Abstract
In the present work, a layer of 75%Cr3C2−25%NiCr with thickness of 260 ± 15 µm was coated onto the API-2H pipeline steel surface using high-velocity oxy-fuel deposition. The effect of 75%Cr3C2−25%NiCr coating on the corrosion of the API steel after 1 h, 24 h, and 48 h exposure in 4.0% sodium chloride solutions is reported. The corrosion tests were performed using potentiodynamic cyclic polarization, electrochemical impedance spectroscopy, and chronoamperometric current–time techniques along with scanning electron microscopy and energy-dispersive X-ray analyses. The curves of polarization indicated that the presence of the coating increases the corrosion resistance of the steel through decreasing its corrosion current and corrosion rate. Impedance data showed that all resistances recorded higher values for the coated API steel. Chronoamperometric current–time measurements confirmed that the coated API steel has lower absolute current values and thus lower corrosion rate. All results proved that the presence of 75%Cr3C2−25%NiCr coating enhances the corrosion resistance of the API steel via the formation of a protective layer of Cr and Ni oxides, which could lead to decreasing the corrosion rate.
1. Introduction
It is well known that API pipeline steels have good toughness, high strength and weldability, low ductile to brittle transition temperature, and a low crack sensitivity coefficient [1,2,3,4,5]. Therefore, API pipeline steels have been widely used in construction, transportation pipelines, oil/gas storage tanks, off-shore rigs, agitators, pumps, and chemical processing [4,5,6,7,8,9]. Due to the exposure of these steels to corrosive media in most of their applications, corrosion takes place. The electrochemical corrosion behavior of different grades of API steels in many harsh environments have been reported by several researchers [1,2,3,4,5,6]. Alizadeh and Bordbar [2] investigated the effect of microstructure on the product layer formed on this steel in chloride media. They [2] have found that the corrosion of the API steel is decreased upon heat treatment due to the formation of a protective corrosion product layer. Bellaouchou et al. [10] have also reported the influence of changing the microstructure on the corrosion behavior of the welded joint in the pipeline steel. The alteration of microstructure on the corrosion of API pipelines was also reported by Hemmingsten et al. [11]. In this regard, changing the microstructure is usually accompanied by a change in the composition of the materials of the API steel and also, the phases. This leads to corrosion via the galvanic form due to the increased dissimilarity and thus the great potential difference between the base metal and the weld metal [11,12].
We have reported the corrosion of two API steel grades, namely API-2H and API-4F steels after being exposed to varied exposure periods of time in sodium chloride solutions using different electrochemical and spectroscopic measurements [13]. It was found that these API steels suffer both uniform and localized corrosion; the severity of the pitting attack was found to decrease by increasing the time of exposure in the test solution. It was also found that the corrosion resistance of API-2H recorded a higher value as compared to API-4F steel [13]. However, the failure of steel pipelines was reported to take place as a result of a combination between uniform corrosion and localized attack [14,15,16,17]. The presence of welds within the steel pipelines has been shown to lead to the occurrence of localized corrosion, either via pitting attack or galvanic corrosion in the area of the welds [14,15,16,17,18].
The protection of pipeline steel by the use of inorganic coatings has been reported by many researchers [19,20,21,22]. Fan et al. [19,20] investigated the protection of pipeline steels enamel coatings, while Chatha et al. [21] studied the effect of the Cr3C2-NiCr coating layer on the corrosion protection of T91 boiler steel under different types of environments. The authors [21] claimed that this coating offers a high melting point and maintains high hardness, strength and wear resistance up to a maximum operating temperature of 900 °C. Tillmann et al. [22] also claimed that the Cr3C2-NiCr coating features a very low porosity, low oxidization and low carbide decomposition or carbide-matrix dissolution, which leads to increasing hardness and abrasion resistance. Thi et al. [23] reported the advantage of using a Cr3C2-NiCr cermet coating, which was applied onto the surface of 410 stainless steel substrate to protect the corrosion of this steel 3.5 wt. % NaCl solution. These authors [23] found that the sprayed layer of Cr3C2-NiCr provides high resistance to corrosion in the sodium chloride solution. This cermet coating was also applied onto the surface of carbon steel (SC45) pipes using high-velocity oxy-fuel (HVOF) and plasma and was found to greatly increase the corrosion resistance in 3.5% NaCl solution [24]. The objective of this study was to manufacture an inorganic Cr3C2-NiCr coating layer to be applied on the surface of a hot-rolled API-2H grade 50 pipeline steel using HVOF deposition. Furthermore, the effect of 75%Cr3C2−25%NiCr coating, whose thickness after its application was 260 ± 15 µm, on the severity of corrosion of this steel after 1 h, 24 h, and 48 h exposure in 4.0% sodium chloride solutions was reported. The work was carried out using potentiodynamic cyclic polarization (PCP), electrochemical impedance spectroscopy (EIS), and chronoamperometric current–time measurements. Scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) surface examinations were employed for surface morphology and analysis. It is expected that this inorganic coating will greatly increase the corrosion resistance of the API pipeline steel as compared to the uncoated steel.
2. Experimental Procedures
2.1. Materials and Solutions
API-2H grade 50 (hot-rolled) steel with the chemical composition (weight percentage, wt%) of 0.14% C, 1.5% Mn, 0.2% Si, 0.04% Nb, 0.03% P, 0.02% Ti, 0.015% S, and the remaining balance of Fe was used in this study. The mechanical parameters of the API-2H steel was 345 MPa yield, 520 MPa ultimate, and 18% elongation. A layer of 75%Cr3C2–25%NiCr coating with thickness of 260 ± 15 µm was applied on the surface of the API-2H steel using high-velocity oxy-fuel (HVOF) deposition. Sodium chloride (NaCl) of 99% purity was purchased from Merck (As Sulimaniyah, Riyadh, Saudi Arabia) and was used as received to prepare the 4.0% NaCl solution.
2.2. Electrochemical Techniques
An electrochemical cell with a three-electrode configuration that accommodated 250 mL of the electrolytic solution was used. A silver/silver chloride (Ag/AgCl, in 3M KCl solution) and a Pt wire were used as the reference and the counter electrodes, respectively. The uncoated and coated API-2H steels were the working electrodes. The working electrodes were prepared by connecting a copper wire to the surface of each electrode. These welded steels with the copper wire as a connector were cold mounted using an epoxy resin and left to dry in air. The uncoated steel electrode surface was polished with different emery papers up to 1000 grit. This electrode was then degreased with acetone and washed by distilled water just before being immersed in the test medium. The corrosion measurements were acquired by the use of an Autolab Potentiostat-Galvanostat (purchased from Metrohm, Utrecht, Netherlands). PCP curves were collected via scanning the potential from −1.200 V in the anodic direction up to −0.200 V and the scan rate of 0.00166 V/s. The potential was then scanned at the same scan rate in the backward direction until the reversed currents intersected with the forward ones. The EIS measurements were performed at the open-circuit potential of the electrode over a change of frequency from 100 kHz to 1 mHz and at an AC wave of ±5 mV peak-to-peak overlaid. The EIS data were collected using PowerSINE software that is already installed within the Autolab. The chronoamperometric current–time curves were obtained after applying a value of −0.7 V (Ag/AgCl) on the working electrodes after their immersions in the chloride test solution for the different exposure times. For ensuring the reproducibility of our measurements, each electrochemical experiment was performed in triplicate and was carried out on a new portion of the chloride solution at room temperature (25 ± 2 °C) and a new polished electrode surface.
2.3. Surface Characterization
The morphology of the surface after being immersed in the chloride solution for 48 h followed by stepping the potential to −0.7 V for 40 min was obtained using a scanning electron microscopy (SEM, JEOL FE-SEM Model 7600 (Tokyo, Japan)), which was operated at 15 kV. An energy-dispersive X-ray analyzer (EDX) was employed to obtain the elemental analysis of corroded surfaces via an EDX (JEOL, Tokyo, Japan) unit attached to the SEM instrument.
3. Results and Discussion
3.1. Potentiodynamic Cyclic Polarization (PCP) Measurements
Evaluating the polarization is a well-known technique in understanding the corrosion and corrosion mitigation of metallic materials in corrosive solutions [25,26,27,28,29]. The PCP curves of (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after 1 h exposure in 4% NaCl solutions are depicted in Figure 1. The same measurements were also carried out for the same materials after 24 h and 48 h immersion in the test solution and the curves are shown in Figure 2 and Figure 3, respectively. The parameters obtained from Figure 1, Figure 2, and Figure 3 are listed in Table 1. Here, βc is the cathodic Tafel slope, ECorr is the corrosion potential, βa is the anodic Tafel slope, jCorr is the corrosion current density, EProt is the protection potential before which the pitting corrosion does not occur, and RCorr is the corrosion rate. The βc, ECorr, βa, and jCorr values were calculated as previously reported [28,29,30]. The values of RCorr, which are mostly expressing the uniformed corrosion, were calculated using the following equation [31]:
where, RCorr is in millimeters per year, k is a constant with a value of 3272 mm/(amp·cm·year)), EW is the equivalent weight in grams, d is the density of the metal or alloy in g/cm3, jCorr is in µA/cm2, and A is the tested surface area in cm2.
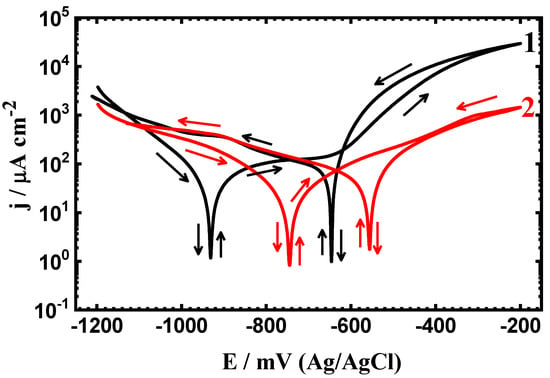
Figure 1.
Cyclic potentiodynamic polarization (PCP) curves of (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after exposure in 4% NaCl solution for 1 h.
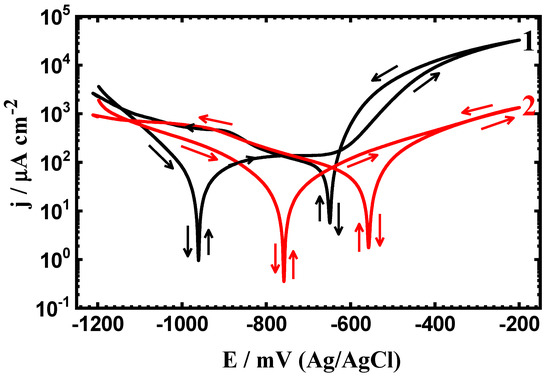
Figure 2.
PCP curves of (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after exposure in 4% NaCl solution for 24 h.
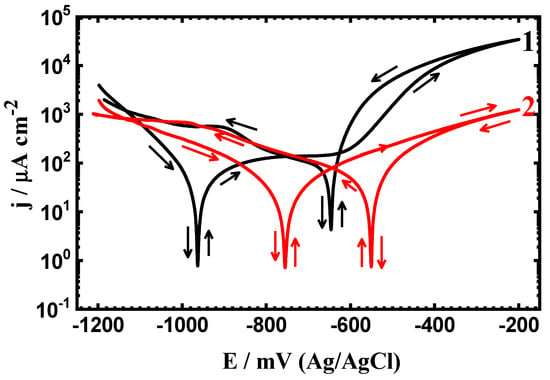
Figure 3.
PCP curves of (1) API-2H steel and (2) 75%Cr3C2 −25%NiCr coated API-2H steel after exposure in 4% NaCl solution for 48 h.

Table 1.
Cyclic potentiodynamic polarization (PCP) data obtained for the API-2H steel and coated API-2H steel samples after the different immersion periods in NaCl solutions.
The polarization curves indicated that the current of both coated and uncoated API-2H decreased in the cathodic branch upon sweeping the potential from the most negative values until it reached the corrosion current. Here, the reaction at the cathode for most metals and/or alloys in sodium chloride solutions is the oxygen reduction [28];
The current then increased again with increasing potential in the positive side as a result of the iron dissolution from the surface as follows [29];
Fe = Fe2+ + 2e−
The Fe2+ cations get further oxidized via potential increases and chloride solutions attack, which leads to the formation of ferric cations, Fe3+, as follows:
Fe2+ = Fe3+ + e‒
For the uncoated API-2H steel, the current has an active–passive behavior. The active region appears as a result of the dissolution of Fe to Fe2+ and Fe3+, while the passivation occurs due to the formation of a corrosion product layer on the steel surface as per the following reaction:
Fe + ½ O2 + H2O → Fe(OH)2
The current continues to increase even in the backward direction to create a hysteresis loop (curve 1), which results from the pitting corrosion. The bigger the area of the hysteresis loop, the more severe the pitting corrosion is.
Coating the steel with 75%Cr3C2−25%NiCr decreases the overall currents in the cathodic and anodic sides and also reduces the possibility of pitting corrosion that could take place as indicated from the presence of a tiny size of the hysteresis loop (curve 2). The values tabulated in Table 1 indicated that the coated API-2H steel has less negative values of ECorr and EProt lower values of jCorr and RCorr. This means that the layer of 75%Cr3C2−25%NiCr protects the steel against uniform corrosion as well as pitting attack.
Prolonging the immersion time from 1 h to 24 h and further, to 48 h greatly decreases its corrosion via increasing the corrosion resistance and decreasing both corrosion currents and corrosion rates, while it slightly shifts the values of Eprot toward more negative values. This effect is further increased with prolonging the time of immersion in the presence of 75%Cr3C2−25%NiCr coated surface. Moreover, the values of EProt shift largely to less negative ones. This is because increasing the time of immersion allows both the API-2H steel and the coated steel one to form an oxide film; this film becomes thick with time and leads to increasing the resistance against corrosion. It is worth mentioning that the percentage of the protection efficiency (PE%) as listed in Table 1 was calculated for the coated API steel samples, as reported in our previous work [32], using this equation;
Where, j1Corr and j2Corr are the corrosion current density obtained for the uncoated and the coated API steels after the different exposure periods of time, respectively. It is seen from Table 1 that the value of PE% recorded only 40.43% after only 1 h immersion in the chloride solution. This value was found to increase to 45.24% and 52.63% when the exposure time was increased to 24 h and 48 h, respectively. This also confirms that the prolonging of the exposure time increases the protection of the surface against corrosion in the present test solution.
3.2. EIS Measurements
EIS experiments have been carried out to understand the impedance parameters for studying the influence of 75%Cr3C−25%NiCr coating and also, the effect of increasing the exposure time on the corrosion of API-2H steel in 4.0% NaCl solutions. Figure 4 shows the Nyquist plots of (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after 1 h immersion in 4% NaCl solutions. The measurements were also obtained for API-2H steel and coated API-2H steel after 24 h and 48 h and the Nyquist plots are depicted in Figure 5 and Figure 6, respectively. All impedance data were analyzed and fitted to the equivalent circuit model shown in Figure 7. The values of the parameters obtained from fitting these data are listed in Table 2. The elements of the equivalent circuit are defined as following; RS the solution resistance, Q the constant phase elements (CPEs), RP the polarization resistance and can be classified as the charge transfer resistance between the surface of the uncoated/coated API-2H steel and the chloride solution interface, and W is the Warburg [33].
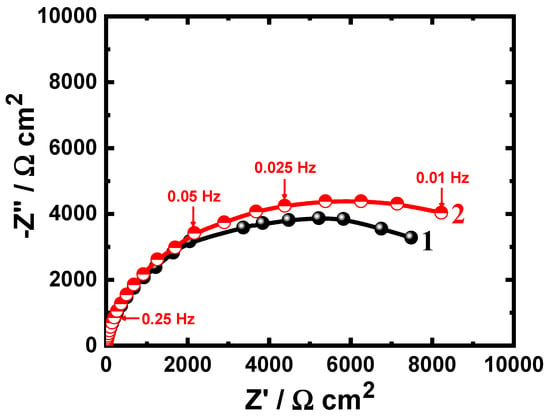
Figure 4.
Nyquist plots of (1) uncoated API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after 1 h immersion in 4% NaCl solutions.
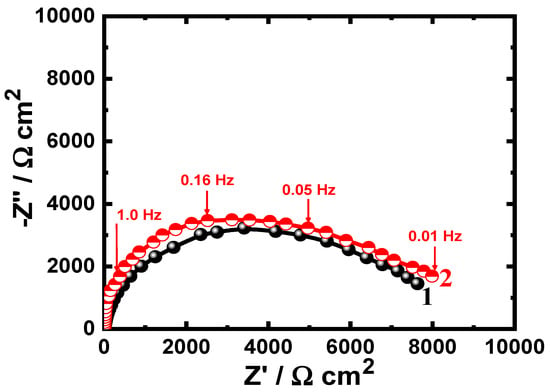
Figure 5.
Nyquist plots of (1) uncoated API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after 24 h immersion in the chloride solutions.
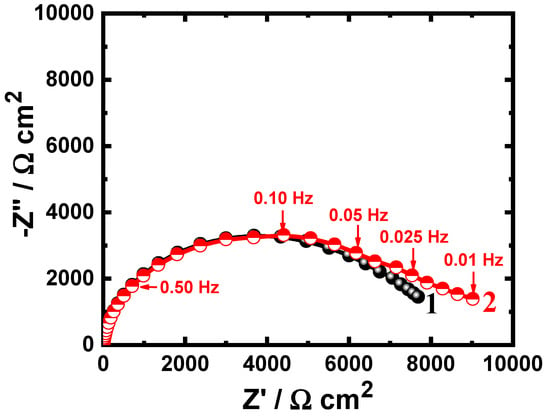
Figure 6.
Nyquist plots of (1) uncoated API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after 48 h immersion in 4% NaCl solutions.
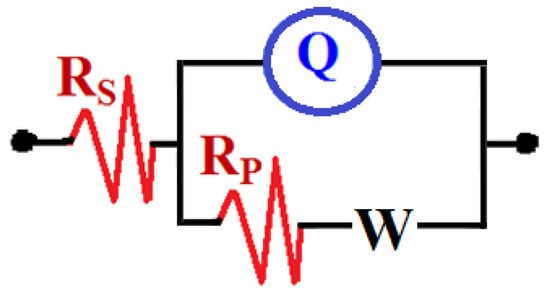
Figure 7.
The equivalent circuit model used for best fitting the EIS experiments.

Table 2.
Impedance (EIS) parameters of the uncoated and coated API steels in NaCl solutions.
The Nyquist plots of all samples after all immersion times showed only one semicircle in the chloride solution. The spectra depicted in Figure 4 prove that the spectrum plotted for the uncoated API-2H steel after 1 h immersion (spectrum 1) has the smallest-diameter semicircle. Coating the surface of the steel with 75%Cr3C2−25%NiCr increased the diameter of the semicircle and confirms that the corrosion resistance increased in the presence of the coating layer. This was further indicated by the parameters listed in Table 2 as the values of RS and RP are higher for the coated API-2H steel compared to their values for the bare API-2H steel.
It is well knowing that the CPE, constant phase elements, (Q) can represent various components thanks to the n-values, where it is a Warburg impedance when n = 0.5, or a double-layer capacitor with some pores when n→1.0, and a resistor when n = 0 [34]. According to this principle, the Q’s in the current work represent double-layer capacitors with some pores. Accordingly, the value of YQ obtained for the coated API-2H steel was lower than that obtained for uncoated API-2H steel, which indicates that the corrosion of coated API-2H steel is lower. The presence of the Warburg (W) impedance in the circuit indicates that the mass transport limits the access of the chloride ions through the formed layer of the corrosion products and/or oxide layers on the surface. The reason for the greater protection obtained for the coated API-2H steel is that the top layer of the coating decreases the dissolution of the steel surface via increasing its resistance against the aggressiveness action of the chloride ions. This is because the presence of the 75%Cr3C2−25%NiCr layer on the surface of API-2H steel electrode allows the formation of chromium oxide that provides more corrosion resistance compared to the formed iron oxide on the surface of the uncoated API-2H steel.
Extending the time of immersion to 24 h is shown to reduce the diameter of the plotted semicircles for both bare and coated API-2H steel electrodes, as can be seen from Figure 5. Further decreasing of the diameter of the semicircle was obtained upon increasing the immersion time to 48 h, as depicted in Figure 6. This means that the increase of immersion time at the same condition decreases the corrosion resistance of the tested electrodes. This was further confirmed by the data shown in Table 2, where the increase in exposure time decreases the values of the surface and polarization resistances, RS and RP. The value of YQ was also found to slightly increase with the increase of immersion time and thus the dissolution of both uncoated and coated API-2H steel increase. In all cases, the corrosion resistance for the coated API-2H steel has a greater value as compared to the uncoated API-2H steel at all exposure times.
In order to further confirm the Nyquist data, the Bode impedance of the interface (|Z|) and the degree of the phase angle (Φ) are also given. Figure 8 shows (a) the Bode impedance of the interface and (b) the degree of phase angle obtained for the steels after 1 h exposure to the NaCl solutions. It is clear from Figure 8a that the obtained values of the impedance of the interface are higher for coated API-2H steel compared to its values for uncoated API-2H steel. Also, the maximum values obtained for coated API-2H steel are higher than those obtained for the uncoated API-2H steel. This proves that coating the carbon steel with 75%Cr3C2−25%NiCr protects its surface form being corroded easily via increasing the resistance to corrosion in 4% NaCl solutions.
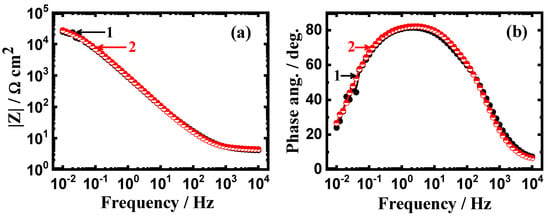
Figure 8.
Change of (a) |Z| and (b) Φ with frequency plots obtained for (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after their immersion in 4% NaCl solution for 1 h.
Figure 9 and Figure 10 depict (a) the Bode impedance of the interface and (b) the degree of phase angle after 24 h and 48 immersions in the chloride solutions. It is obvious that prolonging the time of immersion to 12 h and 24 h does not change the behavior of the Bode plots. However, coating API-2H steel with 75%Cr3C2−25%NiCr allows the steel to provide higher corrosion resistance even after the longer immersion periods of time. It was previously reported [35] that obtaining higher values of |Z| and also, a higher maximum value of Φ, indicate a higher resistance against corrosion in corrosive media.
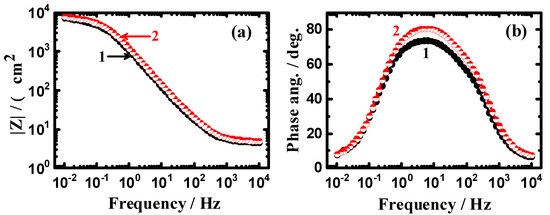
Figure 9.
Change of (a) |Z| and (b) Φ with frequency plots obtained for (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after their immersion in 4% NaCl solution for 24 h.
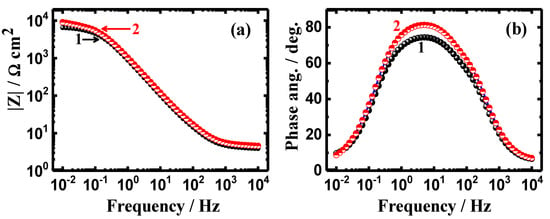
Figure 10.
Change of (a) |Z| and (b) Φ with frequency plots obtained for (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after their immersion in 4% NaCl solution for 48 h.
3.3. Chronoamperometric Current-Time Experiments
The change of potentiostatic current with time after applying a constant value of potential at −0.70 V (Ag/AgCl) was carried out in order to investigate the effect of coating on the general and localized corrosion of the carbon steel. The effect of increasing the exposure periods of time to 24 h and further to 48 h on the corrosion of the uncoated and coated steels was also investigated. Figure 11 depicts the change of current with time curves of (1) uncoated and (2) 75%Cr3C2−25%NiCr coated API-2H steel at −700 mV (Ag/AgCl) after 1 h immersion in 4% NaCl solutions. The initial current values for both uncoated and coated API-2H steel are seen to rapidly decrease with time, which is due to the thickening of an oxide film formed on the surface of the steel. Finally, the current slightly decreased until the end of the experiment, indicating that the film thickening continued to occur. This behavior also indicates that there is no pitting corrosion for coated or uncoated steel. The lower absolute current values for coated API-2H steel confirm that the surface of coated steel has more resistance to corrosion as compared to the uncoated API-2H steel.
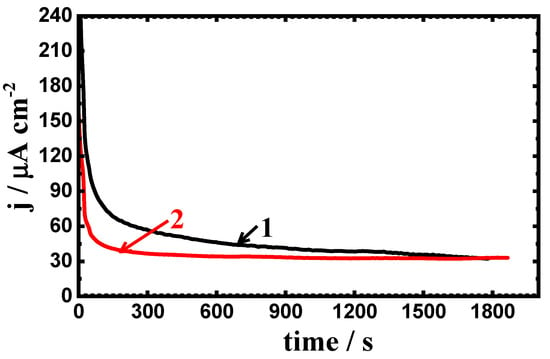
Figure 11.
The obtained current vs. time curves at −700 mV for (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after immersion in 4% NaCl solution for 1 h.
The obtained chronoamperometric current–time curves at −700 mV (Ag/AgCl) after prolonging the exposure time to 24 h for (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel are shown in Figure 12. The current values decreased from the first moment of the measurement with a slight decrease with time. Here, the values of current obtained for uncoated API-2H steel slightly increased, accompanied with little fluctuations after 10 min of stepping the potential and until the end of the experiment, which reveals that the occurrence of pitting attack on the surface of API-2H steel. On the other side, the recorded currents for the coated API-2H steel slightly decreased confirming that there was no pitting corrosion occurred at this potential.
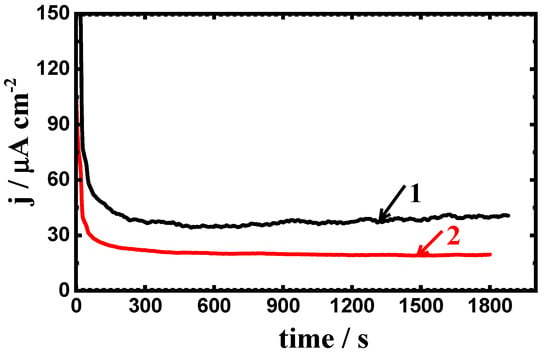
Figure 12.
The obtained current vs. time curves at −700 mV for (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after immersion in 4% NaCl solution for 24 h.
Prolonging the period of time to 48 h prior to measurements as can be seen from Figure 13 has shown almost similar current–time behavior to those obtained after 24 h immersion (Figure 12) but with lower values of the absolute currents for both coated and uncoated steel electrodes. It is also noticed that the absolute currents obtained after immersion 24 h were lower in compared to those collected at a shorter immersion time—1 h. This is because the long immersion periods of time allow the API-2H steel electrodes to form a thick layer of an oxide film onto its surfaces. This confirms that the increase of time before measurements increases the possibility of pitting attack for the uncoated API-2H steel and also, decreases the overall currents obtained for both coated and uncoated API-2H steel at the same value of potential, −0.700 mV.
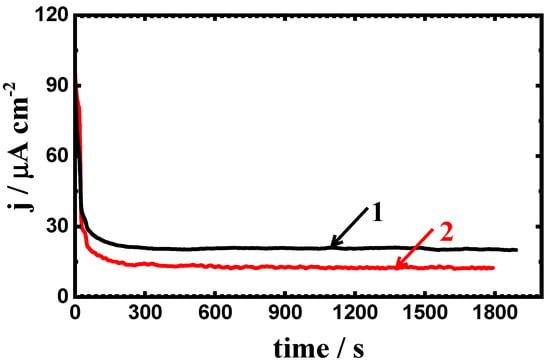
Figure 13.
The obtained current vs. time curves at −700 mV for (1) API-2H steel and (2) 75%Cr3C2−25%NiCr coated API-2H steel after immersion in 4% NaCl solution for 48 h.
3.4. Surface Analysis
The surface morphology investigations for the uncoated and coated API-2H steel have been carried out using SEM and the EDX techniques to report whether the uniform attack and pitting corrosion in 4% NaCl solutions. Figure 14 depicts (a), (b) the SEM images and (c), (d) the corresponding EDX profile analyses for API-2H steel and 75%Cr3C2−25%NiCr coated API-2H steel, respectively, after their immersions for 48 h in 4% NaCl solutions then stepping a value of −0.7 V for 40 min. Although, the values of the recorded currents for both API-2H steel electrodes, the surface of the uncoated API-2H steel (Figure 14a) looks deteriorated due to the attack of chloride ions; the deterioration becomes aggressive at the anodic value of the applied potential of −0.7 V. The surface morphology here indicates that the uniform corrosion is severe as well as the pitting attack that occurred. This confirms the change of current with time behavior we have seen in Figure 13 (curve 1). The weight % (wt%) of the elements found on the surface of the uncoated API-2H steel represented by the EDX spectra of Figure 14c recorded the following: 54.86% Fe, 29.51% O, 12.78% C, and 1.33% Cl. The wt.% of Fe and O are high and confirm that the surface of the uncoated API-2H steel develops a layer that is mainly iron oxide [36].
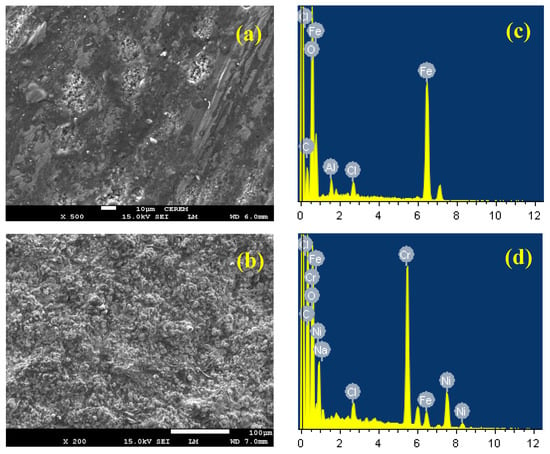
Figure 14.
(a,b) the SEM images and (c,d), the corresponding EDX spectra for API-2H steel and 75%Cr3C2−25%NiCr coated API-2H steel, respectively, after their immersions for 48 h in NaCl solutions before stepping a value of −0.7 V for 40 min.
The image shown in Figure 14c depicts that the uncoated API-2H steel is covered with a thick layer of corrosion products. The wt.% for elements in the case of the 75%Cr3C2−25%NiCr coated API-2H steel, which is depicted in Figure 14d, upon EDX profile analysis, are listed as the following; 32.33% C, 26.48 Cr, 20.26% O, 15.44% Ni, 3.48% Fe, 1.04% Na, and 0.97% Cl. It is evident that the highest wt.% were for C, Cr, and Ni; these elements are the main contents of the coating layer on the steel surface. The presence of high wt.% of O indicates that the compounds formed on the surface are Cr and Ni oxides. Furthermore, the presence of low wt.% of Fe confirms that the steel surface is well coated with Cr3C and NiCr coating. Moreover, both Na and Cl at low wt.% reveals an NaCl salt deposited on the surface. The SEM and EDX investigations are in good agreement with PCP, EIS, and chronoamperometric data.
4. Conclusions
The effect of 75%Cr3C2−25%NiCr coating on the corrosion protection of API-2H pipeline steel after different exposure periods of time in 4 wt. % NaCl solution using varied electrochemical and spectroscopic measurements has been reported. Polarization experiments indicated that coating the steel with 75%Cr3C2−25%NiCr decreased the corrosion current and corrosion rate and increased corrosion resistance. Impedance data showed that the top layer of the inorganic coating increased the solution and polarization resistance for the steel. Chronoamperometric current–time measurements revealed that the presence of coating decreased the absolute currents with increasing time, which reflects on the decreasing of the uniform corrosion and the prevention of the pitting attack. Surface investigations taken by SEM and EDX confirmed that the coating layer protected the steel against uniform and pitting corrosion. Application of 75%Cr3C2−25%NiCr coating onto the steel surface thus highly protects the steel corrosion in 4% NaCl chloride solution. The long immersion of the steel remarkably magnified the corrosion resistance via formation of corrosion products that have the ability to weaken the corrosivity of the chloride test solution. Building on the outcome of this study, 75%Cr3C2−25%NiCr can be used as an inorganic cermet coating to minimize the corrosion of API-2H pipeline steel grade in the chloride, 4% NaCl, solution.
Author Contributions
Conceptualization, E.-S.M.S., H.S.A., and M.M.E.R.; methodology, E.-S.M.S., H.S.A., and M.M.E.R.; validation, E.-S.M.S., H.S.A., and M.M.E.R.; formal analysis, E.-S.M.S., H.S.A., and M.M.E.R.; investigation, E.-S.M.S., H.S.A., and M.M.E.R.; resources, E.-S.M.S., and M.M.E.R.; data curation, E-S.M.S.; writing—original draft preparation, E.-S.M.S.; writing—review and editing, E.-S.M.S.; supervision, E.-S.M.S. and M.M.E.R.; project administration, E.-S.M.S.; funding acquisition, E.-S.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at King Saud University via the Research Group Project No. RGP-160.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-160.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sherif, E.S.; Almajid, A.A.; Khalil, K.A.; Junaedi, H.; Latief, F.H. Electrochemical studies on the corrosion behavior of API X65 pipeline steel in chloride solutions. Int. J. Electrochem. Sci. 2013, 8, 9360–9370. [Google Scholar]
- Alizadeh, M.; Bordbar, S. The influence of microstructure on the protective properties of the corrosion product layer generated on the welded API X70 steel in chloride solution. Corros. Sci. 2013, 70, 170–179. [Google Scholar] [CrossRef]
- Zhao, M.C.; Yang, K.; Shan, Y.Y. Comparison on strength and toughness behaviors of microalloyed pipeline steels with acicular ferrite and ultrafine ferrite. Mater. Lett. 2003, 57, 1496–1500. [Google Scholar] [CrossRef]
- Yakubtsov, I.A.; Poruks, P.; Boyd, J.D. Microstructure and mechanical properties of bainitic low carbon high strength plate steels. Mater. Sci. Eng. A 2008, 480, 109–116. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Almajid, A.A. Anodic dissolution of API X70 pipeline steel in Arabian Gulf seawater after different exposure intervals. J. Chem. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Guenbour, A.; Hajji, M.A.; Jallouli, E.M.; Bachir, A. Ben Study of corrosion-erosion behaviour of stainless alloys in industrial phosphoric acid medium. Appl. Surf. Sci. 2006, 253, 2362–2366. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Almajid, A.A. Electrochemical corrosion behavior of API X-70 5L grade steel in 4.0 wt. % sodium chloride solutions after different immersion periods of time. Int. J. Electrochem. Sci. 2015, 10, 34–45. [Google Scholar]
- Hegazy, M.A.; Ahmed, H.M.; El-Tabei, A.S. Investigation of the inhibitive effect of p-substituted 4-(N,N,N-dimethyldodecylammonium bromide)benzylidene-benzene-2-yl-amine on corrosion of carbon steel pipelines in acidic medium. Corros. Sci. 2011, 53, 671–678. [Google Scholar] [CrossRef]
- Hernández-Espejel, A.; Domínguez-Crespo, M.A.; Cabrera-Sierra, R.; Rodríguez-Meneses, C.; Arce-Estrada, E.M. Investigations of corrosion films formed on API-X52 pipeline steel in acid sour media. Corros. Sci. 2010, 52, 2258–2267. [Google Scholar] [CrossRef]
- Bellaouchou, A.; Kabkab, B.; Guenbour, A.; Ben Bachir, A. Corrosion inhibition under heat transfer of 904L stainless steel in phosphoric acid by benzotriazole. Prog. Org. Coat. 2001, 41, 121–127. [Google Scholar] [CrossRef]
- Hemmingsen, T.; Hovdan, H.; Sanni, P.; Aagotnes, N.O. The influence of electrolyte reduction potential on weld corrosion. Electrochim. Acta 2002, 47, 3949–3955. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Seikh, A.H. Effects of grain refinement on the corrosion behaviour of microalloyed steel in sulphuric acid solutions. Int. J. Electrochem. Sci. 2012, 8, 7567–7578. [Google Scholar]
- Sherif, E.S.M.; El Rayes, M.M. Corrosion behavior of API 2H and API 4F steels in freely aerated 4.0% sodium chloride solutions. Int. J. Electrochem. Sci. 2015, 10, 7493–7504. [Google Scholar]
- Ma, F.Y.; Wang, W.H. Fatigue crack propagation estimation of SUS 630 shaft based on fracture surface analysis under pitting corrosion condition. Mater. Sci. Eng. A 2006, 430, 1–8. [Google Scholar] [CrossRef]
- Kwok, C.T.; Fong, S.L.; Cheng, F.T.; Man, H.C. Pitting and galvanic corrosion behavior of laser-welded stainless steels. J. Mater. Process. Technol. 2006, 176, 168–178. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.G.; Ke, W. Effect of flow velocity and entrained sand on inhibition performances of two inhibitors for CO2 corrosion of N80 steel in 3% NaCl solution. Corros. Sci. 2005, 47, 2636–2658. [Google Scholar] [CrossRef]
- Hardie, D.; Charles, E.A.; Lopez, A.H. Hydrogen embrittlement of high strength pipeline steels. Corros. Sci. 2006, 48, 4378–4385. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Seikh, A.H. Anodic dissolution in sulfuric acid pickling solutions of the API pipeline X70 grade steel. Int. J. Electrochem. Sci. 2015, 10, 209–222. [Google Scholar]
- Fan, L.; Tang, F.; Reis, S.T.; Chen, G.; Koenigstein, M.L. Corrosion resistances of steel pipes internally coated with enamel. Corrosion 2017, 73, 1335–1345. [Google Scholar] [CrossRef]
- Fan, L.; Reis, S.T.; Chen, G.; Koenigstein, M.L. Corrosion resistance of pipeline steel with damaged enamel coating and cathodic protection. Coatings 2018, 8, 185. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. Characterization and corrosion-erosion behaviour of carbide based thermal spray coatings. J. Miner. Mater. Charact. Eng. 2012, 11, 569–586. [Google Scholar]
- Tillmann, W.; Vogli, E.; Baumann, I.; Kopp, G.; Weihs, C. Desirability-based multi-criteria optimization of HVOF spray experiments to manufacture fine structured wear-resistant 75Cr3C2-25(NiCr20) coatings. J. Therm. Spray Technol. 2010, 19, 392–408. [Google Scholar] [CrossRef]
- Thi, H.P.; Van, T.N.; Thu, Q.L.; Nguyen, T.A.; Thi, L.P.; Quoc, C.L.; Bich, T.D. A study on Erosion and corrosion behavior of Cr3C2-NiCr cermet coatings. Vietnam J. Sci. Technol. 2018, 56, 42–49. [Google Scholar]
- Zavareh, M.A.; Sarhan, A.A.D.M.; Zavareh, P.A.; Basirum, W.J. Electrochemical corrosion behavior of carbon steel pipes coated with a protective ceramic layer using plasma and HVOF thermal spray techniques for oil and gas. Ceram. Int. 2016, 42, 3397–3406. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Abdo, H.S.; Latief, F.H.; Alharthi, N.H.; Abedin, S.Z. El Fabrication of Ti-Al-Cu new alloys by inductive sintering, characterization, and corrosion evaluation. J. Mater. Res. Technol. 2019, 8, 4302–4311. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A.; Lgaz, H.; Olasunkanmi, L.O.; Sherif, E.S.M.; Salghi, R.; Ebenso, E.E. Adsorption and anticorrosion behaviour of mild steel treated with 2-((1H-indol-2-yl)thio)-6-amino-4-phenylpyridine-3,5-dicarbonitriles in a hydrochloric acid solution: Experimental and computational studies. J. Mol. Liq. 2019, 283, 491–506. [Google Scholar] [CrossRef]
- Alharthi, N.; Sherif, E.S.M.; Abdo, H.S.; Alharbi, H.F.; Misiolek, W.Z. Effect of extrusion welding locations on the corrosion of AM30 alloy extrudate. J. Mater. Res. Technol. 2019, 8, 2280–2289. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Abdo, H.S.; Zein El Abedin, S. Electrochemical corrosion behavior of Fe64/Ni36 and Fe55/Ni45 alloys in 4.0% sodium chloride solutions. Int. J. Electrochem. Sci. 2017, 12, 1600–1611. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Erasmus, R.M.; Comins, J.D. In situ Raman spectroscopy and electrochemical techniques for studying corrosion and corrosion inhibition of iron in sodium chloride solutions. Electrochim. Acta 2010, 55, 3657–3663. [Google Scholar] [CrossRef]
- Latief, F.H.; Sherif, E.S.M.; Almajid, A.A.; Junaedi, H. Fabrication of exfoliated graphite nanoplatelets-reinforced aluminum composites and evaluating their mechanical properties and corrosion behavior. J. Anal. Appl. Pyrolysis 2011, 92, 485–592. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Sherif, E.S.M.; Potgieter, J.H.; Comins, J.D.; Cornish, L.; Olubambi, P.A.; Machio, C.N. Effects of minor additions of ruthenium on the passivation of duplex stainless-steel corrosion in concentrated hydrochloric acid solutions. J. Appl. Electrochem. 2009, 39, 1385–1392. [Google Scholar] [CrossRef]
- Nabawy, A.M.; Khalil, K.A.; Al-Ahmari, A.M.; Sherif, E.S.M. Melt processing and characterization of Al-SiC nanocomposite, Al, and Mg foam materials. Metals (Basel) 2016, 6, 110. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Abdo, H.S.; Almajed, A.A. Corrosion behavior of cast Iron in freely aerated stagnant arabian gulf seawater. Materials 2015, 8, 2127–2138. [Google Scholar] [CrossRef]
- Mansfeld, F.; Lin, S.; Kim, K.; Shih, H. Pitting and surface modification of SIC/Al. Corros. Sci. 1987, 27, 997. [Google Scholar] [CrossRef]
- Alharthi, N.; Sherif, E.S.M.; Abdo, H.S.; El Abedin, S.Z. Effect of Nickel Content on the Corrosion Resistance of Iron-Nickel Alloys in Concentrated Hydrochloric Acid Pickling Solutions. Adv. Mater. Sci. Eng. 2017, 6, 1–8. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).