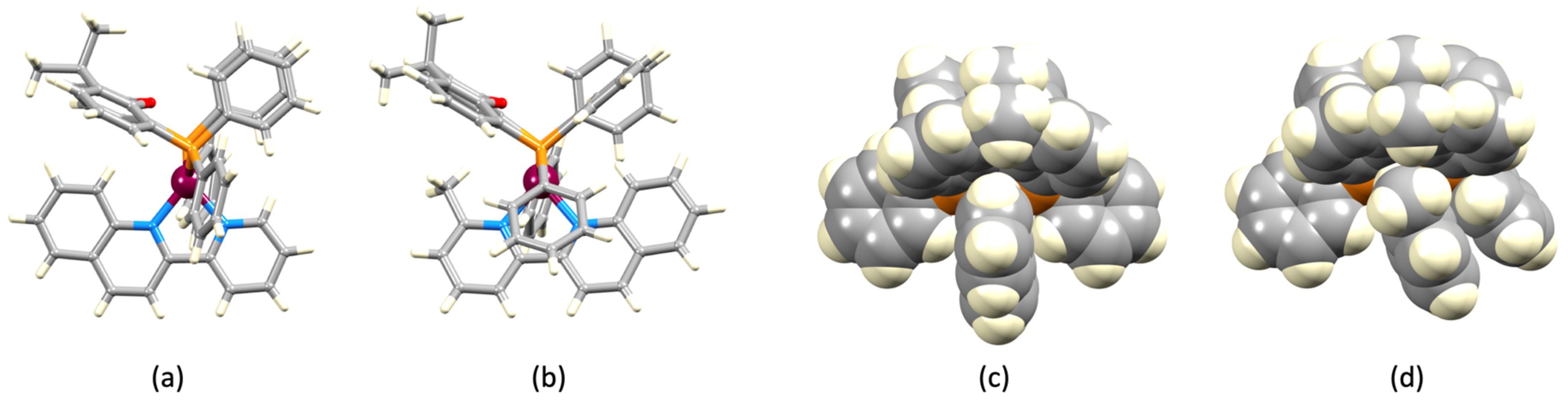
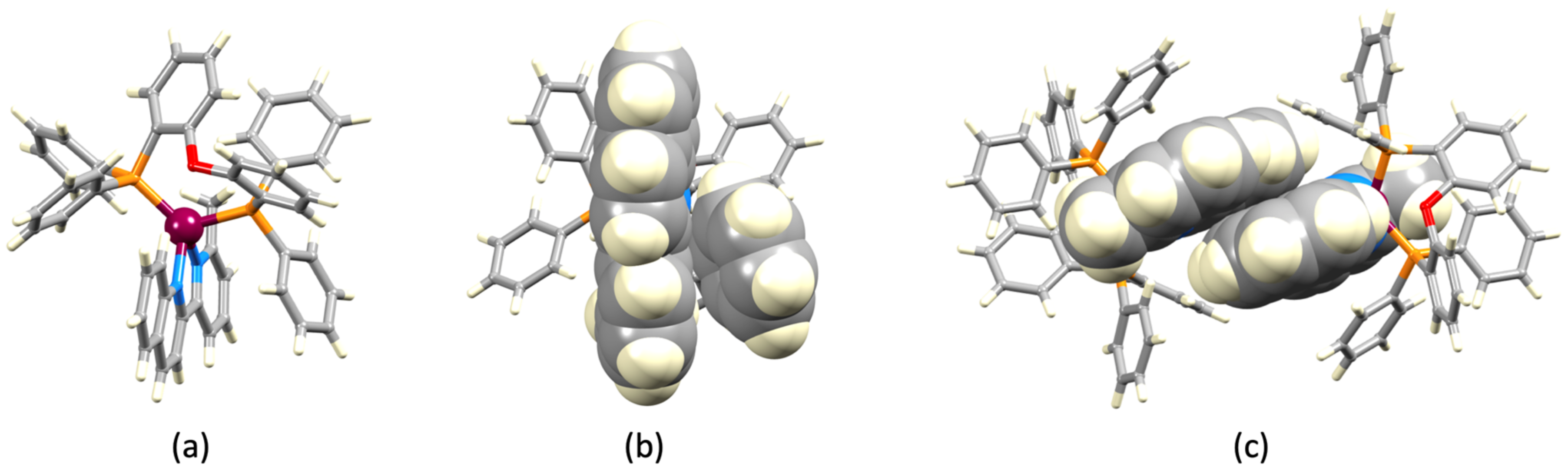

| Parameter | [Cu(xantphos)(1)]+ | [Cu(xantphos)(2)]+ | [Cu(POP)(2)]+ |
|---|---|---|---|
| Cu–N / Å | 2.070(2), 2.083(2) | 2.094(2), 2.096(2) | 2.129(4), 2.043(4) |
| Cu–P / Å | 2.2641(7), 2.2702(7) | 2.2562(8), 2.3153(8) | 2.3032(12), 2.2597(13) |
| P–Cu–P / o | 112.24(3) | 119.38(3) | 117.59(5) |
| N–Cu–N / o | 79.24(8) | 79.05(9) | 79.53(15) |
| P–Cu–N / o | 118.13(6), 117.32(6), 115.99(6), 110.11(6) | 122.31(6), 102.51(6), 125.94(6), 98.99(6) | 129.07(11), 104.52(10), 119.73(11), 98.76(10) |
| τ4 1 | 0.88 | 0.79 | 0.79 |
| Compound | λmax / nm (ε / dm3 mol–1 cm–1) | |
|---|---|---|
| Ligand-Centred π*←π | MLCT | |
| [Cu(POP)(1)][PF6] | 257 (39,800), 273 sh (28,600), 314 sh (15,800), 330 (11,200), 344 (8,500) | 429 (3,200) |
| [Cu(xantphos)(1)][PF6] | 258 (42,800), 270 (36,500), 275 sh (35,000), 293 sh (19,600), 329 (10,950), 344 (8,800) | 429 (3,200) |
| [Cu(POP)(2)][PF6] | 250 (46,500), 275 sh (33,600), 318 sh (15,000), 336 (12,950), 348 (13,000) | 416 (3,400) |
| [Cu(xantphos)(2)][PF6] | 251 (43,200), 258 sh (39,300), 278 (33,700), 332 (11,800), 338 sh (10,400), 347 (11,500) | 419 (2,900) |
| Compound | λexc / nm | λemmax / nm | PLQY / % | τ / μs |
|---|---|---|---|---|
| [Cu(POP)(1)][PF6] | 365 | 610 | 6 | 2.9 |
| [Cu(xantphos)(1)][PF6] | 365 | 602 | 9 | 5.0 |
| [Cu(POP)(2)][PF6] | 365 | 583 | 21 | 5.6 |
| [Cu(xantphos)(2)][PF6] | 365 | 580 | 17 | 10.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keller, S.; Alkan-Zambada, M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Extended π-Systems in Diimine Ligands in [Cu(P^P)(N^N)][PF6] Complexes: From 2,2′-Bipyridine to 2-(Pyridin-2-yl)Quinoline. Crystals 2020, 10, 255. https://doi.org/10.3390/cryst10040255
Keller S, Alkan-Zambada M, Prescimone A, Constable EC, Housecroft CE. Extended π-Systems in Diimine Ligands in [Cu(P^P)(N^N)][PF6] Complexes: From 2,2′-Bipyridine to 2-(Pyridin-2-yl)Quinoline. Crystals. 2020; 10(4):255. https://doi.org/10.3390/cryst10040255
Chicago/Turabian StyleKeller, Sarah, Murat Alkan-Zambada, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2020. "Extended π-Systems in Diimine Ligands in [Cu(P^P)(N^N)][PF6] Complexes: From 2,2′-Bipyridine to 2-(Pyridin-2-yl)Quinoline" Crystals 10, no. 4: 255. https://doi.org/10.3390/cryst10040255
APA StyleKeller, S., Alkan-Zambada, M., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2020). Extended π-Systems in Diimine Ligands in [Cu(P^P)(N^N)][PF6] Complexes: From 2,2′-Bipyridine to 2-(Pyridin-2-yl)Quinoline. Crystals, 10(4), 255. https://doi.org/10.3390/cryst10040255








